Shaping the Innate Immune Response by Dietary Glucans: Any Role in the Control of Cancer?
Abstract
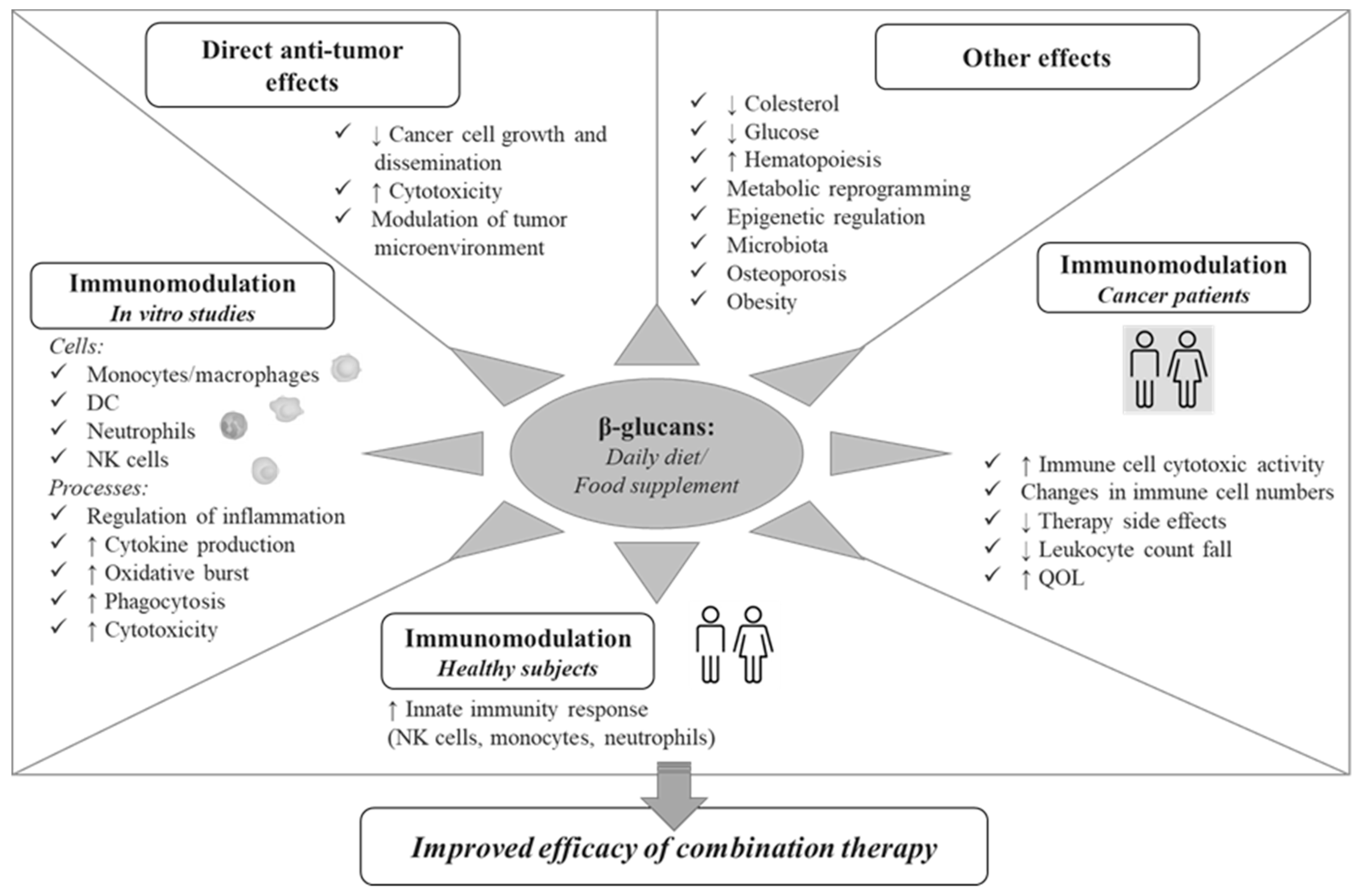
1. Introduction
2. Dietary β-glucans: Main Features and Sources
3. Immunomodulatory Effects of β-glucans
3.1. In Vitro Effects on Innate Immunity Cells
| Compound (Concentration Range 1) | Cell Type | Effects | Molecular Mechanisms | Refs |
|---|---|---|---|---|
| Agaricus brasiliensis acid-treated polysaccharide-rich fraction (50 µg/mL) | Monocytes | ↑ Adherence ↑ Phagocytosis ↑ TNFα , IL-1β, IL-10 | ↑ TLR2 and TLR4 = βGR or MR | [39] |
| Flammulina velutipes extract | Monocytes Macrophages | ↑ Cytokine production ↑ Phagocytosis ↑ ROS | [40] | |
| Agaricus blazei Murill extract (1–15%) | Monocytes | ↑ IL-8, TNFα, IL-1β, IL-6 | [41] | |
| Agaricus blazei Murill extract (0.5–15%) | Monocytes | ↑ Phagocytosis | ↑ CD11b ↓ CD62L | [42] |
| Pleurotus citrinopileatus polysaccharide (PCPS, 0.5 µg/mL) | Monocytes Macrophages | Differentiation of monocytes toward macrophages (IFNγ + LPS) with reduced proinflammatory capacity: ↓ TNFα, IL-6 and CCR2 mRNA ↑ IL-10, CCL2 and CCL8 mRNA | Dectin-1 and TLR2 signaling pathways | [43] |
| Piptoporus betulinus extract | Monocytes | ↓ Apoptosis ↑ IL-8 | [44] | |
| MDDC | ↑ Maturation ↑ IL-8 | |||
| Pleurotus citrinopileatus polysaccharide (PCPS, 0.01–5 µg/mL) | MDDC | ↑ CD80, CD86, HLA-DR ↑ Pro- and anti-inflammatory cytokines (TNFα, IL-1β, IL-6, IL-12, IL-10) ↑ mRNA: CCL2, CCL3, CCL8, CXCL9, CXCL10, and LTA | Dectin-1, TLR2 and TLR4 signaling pathways | [45] |
| Armillariella mellea water-soluble components (2–20 µg/mL) | MDDC | ↑ CD80, CD83, CD86, MHC class I and II, CD205 ↓ CD206 ↓ Endocytic capacity = TNFα, IL-12, IL-10 | [46] | |
| Hericium erinaceum water-soluble components (2–20 µg/mL) | MDDC | ↑ CD80, CD83, CD86, MHC class I and II, CD205 ↓ CD206 ↓ Endocytic capacity = TNFα, IL-12 | [47] | |
| Agaricus blazei Murill Extract (10% ABM = 2.8 g of β-glucan/100 g) | MDDC | ↑ IL-8, G-CSF, TNFα, IL-1β, IL-6, CCL4 | [48] | |
| Various higher Basidiomycetes exctracts (0.0005–5 mg/mL) | Neutrophils | ↑ ROS | [49] | |
| Pleurotus ostreatus β-glucans extracted from fruiting bodies (5 mg/mL) | NK | ↑ Cytotoxic effects against lung and breast cancer cell lines | ↑ KIR2DL genes ↑ NKG2D, IFNγ, NO | [50] |
| Grifola frondosa polysaccharide (10 mg/L), Lentinan (500 mg/L), Yeast glucan (100 mg/L) | NK | ↑ Cytotoxicity, IFNγ, perforin secretion | ↑ NKp30 expression | [51] |
| Saccaromyces cerevisiae glucan from baker’s yeast and zymosan (10 or 100 µg/mL) | Macrophages | ↑ IL-1β transcription and secretion | Dectin 1/Syk signaling pathway NLPR3 activation | [52] |
| Saccaromyces cerevisiae whole β-glucan particles (100 µg/mL) | MDDC | ↑ CD40, CD86, HLA-DR ↑ IL12, IL-2, TNF, IFNγ ↑ CD8 T cell priming ↑ Tumor-specific CTL activity | PI3K/Akt signalling | [53] |
| Saccaromyces cerevisiae baker’s yeast | MDDC | ↑ Th17 cells ↑ Adhesion and migration | IL-1α, IFNγ | [54] |
| Saccharomyces cerevisiae zymosan (1 mg/mL) | MDDC | ↑ IL-23 | LTB4, PAF | [55] |
| Saccharomyces cerevisiae zymosan (1 mg/mL) | MDDC Macrophages | ↑ p-STAT3 ↑ mRNA: IL-10, IL-23, INF1B, CSF1, CSF2 and CSF3 | PGE2 | [56] |
| Saccaromyces cerevisiae Imprime PGG (10 µg/mL) | Monocytes | ↑ ADCP ↑ C5a ↑ IL-8, CCL2 ↑ CD11b ↓ CD62L, CD88, CXCR2 ↑ Phenotypic and functional activation | Formation of an immune complex with naturally occurring ABA | [57] |
| Neutrophils | ↑ ROS ↑ IL-8, CCL2 ↑ CD11b ↓ CD62L, CD88, CXCR2 | |||
| Saccaromyces cerevisiae Betafectin PGG (0–300 µg/mL) | Neutrophils | ↑ Chemotaxis toward C5a ↓ Chemotaxis toward IL-8 | CR3-dependent | [58] |
| Barley polysaccharides (100 µg/mL) | MDDC | ↑ Phenotypic and functional maturation of DC ↑ IL-12, IL-10 | [59] | |
| Barley β-glucan | Umbilical cord blood-generated DC | ↓ CCL2 ↑ CD83 cells | [60] |
3.2. In Vivo Effects in Healthy Subjects
| Compound (Concentration Range 1) | Subjects/Study Type | Control Group | β-glucan Group | Major Findings | Refs. |
