A Comprehensive Review of Calcium Electroporation—A Novel Cancer Treatment Modality
Abstract
1. Introduction
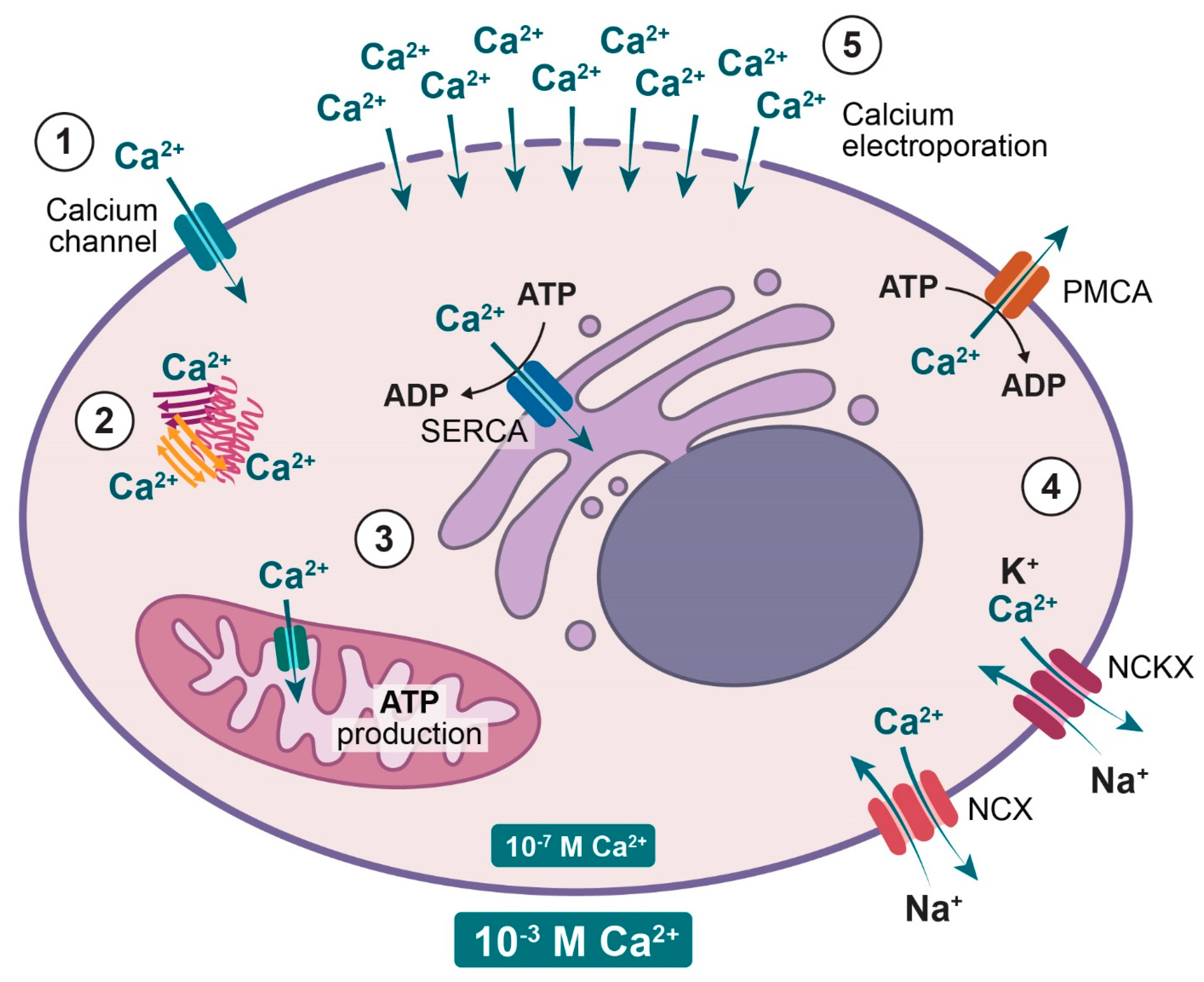
2. Normal Cellular Calcium Homeostasis
3. Normal versus Malignant Calcium Homeostasis
4. Electroporation
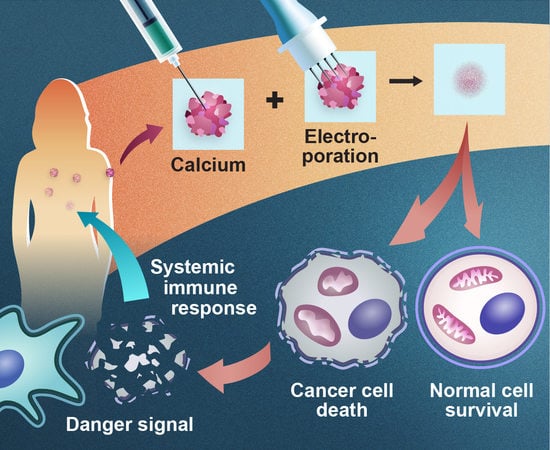
5. Calcium Electroporation
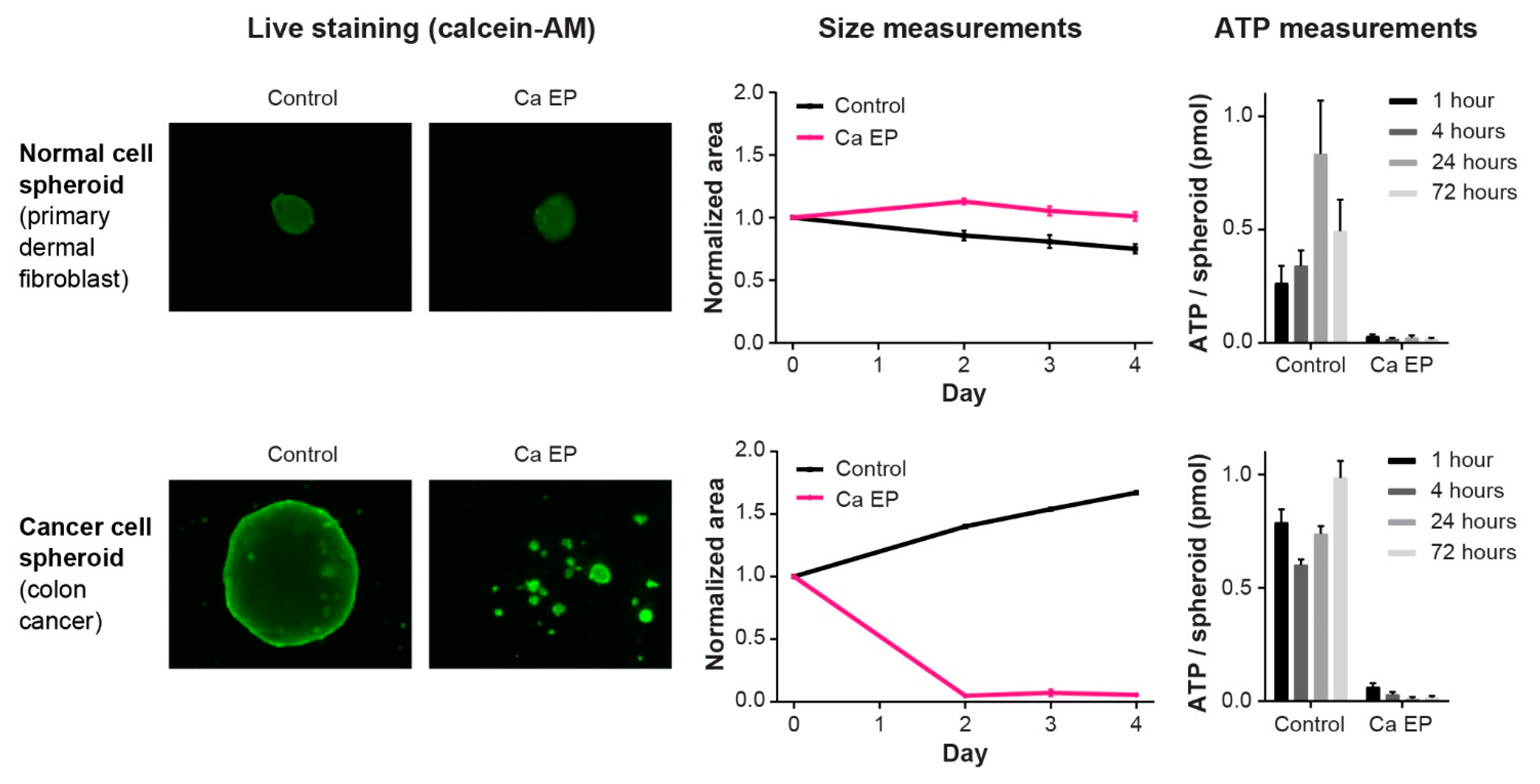
5.1. Effect on Cancer Cells In Vitro and In Vivo
5.2. Effect on Normal Cells and Tissues
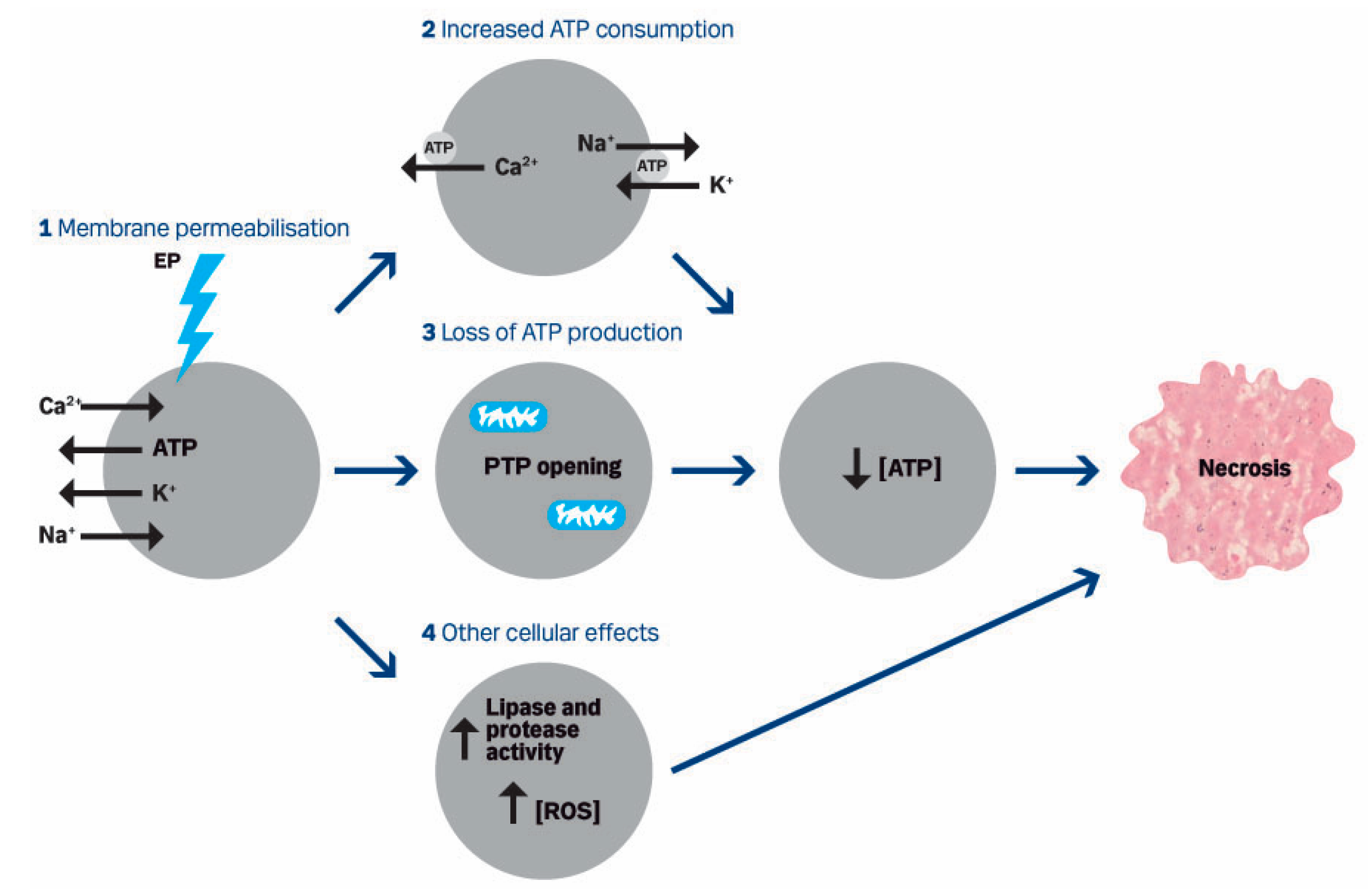
5.3. Mechanisms of Action—Cellular Effects
5.3.1. Cell Death
5.3.2. Calcium Uptake
5.3.3. Calcium Transporters
5.3.4. ATP
5.3.5. Other Cellular Effects
5.4. Mechanisms of Action—Immune Response
5.5. Veterinary Studies
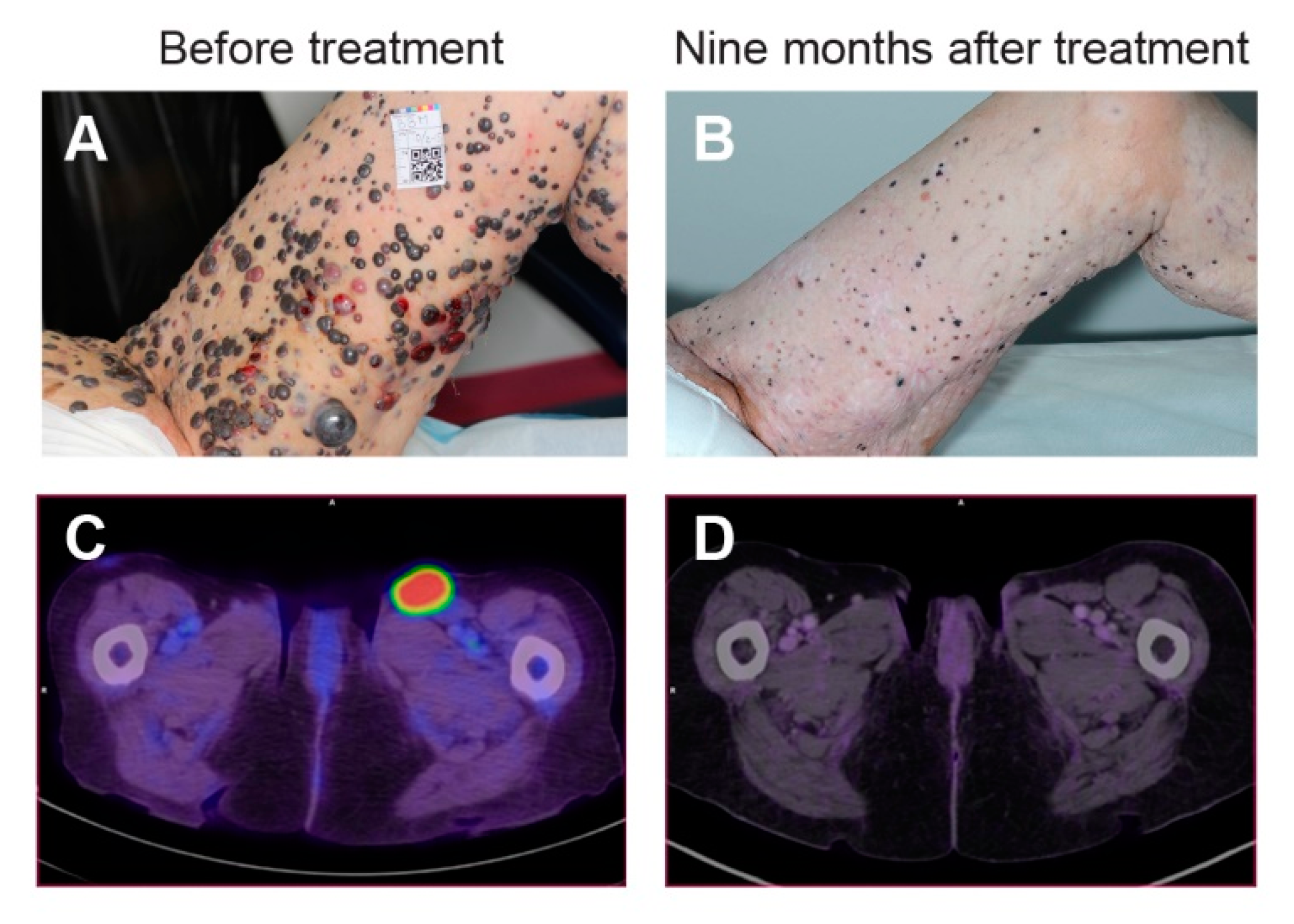
5.6. Clinical Trials
6. Cost and Feasibility of Intervention
7. Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berridge, M.J.; Bootman, M.D.; Lipp, P. Calcium—A life and death signal. Nature 1998, 395, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Carafoli, E. Calcium signalling: A historical