Evaluation of Calcium Electroporation for the Treatment of Cutaneous Metastases: A Double Blinded Randomised Controlled Phase II Trial
Abstract
1. Introduction
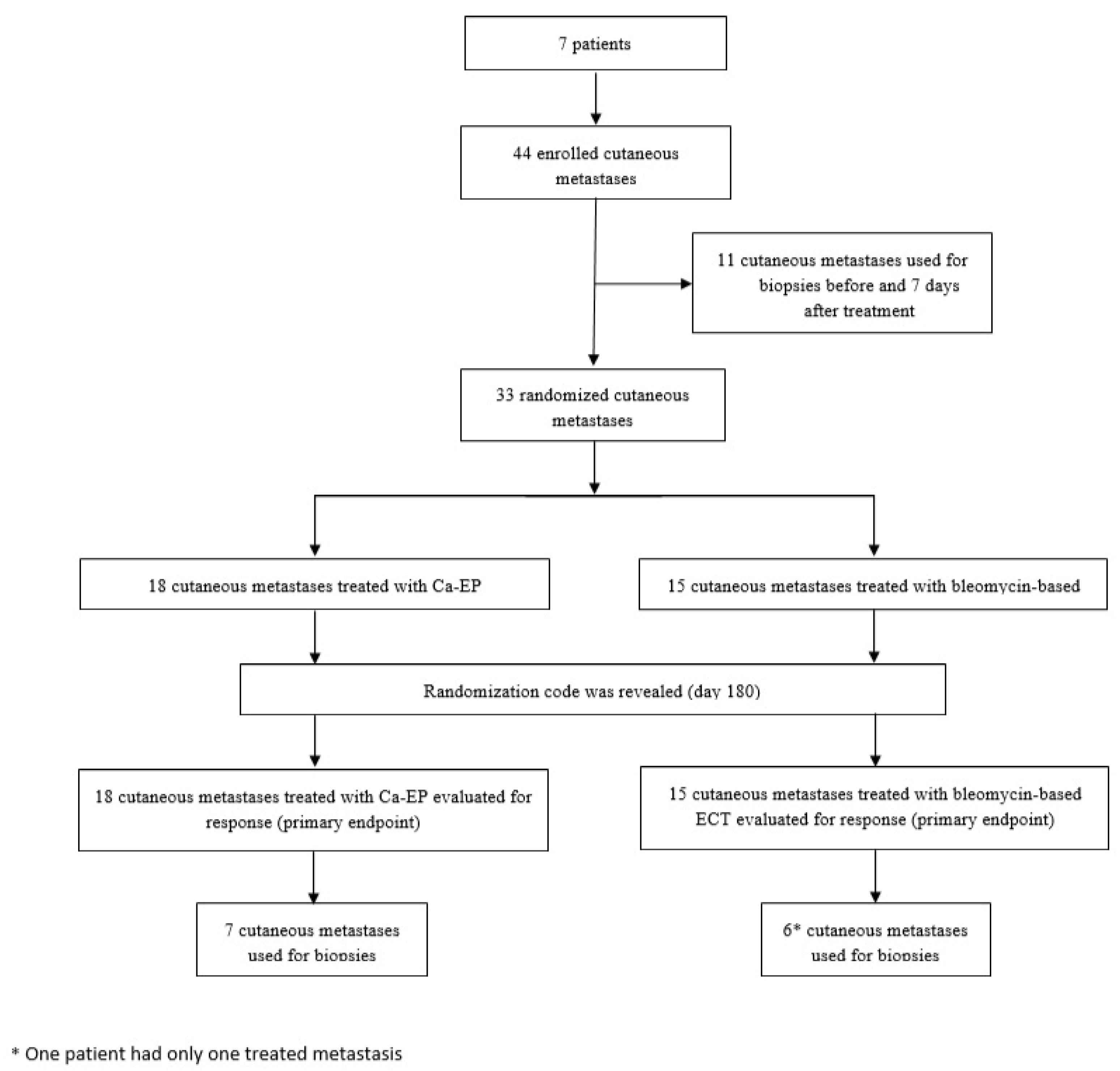
2. Materials and Methods
2.1. Randomization and Blinding
2.2. Procedure
All Participants Assessed the Primary and Safety Analyses
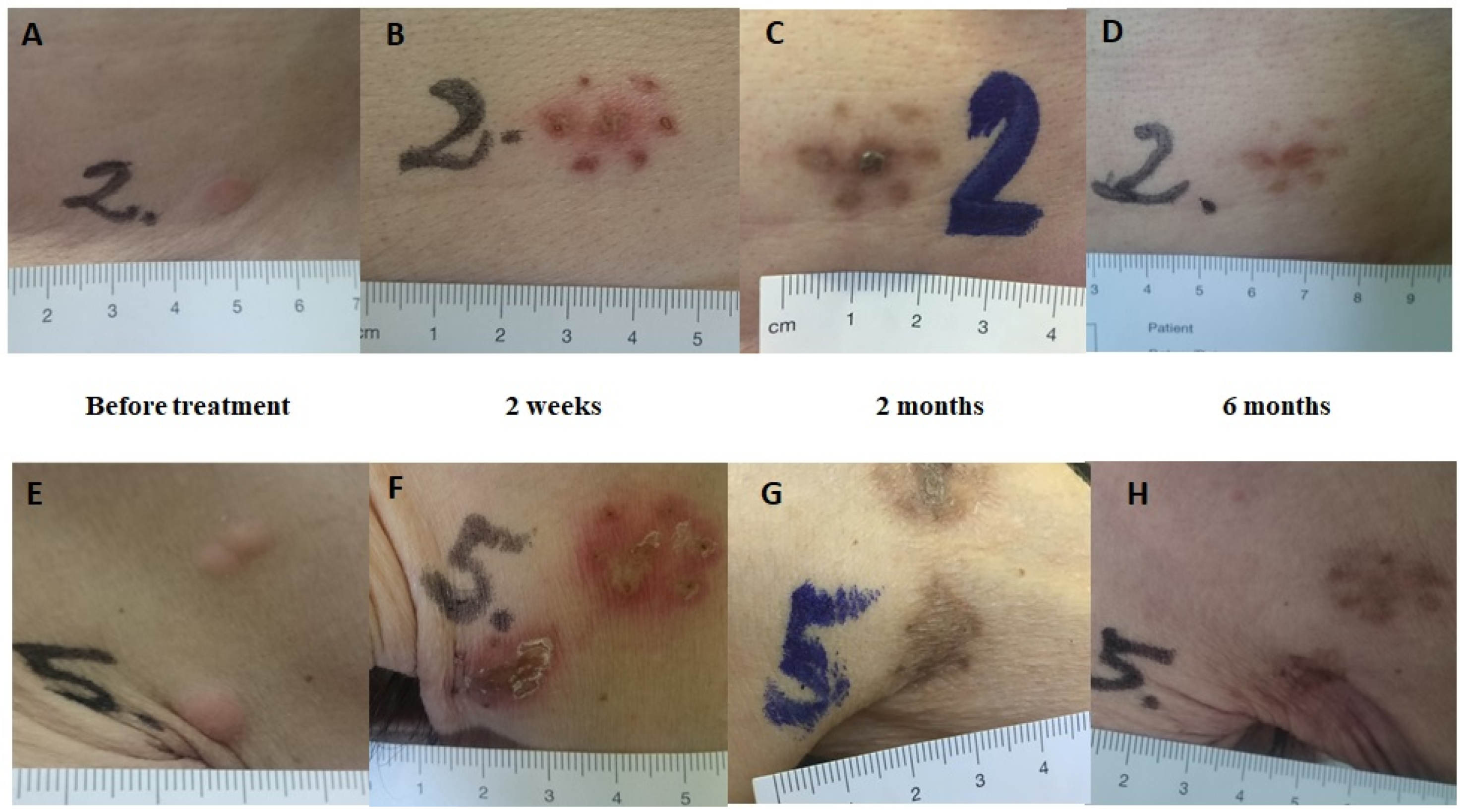
3. Results
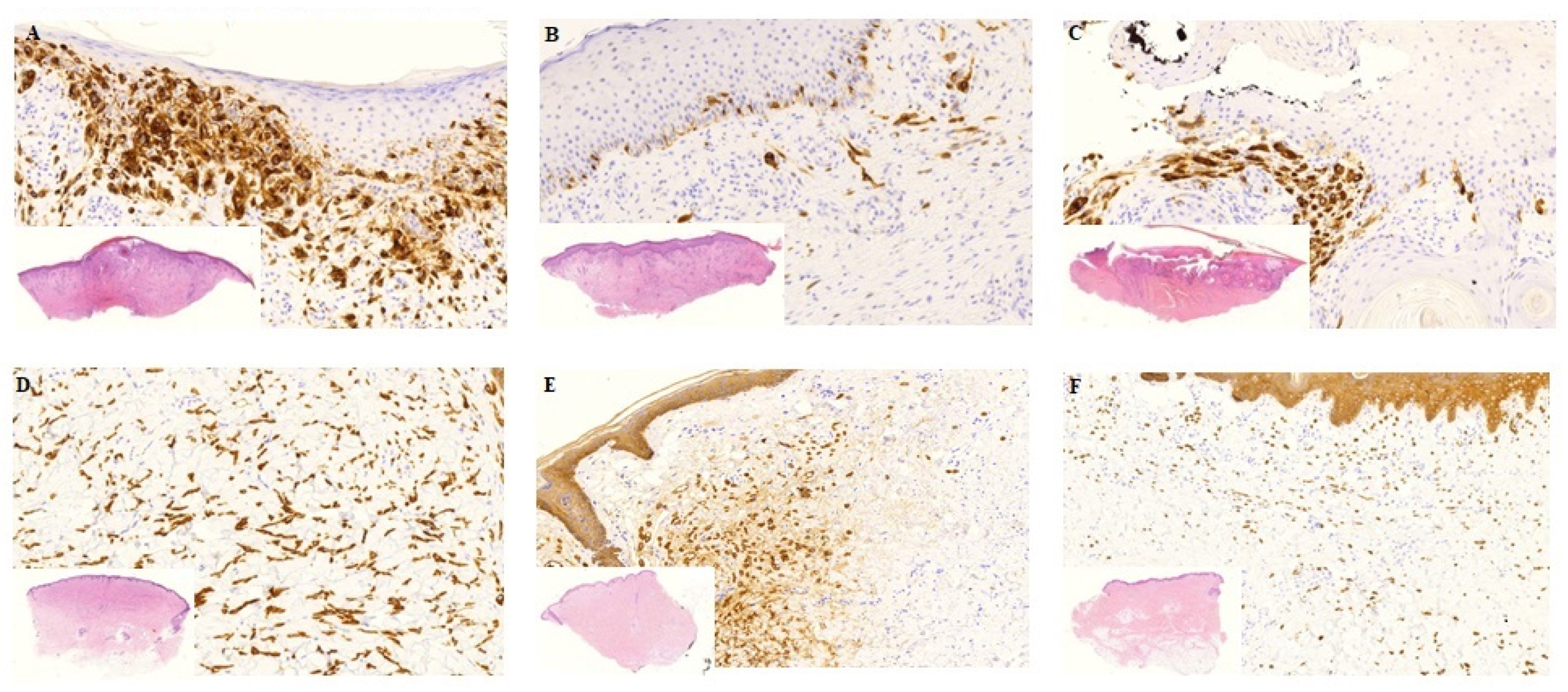
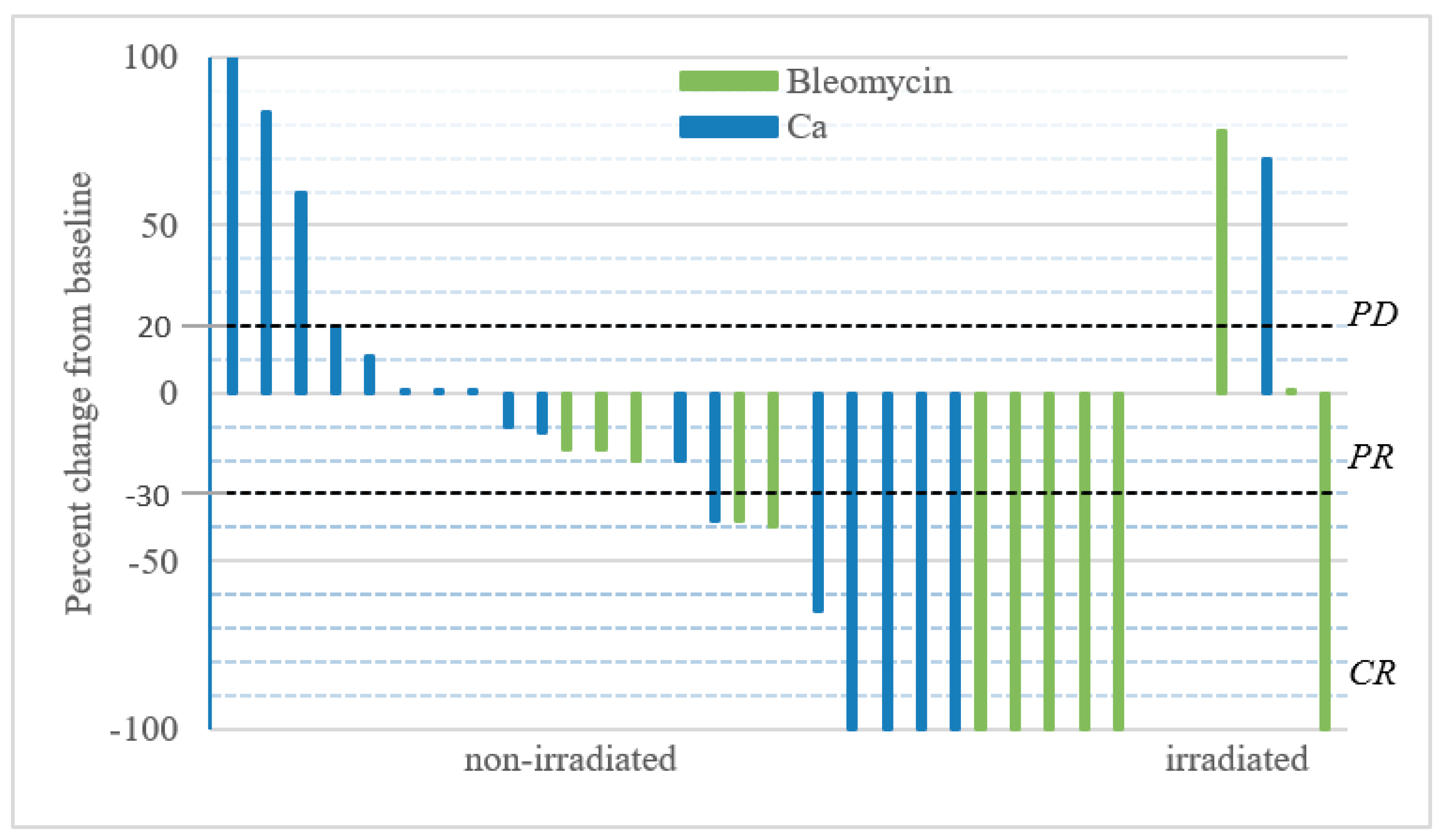
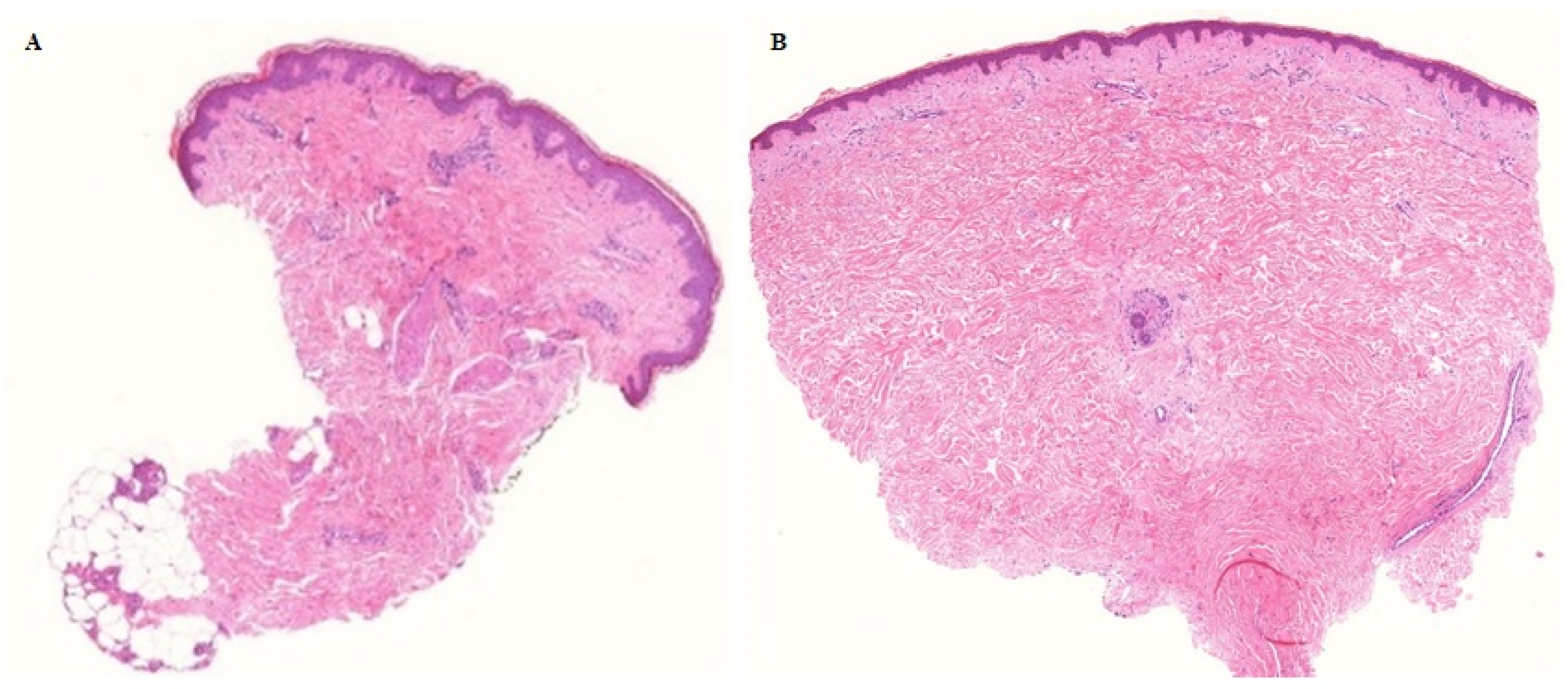
3.1. Tumor Response
3.2. Adverse Events
3.3. Delivered Current
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Campana, L.G.; Valpione, S.; Falci, C.; Mocellin, S.; Basso, M.; Corti, L.; Balestrieri, N.; Marchet, A.; Rossi, C.R. The