|---|---|---|---|---|---|
| Pleurotus ostreatus β-glucan (Pleuran, 100 mg/day) | Regularly training athletes/Randomized | Vitamin C (n = 25) | β-glucan + vitamin C (n = 25) | ↑ NK cell frequency ↑ PMN-mediated phagocytosis ↓ URTI symptom incidence | [63] |
| Pleurotus ostreatus β-glucan (Pleuran, 100 mg/day) | Elite athletes/Randomized | Fructose + vitamin C (n = 11) | β-glucan + vitamin C (n = 9) | Restrained high intensity PA-induced reduction of NK cell number and activity = Monocyte and granulocyte counts | [64] |
| Pleurotus cornucopiae water extract (β-glucan 24 mg/meal) | Healthy volunteers/ Randomized | Water, tea, oyster souce, caffeine-free coffee (n = 21) | β-glucan + water, tea, oyster souce, caffeine-free coffee (n = 20) | ↑ NK cell activity ↑ Th1-type response | [65] |
| Agaricus blazei Murill extract (AndoSan, 60 mL/day = 5.7 g β-glucan) | Healthy volunteers/Intervention study | None | Mushroom extract (n = 10) | ↓ Intracellular ROS in monocytes and granulocytes vs baseline | [66] |
| Grifola frondosa extract (6 mg/kg/day) | Myelodysplastic syndrome patients/ Non randomized phase II trial | None | Mushroom extract (n = 21) | ↑ Neutrophil and monocyte functions (ROS production) | [67] |
| Oat soluble β-glucan (5.6 g) | Trained male cyclists (on intense exercise)/ Randomized | Cornstarch + Gatorade (n = 20) | β-glucan (Oatvantge) + Gatorade (n = 20) | No rescue of NK cell activity No rescue of PNM-RBA No effect on URTI symptom incidence | [68] |
| Saccharomyces cerevisiae β-glucan (Purified, Imuneks, 20 mg/day) | Subjects with seasonal allergic rhinitis (allergen sensitized)/ Randomized | Nihil (n = 12) | β-glucan (n = 12) | ↓ Eosinophil frequency in the nasal fluid lavage | [69] |
| Saccharomyces cerevisiae β-glucan (insoluble, 1 g/day) | Healthy volunteers/ Intervention study | Nihil (n = 5) | β-glucan (n = 10) | No effect on phagocyte functions (cytokine production + microbicide activity) | [70] |
3.3. In Vivo Effects in Cancer Patients
| Compound (Concentration Range 1) | Cancer Type | Conventional Therapy | Treated Patients (N) | Major Findings | Refs. |
|---|---|---|---|---|---|
| Agaricus blazei Murill extract | Gynecological | Yes | 39 | ↑ NK cell activity = LAK and monocyte activity ↓ Chemotherapy-induced side effects | [73] |
| Agaricus blazei Murill extract (AndoSan, 60 mL/day = 5.7 g β-glucan) | Multiple myeloma | Yes | 19 | ↑ Treg and pDC numbers ↑ IL-1Ra, IL-5, IL-7 ↑ Ig, KIR, HLA gene expression | [74] |
| Lentinula edodes mycelia extract (1.8 g/day) | Advanced breast | Yes | 10 | Restrained chemotherapy-induced reduction of NK and LAK cell activity and of white blood cell/neutrophil counts ↑ QOL | [75] |
| Lentinula edodes mycelia extract (1.8 g/day) | Breast, gastric, colorectal, esophageal | Yes | 7 | ↑ NK cell and LAK activity ↑ QOL ↓ IAP | [76] |
| Lentinula edodes β-glucan Lentinan (1 mg/every other day) | Esophageal | Yes | 25 | ↓ Chemotherapy side effects ↑ QOL ↑ IL-12, IL-2, IL-6 ↓IL-4, IL-5, IL-10 | [77] |
| Lentinula edodes β-glucan Lentinan | Gastric | Yes | 20 | ↑ QOL | [78] |
| Lentinula edodes β-glucan Lentinan (2 mg/Kg/week) | Unresectable or recurrent gastric | Yes | 147 | = Leukocyte and neutrophil counts = Side-effects = QOL | [79] |
| Yeast β-glucan (Purified, Imuneks, 20 mg/day) | Advanced breast | Yes | 15 | Restrained chemotherapy-induced reduction of white blood cells = Neutrophil and monocyte counts ↑ IL-12 ↓IL-4 ↑ QOL | [80,81] |
| Yeast β-glucan (Purified, Imuneks, 20 mg/day) | Advanced breast | Yes | 8 | ↑ CD14+ monocyte number ↑ CD95 and CD45RA expression in monocytes | [82] |
| Agaricus bisporus powder (4–14 g/day) | Recurrent prostate | No | 36 | ↓ MDSC numbers ↑ IL-15 | [83] |
| Grifola frondosa D-Fraction (40–150 mg/day) | Advanced lung and breast | No | 10 | ↑ NK cell activity | [84] |
| Grifola frondosa D-Fraction (0.1–5 mg/twice/day | Breast | No | 34 | ↑ NKT and Treg cell numbers ↑ Response of ex-vivo immune cells | [85] |