account, recent developments and future perspectives. Cell. Mol. Life Sci. 2000, 57, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Case, R.M.; Eisner, D.; Gurney, A.; Jones, O.; Muallem, S.; Verkhratsky, A. Evolution of calcium homeostasis: From birth of the first cell to an omnipresent signalling system. Cell Calcium 2007, 42, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Zhivotovsky, B.; Orrenius, S. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium 2011, 50, 211–221. [Google Scholar] [CrossRef]
- Belehradek, M.; Domenge, C.; Luboinski, B.; Orlowski, S.; Belehradek, J., Jr.; Mir, L.M. Electrochemotherapy, a new antitumor treatment. First clinical phase I-II trial. Cancer 1993, 72, 3694–3700. [Google Scholar]
- Heller, R.; Jaroszeski, M.J.; Reintgen, D.S.; Puleo, C.A.; DeConti, R.C.; Gilbert, R.A.; Glass, L.F. Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer 1998, 83, 148–157. [Google Scholar] [CrossRef]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D.; et al. Electrochemotherapy—An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. EJC Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- Matthiessen, L.W.; Chalmers, R.L.; Sainsbury, D.C.; Veeramani, S.; Kessell, G.; Humphreys, A.C.; Bond, J.E.; Muir, T.; Gehl, J. Management of cutaneous metastases using electrochemotherapy. Acta Oncol. 2011, 50, 621–629. [Google Scholar] [CrossRef]
- Curatolo, P.; Quaglino, P.; Marenco, F.; Mancini, M.; Nardo, T.; Mortera, C.; Rotunno, R.; Calvieri, S.; Bernengo, M.G. Electrochemotherapy in the treatment of Kaposi sarcoma cutaneous lesions: A two-center prospective phase II trial. Ann. Surg. Oncol. 2012, 19, 192–198. [Google Scholar] [CrossRef]
- Matthiessen, L.W.; Johannesen, H.H.; Hendel, H.W.; Moss, T.; Kamby, C.; Gehl, J. Electrochemotherapy for large cutaneous recurrence of breast cancer: A phase II clinical trial. Acta Oncol. 2012, 51, 713–721. [Google Scholar] [CrossRef]
- NICE Guidance: Electrochemotherapy for Metastases in the Skin from Tumours of Non-Skin Origin (IPG446). Available online: http://www.nice.org.uk/guidance/IPG446 (accessed on 23 December 2019).
