Systemic Actions of Breast Cancer Facilitate Functional Limitations
Abstract
1. Prevalence of Functional Limitations in Breast Cancer
2. Functional Limitation is Likely Due to Skeletal Muscle Dysfunction in Breast Cancer
3. Altered Cytokines Contribute to Functional Limitations in Breast Cancer
4. Impaired Myogenesis Contributes to Functional Limitations in Breast Cancer
5. Energetic Inefficiency in Skeletal Muscle Contributes to Functional Limitations in Breast Cancer
6. Altered Extracellular Matrix Contributes to Functional Limitations in Breast Cancer
7. STAT3 Signaling Contributes to Muscle Wasting in Cancer Cachexia
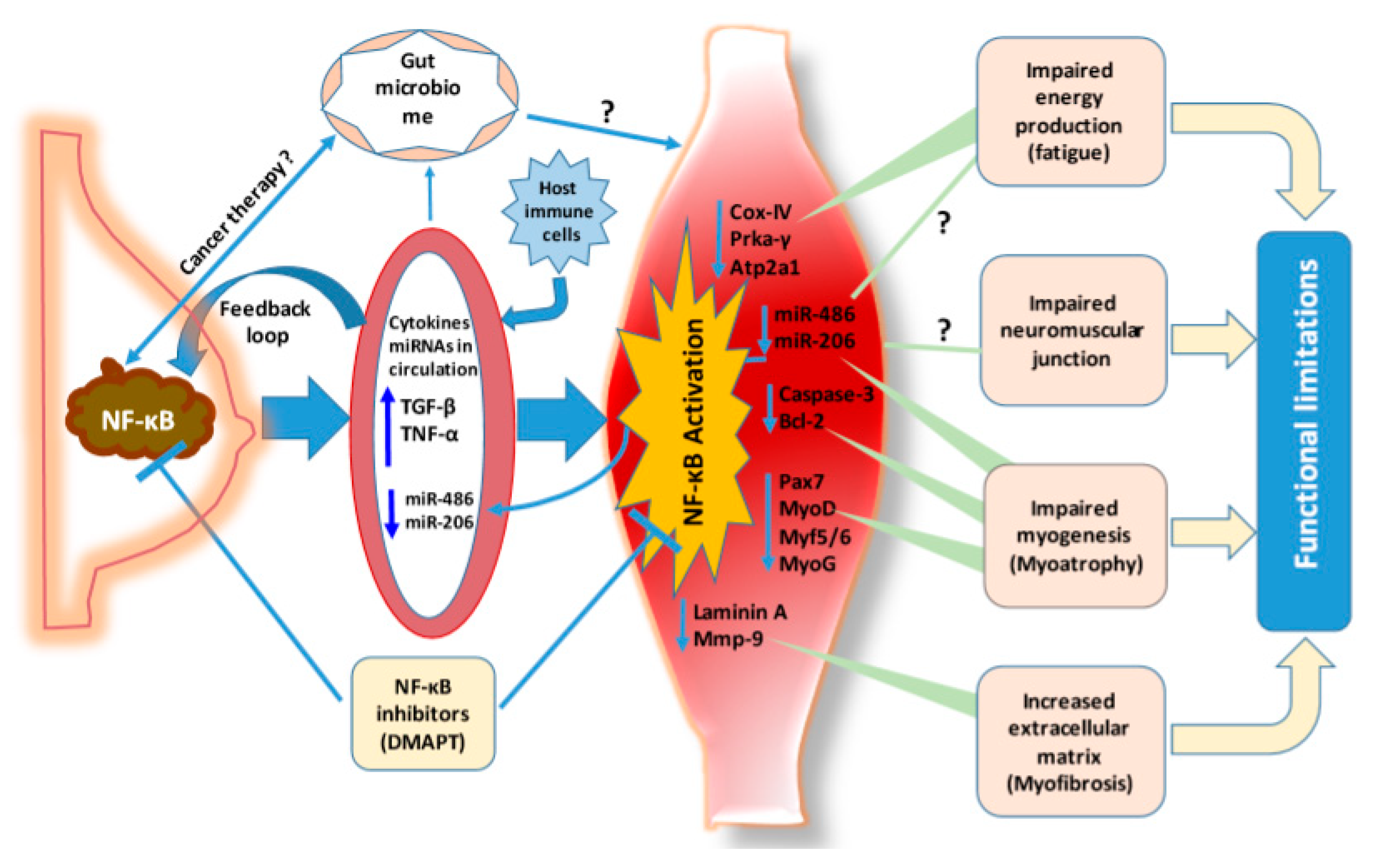
8. NF-κB is a Critical Signaling Relay Engaged in Breast Cancer Associated Functional Limitations
9. Perspective and Conclusions
Funding
Conflicts of Interest
Abbreviations
| AKT | Thymoma viral proto-oncogene, also as known protein kinase B (PKB) |
| AMPK | AMP-activated protein kinase |
| BAK | Bcl-2 homologous antagonist killer |
| BAX | Bcl-2-associated X protein |
| BCL-2 | B-cell lymphoma 2 |
| CCL1, 2, or 5 | C-C motif chemokine ligand 1, 2, or 5 |
| CCR3 | C-C chemokine receptor type 3 |
| CM | Conditioned media |
| COX-IV | Cytochrome c oxidase subunit 4 isoform 1 |
| CXCL1, 2, 5 or 10 | C-X-C motif chemokine ligand 1, 2, 5, or 10 |
| DMAPT | Dimethylaminoparthenolide |
| DMD | Duchenne muscular dystrophy |
| DOCK3 | Dedicator of cytokinesis 3 |
| ECM | Extracellular matrix |
| G-CSF | Granulocyte-colony stimulating factor |
| GM-CSF | Granulocyte-macrophage colony stimulating factor |
| GTex, | Genotype-tissue expression |
| HOXA9 | Homeobox A9 |
| IFN-α, β or γ | Interferon alpha, beta or gamma |
| IKK | Inhibitor of kappaB kinase |
| IL-1, 2, 4, 6 or 9 | Interleukin 1, 2, 4, 6 or 9 |
| IL-1RA | Interleukin 1 receptor antagonist |
| MAPK | Mitogen-activated protein kinase |
| MMPs | Matrix metalloproteinases |
| MMTV | Mouse mammary tumor virus |
| MIP2 | Macrophage inflammatory protein 2 |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| MuSC | Muscle stem cell |
| MYF5 or 6 | Myogenic factor 5 or 6 |
| MyHC | Myosin heavy chain |
| MyoD | Myoblast determination protein 1 |
| MyoG | Myogenin |
| NF-κB | Nuclear factor-kappa B |
| NIK | NF-kappa B-inducing kinase |
| NO | Nitric oxide |
| Nos2 | Nitric oxide synthase |
| Pax3 | Paired box 3 |
| Pax7 | Paired box 7 |
| PDX | Patient-derived xenograft |
| PGC-1β | Peroxisome proliferator-activated receptor gamma co-activator 1 beta |
| PTEN | Phosphatase and tensin homolog |
| PyMT | Polyoma middle tumor antigen |
| ROS | Reactive oxygen species |
| STAT3 | Signal transducer and activator of transcription 3 |
| sTNFRII | Soluble tumor necrosis factor receptor 2 |
| TAK1 | Transforming growth factor beta activated kinase 1 |
| TGF-β | Transforming growth factor beta |
| TIMP1 | TIMP metallopeptidase inhibitor 1 |
| TNF | Tumor necrosis factor |
| TNFR1 | TNF-α receptor type 1 |
| ZIP14 | ZRT/IRT-like protein 14 |
References
- Consul, N.; Guo, X.; Coker, C.; Lopez-Pintado, S.; Hibshoosh, H.; Zhao, B.; Kalinsky, K.; Acharyya, S. Monitoring metastasis and cachexia in a patient with breast cancer: A case study. Clin. Med. Insights Oncol. 2016, 10, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Tomasin, R.; Martin, A.; Cominetti, M.R. Metastasis and cachexia: Alongside in clinics, but not so in animal models. J. Cachexia Sarcopenia Muscle 2019, 10, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Hendren, E.; Vinik, O.; Faragalla, H.; Haq, R. Breast cancer and dermatomyositis: A case study and literature review. Curr. Oncol. 2017, 24, e429–e433. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.Y.; Tan, W.J.; Cheong, Y.T. Breast cancer with dermatomyositis as initial presentation. Med. J. Malays. 2018, 73, 44–45. [Google Scholar]
- Braithwaite, D.; Satariano, W.A.; Sternfeld, B.; Hiatt, R.A.; Ganz, P.A.; Kerlikowske, K.; Moore, D.H.; Slattery, M.L.; Tammemagi, M.; Castillo, A.; et al. Long-term prognostic role of functional limitations among women with breast cancer. J. Natl. Cancer Inst. 2010, 102, 1468–1477. [Google Scholar] [CrossRef]
- Baltgalvis, K.A.; Berger, F.G.; Pena, M.M.; Mark Davis, J.; White, J.P.; Carson, J.A. Activity level, apoptosis, and development of cachexia in apc(min/+) mice. J. Appl. Physiol. 2010, 109, 1155–1161. [Google Scholar] [CrossRef]
- Murphy, K.T.; Chee, A.; Trieu, J.; Naim, T.; Lynch, G.S. Importance of functional and metabolic impairments in the characterization of the c-26 murine model of cancer cachexia. Dis. Model Mech. 2012, 5, 533–545. [Google Scholar] [CrossRef]
- Puppa, M.J.; Gao, S.; Narsale, A.A.; Carson, J.A. Skeletal muscle glycoprotein 130’s role in lewis lung carcinoma-induced cachexia. FASEB J. 2014, 28, 998–1009. [Google Scholar] [CrossRef]
- Roberts, B.M.; Frye, G.S.; Ahn, B.; Ferreira, L.F.; Judge, A.R. Cancer cachexia decreases specific force and accelerates fatigue in limb muscle. Biochem. Biophys. Res. Commun. 2013, 435, 488–492. [Google Scholar] [CrossRef]
- Toth, M.J.; Callahan, D.M.; Miller, M.S.; Tourville, T.W.; Hackett, S.B.; Couch, M.E.; Dittus, K. Skeletal muscle fiber size and fiber type distribution in human cancer: Effects of weight loss and relationship to physical function. Clin. Nutr. 2016, 35, 1359–1365. [Google Scholar] [CrossRef]