| Yeast β-glucan (500 mg/day) | Newly diagnosed NSCLC | No | 23 | ↓ MDSC numbers | [86] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2019, 10.1038/s41577-019-0210-z. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Franco, P.I.; Rodrigues, A.P.; de Menezes, L.B.; Pacheco Miguel, M. Tumor microenvironment components: Allies of cancer progression. Pathol. Res. Pract. 2019, 152729. [Google Scholar] [CrossRef]
- Lei, X.; Lei, Y.; Li, J.K.; Du, W.X.; Li, R.G.; Yang, J.; Li, J.; Li, F.; Tan, H.B. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2019. [Google Scholar] [CrossRef]
- Woo, S.R.; Corrales, L.; Gajewski, T.F. The STING pathway and the T cell-inflamed tumor microenvironment. Trends Immunol. 2015, 36, 250–256. [Google Scholar] [CrossRef]
- Mayne, S.T.; Playdon, M.C.; Rock, C.L. Diet, nutrition, and cancer: Past, present and future. Nat. Rev. Clin. Oncol. 2016, 13, 504–515. [Google Scholar] [CrossRef]
- Fuller, S.; Beck, E.; Salman, H.; Tapsell, L. New Horizons for the Study of Dietary Fiber and Health: A Review. Plant Foods Hum. Nutr. 2016, 71, 1–12. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Choi, J.S. Clinical and Physiological Perspectives of beta-Glucans: The Past, Present, and Future. Int. J. Mol. Sci. 2017, 18, 1906. [Google Scholar] [CrossRef]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelou, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary fiber and health outcomes: An umbrella review of systematic reviews and meta-analyses. Am. J. Clin. Nutr. 2018, 107, 436–444. [Google Scholar] [CrossRef]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. beta-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Dilshad, S.M. Beta glucan: A valuable functional ingredient in foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Sukhithasri, V.; Nisha, N.; Biswas, L.; Anil Kumar, V.; Biswas, R. Innate immune recognition of microbial cell wall components and microbial strategies to evade such recognitions. Microbiol. Res. 2013, 168, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Zekovic, D.B.; Kwiatkowski, S.; Vrvic, M.M.; Jakovljevic, D.; Moran, C.A. Natural and modified (1-->3)-beta-D-glucans in health promotion and disease alleviation. Crit. Rev. Biotechnol. 2005, 25, 205–230. [Google Scholar] [CrossRef]
- Jin, Y.; Li, P.; Wang, F. beta-glucans as potential immunoadjuvants: A review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine 2018, 36, 5235–5244. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Batbayar, S.; Lee, D.H.; Kim, H.W. Immunomodulation of Fungal beta-Glucan in Host Defense Signaling by Dectin-1. Biomol. Ther. 2012, 20, 433–445. [Google Scholar] [CrossRef]
- Rice, P.J.; Adams, E.L.; Ozment-Skelton, T.; Gonzalez, A.J.; Goldman, M.P.; Lockhart, B.E.; Barker, L.A.; Breuel, K.F.; Deponti, W.K.; Kalbfleisch, J.H.; et al. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J. Pharmacol. Exp. Ther. 2005, 314, 1079–1086. [Google Scholar] [CrossRef]
- Hong, F.; Yan, J.; Baran, J.T.; Allendorf, D.J.; Hansen, R.D.; Ostroff, G.R.; Xing, P.X.; Cheung, N.K.; Ross, G.D. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J. Immunol. 2004, 173, 797–806. [Google Scholar] [CrossRef]
- Sandvik, A.; Wang, Y.Y.; Morton, H.C.; Aasen, A.O.; Wang, J.E.; Johansen, F.E. Oral and systemic administration of beta-glucan protects against lipopolysaccharide-induced shock and organ injury in rats. Clin. Exp. Immunol. 2007, 148, 168–177. [Google Scholar] [CrossRef]
- Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrieres, V. Molecular Interactions of beta-(1-->3)-Glucans with Their Receptors. Molecules 2015, 20, 9745–9766. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.R.; Brown, G.D.; Reid, D.M.; Willment, J.A.; Martinez-Pomares, L.; Gordon, S.; Wong, S.Y. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 2002, 169, 3876–3882. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Pang, L.; Yao, L.; ShangGuan, Z.; Pan, Y. Agaricus bisporus-derived beta-glucan enter macrophages and adipocytes by CD36 receptor. Nat. Prod. Res. 2019, 1–4. [Google Scholar] [CrossRef]