- Curatolo, P.; Miraglia, E.; Rotunno, R.; Calvieri, S.; Giustini, S. Electrochemotherapy: A valid treatment for Gorlin-Goltz syndrome. Acta Derm. Croat. 2013, 21, 132–133. [Google Scholar]
- Campana, L.G.; Testori, A.; Mozzillo, N.; Rossi, C.R. Treatment of metastatic melanoma with electrochemotherapy. J. Surg. Oncol. 2014, 109, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Landstrom, F.J.; Reizenstein, J.; Adamsson, G.B.; Beckerath, M.; Moller, C. Long-term follow-up in patients treated with curative electrochemotherapy for cancer in the oral cavity and oropharynx. Acta Oto Laryngol. 2015, 135, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Mozzillo, N.; Simeone, E.; Benedetto, L.; Curvietto, M.; Giannarelli, D.; Gentilcore, G.; Camerlingo, R.; Capone, M.; Madonna, G.; Festino, L.; et al. Assessing a novel immuno-oncology-based combination therapy: Ipilimumab plus electrochemotherapy. Oncoimmunology 2015, 4, e1008842. [Google Scholar] [CrossRef] [PubMed]
- Gehl, J.; Skovsgaard, T.; Mir, L.M. Enhancement of cytotoxicity by electropermeabilization: An improved method for screening drugs. Anti Cancer Drugs 1998, 9, 319–325. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Gissel, H.; Hojman, P.; Tramm, T.; Eriksen, J.; Gehl, J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012, 72, 1336–1341. [Google Scholar] [CrossRef]
- Falk, H.; Matthiessen, L.W.; Wooler, G.; Gehl, J. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2018, 57, 311–319. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Carafoli, E. Calcium signaling: A tale for all seasons. Proc. Natl. Acad. Sci. USA 2002, 99, 1115–1122. [Google Scholar] [CrossRef]
- Lytton, J.; Westlin, M.; Burk, S.E.; Shull, G.E.; MacLennan, D.H. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J. Biol. Chem. 1992, 267, 14483–14489. [Google Scholar] [PubMed]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabo, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.S. The role of the mitochondrial permeability transition in cell death. Mitochondrion 2006, 6, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Decuypere, J.P.; Monaco, G.; Bultynck, G.; Missiaen, L.; De Smedt, H.; Parys, J.B. The IP(3) receptor-mitochondria connection in apoptosis and autophagy. Biochim. Biophys. Acta 2011, 1813, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Cali, T.; Ottolini, D.; Carafoli, E. The plasma membrane calcium pump in health and disease. FEBS J. 2013, 280, 5385–5397. [Google Scholar] [CrossRef]
- Brini, M.; Carafoli, E. The plasma membrane Ca(2)+ ATPase and the plasma membrane sodium calcium exchanger cooperate in the regulation of cell calcium. Cold Spring Harb. Perspect. Biol. 2011, 3, a004816. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Monteith, G.R.; McAndrew, D.; Faddy, H.M.; Roberts-Thomson, S.J. Calcium and cancer: Targeting Ca2+ transport. Nat. Rev. Cancer 2007, 7, 519–530. [Google Scholar] [CrossRef]
- Roderick, H.L.; Cook, S.J. Ca2+ signalling checkpoints in cancer: Remodelling Ca2+ for cancer cell proliferation and survival. Nat. Rev. Cancer 2008, 8, 361–375. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Ouadid-Ahidouch, H.; Skryma, R.; Shuba, Y. Remodelling of Ca2+ transport in cancer: How it contributes to cancer hallmarks? Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130097. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Uzawa, K.; Mochida, Y.; Shiiba, M.; Bukawa, H.; Yokoe, H.; Tanzawa, H. Sarcoendoplasmic reticulum Ca(2+) ATPase type 2 downregulated in human oral squamous cell carcinoma. Int. J. Cancer 2004, 110, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Bergner, A.; Kellner, J.; Tufman, A.; Huber, R.M. Endoplasmic reticulum Ca2+-homeostasis is altered in Small and non-small Cell Lung Cancer cell lines. J. Exp. Clin. Cancer Res. 2009, 28, 25. [Google Scholar] [CrossRef] [PubMed]
- Gelebart, P.; Kovacs, T.; Brouland, J.P.; van Gorp, R.; Grossmann, J.; Rivard, N.; Panis, Y.; Martin, V.; Bredoux, R.; Enouf, J.; et al. Expression of endomembrane calcium pumps in colon and gastric cancer cells. Induction of SERCA3 expression during differentiation. J. Biol. Chem. 2002, 277, 26310–26320. [Google Scholar] [CrossRef] [PubMed]
- Brouland, J.P.; Gelebart, P.; Kovacs, T.; Enouf, J.; Grossmann, J.; Papp, B. The loss of sarco/endoplasmic reticulum calcium transport ATPase 3 expression is an early event during the multistep process of colon carcinogenesis. Am. J. Pathol. 2005, 167, 233–242. [Google Scholar] [CrossRef]
- Papp, B.; Brouland, J.P. Altered Endoplasmic Reticulum Calcium Pump Expression during Breast Tumorigenesis. Breast Cancer 2011, 5, 163–174. [Google Scholar] [CrossRef]
- Korosec, B.; Glavac, D.; Rott, T.; Ravnik-Glavac, M. Alterations in the ATP2A2 gene in correlation with colon and lung cancer. Cancer Genet. Cytogenet. 2006, 171, 105–111. [Google Scholar] [CrossRef]
- Korosec, B.; Glavac, D.; Volavsek, M.; Ravnik-Glavac, M. Alterations in genes encoding sarcoplasmic-endoplasmic reticulum Ca(2+) pumps in association with head and neck squamous cell carcinoma. Cancer Genet. Cytogenet. 2008, 181, 112–118. [Google Scholar] [CrossRef]
- Korosec, B.; Glavac, D.; Volavsek, M.; Ravnik-Glavac, M. ATP2A3 gene is involved in cancer susceptibility. Cancer Genet. Cytogenet. 2009, 188, 88–94. [Google Scholar] [CrossRef]
- Aung, C.S.; Kruger, W.A.; Poronnik, P.; Roberts-Thomson, S.J.; Monteith, G.R. Plasma membrane Ca2+-ATPase expression during colon cancer cell line differentiation. Biochem. Biophys. Res. Commun. 2007, 355, 932–936. [Google Scholar] [CrossRef]