- Sweeney, C.; Schmitz, K.H.; Lazovich, D.; Virnig, B.A.; Wallace, R.B.; Folsom, A.R. Functional limitations in elderly female cancer survivors. J. Natl. Cancer Inst. 2006, 98, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Jiang, L.; Stefanick, M.L.; Johnson, K.C.; Lane, D.S.; LeBlanc, E.S.; Prentice, R.; Rohan, T.E.; Snively, B.M.; Vitolins, M.; et al. Duration of adulthood overweight, obesity, and cancer risk in the women’s health initiative: A longitudinal study from the united states. PLoS Med. 2016, 13, e1002081. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Bhat-Nakshatri, P.; Padua, M.B.; Prasad, M.S.; Anjanappa, M.; Jacobson, M.; Finnearty, C.; Sefcsik, V.; McElyea, K.; Redmond, R.; et al. Pharmacological dual inhibition of tumor and tumor-induced functional limitations in transgenic model of breast cancer. Mol. Cancer Ther. 2017, 16, 2747–2758. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Small, E.M.; O’Rourke, J.R.; Moresi, V.; Sutherland, L.B.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. Regulation of pi3-kinase/akt signaling by muscle-enriched microrna-486. Proc. Natl. Acad. Sci. USA 2010, 107, 4218–4223. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Rowland, L.A.; Bal, N.C.; Periasamy, M. The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol. Rev. Camb. Philos. Soc. 2015, 90, 1279–1297. [Google Scholar] [CrossRef]
- Al-Majid, S.; McCarthy, D.O. Cancer-induced fatigue and skeletal muscle wasting: The role of exercise. Biol. Res. Nurs. 2001, 2, 186–197. [Google Scholar] [CrossRef]
- Barreiro, E.; Gea, J. Respiratory and limb muscle dysfunction in copd. COPD 2015, 12, 413–426. [Google Scholar] [CrossRef]
- Siegel, I.M. Update on duchenne muscular dystrophy. Compr. Ther. 1989, 15, 45–52. [Google Scholar]
- Winningham, M.L.; Nail, L.M.; Burke, M.B.; Brophy, L.; Cimprich, B.; Jones, L.S.; Pickard-Holley, S.; Rhodes, V.; St Pierre, B.; Beck, S.; et al. Fatigue and the cancer experience: The state of the knowledge. Oncol. Nurs. Forum 1994, 21, 23–36. [Google Scholar] [PubMed]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Guigni, B.A.; Callahan, D.M.; Tourville, T.W.; Miller, M.S.; Fiske, B.; Voigt, T.; Korwin-Mihavics, B.; Anathy, V.; Dittus, K.; Toth, M.J. Skeletal muscle atrophy and dysfunction in breast cancer patients: Role for chemotherapy-derived oxidant stress. Am. J. Physiol. Cell Physiol. 2018, 315, C744–C756. [Google Scholar] [CrossRef] [PubMed]
- Caan, B.J.; Cespedes Feliciano, E.M.; Prado, C.M.; Alexeeff, S.; Kroenke, C.H.; Bradshaw, P.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Olobatuyi, T.A.; et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018, 4, 798–804. [Google Scholar] [CrossRef]
- Shiroyama, T.; Nagatomo, I.; Koyama, S.; Hirata, H.; Nishida, S.; Miyake, K.; Fukushima, K.; Shirai, Y.; Mitsui, Y.; Takata, S.; et al. Impact of sarcopenia in patients with advanced non-small cell lung cancer treated with pd-1 inhibitors: A preliminary retrospective study. Sci. Rep. 2019, 9, 2447. [Google Scholar] [CrossRef]
- Cespedes Feliciano, E.; Chen, W.Y. Clinical implications of low skeletal muscle mass in early-stage breast and colorectal cancer. Proc. Nutr. Soc. 2018, 77, 382–387. [Google Scholar] [CrossRef]
- Waning, D.L.; Mohammad, K.S.; Reiken, S.; Xie, W.; Andersson, D.C.; John, S.; Chiechi, A.; Wright, L.E.; Umanskaya, A.; Niewolna, M.; et al. Excess tgf-beta mediates muscle weakness associated with bone metastases in mice. Nat. Med. 2015, 21, 1262–1271. [Google Scholar] [CrossRef]
- Zhang, Y.; Pilon, G.; Marette, A.; Baracos, V.E. Cytokines and endotoxin induce cytokine receptors in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E196–E205. [Google Scholar] [CrossRef]
- Peake, J.M.; Della Gatta, P.; Suzuki, K.; Nieman, D.C. Cytokine expression and secretion by skeletal muscle cells: Regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015, 21, 8–25. [Google Scholar]
- Argiles, J.M.; Busquets, S.; Lopez-Soriano, F.J. Cytokines in the pathogenesis of cancer cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 401–406. [Google Scholar] [CrossRef]
- Fearon, K.; Arends, J.; Baracos, V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 2013, 10, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, M.J. Cancer cachexia. Curr. Opin. Gastroenterol. 2010, 26, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Hogan, K.A.; Cho, D.S.; Arneson, P.C.; Samani, A.; Palines, P.; Yang, Y.; Doles, J.D. Tumor-derived cytokines impair myogenesis and alter the skeletal muscle immune microenvironment. Cytokine 2018, 107, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Ganz, P.A.; Aziz, N.; Fahey, J.L. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom. Med. 2002, 64, 604–611. [Google Scholar] [CrossRef]
- Arend, W.P.; Malyak, M.; Guthridge, C.J.; Gabay, C. Interleukin-1 receptor antagonist: Role in biology. Annu. Rev. Immunol. 1998, 16, 27–55. [Google Scholar] [CrossRef]
- Diez-Ruiz, A.; Tilz, G.P.; Zangerle, R.; Baier-Bitterlich, G.; Wachter, H.; Fuchs, D. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur. J. Haematol. 1995, 54, 1–8. [Google Scholar] [CrossRef]
- Fuchs, D.; Hausen, A.; Reibnegger, G.; Werner, E.R.; Dierich, M.P.; Wachter, H. Neopterin as a marker for activated cell-mediated immunity: Application in hiv infection. Immunol. Today 1988, 9, 150–155. [Google Scholar] [CrossRef]
- Chen, D.; Goswami, C.P.; Burnett, R.M.; Anjanappa, M.; Bhat-Nakshatri, P.; Muller, W.; Nakshatri, H. Cancer affects microrna expression, release, and function in cardiac and skeletal muscle. Cancer Res. 2014, 74, 4270–4281. [Google Scholar] [CrossRef]