- Li, S.S.; Ogbomo, H.; Mansour, M.K.; Xiang, R.F.; Szabo, L.; Munro, F.; Mukherjee, P.; Mariuzza, R.A.; Amrein, M.; Vyas, J.M.; et al. Identification of the fungal ligand triggering cytotoxic PRR-mediated NK cell killing of Cryptococcus and Candida. Nat. Commun. 2018, 9, 751. [Google Scholar] [CrossRef]
- de Graaff, P.; Govers, C.; Wichers, H.J.; Debets, R. Consumption of beta-glucans to spice up T cell treatment of tumors: A review. Expert Opin. Biol. Ther. 2018, 18, 1023–1040. [Google Scholar] [CrossRef]
- Geller, A.; Shrestha, R.; Yan, J. Yeast-Derived beta-Glucan in Cancer: Novel Uses of a Traditional Therapeutic. Int. J. Mol. Sci. 2019, 20, 3618. [Google Scholar] [CrossRef]
- Pan, P.; Huang, Y.W.; Oshima, K.; Yearsley, M.; Zhang, J.; Arnold, M.; Yu, J.; Wang, L.S. The immunomodulatory potential of natural compounds in tumor-bearing mice and humans. Crit. Rev. Food Sci. Nutr. 2019, 59, 992–1007. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P. beta-glucan as a new tool in vaccine development. Scand. J. Immunol. 2019, e12833. [Google Scholar] [CrossRef]
- Halstenson, C.E.; Shamp, T.; Gargano, M.A.; Walsh, R.M.; Patchen, M.L. Two randomized, double-blind, placebo-controlled, dose-escalation phase 1 studies evaluating BTH1677, a 1, 3-1,6 beta glucan pathogen associated molecular pattern, in healthy volunteer subjects. Investig. New Drug 2016, 34, 202–215. [Google Scholar] [CrossRef]
- Zent, C.S.; Call, T.G.; Bowen, D.A.; Conte, M.J.; LaPlant, B.R.; Witzig, T.E.; Ansell, S.M.; Weiner, G.J. Early treatment of high risk chronic lymphocytic leukemia with alemtuzumab, rituximab and poly-(1-6)-beta-glucotriosyl-(1-3)-beta- glucopyranose beta- glucan is well tolerated and achieves high complete remission rates. Leuk Lymphoma 2015, 56, 2373–2378. [Google Scholar] [CrossRef]
- Weitberg, A.B. A phase I/II trial of beta-(1,3)/(1,6) D-glucan in the treatment of patients with advanced malignancies receiving chemotherapy. J. Exp. Clin. Cancer Res. 2008, 27. [Google Scholar] [CrossRef] [PubMed]
- Lehne, G.; Haneberg, B.; Gaustad, P.; Johansen, P.W.; Preus, H.; Abrahamsen, T.G. Oral administration of a new soluble branched beta-1,3-D-glucan is well tolerated and can lead to increased salivary concentrations of immunoglobulin A in healthy volunteers. Clin. Exp. Immunol. 2006, 143, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.; Bihari, B.; Goosby, E.; Gorter, R.; Greco, M.; Guralnik, M.; Mimura, T.; Rudinicki, V.; Wong, R.; Kaneko, Y. A placebo-controlled trial of the immune modulator, lentinan, in HIV-positive patients: A phase I/II trial. J. Med. 1998, 29, 305–330. [Google Scholar] [PubMed]
- Ohno, S.; Sumiyoshi, Y.; Hashine, K.; Shirato, A.; Kyo, S.; Inoue, M. Phase I Clinical Study of the Dietary Supplement, Agaricus blazei Murill, in Cancer Patients in Remission. Evid. Based Compl. Alt. 2011, 1–9. [Google Scholar] [CrossRef]
- Synytsya, A.; Novak, M. Structural diversity of fungal glucans. Carbohydr. Polym. 2013, 92, 792–809. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.; Raptis, J.; Rice, P.J.; Kalbfleisch, J.H.; Stout, R.D.; Ensley, H.E.; Browder, W.; Williams, D.L. The influence of glucan polymer structure and solution conformation on binding to (1 -> 3)-beta-D-glucan receptors in a human monocyte-like cell line. Glycobiology 2000, 10, 339–346. [Google Scholar] [CrossRef]
- Adams, E.L.; Rice, P.J.; Graves, B.; Ensley, H.E.; Yu, H.; Brown, G.D.; Gordon, S.; Monteiro, M.A.; Papp-Szabo, E.; Lowman, D.W.; et al. Differential high-affinity interaction of dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branching. J. Pharmacol. Exp. Ther. 2008, 325, 115–123. [Google Scholar] [CrossRef]
- Williams, D.L. Overview of (1-->3)-beta-D-glucan immunobiology. Mediat. Inflamm. 1997, 6, 247–250. [Google Scholar] [CrossRef]