activity and safety of electrochemotherapy in persistent chest wall recurrence from breast cancer after mastectomy: A phase-II study. Breast Cancer Res. Treat. 2012, 134, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Kunte, C.; Letulé, V.; Gehl, J.; Dahlstroem, K.; Curatolo, P.; Rotunno, R.; Muir, R.; Occhini, A.; Bertino, G.; Powell, B.; et al. Electrochemotherapy in the treatment of metastatic malignant melanoma: A prospective cohort study by InspECT. Br. J. Dermatol. 2017, 176, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, I.; et al. Electrochemotherapy—An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. EJC Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- Matthiessen, L.W.; Chalmers, R.L.; Sainsbury, D.C.; Veeramani, S.; Kessell, G.; Humphreys, A.C.; Bond, J.E.; Muir, T.; Gehl, J. Management of cutaneous metastases using electrochemotherapy. Acta Oncol. 2011, 50, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Solari, N.; Spagnolo, F.; Ponte, E.; Quaglia, A.; Lillini, R.; Battista, M.; Queirolo, P.; Cafiero, F. Electrochemotherapy for the management of cutaneous and subcutaneous metastasis: A series of 39 patients treated with palliative intent. J. Surg. Oncol. 2014, 109, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Curatolo, P.; Quaglino, P.; Marenco, F.; Mancini, M.; Nardò, T.; Mortera, C.; Rotunno, R.; Calvieri, S.; Bernengo, M.G. Electrochemotherapy