- Hara, M.; Yuasa, S.; Shimoji, K.; Onizuka, T.; Hayashiji, N.; Ohno, Y.; Arai, T.; Hattori, F.; Kaneda, R.; Kimura, K.; et al. G-csf influences mouse skeletal muscle development and regeneration by stimulating myoblast proliferation. J. Exp. Med. 2011, 208, 715–727. [Google Scholar] [CrossRef]
- Ismaeel, A.; Kim, J.S.; Kirk, J.S.; Smith, R.S.; Bohannon, W.T.; Koutakis, P. Role of transforming growth factor-beta in skeletal muscle fibrosis: A review. Int. J. Mol. Sci. 2019, 20, 2446. [Google Scholar] [CrossRef]
- Reid, M.B.; Li, Y.P. Tumor necrosis factor-alpha and muscle wasting: A cellular perspective. Respir. Res. 2001, 2, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Mendias, C.L.; Gumucio, J.P.; Davis, M.E.; Bromley, C.W.; Davis, C.S.; Brooks, S.V. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve 2012, 45, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.N.; Trivedi, T.; Guise, T.A.; Waning, D.L. The role of tgfbeta in bone-muscle crosstalk. Curr. Osteoporos Rep. 2017, 15, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Biswas, A.K.; Ma, W.; Kandpal, M.; Coker, C.; Grandgenett, P.M.; Hollingsworth, M.A.; Jain, R.; Tanji, K.; Lomicronpez-Pintado, S.; et al. Metastatic cancers promote cachexia through zip14 upregulation in skeletal muscle. Nat. Med. 2018, 24, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Feige, P.; Brun, C.E.; Ritso, M.; Rudnicki, M.A. Orienting muscle stem cells for regeneration in homeostasis, aging, and disease. Cell Stem Cell 2018, 23, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Rayagiri, S.S.; Ranaldi, D.; Raven, A.; Mohamad Azhar, N.I.F.; Lefebvre, O.; Zammit, P.S.; Borycki, A.G. Basal lamina remodeling at the skeletal muscle stem cell niche mediates stem cell self-renewal. Nat. Commun. 2018, 9, 1075. [Google Scholar] [CrossRef]
- Dumont, N.A.; Wang, Y.X.; Rudnicki, M.A. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 2015, 142, 1572–1581. [Google Scholar] [CrossRef]
- Dueweke, J.J.; Awan, T.M.; Mendias, C.L. Regeneration of skeletal muscle after eccentric injury. J. Sport Rehabil. 2017, 26, 171–179. [Google Scholar] [CrossRef]
- Zammit, P.S.; Relaix, F.; Nagata, Y.; Ruiz, A.P.; Collins, C.A.; Partridge, T.A.; Beauchamp, J.R. Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Sci. 2006, 119, 1824–1832. [Google Scholar] [CrossRef]
- He, W.A.; Berardi, E.; Cardillo, V.M.; Acharyya, S.; Aulino, P.; Thomas-Ahner, J.; Wang, J.; Bloomston, M.; Muscarella, P.; Nau, P.; et al. Nf-kappab-mediated pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J. Clin. Investig. 2013, 123, 4821–4835. [Google Scholar] [CrossRef]
- Von Maltzahn, J.; Jones, A.E.; Parks, R.J.; Rudnicki, M.A. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc. Natl. Acad. Sci. USA 2013, 110, 16474–16479. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Sabourin, L.A.; Girgis-Gabardo, A.; Mansouri, A.; Gruss, P.; Rudnicki, M.A. Pax7 is required for the specification of myogenic satellite cells. Cell 2000, 102, 777–786. [Google Scholar] [CrossRef]
- Bajard, L.; Relaix, F.; Lagha, M.; Rocancourt, D.; Daubas, P.; Buckingham, M.E. A novel genetic hierarchy functions during hypaxial myogenesis: Pax3 directly activates myf5 in muscle progenitor cells in the limb. Genes Dev. 2006, 20, 2450–2464. [Google Scholar] [CrossRef] [PubMed]
- McKinnell, I.W.; Ishibashi, J.; Le Grand, F.; Punch, V.G.; Addicks, G.C.; Greenblatt, J.F.; Dilworth, F.J.; Rudnicki, M.A. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat. Cell. Biol. 2008, 10, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S. Function of the myogenic regulatory factors myf5, myod, myogenin and mrf4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Palacios, D.; Mozzetta, C.; Consalvi, S.; Caretti, G.; Saccone, V.; Proserpio, V.; Marquez, V.E.; Valente, S.; Mai, A.; Forcales, S.V.; et al. Tnf/p38alpha/polycomb signaling to pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell 2010, 7, 455–469. [Google Scholar] [CrossRef]
- Rigamonti, E.; Touvier, T.; Clementi, E.; Manfredi, A.A.; Brunelli, S.; Rovere-Querini, P. Requirement of inducible nitric oxide synthase for skeletal muscle regeneration after acute damage. J. Immunol. 2013, 190, 1767–1777. [Google Scholar] [CrossRef]
- De Palma, C.; Clementi, E. Nitric oxide in myogenesis and therapeutic muscle repair. Mol. Neurobiol. 2012, 46, 682–692. [Google Scholar] [CrossRef]
- Filippin, L.I.; Moreira, A.J.; Marroni, N.P.; Xavier, R.M. Nitric oxide and repair of skeletal muscle injury. Nitric Oxide 2009, 21, 157–163. [Google Scholar] [CrossRef]
- Stamler, J.S.; Meissner, G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 2001, 81, 209–237. [Google Scholar] [CrossRef]
- Nisoli, E.; Clementi, E.; Carruba, M.O.; Moncada, S. Defective mitochondrial biogenesis: A hallmark of the high cardiovascular risk in the metabolic syndrome? Circ. Res. 2007, 100, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Buono, R.; Vantaggiato, C.; Pisa, V.; Azzoni, E.; Bassi, M.T.; Brunelli, S.; Sciorati, C.; Clementi, E. Nitric oxide sustains long-term skeletal muscle regeneration by regulating fate of satellite cells via signaling pathways requiring vangl2 and cyclic gmp. Stem Cells 2012, 30, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Janicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y. Role of bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Edlich, F. Bcl-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; El Meslemani, A.H.; Sandri, C.; Schjerling, P.; Vissing, K.; Andersen, J.L.; Rossini, K.; Carraro, U.; Angelini, C. Caspase 3 expression correlates with skeletal muscle apoptosis in duchenne and facioscapulo human muscular dystrophy. A potential target for pharmacological treatment? J. Neuropathol. Exp. Neurol. 2001, 60, 302–312. [Google Scholar] [CrossRef]