- Martins, P.R.; de Campos Soares, A.M.V.; da Silva Pinto Domeneghini, A.V.; Golim, M.A.; Kaneno, R. Agaricus brasiliensis polysaccharides stimulate human monocytes to capture Candida albicans, express toll-like receptors 2 and 4, and produce pro-inflammatory cytokines. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 17. [Google Scholar] [CrossRef]
- Kashina, S.; Villavicencio, L.L.; Zaina, S.; Ordaz, M.B.; Sabanero, G.B.; Fujiyoshi, V.T.; Lopez, M.S. Activity of Extracts from Submerged Cultured Mycelium of Winter Mushroom, Flammulina velutipes (Agaricomycetes), on the Immune System In Vitro. Int. J. Med. Mushrooms 2016, 18, 49–57. [Google Scholar] [CrossRef]
- Bernardshaw, S.; Hetland, G.; Ellertsen, L.K.; Tryggestad, A.M.; Johnson, E. An extract of the medicinal mushroom Agaricus blazei Murill differentially stimulates production of pro-inflammatory cytokines in human monocytes and human vein endothelial cells in vitro. Inflammation 2005, 29, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Bernardshaw, S.; Lyberg, T.; Hetland, G.; Johnson, E. Effect of an extract of the mushroom Agaricus blazei Murill on expression of adhesion molecules and production of reactive oxygen species in monocytes and granulocytes in human whole blood ex vivo. Apmis 2007, 115, 719–725. [Google Scholar] [CrossRef]
- Minato, K.I.; Laan, L.C.; van Die, I.; Mizuno, M. Pleurotus citrinopileatus polysaccharide stimulates anti-inflammatory properties during monocyte-to-macrophage differentiation. Int. J. Biol. Macromol. 2019, 122, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, F.; Steinborn, C.; Huber, R.; Wille, R.; Meier, S.; Alresly, Z.; Lindequist, U.; Grundemann, C. Effects of Birch Polypore Mushroom, Piptoporus betulinus (Agaricomycetes), the “Iceman’s Fungus”, on Human Immune Cells. Int. J. Med. Mushrooms 2018, 20, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Minato, K.I.; Laan, L.C.; Ohara, A.; van Die, I. Pleurotus citrinopileatus polysaccharide induces activation of human dendritic cells through multiple pathways. Int. Immunopharmacol. 2016, 40, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Im, J.; Yun, C.H.; Son, J.Y.; Son, C.G.; Park, D.K.; Han, S.H. Armillariella mellea induces maturation of human dendritic cells without induction of cytokine expression. J. Ethnopharmacol. 2008, 119, 153–159. [Google Scholar] [CrossRef]
- Kim, S.K.; Son, C.G.; Yun, C.H.; Han, S.H. Hericium erinaceum induces maturation of dendritic cells derived from human peripheral blood monocytes. Phytother. Res. 2010, 24, 14–19. [Google Scholar] [CrossRef]
- Forland, D.T.; Johnson, E.; Tryggestad, A.M.; Lyberg, T.; Hetland, G. An extract based on the medicinal mushroom Agaricus blazei Murill stimulates monocyte-derived dendritic cells to cytokine and chemokine production in vitro. Cytokine 2010, 49, 245–250. [Google Scholar] [CrossRef]
- Shamtsyan, M.; Konusova, V.; Maksimova, Y.; Goloshchev, A.; Panchenko, A.; Simbirtsev, A.; Petrishchev, N.; Denisova, N. Immunomodulating and anti-tumor action of extracts of several mushrooms. J. Biotechnol. 2004, 113, 77–83. [Google Scholar] [CrossRef]
- El-Deeb, N.M.; El-Adawi, H.I.; El-Wahab, A.E.A.; Haddad, A.M.; El Enshasy, H.A.; He, Y.W.; Davis, K.R. Modulation of NKG2D, KIR2DL and Cytokine Production by Pleurotus ostreatus Glucan Enhances Natural Killer Cell Cytotoxicity Toward Cancer Cells. Front. Cell Dev. Biol. 2019, 7, 165. [Google Scholar] [CrossRef]