- Ribiczey, P.; Tordai, A.; Andrikovics, H.; Filoteo, A.G.; Penniston, J.T.; Enouf, J.; Enyedi, A.; Papp, B.; Kovacs, T. Isoform-specific up-regulation of plasma membrane Ca2+ATPase expression during colon and gastric cancer cell differentiation. Cell Calcium 2007, 42, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Aung, C.S.; Ye, W.; Plowman, G.; Peters, A.A.; Monteith, G.R.; Roberts-Thomson, S.J. Plasma membrane calcium ATPase 4 and the remodeling of calcium homeostasis in human colon cancer cells. Carcinogenesis 2009, 30, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Ruschoff, J.H.; Brandenburger, T.; Strehler, E.E.; Filoteo, A.G.; Heinmoller, E.; Aumuller, G.; Wilhelm, B. Plasma membrane calcium ATPase expression in human colon multistep carcinogenesis. Cancer Invest. 2012, 30, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Roberts-Thomson, S.J.; Monteith, G.R. Plasma membrane calcium-ATPase 2 and 4 in human breast cancer cell lines. Biochem. Biophys. Res. Commun. 2005, 337, 779–783. [Google Scholar] [CrossRef]
- Peters, A.A.; Milevskiy, M.J.; Lee, W.C.; Curry, M.C.; Smart, C.E.; Saunus, J.M.; Reid, L.; da Silva, L.; Marcial, D.L.; Dray, E.; et al. The calcium pump plasma membrane Ca(2+)-ATPase 2 (PMCA2) regulates breast cancer cell proliferation and sensitivity to doxorubicin. Sci. Rep. 2016, 6, 25505. [Google Scholar] [CrossRef]
- Orlowski, S.; Belehradek, J.; Paoletti, C.; Mir, L.M. Transient Electropermeabilization of Cells in Culture—Increase of the Cyto-Toxicity of Anticancer Drugs. Biochem. Pharmacol. 1988, 37, 4727–4733. [Google Scholar] [CrossRef]
- Sersa, G.; Cemazar, M.; Miklavcic, D. Antitumor effectiveness of electrochemotherapy with cis-diamminedichloroplatinum(II) in mice. Cancer Res. 1995, 55, 3450–3455. [Google Scholar]
- Jaroszeski, M.J.; Dang, V.; Pottinger, C.; Hickey, J.; Gilbert, R.; Heller, R. Toxicity of anticancer agents mediated by electroporation in vitro. Anti Cancer Drugs 2000, 11, 201–208. [Google Scholar] [CrossRef]
- Gehl, J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef]
- Vasquez, J.L.; Ibsen, P.; Lindberg, H.; Gehl, J. In vitro and in vivo experiments on electrochemotherapy for bladder cancer. J. Urol. 2015, 193, 1009–1015. [Google Scholar] [CrossRef]
- Josserand, V.; Keramidas, M.; Lavaud, J.; Righini, C.; Vollaire, J.; Bellard, E.; Rols, M.P.; Teissie, J.; Coll, J.L.; Golzio, M. Electrochemotherapy guided by intraoperative fluorescence imaging for the treatment of inoperable peritoneal micro-metastases. J. Control Release 2016, 233, 81–87. [Google Scholar] [CrossRef]
- Ho, M.C.; Levine, Z.A.; Vernier, P.T. Nanoscale, electric field-driven water bridges in vacuum gaps and lipid bilayers. J. Membr. Biol. 2013, 246, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Venslauskas, M.S.; Satkauskas, S. Mechanisms of transfer of bioactive molecules through the cell membrane by electroporation. Eur. Biophys. J. 2015, 44, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, S.; Mir, L.M. Cell electropermeabilization: A new tool for biochemical and pharmacological studies. Biochim. Biophys. Acta 1993, 1154, 51–63. [Google Scholar] [CrossRef]
- Frandsen, S.K.; McNeil, A.K.; Novak, I.; McNeil, P.L.; Gehl, J. Difference in Membrane Repair Capacity Between Cancer Cell Lines and a Normal Cell Line. J. Membr. Biol. 2016, 249, 569–576. [Google Scholar] [CrossRef] [PubMed]
- McNeil, P.L.; Steinhardt, R.A. Plasma membrane disruption: Repair, prevention, adaptation. Annu. Rev. Cell Dev. Biol. 2003, 19, 697–731. [Google Scholar] [CrossRef]
- Ciobanu, F.; Golzio, M.; Kovacs, E.; Teissie, J. Control by Low Levels of Calcium of Mammalian Cell Membrane Electropermeabilization. J. Membr. Biol. 2018, 251, 221–228. [Google Scholar] [CrossRef]
- Bansal, D.; Miyake, K.; Vogel, S.S.; Groh, S.; Chen, C.C.; Williamson, R.; McNeil, P.L.; Campbell, K.P. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 2003, 423, 168–172. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Kruger, M.B.; Mangalanathan, U.M.; Tramm, T.; Mahmood, F.; Novak, I.; Gehl, J. Normal and Malignant Cells Exhibit Differential Responses to Calcium Electroporation. Cancer Res. 2017, 77, 4389–4401. [Google Scholar] [CrossRef]
- Levine, Z.A.; Vernier, P.T. Calcium and phosphatidylserine inhibit lipid electropore formation and reduce pore lifetime. J. Membr. Biol. 2012, 245, 599–610. [Google Scholar] [CrossRef]
- Vernier, P.T.; Ziegler, M.J.; Dimova, R. Calcium binding and head group dipole angle in phosphatidylserine-phosphatidylcholine bilayers. Langmuir 2009, 25, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Kunte, C.; Letule, V.; Gehl, J.; Dahlstroem, K.; Curatolo, P.; Rotunno, R.; Muir, T.; Occhini, A.; Bertino, G.; Powell, B.; et al. Electrochemotherapy in the treatment of metastatic malignant melanoma: A prospective cohort study by InspECT. Br. J. Derm. 2017, 176, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Sersa, G.; Cufer, T.; Paulin, S.M.; Cemazar, M.; Snoj, M. Electrochemotherapy of chest wall breast cancer recurrence. Cancer Treat. Rev. 2012, 38, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Campanacci, L.; Ronchetti, M.; Donati, D. Electrochemotherapy in the Treatment of Bone Metastases: A Phase II Trial. World J. Surg. 2016, 40, 3088–3094. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Leongito, M.; Granata, V.; Barbieri, A.; Del Vecchio, V.; Falco, M.; Nasto, A.; Albino, V.; Piccirillo, M.; Palaia, R.; et al. Electrochemotherapy in pancreatic adenocarcinoma treatment: Pre-clinical and clinical studies. Radiol. Oncol. 2016, 50, 14–20. [Google Scholar] [CrossRef]
- Edhemovic, I.; Gadzijev, E.M.; Brecelj, E.; Miklavcic, D.; Kos, B.; Zupanic, A.; Mali, B.; Jarm, T.; Pavliha, D.; Marcan, M.; et al. Electrochemotherapy: A new technological approach in treatment of metastases in the liver. Technol. Cancer Res. Treat. 2011, 10, 475–485. [Google Scholar] [CrossRef]