- Fernando, P.; Kelly, J.F.; Balazsi, K.; Slack, R.S.; Megeney, L.A. Caspase 3 activity is required for skeletal muscle differentiation. Proc. Natl. Acad. Sci. USA 2002, 99, 11025–11030. [Google Scholar] [CrossRef]
- Arnon-Friedman, R.; Dupuis, F.; Fawzi, O.; Renner, R.; Vidick, T. Practical device-independent quantum cryptography via entropy accumulation. Nat. Commun. 2018, 9, 459. [Google Scholar] [CrossRef]
- Dominov, J.A.; Dunn, J.J.; Miller, J.B. Bcl-2 expression identifies an early stage of myogenesis and promotes clonal expansion of muscle cells. J. Cell Biol. 1998, 142, 537–544. [Google Scholar] [CrossRef]
- Sohi, G.; Dilworth, F.J. Noncoding rnas as epigenetic mediators of skeletal muscle regeneration. FEBS J. 2015, 282, 1630–1646. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Negri, I.; Oliveira, W.; Brown, C.; Asiimwe, P.; Sammons, B.; Horak, M.; Jiang, C.; Carson, D. Transportable data from non-target arthropod field studies for the environmental risk assessment of genetically modified maize expressing an insecticidal double-stranded rna. Transgenic Res. 2016, 25, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Simionescu-Bankston, A.; Kumar, A. Noncoding rnas in the regulation of skeletal muscle biology in health and disease. J. Mol. Med. 2016, 94, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, R.; Inoue, K. Developmental regulation and evolution of muscle-specific micrornas. Semin. Cell Dev. Biol. 2015, 47–48, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Black, B.L.; Derynck, R. Tgf-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by smad3. Genes Dev. 2001, 15, 2950–2966. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microrna biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Catalanotto, C.; Cogoni, C.; Zardo, G. Microrna in control of gene expression: An overview of nuclear functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef]
- Cai, L.; Chang, H.; Fang, Y.; Li, G. A comprehensive characterization of the function of lincrnas in transcriptional regulation through long-range chromatin interactions. Sci. Rep. 2016, 6, 36572. [Google Scholar] [CrossRef]
- Goncalves, T.J.M.; Armand, A.S. Non-coding rnas in skeletal muscle regeneration. Noncoding RNA Res. 2017, 2, 56–67. [Google Scholar] [CrossRef]
- D’Souza, R.F.; Bjornsen, T.; Zeng, N.; Aasen, K.M.M.; Raastad, T.; Cameron-Smith, D.; Mitchell, C.J. Micrornas in muscle: Characterizing the powerlifter phenotype. Front. Physiol. 2017, 8, 383. [Google Scholar] [CrossRef]
- Li, L.; Sarver, A.L.; Alamgir, S.; Subramanian, S. Downregulation of micrornas mir-1, -206 and -29 stabilizes pax3 and ccnd2 expression in rhabdomyosarcoma. Lab. Investig. 2012, 92, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, A.; Ghosh, S.; Stretch, C.; Greiner, R.; Bathe, O.F.; Baracos, V.; Damaraju, S. Small rnaome profiling from human skeletal muscle: Novel mirnas and their targets associated with cancer cachexia. J. Cachexia Sarcopenia Muscle 2017, 8, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, C.; Huang, B.; Li, H.; Zhang, R.; Huang, Y.; Wang, J. Downregulation of microrna-206 is a potent prognostic marker for patients with gastric cancer. Eur. J. Gastroenterol. Hepatol. 2013, 25, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Brown, J.L.; Rosa-Caldwell, M.E.; Blackwell, T.A.; Perry, R.A., Jr.; Brown, L.A.; Khatri, B.; Seo, D.; Bottje, W.G.; Washington, T.A.; et al. Cancer cachexia-induced muscle atrophy: Evidence for alterations in micrornas important for muscle size. Physiol. Genom. 2017, 49, 253–260. [Google Scholar] [CrossRef]
- Buckingham, M.; Rigby, P.W. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 2014, 28, 225–238. [Google Scholar] [CrossRef]
- Song, L.; Lin, C.; Gong, H.; Wang, C.; Liu, L.; Wu, J.; Tao, S.; Hu, B.; Cheng, S.Y.; Li, M.; et al. Mir-486 sustains nf-kappab activity by disrupting multiple nf-kappab-negative feedback loops. Cell Res. 2013, 23, 274–289. [Google Scholar] [CrossRef]
- Hitachi, K.; Nakatani, M.; Tsuchida, K. Myostatin signaling regulates akt activity via the regulation of mir-486 expression. Int. J. Biochem. Cell Biol. 2014, 47, 93–103. [Google Scholar] [CrossRef]
- Alexander, M.S.; Casar, J.C.; Motohashi, N.; Vieira, N.M.; Eisenberg, I.; Marshall, J.L.; Gasperini, M.J.; Lek, A.; Myers, J.A.; Estrella, E.A.; et al. Microrna-486-dependent modulation of dock3/pten/akt signaling pathways improves muscular dystrophy-associated symptoms. J. Clin. Investig. 2014, 124, 2651–2667. [Google Scholar] [CrossRef]
- Ma, Y.; Ren, Y.; Dai, Z.J.; Wu, C.J.; Ji, Y.H.; Xu, J. Il-6, il-8 and tnf-alpha levels correlate with disease stage in breast cancer patients. Adv. Clin. Exp. Med. 2017, 26, 421–426. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, Y.S.; Sivaprasad, U.; Malhotra, A.; Dutta, A. Muscle-specific microrna mir-206 promotes muscle differentiation. J. Cell Biol. 2006, 174, 677–687. [Google Scholar] [CrossRef]