in the treatment of Kaposi sarcoma cutaneous lesions: A two-center prospective phase II trial. Ann. Surg. Oncol. 2012, 19, 192–198. [Google Scholar] [CrossRef]
- Morley, J.; Grocott, P.; Purssell, E.; Murrells, T. Electrochemotherapy for the palliative management of cutaneous metastases: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019, 45, 2257–2267. [Google Scholar] [CrossRef]
- Zhivotovsky, B.; Orrenius, S. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium 2011, 50, 211–221. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Gissel, H.; Hojman, P.; Eriksen, J.; Gehl, J. Calcium electroporation in three cell lines: A comparison of bleomycin and calcium, calcium compounds, and pulsing conditions. Biochim. Biophys. Acta 2014, 1840, 1204–1208. [Google Scholar] [CrossRef]
- Staresinic, B.; Jesenko, T.; Kamensek, U.; Krog Frandsen, S.; Sersa, G.; Gehl, J.; Cemazar, M. Effect of calcium electroporation on tumour vasculature. Sci. Rep. 2018, 8, 9412. [Google Scholar] [CrossRef]
- Falk, H.; Matthiessen, L.W.; Wooler, G.; Gehl, J. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2018, 57, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Philadelphia: ECOG-Acrin Cancer Research Group. Available online: ecog-acrin.org/resources/ecog-performance-status (accessed on 13 November 2019).