- Egeland, C.; Baeksgaard, L.; Johannesen, H.H.; Lofgren, J.; Plaschke, C.C.; Svendsen, L.B.; Gehl, J.; Achiam, M.P. Endoscopic electrochemotherapy for esophageal cancer: A phase I clinical study. Endosc. Int. Open 2018, 6, E727–E734. [Google Scholar] [CrossRef]
- Plaschke, C.C.; Bertino, G.; McCaul, J.A.; Grau, J.J.; de Bree, R.; Sersa, G.; Occhini, A.; Groselj, A.; Langdon, C.; Heuveling, D.A.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results from the treatment of mucosal cancers. Eur. J. Cancer 2017, 87, 172–181. [Google Scholar] [CrossRef]
- Sallberg, M.; Frelin, L.; Ahlen, G.; Sallberg-Chen, M. Electroporation for therapeutic DNA vaccination in patients. Med. Microbiol. Immunol. 2015, 204, 131–135. [Google Scholar] [CrossRef]
- Heller, L.; Todorovic, V.; Cemazar, M. Electrotransfer of single-stranded or double-stranded DNA induces complete regression of palpable B16.F10 mouse melanomas. Cancer Gene Ther. 2013, 20, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Spanggaard, I.; Dahlstroem, K.; Laessoee, L.; Hansen, R.H.; Johannesen, H.H.; Hendel, H.W.; Bouquet, C.; Attali, P.; Gehl, J. Gene therapy for patients with advanced solid tumors: A phase I study using gene electrotransfer to muscle with the integrin inhibitor plasmid AMEP. Acta Oncol. 2017, 56, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Golzio, M.; Gabriel, B.; Boissier, F.; Deuwille, J.; Rols, M.P.; Teissi, J. Calcium and electropermeabilized cells. J. De La Soc. De Biol. 2003, 197, 301–310. [Google Scholar] [CrossRef]
- Hojman, P.; Spanggaard, I.; Olsen, C.H.; Gehl, J.; Gissel, H. Calcium electrotransfer for termination of transgene expression in muscle. Hum. Gene Ther. 2011, 22, 753–760. [Google Scholar] [CrossRef]
- Hansen, E.L.; Sozer, E.B.; Romeo, S.; Frandsen, S.K.; Vernier, P.T.; Gehl, J. Dose-dependent ATP depletion and cancer cell death following calcium electroporation, relative effect of calcium concentration and electric field strength. PLoS ONE 2015, 10, e0122973. [Google Scholar]
- Zielichowska, A.; Daczewska, M.; Saczko, J.; Michel, O.; Kulbacka, J. Applications of calcium electroporation to effective apoptosis induction in fibrosarcoma cells and stimulation of normal muscle cells. Bioelectrochemistry 2016, 109, 70–78. [Google Scholar] [CrossRef]
- Falk, H.; Forde, P.F.; Bay, M.L.; Mangalanathan, U.M.; Hojman, P.; Soden, D.M.; Gehl, J. Calcium Electroporation Induces Tumor Eradication, Long-lasting Immunity and Cytokine Responses in the CT26 Colon Cancer Mouse Model. Oncoimmunology 2017, 6, e1301332. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Gehl, J. Effect of calcium electroporation in combination with metformin in vivo and correlation between viability and intracellular ATP level after calcium electroporation in vitro. PLoS ONE 2017, 12, e0181839. [Google Scholar] [CrossRef]
- Staresinic, B.; Jesenko, T.; Kamensek, U.; Krog Frandsen, S.; Sersa, G.; Gehl, J.; Cemazar, M. Effect of calcium electroporation on tumour vasculature. Sci. Rep. 2018, 8, 9412. [Google Scholar] [CrossRef]
- Szewczyk, A.; Gehl, J.; Daczewska, M.; Saczko, J.; Frandsen, S.K.; Kulbacka, J. Calcium electroporation for treatment of sarcoma in preclinical studies. Oncotarget 2018, 9, 11604–11618. [Google Scholar] [CrossRef]
- Hoejholt, K.L.; Muzic, T.; Jensen, S.D.; Dalgaard, L.T.; Bilgin, M.; Nylandsted, J.; Heimburg, T.; Frandsen, S.K.; Gehl, J. Calcium electroporation and electrochemotherapy for cancer treatment: Importance of cell membrane composition investigated by lipidomics, calorimetry and in vitro efficacy. Sci. Rep. 2019, 9, 4758. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, S.K.; Gissel, H.; Hojman, P.; Eriksen, J.; Gehl, J. Calcium electroporation in three cell lines: A comparison of bleomycin and calcium, calcium compounds, and pulsing conditions. Biochim. Biophys. Acta 2014, 1840, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, S.K.; Gibot, L.; Madi, M.; Gehl, J.; Rols, M.P. Calcium Electroporation: Evidence for Differential Effects in Normal and Malignant Cell Lines, Evaluated in a 3D Spheroid Model. PLoS ONE 2015, 10, e0144028. [Google Scholar] [CrossRef] [PubMed]
- Landstrom, F.; Ivarsson, M.; von Sydow, A.K.; Magnuson, A.; von Beckerath, M.; Moller, C. Electrochemotherapy—Evidence for Cell-type Selectivity In Vitro. Anticancer Res. 2015, 35, 5813–5820. [Google Scholar] [PubMed]
- Jarm, T.; Cemazar, M.; Miklavcic, D.; Sersa, G. Antivascular effects of electrochemotherapy: Implications in treatment of bleeding metastases. Expert Rev. Anticancer Ther. 2010, 10, 729–746. [Google Scholar] [CrossRef] [PubMed]
- Markelc, B.; Sersa, G.; Cemazar, M. Differential mechanisms associated with vascular disrupting action of electrochemotherapy: Intravital microscopy on the level of single normal and tumor blood vessels. PLoS ONE 2013, 8, e59557. [Google Scholar] [CrossRef]
- Morotomi-Yano, K.; Akiyama, H.; Yano, K. Different involvement of extracellular calcium in two modes of cell death induced by nanosecond pulsed electric fields. Arch. Biochem. Biophys. 2014, 555, 47–54. [Google Scholar] [CrossRef]
- Pakhomova, O.N.; Gregory, B.; Semenov, I.; Pakhomov, A.G. Calcium-mediated pore expansion and cell death following nanoelectroporation. Biochim. Biophys. Acta 2014, 1838, 2547–2554. [Google Scholar] [CrossRef]
- Chopinet, L.; Rols, M.P. Nanosecond electric pulses: A mini-review of the present state of the art. Bioelectrochemistry 2015, 103, 2–6. [Google Scholar] [CrossRef]
