Polyclonal Immunoglobulin Recovery after Autologous Stem Cell Transplantation Is an Independent Prognostic Factor for Survival Outcome in Patients with Multiple Myeloma
Abstract
:1. Introduction
2. Results
2.1. Patients’ Characteristics
2.2. Polyclonal Ig Recovery after ASCT
2.3. Polyclonal Ig Recovery and Bone Marrow Plasma Cells
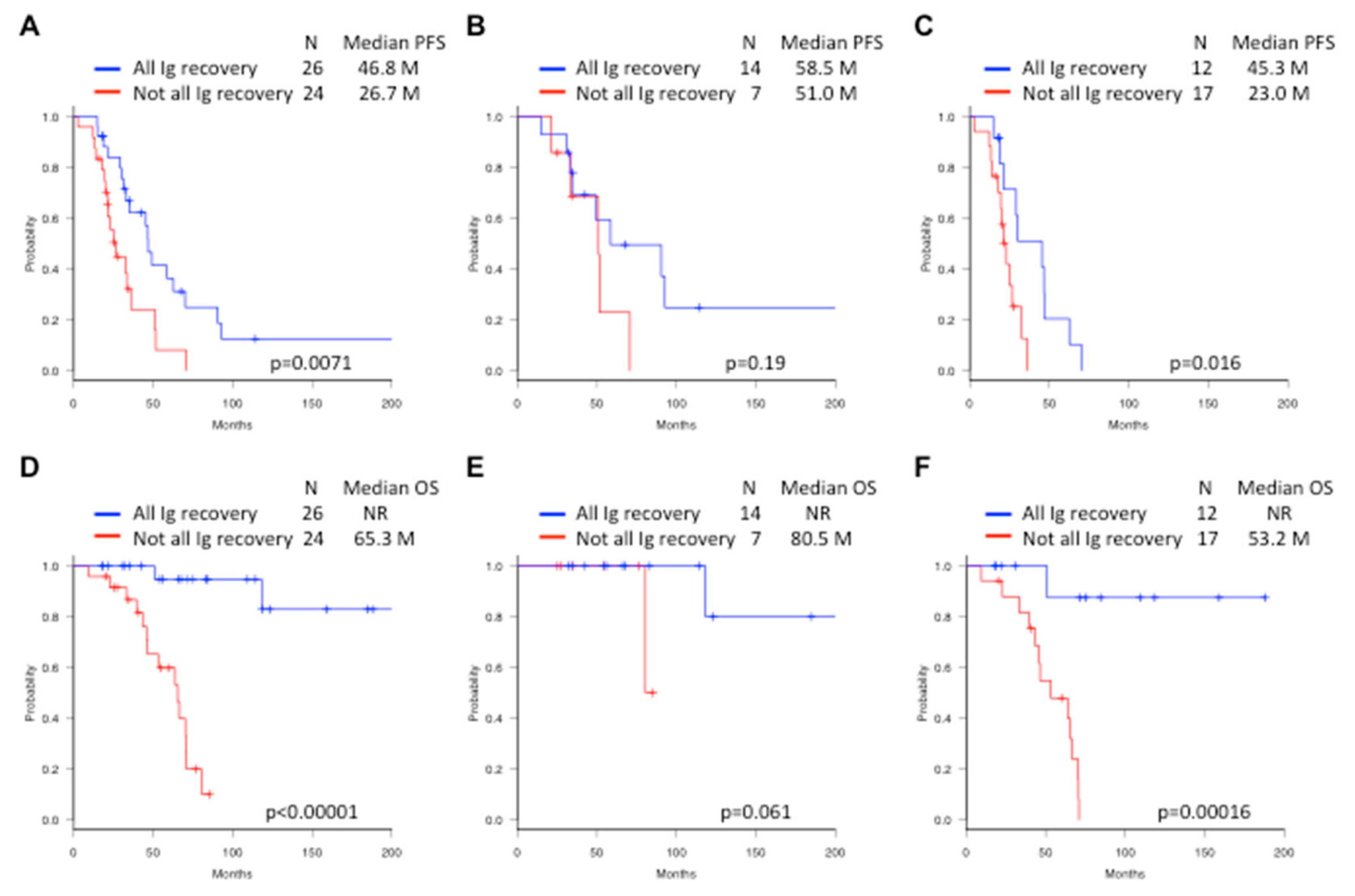
2.4. Survival Outcome
2.5. Univariate and Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Patients and Treatment
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br. J. Haematol. 2003, 121, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Moreau, P.; Cavo, M.; Sonneveld, P.; Rosinol, L.; Attal, M.; Pezzi, A.; Goldschmidt, H.; Lahuerta, J.J.; Marit, G.; Palumbo, A.; et al. Combination of International Scoring System 3, high lactate dehydrogenase, and t(4;14) and/or del(17p) identifies patients with multiple myeloma (MM) treated with front-line autologous stem-cell transplantation at high risk of early MM progression-related death. J. Clin. Oncol. 2014, 32, 2173–2180. [Google Scholar]
- Anderson, K.C. Progress and Paradigms in Multiple Myeloma. Clin. Cancer Res. 2016, 22, 5419–5427. [Google Scholar] [CrossRef] [Green Version]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Leleu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Belhadj, K.; Garderet, L.; et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N. Engl. J. Med. 2017, 376, 1311–1320. [Google Scholar] [CrossRef]
- Munshi, N.C.; Avet-Loiseau, H.; Rawstron, A.C.; Owen, R.G.; Child, J.A.; Thakurta, A.; Sherrington, P.; Samur, M.K.; Georgieva, A.; Anderson, K.C.; et al. Association of Minimal Residual Disease With Superior Survival Outcomes in Patients with Multiple Myeloma: A Meta-analysis. JAMA Oncol. 2017, 3, 28–35. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Palumbo, A.; Gay, F.; Cavallo, F.; Di Raimondo, F.; Larocca, A.; Hardan, I.; Nagler, A.; Petrucci, M.T.; Hajek, R.; Pezzatti, S.; et al. Continuous therapy versus fixed duration of therapy in patients with newly diagnosed multiple myeloma. J. Clin. Oncol. 2015, 33, 3459–3466. [Google Scholar] [CrossRef]
- McCarthy, P.L.; Holstein, S.A.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Bringhen, S.; Musto, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J. Clin. Oncol. 2017, 35, 3279–3289. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Bringhen, S.; Kumar, S.K.; Lupparelli, G.; Usmani, S.; Waage, A.; Larocca, A.; Van Der Holt, B.; Musto, P.; Offidani, M.; et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. Lancet Oncol. 2014, 15, 333–342. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Gay, F.; Schjesvold, F.; Beksac, M.; Hajek, R.; Weisel, K.C.; Goldschmidt, H.; Maisnar, V.; Moreau, P.; Min, C.K.; et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): A double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2019, 393, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Rajkumar, S.V. Value and Cost of Myeloma Therapy. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Barosi, G.; Gale, R.P. Is lenalidomide the standard-of-care after an autotransplant for plasma cell myeloma? Leukemia 2019, 33, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Sørrig, R.; Klausen, T.W.; Salomo, M.; Vangsted, A.J.; Frølund, U.C.; Andersen, K.T.; Klostergaard, A.; Helleberg, C.; Pedersen, R.S.; Pedersen, P.T.; et al. Immunoparesis in newly diagnosed Multiple Myeloma patients: Effects on overall survival and progression free survival in the Danish population. PLoS ONE 2017, 12, e0188988. [Google Scholar] [CrossRef]