- Frandsen, S.K.; Krüger, M.B.; Mangalanathan, U.M.; Tramm, T.; Mahmood, F.; Novak, I.; Gehl, J. Normal and Malignant Cells Exhibit Differential Responses to Calcium Electroporation. Cancer Res. 2017, 77, 4389–4401. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, S.K.; Gissel, H.; Hojman, P.; Tramm, T.; Eriksen, J.; Gehl, J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012, 72, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, P.; Matthiessen, L.W.; Curatolo, P.; Muir, T.; Bertino, G.; Kunte, C.; Odili, J.; Rotunno, R.; Humphreys, A.C.; Letulé, V.; et al. Predicting patients at risk for pain associated with electrochemotherapy. Acta Oncol. 2015, 54, 298–306. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0; v4.03: 14 June 2010; U.S. Department of Health and Human Services, National Cancer Institute: Washington, DC, USA, 2009. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (accessed on 13 November 2019).
- Walker, E.; Nowacki, A.S. Understanding Equivalence and Noninferiority Testing. J. Gen. Intern. Med. 2011, 26, 192–196. [Google Scholar] [CrossRef]
- Hong Kong: Center for Clinical Research and Biostatistics. Available online: www2.ccrb.cuhk.edu.hk/stat/proportion/tspp_sup.htm (accessed on 13 November 2019).
- Plaschke, C.C.; Gehl, J.; Johannesen, H.H.; Fischer, B.M.; Kjaer, A.; Lomholt, A.F.; Wessel, I. Calcium electroporation for recurrent head and neck cancer: A clinical phase I study. Laryngoscope Investig. Otolaryngol. 2019, 4, 49–56. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Gehl, J. Effect of calcium electroporation in combination with metformin In Vivo and correlation between viability and intracellular ATP level after calcium electroporation In Vitro. PLoS ONE 2017, 12, e0181839. [Google Scholar] [CrossRef]
- Hoejholt, K.L.; Mužić, T.; Jensen, S.D.; Dalgaard, L.T.; Bilgin, M.; Nylandsted, J.; Heimburg, T.; Frandsen, S.K.; Gehl, J. Calcium electroporation and electrochemotherapy for cancer treatment: Importance of cell membrane composition investigated by lipidomics, calorimetry and in vitro efficacy. Sci. Rep. 2019, 9, 4758. [Google Scholar] [CrossRef]
- Hansen, E.L.; Sozer, E.B.; Romeo, S.; Frandsen, S.K.; Vernier, P.T.; Gehl, J. Correction: Dose-dependent ATP depletion and cancer cell death following calcium electroporation, relative effect of calcium concentration and electric field strength. PLoS ONE 2015, 10, e0128034. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Gibot, L.; Madi, M.; Gehl, J.; Rols, M.P. Calcium Electroporation: Evidence for Differential Effects in Normal and Malignant Cell Lines, Evaluated in a 3D Spheroid Model. PLoS ONE 2015, 10, e0144028. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, S.K.; McNeil, A.K.; Novak, I.; McNeil, P.L.; Gehl, J. Difference in Membrane Repair Capacity Between Cancer Cell Lines and a Normal Cell Line. J. Membr. Biol. 2016, 249, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Sannino, A.; Scarfi, M.R.; Vernier, P.T.; Cadossi, R.; Gehl, J.; Zeni, O. ESOPE-Equivalent Pulsing Protocols for Calcium Electroporation: An In Vitro Optimization Study on 2 Cancer Cell Models. Technol. Cancer Res. Treat. 2018, 17, 1533033818788072. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, A.; Gehl, J.; Daczewska, M.; Saczko, J.; Frandsen, S.K.; Kulbacka, J. Calcium electroporation for treatment of sarcoma in preclinical studies. Oncotarget 2018, 9, 11604–11618. [Google Scholar] [CrossRef]