- Adams, B.D.; Cowee, D.M.; White, B.A. The role of mir-206 in the epidermal growth factor (egf) induced repression of estrogen receptor-alpha (eralpha) signaling and a luminal phenotype in mcf-7 breast cancer cells. Mol. Endocrinol. 2009, 23, 1215–1230. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Tao, Y.; Li, J.; Deng, Z.; Yan, Z.; Xiao, X.; Wang, D.Z. Microrna-1 and microrna-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing pax7. J. Cell Biol. 2010, 190, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Verma, M.; Watanabe, S.; Tastad, C.; Asakura, Y.; Asakura, A. Myod regulates apoptosis of myoblasts through microrna-mediated down-regulation of pax3. J. Cell Biol. 2010, 191, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.I.; Georges, S.A.; Asawachaicharn, A.; Analau, E.; Tapscott, S.J. Myod inhibits fstl1 and utrn expression by inducing transcription of mir-206. J. Cell Biol. 2006, 175, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Winbanks, C.E.; Wang, B.; Beyer, C.; Koh, P.; White, L.; Kantharidis, P.; Gregorevic, P. Tgf-beta regulates mir-206 and mir-29 to control myogenic differentiation through regulation of hdac4. J. Biol. Chem. 2011, 286, 13805–13814. [Google Scholar] [CrossRef] [PubMed]
- Rachagani, S.; Cheng, Y.; Reecy, J.M. Myostatin genotype regulates muscle-specific mirna expression in mouse pectoralis muscle. BMC Res. Notes 2010, 3, 297. [Google Scholar] [CrossRef]
- Nakasa, T.; Ishikawa, M.; Shi, M.; Shibuya, H.; Adachi, N.; Ochi, M. Acceleration of muscle regeneration by local injection of muscle-specific micrornas in rat skeletal muscle injury model. J. Cell Mol. Med. 2010, 14, 2495–2505. [Google Scholar] [CrossRef]
- Samaeekia, R.; Adorno-Cruz, V.; Bockhorn, J.; Chang, Y.F.; Huang, S.; Prat, A.; Ha, N.; Kibria, G.; Huo, D.; Zheng, H.; et al. Mir-206 inhibits stemness and metastasis of breast cancer by targeting mkl1/il11 pathway. Clin. Cancer Res. 2017, 23, 1091–1103. [Google Scholar] [CrossRef]
- Heinemann, F.G.; Tolkach, Y.; Deng, M.; Schmidt, D.; Perner, S.; Kristiansen, G.; Muller, S.C.; Ellinger, J. Serum mir-122-5p and mir-206 expression: Non-invasive prognostic biomarkers for renal cell carcinoma. Clin. Epigenet. 2018, 10, 11. [Google Scholar] [CrossRef]
- Tian, R.; Liu, T.; Qiao, L.; Gao, M.; Li, J. Decreased serum microrna-206 level predicts unfavorable prognosis in patients with melanoma. Int. J. Clin. Exp. Pathol. 2015, 8, 3097–3103. [Google Scholar]
- Zhang, C.; Yao, C.; Li, H.; Wang, G.; He, X. Serum levels of microrna-133b and microrna-206 expression predict prognosis in patients with osteosarcoma. Int. J. Clin. Exp. Pathol. 2014, 7, 4194–4203. [Google Scholar] [PubMed]
- Argiles, J.M.; Fontes-Oliveira, C.C.; Toledo, M.; Lopez-Soriano, F.J.; Busquets, S. Cachexia: A problem of energetic inefficiency. J. Cachexia Sarcopenia Muscle 2014, 5, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Penna, F.; Ballaro, R.; Beltra, M.; De Lucia, S.; Costelli, P. Modulating metabolism to improve cancer-induced muscle wasting. Oxid. Med. Cell Longev. 2018, 2018, 7153610. [Google Scholar] [CrossRef] [PubMed]
- Hardee, J.P.; Montalvo, R.N.; Carson, J.A. Linking cancer cachexia-induced anabolic resistance to skeletal muscle oxidative metabolism. Oxid. Med. Cell Longev. 2017, 2017, 8018197. [Google Scholar] [CrossRef] [PubMed]
- Harfmann, B.D.; Schroder, E.A.; Kachman, M.T.; Hodge, B.A.; Zhang, X.; Esser, K.A. Muscle-specific loss of bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet. Muscle 2016, 6, 12. [Google Scholar] [CrossRef]
- Leskawa, K.C.; Erwin, R.E.; Buse, P.E.; Hogan, E.L. Glycosphingolipid biosynthesis during myogenesis of rat l6 cells in vitro. Mol. Cell Biochem. 1988, 83, 47–54. [Google Scholar] [CrossRef]
- Anastasia, L.; Papini, N.; Colazzo, F.; Palazzolo, G.; Tringali, C.; Dileo, L.; Piccoli, M.; Conforti, E.; Sitzia, C.; Monti, E.; et al. Neu3 sialidase strictly modulates gm3 levels in skeletal myoblasts c2c12 thus favoring their differentiation and protecting them from apoptosis. J. Biol. Chem. 2008, 283, 36265–36271. [Google Scholar] [CrossRef]
- Gehlert, S.; Bloch, W.; Suhr, F. Ca2+-dependent regulations and signaling in skeletal muscle: From electro-mechanical coupling to adaptation. Int. J. Mol. Sci. 2015, 16, 1066–1095. [Google Scholar] [CrossRef]
- Barrey, E.; Saint-Auret, G.; Bonnamy, B.; Damas, D.; Boyer, O.; Gidrol, X. Pre-microrna and mature microrna in human mitochondria. PLoS ONE 2011, 6, e20220. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, W.; Yan, W.; Li, X.; Wang, X.; Zhao, Z.; Hui, J.; Shen, Z.; Yang, J. Microrna-206 is involved in survival of hypoxia preconditioned mesenchymal stem cells through targeting pim-1 kinase. Stem Cell Res. Ther. 2016, 7, 61. [Google Scholar] [CrossRef]
- Sun, Y.; Su, Q.; Li, L.; Wang, X.; Lu, Y.; Liang, J. Mir-486 regulates cardiomyocyte apoptosis by p53-mediated bcl-2 associated mitochondrial apoptotic pathway. BMC Cardiovasc. Disord. 2017, 17, 119. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Qin, Y.; Chen, X.; Wang, Q.; Wang, J. Mir-206 inhibits proliferation, migration, and invasion of gastric cancer cells by targeting the muc1 gene. Onco Targets Ther. 2019, 12, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Luo, W.; Bai, M.; Li, J.; Bai, X.; Guo, J.; Wu, J.; Wang, M. Microrna-206 inhibited the progression of glioblastoma through bcl-2. J. Mol. Neurosci. 2016, 60, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Deries, M.; Cachaco, A.S.; Bajanca, F. The extracellular matrix dimension of skeletal muscle development. Dev. Biol. 2011, 354, 191–207. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef]