- Huyan, T.; Li, Q.; Yang, H.; Jin, M.L.; Zhang, M.J.; Ye, L.J.; Li, J.; Huang, Q.S.; Yin, D.C. Protective effect of polysaccharides on simulated microgravity-induced functional inhibition of human NK cells. Carbohydr. Polym. 2014, 101, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Kankkunen, P.; Teirila, L.; Rintahaka, J.; Alenius, H.; Wolff, H.; Matikainen, S. (1,3)-beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J. Immunol. 2010, 184, 6335–6342. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Feng, T.; Ning, Y.; Li, W.; Wu, Q.; Qian, K.; Wang, Y.; Qi, C. beta-Glucan enhances cytotoxic T lymphocyte responses by activation of human monocyte-derived dendritic cells via the PI3K/AKT pathway. Hum. Immunol. 2015, 76, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Cardone, M.; Dzutsev, A.K.; Li, H.; Riteau, N.; Gerosa, F.; Shenderov, K.; Winkler-Pickett, R.; Provezza, L.; Riboldi, E.; Leighty, R.M.; et al. Interleukin-1 and interferon-gamma orchestrate beta-glucan-activated human dendritic cell programming via IkappaB-zeta modulation. PLoS ONE 2014, 9, e114516. [Google Scholar] [CrossRef]
- Rodriguez, M.; Marquez, S.; Montero, O.; Alonso, S.; Frade, J.G.; Crespo, M.S.; Fernandez, N. Pharmacological inhibition of eicosanoids and platelet-activating factor signaling impairs zymosan-induced release of IL-23 by dendritic cells. Biochem. Pharmacol. 2016, 102, 78–96. [Google Scholar] [CrossRef]
- Rodriguez, M.; Marquez, S.; de la Rosa, J.V.; Alonso, S.; Castrillo, A.; Sanchez Crespo, M.; Fernandez, N. Fungal pattern receptors down-regulate the inflammatory response by a cross-inhibitory mechanism independent of interleukin-10 production. Immunology 2017, 150, 184–198. [Google Scholar] [CrossRef]
- Chan, A.S.; Jonas, A.B.; Qiu, X.; Ottoson, N.R.; Walsh, R.M.; Gorden, K.B.; Harrison, B.; Maimonis, P.J.; Leonardo, S.M.; Ertelt, K.E.; et al. Imprime PGG-Mediated Anti-Cancer Immune Activation Requires Immune Complex Formation. PLoS ONE 2016, 11, e0165909. [Google Scholar] [CrossRef]
- Tsikitis, V.L.; Albina, J.E.; Reichner, J.S. Beta-glucan affects leukocyte navigation in a complex chemotactic gradient. Surgery 2004, 136, 384–389. [Google Scholar] [CrossRef]
- Chan, W.K.; Law, H.K.; Lin, Z.B.; Lau, Y.L.; Chan, G.C. Response of human dendritic cells to different immunomodulatory polysaccharides derived from mushroom and barley. Int. Immunol. 2007, 19, 891–899. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Sahasrabudhe, N.M.; Rosch, C.; Schols, H.A.; Faas, M.M.; de Vos, P. The impact of dietary fibers on dendritic cell responses in vitro is dependent on the differential effects of the fibers on intestinal epithelial cells. Mol. Nutr. Food Res. 2015, 59, 698–710. [Google Scholar] [CrossRef]
- Wakshull, E.; Brunke-Reese, D.; Lindermuth, J.; Fisette, L.; Nathans, R.S.; Crowley, J.J.; Tufts, J.C.; Zimmerman, J.; Mackin, W.; Adams, D.S. PGG-glucan, a soluble beta-(1,3)-glucan, enhances the oxidative burst response, microbicidal activity, and activates an NF-kappa B-like factor in human PMN: Evidence for a glycosphingolipid beta-(1,3)-glucan receptor. Immunopharmacology 1999, 41, 89–107. [Google Scholar] [CrossRef]
- Bose, N.; Chan, A.S.; Guerrero, F.; Maristany, C.M.; Qiu, X.; Walsh, R.M.; Ertelt, K.E.; Jonas, A.B.; Gorden, K.B.; Dudney, C.M.; et al. Binding of Soluble Yeast beta-Glucan to Human Neutrophils and Monocytes is Complement-Dependent. Front. Immunol. 2013, 4, 230. [Google Scholar] [CrossRef] [PubMed]
- Bergendiova, K.; Tibenska, E.; Majtan, J. Pleuran (beta-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur. J. Appl. Physiol. 2011, 111, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Bobovcak, M.; Kuniakova, R.; Gabriz, J.; Majtan, J. Effect of Pleuran (beta-glucan from Pleurotus ostreatus) supplementation on cellular immune response after intensive exercise in elite athletes. Appl. Physiol. Nutr. Metab. 2010, 35, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nishimura, M.; Sato, Y.; Sato, H.; Nishihira, J. Enhancement of the Th1-phenotype immune system by the intake of Oyster mushroom (Tamogitake) extract in a double-blind, placebo-controlled study. J. Tradit. Complementary Med. 2016, 6, 424–430. [Google Scholar] [CrossRef]
- Johnson, E.; Forland, D.T.; Hetland, G.; Saetre, L.; Olstad, O.K.; Lyberg, T. Effect of AndoSan on expression of adhesion molecules and production of reactive oxygen species in human monocytes and granulocytes in vivo. Scand. J. Gastroenterol. 2012, 47, 984–992. [Google Scholar] [CrossRef]