- Falk, H.; Lambaa, S.; Johannesen, H.H.; Wooler, G.; Venzo, A.; Gehl, J. Electrochemotherapy and calcium electroporation inducing a systemic immune response with local and distant remission of tumors in a patient with malignant melanoma—A case report. Acta Oncol. 2017, 56, 1126–1131. [Google Scholar] [CrossRef]
- Dolinsek, T.; Prosen, L.; Cemazar, M.; Potocnik, T.; Sersa, G. Electrochemotherapy with bleomycin is effective in BRAF mutated melanoma cells and interacts with BRAF inhibitors. Radiol. Oncol. 2016, 50, 274–279. [Google Scholar] [CrossRef][Green Version]
- Gehl, J.; Sorensen, T.H.; Nielsen, K.; Raskmark, P.; Nielsen, S.L.; Skovsgaard, T.; Mir, L.M. In vivo electroporation of skeletal muscle: Threshold, efficacy and relation to electric field distribution. Biochim. Et Biophys. Acta 1999, 1428, 233–240. [Google Scholar] [CrossRef]
- Miklavcic, D.; Beravs, K.; Semrov, D.; Cemazar, M.; Demsar, F.; Sersa, G. The importance of electric field distribution for effective in vivo electroporation of tissues. Biophys. J. 1998, 74, 2152–2158. [Google Scholar] [CrossRef]
| Patient | Sex Age (year) | Primary Tumour Characteristics | Location of Cutan Metastases | Number of Metastases Included/Evaluated | Years Since Diagnosis | Previous Therapy | Concomitant Treatment |
|---|---|---|---|---|---|---|---|
| 1. | Male 76 | pT3b, ALM, BRAF-WT | lower extr. | 1/1 | 1 | - | - |
| 2. | Female 62 | Breast: HER2-, ER+, PgR+ | trunk | 10/6 | 5.6 | Epi-txt, Letrozole, mTORi | Letrozole |
| 3. | Female 83 | pT3b, NM, BRAF-WT, satellite met. Ing. sentinel: pos. BD: negative | lower extr. | 9/6 | 7 | adj. IFN, radiotherapy, ECT | - |
| 4. | Female 49 | pT3b, SSM | lower extr. | 3/3 | 2 | adj. IFN | - |
| 5. | Female 83 | pT3a, ALM, BRAF-WT Ing. sentinel: neg. | lower extr. | 10/6 | 4.5 | ECT | - |
| 6. | Female 64 | pT2a, SSM, BRAF-WT | lower extr. | 6/6 | 2.75 | - | - |
| 7. | Male 73 | pT3a, ALM, BRAF-WT | lower extr. | 5/5 | 3.8 | adj. IFN, radiotherapy | - |
| Total | 5 Females, 2 Males mean: 70 (σ = 11.4891) | MM: 6, BRAF-WT: 5/6 Breast: 1 | 6 lower extr. 1 trunk | 44/33 | Mean 3.8 (σ = 1.9329) | various |
| Treatment Arm | Calcium-Electroporation | Bleomycin-Based Electrochemotherapy | ||||
|---|---|---|---|---|---|---|
| Current Study | 2018 Study | Total | Current Study | 2018 Study | Total | |
| Lesion characteristics | ||||||
| Tumour size | ||||||
| Median of the largest diameter, mm | 6.5 (5–30) | 9.5 (5–18) | 7 (5–25) | 11 (4–25) | ||
| Tumour type | ||||||
| Malignant melanoma | 15 | 1 | 16 | 12 | 1 | 13 |
| Breast cancer | 3 | 17 | 20 | 3 | 18 | 21 |
| Previously irradiated lesions, n | 2 | 8 | 10 | 4 | 7 | 11 |
| Location | ||||||
| Lower extremity | 15 | 4 | 19 | 12 | 4 | 16 |