- Desiderio, M.A. Hepatocyte growth factor in invasive growth of carcinomas. Cell Mol. Life Sci. 2007, 64, 1341–1354. [Google Scholar] [CrossRef]
- Shiba, N.; Miyazaki, D.; Yoshizawa, T.; Fukushima, K.; Shiba, Y.; Inaba, Y.; Imamura, M.; Takeda, S.; Koike, K.; Nakamura, A. Differential roles of mmp-9 in early and late stages of dystrophic muscles in a mouse model of duchenne muscular dystrophy. Biochim. Biophys. Acta 2015, 1852, 2170–2182. [Google Scholar] [CrossRef]
- Dahiya, S.; Bhatnagar, S.; Hindi, S.M.; Jiang, C.; Paul, P.K.; Kuang, S.; Kumar, A. Elevated levels of active matrix metalloproteinase-9 cause hypertrophy in skeletal muscle of normal and dystrophin-deficient mdx mice. Hum. Mol. Genet. 2011, 20, 4345–4359. [Google Scholar] [CrossRef]
- Mehan, R.S.; Greybeck, B.J.; Emmons, K.; Byrnes, W.C.; Allen, D.L. Matrix metalloproteinase-9 deficiency results in decreased fiber cross-sectional area and alters fiber type distribution in mouse hindlimb skeletal muscle. Cells Tissues Organs 2011, 194, 510–520. [Google Scholar] [CrossRef]
- Winer, A.; Adams, S.; Mignatti, P. Matrix metalloproteinase inhibitors in cancer therapy: Turning past failures into future successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Newby, A.C. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc. Res. 2006, 69, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Phanish, M.K.; Winn, S.K.; Dockrell, M.E. Connective tissue growth factor-(ctgf, ccn2)—A marker, mediator and therapeutic target for renal fibrosis. Nephron Exp. Nephrol. 2010, 114, e83–e92. [Google Scholar] [CrossRef] [PubMed]
- Leask, A.; Parapuram, S.K.; Shi-Wen, X.; Abraham, D.J. Connective tissue growth factor (ctgf, ccn2) gene regulation: A potent clinical bio-marker of fibroproliferative disease? J. Cell Commun. Signal. 2009, 3, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Rameshwar, P.; Narayanan, R.; Qian, J.; Denny, T.N.; Colon, C.; Gascon, P. Nf-kappa b as a central mediator in the induction of tgf-beta in monocytes from patients with idiopathic myelofibrosis: An inflammatory response beyond the realm of homeostasis. J. Immunol. 2000, 165, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Grande, J.P.; Melder, D.C.; Zinsmeister, A.R. Modulation of collagen gene expression by cytokines: Stimulatory effect of transforming growth factor-beta1, with divergent effects of epidermal growth factor and tumor necrosis factor-alpha on collagen type i and collagen type iv. J. Lab. Clin. Med. 1997, 130, 476–486. [Google Scholar] [CrossRef]
- Ignotz, R.A.; Massague, J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. 1986, 261, 4337–4345. [Google Scholar]
- Micallef, L.; Vedrenne, N.; Billet, F.; Coulomb, B.; Darby, I.A.; Desmouliere, A. The myofibroblast, multiple origins for major roles in normal and pathological tissue repair. Fibrogenes. Tissue Repair. 2012, 5, S5. [Google Scholar] [CrossRef]
- Massague, J. Tgfbeta signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef]
- Leask, A.; Abraham, D.J. Tgf-beta signaling and the fibrotic response. FASEB J. 2004, 18, 816–827. [Google Scholar] [CrossRef]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates tgf-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar] [PubMed]
- Imai, K.; Hiramatsu, A.; Fukushima, D.; Pierschbacher, M.D.; Okada, Y. Degradation of decorin by matrix metalloproteinases: Identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release. Biochem. J. 1997, 322, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Zimmers, T.A.; Fishel, M.L.; Bonetto, A. Stat3 in the systemic inflammation of cancer cachexia. Semin. Cell Dev. Biol. 2016, 54, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Guadagnin, E.; Mazala, D.; Chen, Y.W. Stat3 in skeletal muscle function and disorders. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, A.; Aydogdu, T.; Kunzevitzky, N.; Guttridge, D.C.; Khuri, S.; Koniaris, L.G.; Zimmers, T.A. Stat3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS ONE 2011, 6, e22538. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, A.; Aydogdu, T.; Jin, X.; Zhang, Z.; Zhan, R.; Puzis, L.; Koniaris, L.G.; Zimmers, T.A. Jak/stat3 pathway inhibition blocks skeletal muscle wasting downstream of il-6 and in experimental cancer cachexia. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E410–E421. [Google Scholar] [CrossRef]
- Ma, J.F.; Sanchez, B.J.; Hall, D.T.; Tremblay, A.K.; Di Marco, S.; Gallouzi, I.E. Stat3 promotes ifngamma/tnfalpha-induced muscle wasting in an nf-kappab-dependent and il-6-independent manner. EMBO Mol. Med. 2017, 9, 622–637. [Google Scholar] [CrossRef]
- Price, F.D.; von Maltzahn, J.; Bentzinger, C.F.; Dumont, N.A.; Yin, H.; Chang, N.C.; Wilson, D.H.; Frenette, J.; Rudnicki, M.A. Inhibition of jak-stat signaling stimulates adult satellite cell function. Nat. Med. 2014, 20, 1174–1181. [Google Scholar] [CrossRef]
- Tierney, M.T.; Aydogdu, T.; Sala, D.; Malecova, B.; Gatto, S.; Puri, P.L.; Latella, L.; Sacco, A. Stat3 signaling controls satellite cell expansion and skeletal muscle repair. Nat. Med. 2014, 20, 1182–1186. [Google Scholar] [CrossRef]