- Heaney, J.L.; Campbell, J.P.; Iqbal, G.; Cairns, D.; Richter, A.; Child, J.A.; Gregory, W.; Jackson, G.; Kaiser, M.; Owen, R.; et al. Characterisation of immunoparesis in newly diagnosed myeloma and its impact on progression-free and overall survival in both old and recent myeloma trials. Leukemia 2018, 32, 1727–1738. [Google Scholar] [CrossRef]
- Gao, W.; Li, J.; Jian, Y.; Yang, G.; Wu, Y.; Li, Y.; Len, Y.; Liu, A.; Tian, Y.; Wang, H.; et al. Immunoparesis in symptomatic multiple myeloma at diagnosis affects PFS with bortezomib-containing induction therapy, but not ASCT consolidation. Int. J. Hematol. 2019, 109, 169–174. [Google Scholar] [CrossRef]
- González-Calle, V.; Cerdá, S.; Labrador, J.; Sobejano, E.; González-Mena, B.; Aguilera, C.; Ocio, E.M.; Vidriales, M.B.; Puig, N.; Gutiérrez, N.C.; et al. Recovery of polyclonal immunoglobulins one year after autologous stem cell transplantation as a long-term predictor marker of progression and survival in multiple myeloma. Haematologica 2017, 102, 922–931. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Zepeda, V.H.; Duggan, P.; Neri, P.; Chaudhry, A.; Tay, J.; Bahlis, N. Immunoparesis and polyclonal immunoglobulin recovery after auto-SCT for patients with multiple myeloma treated at a single institution. Leuk Lymphoma 2018, 59, 1920–1926. [Google Scholar] [CrossRef]
- Gao, W.; Li, J.; Wu, Y.; Li, Y.; Leng, Y.; Liu, A.; Yang, G.; Tian, Y.; Wang, H.; Wang, G.; et al. Immunoparesis recovery 1 year after ASCT is independently associated with favorable survival in patients with symptomatic multiple myeloma who undergo autologous stem cell transplantation. Ann. Hematol. 2019, 98, 1177–1184. [Google Scholar] [CrossRef]
- Arteche-López, A.; Kreutzman, A.; Alegre, A.; Martín, P.S.; Aguado, B.; González-Pardo, M.; Espiño, M.; Villar, L.M.; Belmonte, D.G.; de la Cámara, R.; et al. Multiple myeloma patients in long-term complete response after autologous stem cell transplantation express a particular immune signature with potential prognostic implication. Bone Marrow Transplant. 2017, 52, 832–838. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.M.; McCarthy, P.L.; Wallace, P.K.; Zhang, Y.; Fora, A.; Mellors, P.; Tario, J.D.; McCarthy, B.L.; Chen, G.L.; Holstein, S.A.; et al. Immune signatures associated with improved progression-free and overall survival for myeloma patients treated with AHSCT. Blood Adv. 2017, 1, 1056–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Larrea, C.F.; Tovar, N.; Cibeira, M.T.; Aróstegui, J.I.; Rosiñol, L.; Elena, M.; Filella, X.; Yagüe, J.; Blade, J. Emergence of oligoclonal bands in patients with multiple myeloma in complete remission after induction chemotherapy: Association with the use of novel agents. Haematologica 2011, 96, 171–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujisawa, M.; Seike, K.; Fukumoto, K.; Suehara, Y.; Fukaya, M.; Sugihara, H.; Takeuchi, M.; Matsue, K. Oligoclonal bands in patients with multiple myeloma: its emergence per se could not be translated to improved survival. Cancer Sci. 2014, 105, 1442–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Zepeda, V.H.; Reece, D.E.; Trudel, S.; Franke, N.; Winter, A.; Chen, C.; Tiedemann, R.; Kukreti, V. Oligoclonal and monoclonal bands after single autologous stem cell transplant in patients with multiple myeloma: impact on overall survival and progression-free survival. Leuk Lymphoma 2014, 55, 2284–2289. [Google Scholar] [CrossRef] [PubMed]