- Semenov, I.; Xiao, S.; Pakhomov, A.G. Primary pathways of intracellular Ca(2+) mobilization by nanosecond pulsed electric field. Biochim. Biophys. Acta 2013, 1828, 981–989. [Google Scholar] [CrossRef]
- Vernier, P.T.; Sun, Y.; Chen, M.T.; Gundersen, M.A.; Craviso, G.L. Nanosecond electric pulse-induced calcium entry into chromaffin cells. Bioelectrochemistry 2008, 73, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Beebe, S.J.; Chen, X.; Liu, J.A.; Schoenbach, K.H. Nanosecond pulsed electric field ablation of hepatocellular carcinoma. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 6861–6865. [Google Scholar]
- Nuccitelli, R.; Wood, R.; Kreis, M.; Athos, B.; Huynh, J.; Lui, K.; Nuccitelli, P.; Epstein, E.H., Jr. First-in-human trial of nanoelectroablation therapy for basal cell carcinoma: Proof of method. Exp. Derm. 2014, 23, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.E.; Rossmeisl, J.H., Jr.; Garcia, P.A.; Lanz, O.I.; Henao-Guerrero, N.; Davalos, R.V. Successful treatment of a large soft tissue sarcoma with irreversible electroporation. J. Clin. Oncol. 2011, 29, e372–e377. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Davalos, R.V.; Bischof, J.C. A review of basic to clinical studies of irreversible electroporation therapy. IEEE Trans. Biomed. Eng. 2015, 62, 4–20. [Google Scholar] [CrossRef]
- Martin, R.C., 2nd; Kwon, D.; Chalikonda, S.; Sellers, M.; Kotz, E.; Scoggins, C.; McMasters, K.M.; Watkins, K. Treatment of 200 Locally Advanced (Stage III) Pancreatic Adenocarcinoma Patients With Irreversible Electroporation: Safety and Efficacy. Ann. Surg. 2015, 262, 486–494. [Google Scholar] [CrossRef]
- Wasson, E.M.; Ivey, J.W.; Verbridge, S.S.; Davalos, R.V. The Feasibility of Enhancing Susceptibility of Glioblastoma Cells to IRE Using a Calcium Adjuvant. Ann. Biomed. Eng. 2017, 45, 2535–2547. [Google Scholar] [CrossRef]
- Chen, Y.; Moser, M.A.J.; Luo, Y.; Zhang, W.; Zhang, B. Chemical Enhancement of Irreversible Electroporation: A Review and Future Suggestions. Technol. Cancer Res. Treat. 2019, 18, 1533033819874128. [Google Scholar] [CrossRef]
- Novickij, V.; Cesna, R.; Perminaite, E.; Zinkeviciene, A.; Characiejus, D.; Novickij, J.; Satkauskas, S.; Ruzgys, P.; Girkontaite, I. Antitumor Response and Immunomodulatory Effects of Sub-Microsecond Irreversible Electroporation and Its Combination with Calcium Electroporation. Cancers 2019, 11, 1763. [Google Scholar] [CrossRef]
- Wasson, E.M.; Alinezhadbalalami, N.; Brock, R.M.; Allen, I.C.; Verbridge, S.S.; Davalos, R.V. Understanding the role of calcium-mediated cell death in high-frequency irreversible electroporation. Bioelectrochemistry 2020, 131, 107369. [Google Scholar] [CrossRef]
- Hanna, H.; Denzi, A.; Liberti, M.; Andre, F.M.; Mir, L.M. Electropermeabilization of Inner and Outer Cell Membranes with Microsecond Pulsed Electric Fields: Quantitative Study with Calcium Ions. Sci. Rep. 2017, 7, 13079. [Google Scholar] [CrossRef]
- Guionet, A.; Moosavi Nejad, S.; Teissie, J.; Sakugawa, T.; Katsuki, S.; Akiyama, H.; Hosseini, H. Spatio-temporal dynamics of calcium electrotransfer during cell membrane permeabilization. Drug Deliv. Transl. Res. 2018, 8, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Sannino, A.; Scarfi, M.R.; Vernier, P.T.; Cadossi, R.; Gehl, J.; Zeni, O. ESOPE-Equivalent Pulsing Protocols for Calcium Electroporation: An In Vitro Optimization Study on 2 Cancer Cell Models. Technol. Cancer Res. Treat. 2018, 17, 1533033818788072. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wu, Y.H.; Yin, D.; Koeffler, H.P.; Sawcer, D.E.; Vernier, P.T.; Gundersen, M.A. Differential sensitivities of malignant and normal skin cells to nanosecond pulsed electric fields. Technol. Cancer Res. Treat. 2011, 10, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Guerini, D.; Garcia-Martin, E.; Zecca, A.; Guidi, F.; Carafoli, E. The calcium pump of the plasma membrane: Membrane targeting, calcium binding sites, tissue-specific isoform expression. Acta Physiol. Scandsuppl. 1998, 643, 265–273. [Google Scholar]
- Guerini, D. The significance of the isoforms of plasma membrane calcium ATPase. Cell Tissue Res. 1998, 292, 191–197. [Google Scholar] [CrossRef]
- Curry, M.C.; Luk, N.A.; Kenny, P.A.; Roberts-Thomson, S.J.; Monteith, G.R. Distinct regulation of cytoplasmic calcium signals and cell death pathways by different plasma membrane calcium ATPase isoforms in MDA-MB-231 breast cancer cells. J. Biol. Chem. 2012, 287, 28598–28608. [Google Scholar] [CrossRef]
- Kosowski, H.M.R.; Schild, L.; Halangk, W. Electropulsing of acinar cells isolated from rat pancreas: Dependence of reversible membrane perforation on cellular energy state. Bioelectro. Bioenerg. 1995, 38, 377–381. [Google Scholar] [CrossRef]
- Rols, M.P.; Teissie, J. Electropermeabilization of mammalian cells. Quantitative analysis of the phenomenon. Biophys. J. 1990, 58, 1089–1098. [Google Scholar] [CrossRef]
- Cerella, C.; Diederich, M.; Ghibelli, L. The dual role of calcium as messenger and stressor in cell damage, death, and survival. Int. J. Cell Biol. 2010, 2010, 546163–546176. [Google Scholar] [CrossRef]
- Gibot, L.A.M.; Rols, M.P. Calcium delivery by electroporation induces in vitro cell death through mitochondrial dysfunction without genetoxicity. In Proceedings of the 3rd World Congress on Electroporation and Pulsed Electric Fields in Biology, Medicine and Food & Environmental Technologies, Toulouse, France, 3–6 September 2019. [Google Scholar]
- Viollet, B.; Guigas, B.; Sanz Garcia, N.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and molecular mechanisms of metformin: An overview. Clin. Sci. 2012, 122, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Calvet, C.Y.; Famin, D.; Andre, F.M.; Mir, L.M. Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells. Oncoimmunology 2014, 3, e28131. [Google Scholar] [CrossRef] [PubMed]