- Sala, D.; Cunningham, T.J.; Stec, M.J.; Etxaniz, U.; Nicoletti, C.; Dall’Agnese, A.; Puri, P.L.; Duester, G.; Latella, L.; Sacco, A. The stat3-fam3a axis promotes muscle stem cell myogenic lineage progression by inducing mitochondrial respiration. Nat. Commun. 2019, 10, 1796. [Google Scholar] [CrossRef]
- Qaed, E.; Wang, J.; Almoiliqy, M.; Song, Y.; Liu, W.; Chu, P.; Alademi, S.; Alademi, M.; Li, H.; Alshwmi, M.; et al. Phosphocreatine improves cardiac dysfunction by normalizing mitochondrial respiratory function through jak2/stat3 signaling pathway in vivo and in vitro. Oxid. Med. Cell Longev. 2019, 2019, 6521218. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Ungefug, E.; Heusch, G.; Schulz, R. The stat3 inhibitor stattic impairs cardiomyocyte mitochondrial function through increased reactive oxygen species formation. Curr. Pharm. Des. 2013, 19, 6890–6895. [Google Scholar] [CrossRef] [PubMed]
- Le Moal, E.; Pialoux, V.; Juban, G.; Groussard, C.; Zouhal, H.; Chazaud, B.; Mounier, R. Redox control of skeletal muscle regeneration. Antioxid. Redox. Signal 2017, 27, 276–310. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar] [PubMed]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef]
- Gianotti, T.F.; Castano, G.; Gemma, C.; Burgueno, A.L.; Rosselli, M.S.; Pirola, C.J.; Sookoian, S. Mitochondrial DNA copy number is modulated by genetic variation in the signal transducer and activator of transcription 3 (stat3). Metabolism 2011, 60, 1142–1149. [Google Scholar] [CrossRef]
- Vassilev, A.O.; Lorenz, D.R.; Tibbles, H.E.; Uckun, F.M. Role of the leukemia-associated transcription factor stat3 in platelet physiology. Leuk. Lymphoma 2002, 43, 1461–1467. [Google Scholar] [CrossRef]
- Phillips, D.; Reilley, M.J.; Aponte, A.M.; Wang, G.; Boja, E.; Gucek, M.; Balaban, R.S. Stoichiometry of stat3 and mitochondrial proteins: Implications for the regulation of oxidative phosphorylation by protein-protein interactions. J. Biol. Chem. 2010, 285, 23532–23536. [Google Scholar] [CrossRef]
- Wegrzyn, J.; Potla, R.; Chwae, Y.J.; Sepuri, N.B.; Zhang, Q.; Koeck, T.; Derecka, M.; Szczepanek, K.; Szelag, M.; Gornicka, A.; et al. Function of mitochondrial stat3 in cellular respiration. Science 2009, 323, 793–797. [Google Scholar] [CrossRef]
- Gough, D.J.; Corlett, A.; Schlessinger, K.; Wegrzyn, J.; Larner, A.C.; Levy, D.E. Mitochondrial stat3 supports ras-dependent oncogenic transformation. Science 2009, 324, 1713–1716. [Google Scholar] [CrossRef]
- Boengler, K.; Hilfiker-Kleiner, D.; Heusch, G.; Schulz, R. Inhibition of permeability transition pore opening by mitochondrial stat3 and its role in myocardial ischemia/reperfusion. Basic Res. Cardiol. 2010, 105, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.E. Could stat3 provide a link between respiration and cell cycle progression? Cell Cycle 2010, 9, 4294–4296. [Google Scholar] [CrossRef] [PubMed]
- Acharyya, S.; Villalta, S.A.; Bakkar, N.; Bupha-Intr, T.; Janssen, P.M.; Carathers, M.; Li, Z.W.; Beg, A.A.; Ghosh, S.; Sahenk, Z.; et al. Interplay of ikk/nf-kappab signaling in macrophages and myofibers promotes muscle degeneration in duchenne muscular dystrophy. J. Clin. Investig. 2007, 117, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Guttridge, D.C.; Mayo, M.W.; Madrid, L.V.; Wang, C.Y.; Baldwin, A.S., Jr. Nf-kappab-induced loss of myod messenger rna: Possible role in muscle decay and cachexia [see comments]. Science 2000, 289, 2363–2366. [Google Scholar] [CrossRef]
- Cai, D.; Frantz, J.D.; Tawa, N.E., Jr.; Melendez, P.A.; Oh, B.C.; Lidov, H.G.; Hasselgren, P.O.; Frontera, W.R.; Lee, J.; Glass, D.J.; et al. Ikkbeta/nf-kappab activation causes severe muscle wasting in mice. Cell 2004, 119, 285–298. [Google Scholar] [CrossRef]
- Mourkioti, F.; Kratsios, P.; Luedde, T.; Song, Y.H.; Delafontaine, P.; Adami, R.; Parente, V.; Bottinelli, R.; Pasparakis, M.; Rosenthal, N. Targeted ablation of ikk2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J. Clin. Investig. 2006, 116, 2945–2954. [Google Scholar] [CrossRef]
- Mourkioti, F.; Rosenthal, N. Nf-kappab signaling in skeletal muscle: Prospects for intervention in muscle diseases. J. Mol. Med. 2008, 86, 747–759. [Google Scholar] [CrossRef]
- Li, H.; Malhotra, S.; Kumar, A. Nuclear factor-kappa b signaling in skeletal muscle atrophy. J. Mol. Med. 2008, 86, 1113–1126. [Google Scholar] [CrossRef]
- Dogra, C.; Changotra, H.; Mohan, S.; Kumar, A. Tumor necrosis factor-like weak inducer of apoptosis inhibits skeletal myogenesis through sustained activation of nuclear factor-kappab and degradation of myod protein. J. Biol. Chem. 2006, 281, 10327–10336. [Google Scholar] [CrossRef]
- Kong, F.M.; Anscher, M.S.; Murase, T.; Abbott, B.D.; Iglehart, J.D.; Jirtle, R.L. Elevated plasma transforming growth factor-beta 1 levels in breast cancer patients decrease after surgical removal of the tumor. Ann. Surg. 1995, 222, 155–162. [Google Scholar] [CrossRef]