- Tovar, N.; de Larrea, C.F.; Aróstegui, J.I.; Cibeira, M.T.; Rosiñol, L.; Rovira, M.; Elena, M.; Filella, X.; Yagüe, J.; Bladé, J. Natural history and prognostic impact of oligoclonal humoral response in patients with multiple myeloma after autologous stem cell transplantation: long-term results from a single institution. Haematologica 2013, 98, 1142–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradwell, A.; Harding, S.; Fourrier, N.; Mathiot, C.; Attal, M.; Moreau, P.; Harousseau, J.L.; Avet-Loiseau, H. Prognostic utility of intact immunoglobulin Ig’kappa/Ig’lambda ratios in multiple myeloma patients. Leukemia 2013, 27, 202–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, H.; Milosavljevic, D.; Zojer, N.; Faint, J.M.; Bradwell, A.R.; Hübl, W.; Harding, S.J. Immunoglobulin heavy/light chain ratios improve paraprotein detection and monitoring, identify residual disease and correlate with survival in multiple myeloma patients. Leukemia 2013, 27, 213–219. [Google Scholar] [CrossRef]
- Kamiya, Y.; Chou, T.; Murakami, H.; Handa, H.; Ozaki, S.; Shimazaki, C.; Fuchida, S.I.; Okada, J.; Itoh, J.; Sugiyama, S.; et al. Patients assigned to VGPR, PR, and SD in the IMWG response category are composed of heterogeneous population when assessed by the heavy/light chain assay. Hematol. Oncol. 2019, 37, 316–318. [Google Scholar] [CrossRef]
- Ludwig, H.; Milosavljevic, D.; Berlanga, O.; Zojer, N.; Hübl, W.; Fritz, V.; Harding, S. Suppression of the noninvolved pair of the myeloma isotype correlates with poor survival in newly diagnosed and relapsed/refractory patients with myeloma. Am. J. Hematol. 2016, 91, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Tovar, N.; de Larrea, C.F.; Elena, M.; Cibeira, M.T.; Aróstegui, J.I.; Rosinol, L.; Filella, X.; Yagüe, J.; Bladé, J. Prognostic impact of serum immunoglobulin heavy/light chain ratio in patients with multiple myeloma in complete remission after autologous stem cell transplantation. Biol. Blood Marrow Transplant. 2012, 18, 1076–1079. [Google Scholar] [CrossRef] [Green Version]
- Michallet, M.; Chapuis-Cellier, C.; Dejoie, T.; Lombard, C.; Caillon, H.; Sobh, M.; Moreau, P.; Attal, M.; Avet-Loiseau, H. Heavy + light chain monitoring correlates with clinical outcome in multiple myeloma patients. Leukemia 2018, 32, 376–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva, B.; Vidriales, M.B.; Rosinol, L.; Martinez-Lopez, J.; Mateos, M.V.; Ocio, E.M.; Montalbán, M.