- Kulbacka, J.P.J.; Rembiałkowska, N.; Saczko, J.; Kiełbowicz, Z.; Kinda, W.; Liszka, B.; Kotulska, M.; Kos, B.; Miklavčič, D.; Tozon, N.; et al. Electrochemotherapy combined with standard and CO2 laser surgeries in canine oral melanoma. Slov. Vet. Res. 2017, 54, 181–186. [Google Scholar] [CrossRef]
- Dos Anjos, D.S.; Rodrigues, C.G.; Silva, N.C.; De Nardi, A.B.; Fonseca-Alves, C.E. Electrochemotherapy Associated with Calcium Electroporation in Metastatic Feline Cutaneous Malignant Melanoma. Acta Sci. Vet. 2019, 47, 435. [Google Scholar]
- Galant, L.; Delverdier, M.; Lucas, M.N.; Raymond-Letron, I.; Teissie, J.; Tamzali, Y. Calcium electroporation: The bioelectrochemical treatment of spontaneous equine skin tumors results in a local necrosis. Bioelectrochemistry 2019, 129, 251–258. [Google Scholar] [CrossRef]
- Falk, H.; Lambaa, S.; Johannesen, H.H.; Wooler, G.; Venzo, A.; Gehl, J. Electrochemotherapy and calcium electroporation inducing a systemic immune response with local and distant remission of tumors in a patient with malignant melanoma—a case report. Acta Oncol. 2017, 56, 1126–1131. [Google Scholar] [CrossRef]
- Plaschke, C.C.; Gehl, J.; Johannesen, H.H.; Fischer, B.M.; Kjaer, A.; Lomholt, A.F.; Wessel, I. Calcium electroporation for recurrent head and neck cancer: A clinical phase I study. Laryngoscope Investig. Otolaryngol. 2019, 4, 49–56. [Google Scholar] [CrossRef]
- Agoston, D.; Baltas, E.; Ocsai, H.; Ratkai, S.; Lazar, P.G.; Korom, I.; Varga, E.; Nemeth, I.B.; Dosa-Racz Viharosne, E.; Gehl, J.; et al. Evaluation of Calcium Electroporation for the Treatment of Cutaneous Metastases: A Double Blinded Randomised Controlled Phase II Trial. Cancers (Basel) 2020, 12, 179. [Google Scholar] [CrossRef]
- WHO—Cancer. Available online: http://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 23 December 2019).
- Gyawali, B.; Sullivan, R. Economics of Cancer Medicines: For Whose Benefit? New Bioeth. 2017, 23, 95–104. [Google Scholar] [CrossRef]
- Sullivan, R.; Peppercorn, J.; Sikora, K.; Zalcberg, J.; Meropol, N.J.; Amir, E.; Khayat, D.; Boyle, P.; Autier, P.; Tannock, I.F.; et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011, 12, 933–980. [Google Scholar] [CrossRef]
- Kerr, D.J.; Midgley, R. Can we treat cancer for a dollar a day? Guidelines for low-income countries. N. Engl. J. Med. 2010, 363, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Knaul, F.M.; Atun, R.; Farmer, P.; Frenk, J. Seizing the opportunity to close the cancer divide. Lancet 2013, 381, 2238–2239. [Google Scholar] [CrossRef]
- Son, R.S.; Gowrishankar, T.R.; Smith, K.C.; Weaver, J.C. Modeling a Conventional Electroporation Pulse Train: Decreased Pore Number, Cumulative Calcium Transport and an Example of Electrosensitization. IEEE Trans. Biomed. Eng. 2016, 63, 571–580. [Google Scholar] [CrossRef] [PubMed]

| Model | Author | Investigated Cell Types | Cell Condition | Observation |
|---|---|---|---|---|
| In Vitro | Zielichowska et al. 2016 [78] | Murine normal muscle cells; murine sarcoma cells | Suspension | Less cell death in normal cells than malignant cells |
| Szewczyk et al. 2017 [82] | Murine normal muscle cells; murine sarcoma cells | Suspension and attached (differentiated and undifferentiated) | Less cell death in normal cells than malignant cells | |
| Frandsen et al. 2018 [80] | Human primary dermal fibroblasts | Suspension | Induced cell death in normal cells | |
| Staresinic et al. 2018 [81] | Human umbilical endothelial cells; Chinese hamster ovary cells | Suspension | Induced cell death in normal cells | |
| 3D Spheroid | Frandsen et al. 2015 [85] | Human breast-, bladder-, and colon cancer and primary dermal fibroblasts | Spheroids | Cell death induced in all three cancer cell lines but affected normal cells less |
| In Vivo | Frandsen et al. 2017 [61] | Human SCLC *; breast-; bladder-; colon cancer tumors; normal skin and normal muscle | Tissue | Induced necrosis in all tumor types but limited effect on normal tissue |
| Clinicaltrials.gov | Condition | Palliative/Neoadjuvant | Published/Ongoing |
|---|---|---|---|
| NCT01941901 | Cutaneous metastases from breast cancer and malignant melanoma | Palliative | Falk et al., 2018 [19] |
| NCT03628417 | Cutaneous metastases from breast cancer and malignant melanoma | Palliative | Ágoston et al, 2020 [121] |
| NCT04225767 | Cutaneous tumors | Palliative | Ready to start accrual Feb. 2020 |
| NCT03051269 | Recurrent head and neck cancer | Palliative | Plaschke et al., 2019 [120] |
| NCT03542214 | Colorectal cancer | Palliative | Ongoing |
| NCT03694080 | Colorectal cancer | Neoadjuvant | Ongoing |
| NCT01941914 | Keloid | -- | Inclusion completed (7 patients) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frandsen, S.K.; Vissing, M.; Gehl, J. A Comprehensive Review of Calcium Electroporation—A Novel Cancer Treatment Modality. Cancers 2020, 12, 290. https://doi.org/10.3390/cancers12020290
Frandsen SK, Vissing M, Gehl J. A Comprehensive Review of Calcium Electroporation—A Novel Cancer Treatment Modality. Cancers. 2020; 12(2):290. https://doi.org/10.3390/cancers12020290
Chicago/Turabian StyleFrandsen, Stine K., Mille Vissing, and Julie Gehl. 2020. "A Comprehensive Review of Calcium Electroporation—A Novel Cancer Treatment Modality" Cancers 12, no. 2: 290. https://doi.org/10.3390/cancers12020290
APA StyleFrandsen, S. K., Vissing, M., & Gehl, J. (2020). A Comprehensive Review of Calcium Electroporation—A Novel Cancer Treatment Modality. Cancers, 12(2), 290. https://doi.org/10.3390/cancers12020290