- Nakano, H.; Shindo, M.; Sakon, S.; Nishinaka, S.; Mihara, M.; Yagita, H.; Okumura, K. Differential regulation of ikappab kinase alpha and beta by two upstream kinases, nf-kappab-inducing kinase and mitogen-activated protein kinase/erk kinase kinase-1. Proc. Natl. Acad. Sci. USA 1998, 95, 3537–3542. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Lee, F.S. Mitogen-activated protein kinase/erk kinase kinases 2 and 3 activate nuclear factor-kappab through ikappab kinase-alpha and ikappab kinase-beta. J. Biol. Chem. 1999, 274, 8355–8358. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya-Tsuji, J.; Kishimoto, K.; Hiyama, A.; Inoue, J.; Cao, Z.; Matsumoto, K. The kinase tak1 can activate the nik-i kappab as well as the map kinase cascade in the il-1 signalling pathway. Nature 1999, 398, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Deng, L.; Hong, M.; Akkaraju, G.R.; Inoue, J.; Chen, Z.J. Tak1 is a ubiquitin-dependent kinase of mkk and ikk. Nature 2001, 412, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Furutani, Y.; Umemoto, T.; Murakami, M.; Matsui, T.; Funaba, M. Role of endogenous tgf-beta family in myogenic differentiation of c2c12 cells. J. Cell Biochem. 2011, 112, 614–624. [Google Scholar] [CrossRef]
- Wang, H.; Hertlein, E.; Bakkar, N.; Sun, H.; Acharyya, S.; Wang, J.; Carathers, M.; Davuluri, R.; Guttridge, D.C. Nf-kappab regulation of yy1 inhibits skeletal myogenesis through transcriptional silencing of myofibrillar genes. Mol. Cell Biol. 2007, 27, 4374–4387. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Siriett, V.; Salerno, M.S.; Berry, C.; Nicholas, G.; Bower, R.; Kambadur, R.; Sharma, M. Antagonism of myostatin enhances muscle regeneration during sarcopenia. Mol. Ther. 2007, 15, 1463–1470. [Google Scholar] [CrossRef]
- Dey, B.K.; Gagan, J.; Dutta, A. Mir-206 and -486 induce myoblast differentiation by downregulating pax7. Mol. Cell Biol. 2011, 31, 203–214. [Google Scholar] [CrossRef]
- Rathnasamy, G.; Sivakumar, V.; Rangarajan, P.; Foulds, W.S.; Ling, E.A.; Kaur, C. Nf-kappab-mediated nitric oxide production and activation of caspase-3 cause retinal ganglion cell death in the hypoxic neonatal retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5878–5889. [Google Scholar] [CrossRef]
- Gil da Costa, R.M.; Aragao, S.; Moutinho, M.; Alvarado, A.; Carmo, D.; Casaca, F.; Silva, S.; Ribeiro, J.; Sousa, H.; Ferreira, R.; et al. Hpv16 induces a wasting syndrome in transgenic mice: Amelioration by dietary polyphenols via nf-kappab inhibition. Life Sci. 2017, 169, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tang, H.; Kou, Y.; Li, R.; Zheng, Y.; Wang, Q.; Zhou, X.; Jin, L. Mg132-mediated inhibition of the ubiquitin-proteasome pathway ameliorates cancer cachexia. J. Cancer Res. Clin. Oncol. 2013, 139, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Moore-Carrasco, R.; Busquets, S.; Almendro, V.; Palanki, M.; Lopez-Soriano, F.J.; Argiles, J.M. The ap-1/nf-kappab double inhibitor sp100030 can revert muscle wasting during experimental cancer cachexia. Int. J. Oncol. 2007, 30, 1239–1245. [Google Scholar] [PubMed]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.R.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef]
- Sheng, J.Y.; Sharma, D.; Jerome, G.; Santa-Maria, C.A. Obese breast cancer patients and survivors: Management considerations. Oncology 2018, 32, 410–417. [Google Scholar]
- Vegiopoulos, A.; Rohm, M.; Herzig, S. Adipose tissue: Between the extremes. EMBO J. 2017, 36, 1999–2017. [Google Scholar] [CrossRef]
- Baraldo, M.; Geremia, A.; Pirazzini, M.; Nogara, L.; Solagna, F.; Turk, C.; Nolte, H.; Romanello, V.; Megighian, A.; Boncompagni, S.; et al. Skeletal muscle mtorc1 regulates neuromuscular junction stability. J. Cachexia Sarcopenia Muscle 2019. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Ito, A.; Ikeda, K.; Kawabe, Y.; Kamihira, M. Fabricating muscle-neuron constructs with improved contractile force generation. Tissue Eng. Part A 2019, 25, 563–574. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: Biological basis for a gut-muscle axis. Calcif. Tissue Int. 2018, 102, 433–442. [Google Scholar] [CrossRef]
- Ticinesi, A.; Lauretani, F.; Tana, C.; Nouvenne, A.; Ridolo, E.; Meschi, T. Exercise and immune system as modulators of intestinal microbiome: Implications for the gut-muscle axis hypothesis. Exerc. Immunol. Rev. 2019, 25, 84–95. [Google Scholar]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, C.; Neo, S.Y.; McShane, L.M.; Korn, E.L.; Long, P.M.; Jazaeri, A.; Martiat, P.; Fox, S.B.; Harris, A.L.; Liu, E.T. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc. Natl. Acad. Sci. USA 2003, 100, 10393–10398. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.N.; Dave, R.; Sanadya, J.; Sharma, P.; Sharma, K.K. Various types and management of breast cancer: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 109–126. [Google Scholar] [PubMed]
- Advani, S.M.; Advani, P.G.; VonVille, H.M.; Jafri, S.H. Pharmacological management of cachexia in adult cancer patients: A systematic review of clinical trials. BMC Cancer 2018, 18, 1174. [Google Scholar] [CrossRef]
- Penna, F.; Ballaro, R.; Beltra, M.; De Lucia, S.; Garcia Castillo, L.; Costelli, P. The skeletal muscle as an active player against cancer cachexia. Front. Physiol. 2019, 10, 41. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Nakshatri, H. Systemic Actions of Breast Cancer Facilitate Functional Limitations. Cancers 2020, 12, 194. https://doi.org/10.3390/cancers12010194
Wang R, Nakshatri H. Systemic Actions of Breast Cancer Facilitate Functional Limitations. Cancers. 2020; 12(1):194. https://doi.org/10.3390/cancers12010194
Chicago/Turabian StyleWang, Ruizhong, and Harikrishna Nakshatri. 2020. "Systemic Actions of Breast Cancer Facilitate Functional Limitations" Cancers 12, no. 1: 194. https://doi.org/10.3390/cancers12010194
APA StyleWang, R., & Nakshatri, H. (2020). Systemic Actions of Breast Cancer Facilitate Functional Limitations. Cancers, 12(1), 194. https://doi.org/10.3390/cancers12010194