| Trunk | 3 | 14 | 17 | 0 | 15 | 15 |
| Upper extremity | 0 | 0 | 0 | 3 | 0 | 3 |
| Treatment | ||||||
| Median doses (range), mL | 0.085 (0.042–3.14) | 0.24 (0.03–1.21) | 0.132 (0.065–0.475) | 0.21 (0.03–0.55) | ||
| Median delivered current (range), A | 3.85 (1.4–9) | 3.4 (0.9–8.2) | 4 (1.4–6.5) | 2.8 (1–9.6) | ||
| Median delivered current with linear electrodes (range), A | 4 (2.25–9) | 3.4 (0.9–8.3) | 5.05 (4–6.1) | 2.8 (1–9.6) | ||
| Median delivered current with hexagonal electrodes (range), A | 2.5 (1.4–4.2) | NA | 2.75 (1.4–3.6) | NA | ||
| Median number of applications (range), n | 1 (1–6) | 3 (1–7) | 1 (1–3) | 3 (1–7) | ||
| Electrodes | ||||||
| Linear | 39% (7) | 100% (18) | 33% (5) | 100% (19) | ||
| Response (CR) for linear electrode subgroup | 14% (1) | 66% (12) | 0 | 68% (13) | ||
| Hexagonal | 61% (11) | 0 | 67% (10) | 0 | ||
| Response (CR) for hexagonal electrodes subgroup | 27% (3) | NA | 60% (6) | NA | ||
| Response | ||||||
| Complete response, percent (n) | 4 | 12 | 44.44% (16) | 6 | 13 | 55.88% (19) |
| Partial response, percent (n) | 2 | 1 | 8.33% (3) | 2 | 3 | 14.7% (5) |
| Stable disease, percent (n) | 6 | 3 | 25% (9) | 5 | 0 | 14.7% (5) |
| Progressive disease, percent (n) | 6 | 2 | 22.22% (8) | 2 | 3 | 14.7% (5) |
| Adverse events | ||||||
| Ulceration, percent (n) | 2 | 7 | 25% (9) | 3 | 13 | 47.05% (16) |
| Itch, percent (n) | 0 | 1 | 2.77% (1) | 0 | 5 | 14.7% (5) |
| Hyperpigmentation, percent (n) | 2 | 0 | 5.55% (2) | 6 | 5 | 32.35% (11) |
| Exuding, percent (n) | 0 | 2 | 5.55% (2) | 0 | 2 | 5.88% (2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ágoston, D.; Baltás, E.; Ócsai, H.; Rátkai, S.; Lázár, P.G.; Korom, I.; Varga, E.; Németh, I.B.; Dósa-Rácz Viharosné, É.; Gehl, J.; et al. Evaluation of Calcium Electroporation for the Treatment of Cutaneous Metastases: A Double Blinded Randomised Controlled Phase II Trial. Cancers 2020, 12, 179. https://doi.org/10.3390/cancers12010179
Ágoston D, Baltás E, Ócsai H, Rátkai S, Lázár PG, Korom I, Varga E, Németh IB, Dósa-Rácz Viharosné É, Gehl J, et al. Evaluation of Calcium Electroporation for the Treatment of Cutaneous Metastases: A Double Blinded Randomised Controlled Phase II Trial. Cancers. 2020; 12(1):179. https://doi.org/10.3390/cancers12010179
Chicago/Turabian StyleÁgoston, Dóra, Eszter Baltás, Henriette Ócsai, Sándor Rátkai, Péter Gy Lázár, Irma Korom, Erika Varga, István Balázs Németh, Éva Dósa-Rácz Viharosné, Julie Gehl, and et al. 2020. "Evaluation of Calcium Electroporation for the Treatment of Cutaneous Metastases: A Double Blinded Randomised Controlled Phase II Trial" Cancers 12, no. 1: 179. https://doi.org/10.3390/cancers12010179
APA StyleÁgoston, D., Baltás, E., Ócsai, H., Rátkai, S., Lázár, P. G., Korom, I., Varga, E., Németh, I. B., Dósa-Rácz Viharosné, É., Gehl, J., Oláh, J., Kemény, L., & Kis, E. G. (2020). Evaluation of Calcium Electroporation for the Treatment of Cutaneous Metastases: A Double Blinded Randomised Controlled Phase II Trial. Cancers, 12(1), 179. https://doi.org/10.3390/cancers12010179