- Wesa, K.M.; Cunningham-Rundles, S.; Klimek, V.M.; Vertosick, E.; Coleton, M.I.; Yeung, K.S.; Lin, H.; Nimer, S.; Cassileth, B.R. Maitake mushroom extract in myelodysplastic syndromes (MDS): A phase II study. Cancer Immunol. Immunother. 2015, 64, 237–247. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; McMahon, M.; Wrieden, J.L.; Davis, J.M.; Murphy, E.A.; Gross, S.J.; McAnulty, L.S.; Dumke, C.L. Beta-glucan, immune function, and upper respiratory tract infections in athletes. Med. Sci. Sports Exerc. 2008, 40, 1463–1471. [Google Scholar] [CrossRef]
- Kirmaz, C.; Bayrak, P.; Yilmaz, O.; Yuksel, H. Effects of glucan treatment on the Th1/Th2 balance in patients with allergic rhinitis: A double-blind placebo-controlled study. Eur. Cytokine Netw. 2005, 16, 128–134. [Google Scholar]
- Leentjens, J.; Quintin, J.; Gerretsen, J.; Kox, M.; Pickkers, P.; Netea, M.G. The effects of orally administered Beta-glucan on innate immune responses in humans, a randomized open-label intervention pilot-study. PLoS ONE 2014, 9, e108794. [Google Scholar] [CrossRef]
- Nieman, D.C. Immunonutrition support for athletes. Nutr. Rev. 2008, 66, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Stanilka, J.M.; Rowe, C.A.; Esteves, E.A.; Nieves, C., Jr.; Spaiser, S.J.; Christman, M.C.; Langkamp-Henken, B.; Percival, S.S. Consuming Lentinula edodes (Shiitake) Mushrooms Daily Improves Human Immunity: A Randomized Dietary Intervention in Healthy Young Adults. J. Am. Coll. Nutr. 2015, 34, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.S.; Kim, D.J.; Chae, G.T.; Lee, J.M.; Bae, S.M.; Sin, J.I.; Kim, Y.W.; Namkoong, S.E.; Lee, I.P. Natural killer cell activity and quality of life were improved by consumption of a mushroom extract, Agaricus blazei Murill Kyowa, in gynecological cancer patients undergoing chemotherapy. Int. J. Gynecol. Cancer: Off. J. Int. Gynecol. Cancer Soc. 2004, 14, 589–594. [Google Scholar] [CrossRef]
- Tangen, J.M.; Tierens, A.; Caers, J.; Binsfeld, M.; Olstad, O.K.; Troseid, A.M.; Wang, J.; Tjonnfjord, G.E.; Hetland, G. Immunomodulatory effects of the Agaricus blazei Murrill-based mushroom extract AndoSan in patients with multiple myeloma undergoing high dose chemotherapy and autologous stem cell transplantation: A randomized, double blinded clinical study. BioMed Res. Int. 2015, 2015, 718539. [Google Scholar] [CrossRef]
- Nagashima, Y.; Maeda, N.; Yamamoto, S.; Yoshino, S.; Oka, M. Evaluation of host quality of life and immune function in breast cancer patients treated with combination of adjuvant chemotherapy and oral administration of Lentinula edodes mycelia extract. Oncotargets Ther. 2013, 6, 853–859. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Miyahara, E.; Hihara, J. Efficacy and safety of orally administered Lentinula edodes mycelia extract for patients undergoing cancer chemotherapy: A pilot study. Am. J. Chin. Med. 2011, 39, 451–459. [Google Scholar] [CrossRef]
- Wang, J.L.; Bi, Z.; Zou, J.W.; Gu, X.M. Combination therapy with lentinan improves outcomes in patients with esophageal carcinoma. Mol. Med. Rep. 2012, 5, 745–748. [Google Scholar] [CrossRef]
- Kataoka, H.; Shimura, T.; Mizoshita, T.; Kubota, E.; Mori, Y.; Mizushima, T.; Wada, T.; Ogasawara, N.; Tanida, S.; Sasaki, M.; et al. Lentinan with S-1 and paclitaxel for gastric cancer chemotherapy improve patient quality of life. Hepato Gastroenterol. 2009, 56, 547–550. [Google Scholar]
- Yoshino, S.; Nishikawa, K.; Morita, S.; Takahashi, T.; Sakata, K.; Nagao, J.; Nemoto, H.; Murakami, N.; Matsuda, T.; Hasegawa, H.; et al. Randomised phase III study of S-1 alone versus S-1 plus lentinan for unresectable or recurrent gastric cancer (JFMC36-0701). Eur. J. Cancer 2016, 65, 164–171. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Ziaei, J.E.; Esfahani, A.; Jafarabadi, M.A.; Movassaghpourakbari, A.; Farrin, N. Effect of beta glucan on white blood cell counts and serum levels of IL-4 and IL-12 in women with breast cancer undergoing chemotherapy: A randomized double-blind placebo-controlled clinical trial. Asian Pac. J. Cancer Prev. 2014, 15, 5733–5739. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Esfahani, A.; Asghari Jafarabadi, M.; Eivazi Ziaei, J.; Movassaghpourakbari, A.; Farrin, N. Effect of Beta glucan on quality of life in women with breast cancer undergoing chemotherapy: A randomized double-blind placebo-controlled clinical trial. Adv. Pharm. Bull. 2014, 4, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Demir, G.; Klein, H.O.; Mandel-Molinas, N.; Tuzuner, N. Beta glucan induces proliferation and activation of monocytes in peripheral blood of patients with advanced breast cancer. Int. Immunopharmacol. 2007, 7, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Twardowski, P.; Kanaya, N.; Frankel, P.; Synold, T.; Ruel, C.; Pal, S.K.; Junqueira, M.; Prajapati, M.; Moore, T.; Tryon, P.; et al. A phase I trial of mushroom powder in patients with biochemically recurrent prostate cancer: Roles of cytokines and myeloid-derived suppressor cells for Agaricus bisporus-induced prostate-specific antigen responses. Cancer 2015, 121, 2942–2950. [Google Scholar] [CrossRef] [PubMed]
- Kodama, N.; Komuta, K.; Nanba, H. Effect of Maitake (Grifola frondosa) D-Fraction on the activation of NK cells in cancer patients. J. Med. Food 2003, 6, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Lin, H.; Seidman, A.; Fornier, M.; D’Andrea, G.; Wesa, K.; Yeung, S.; Cunningham-Rundles, S.; Vickers, A.J.; Cassileth, B. A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: Immunological effects. J. Cancer Res. Clin. Oncol. 2009, 135, 1215–1221. [Google Scholar] [CrossRef]
- Albeituni, S.H.; Ding, C.; Liu, M.; Hu, X.; Luo, F.; Kloecker, G.; Bousamra, M., 2nd; Zhang, H.G.; Yan, J. Yeast-Derived Particulate beta-Glucan Treatment Subverts the Suppression of Myeloid-Derived Suppressor Cells (MDSC) by Inducing Polymorphonuclear MDSC Apoptosis and Monocytic MDSC Differentiation to APC in Cancer. J. Immunol. 2016, 196, 2167–2180. [Google Scholar] [CrossRef]
- Magnani, M.; Castro-Gomez, R.H.; Aoki, M.N.; Gregorio, E.P.; Libos, F.; Watanabe, M.A.E. Effects of carboxymethyl-glucan from Saccharomyces cerevisiae on the peripheral blood cells of patients with advanced prostate cancer. Exp. Ther. Med. 2010, 1, 859–862. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Zhang, L.; Tian, Q. Mushroom polysaccharide lentinan for treating different types of cancers: A review of 12 years clinical studies in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 297–328. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, L.; Kim, J.A. Modulating mammary tumor growth, metastasis and immunosuppression by siRNA-induced MIF reduction in tumor microenvironment. Cancer Gene Ther. 2015, 22, 463–474. [Google Scholar] [CrossRef]
- Liu, M.; Luo, F.; Ding, C.; Albeituni, S.; Hu, X.; Ma, Y.; Cai, Y.; McNally, L.; Sanders, M.A.; Jain, D.; et al. Dectin-1 activation by a natural product β-glucan converts immunosuppressive macrophages into an M1-like phenotype. J. Immunol. 2015, 195, 5055–5065. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Cornò, M.; Gessani, S.; Conti, L. Shaping the Innate Immune Response by Dietary Glucans: Any Role in the Control of Cancer? Cancers 2020, 12, 155. https://doi.org/10.3390/cancers12010155
Del Cornò M, Gessani S, Conti L. Shaping the Innate Immune Response by Dietary Glucans: Any Role in the Control of Cancer? Cancers. 2020; 12(1):155. https://doi.org/10.3390/cancers12010155
Chicago/Turabian StyleDel Cornò, Manuela, Sandra Gessani, and Lucia Conti. 2020. "Shaping the Innate Immune Response by Dietary Glucans: Any Role in the Control of Cancer?" Cancers 12, no. 1: 155. https://doi.org/10.3390/cancers12010155
APA StyleDel Cornò, M., Gessani, S., & Conti, L. (2020). Shaping the Innate Immune Response by Dietary Glucans: Any Role in the Control of Cancer? Cancers, 12(1), 155. https://doi.org/10.3390/cancers12010155