Á.; Cordón, L.; Gutiérrez, N.C.; Corchete, L.; et al. A multiparameter flow cytometry immunophenotypic algorithm for the identification of newly diagnosed symptomatic myeloma with an MGUS-like signature and long-term disease control. Leukemia 2013, 27, 2056–2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung-Hagesteijn, C.; Erdmann, N.; Cheung, G.; Keats, J.J.; Stewart, A.K.; Reece, D.E.; Chung, K.C.; Tiedemann, R.E. Xbp1s-negative tumor B cells and pre-plasmablasts mediate therapeutic proteasome inhibitor resistance in multiple myeloma. Cancer Cell 2013, 24, 289–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanan-Khan, A.; Sonneveld, P.; Schuster, M.W.; Stadtmauer, E.A.; Facon, T.; Harousseau, J.L.; Ben-Yehuda, D.; Lonial, S.; Goldschmidt, H.; Reece, D.; et al. Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J. Clin. Oncol. 2008, 26, 4784–4790. [Google Scholar] [CrossRef] [PubMed]
- Quach, H.; Ritchie, D.; Stewart, A.K.; Neeson, P.; Harrison, S.; Smyth, M.J.; Prince, H.M. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 2010, 24, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimopoulos, M.A.; Swern, A.S.; Li, J.S.; Hussein, M.; Weiss, L.; Nagarwala, Y.; Baz, R. Efficacy and safety of long-term treatment with lenalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma. Blood Cancer J. 2014, 4, e257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamarin, D.; Devlin, S.M.; Arcila, M.E.; Landau, H.; Lesokhin, A.; Lendvai, N.; Chung, D.J.; Chimento, D.; Weltz, J.; Babu, D.; et al. Polyclonal immune activation and marrow plasmacytosis in multiple myeloma patients receiving long-term lenalidomide therapy: incidence and prognostic significance. Leukemia 2013, 27, 2422–2424. [Google Scholar] [CrossRef] [Green Version]
- Krejcik, J.; Casneuf, T.; Nijhof, I.S.; Verbist, B.; Bald, J.; Plesner, T.; Syed, K.; Liu, K.; van de Donk, N.W.; Weiss, B.M.; et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016, 128, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Greipp, P.R.; Miguel, J.S.; Durie, B.G.; Crowley, J.J.; Barlogie, B.; Bladé, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International staging system for multiple myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for multiple myeloma: A report from International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Ichihara, K.; Yomamoto, Y.; Hotta, T.; Hosogaya, S.; Miyachi, H.; Itoh, Y.; Ishibashi, M.; Kang, D.; Committee on Common Reference Intervals; Japan Society of Clinical Chemistry. Collaborative derivation of reference intervals for major clinical laboratory tests in Japan. Ann. Clin. Biochem. 2016, 53, 347–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durie, B.G.; Harousseau, J.L.; Miguel, J.S.; Blade, J.; Barlogie, B.; Anderson, K.; Gertz, M.; Dimopoulos, M.; Westin, J.; Sonneveld, P.; et al. International uniform response criteria for multiple myeloma. Leukemia 2006, 20, 1467–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | All Ig recovery (n = 26) | Not all Ig recovery (n = 24) | Total (n = 50) | p |
|---|---|---|---|---|
| Median age (range) | 58 (39–71) yrs | 56 (35–69) yrs | 57 (35–71) yrs | 0.16 |
| Gender (M/F) | 11/15 | 12/12 | 23/27 | 0.78 |
| M protein | 0.89 | |||
| IgG | 11 | 13 | 24 | |
| IgA | 6 | 3 | 9 | |
| IgD | 1 | 1 | 2 | |
| BJP | 7 | 6 | 13 | |
| Non-secretory | 1 | 1 | 2 | |
| Hemoglobin | 0.74 | |||
| Normal | 12 | 10 | 22 | |
| Low (<10g/dL) | 7 | 9 | 16 | |
| Unknown | 7 | 5 | 12 | |
| Serum creatinine | 1.00 | |||
| Normal | 19 | 21 | 40 | |
| High (>2mg/dL) | 1 | 1 | 2 | |
| Unknown | 6 | 2 | 8 | |
| Serum calcium | 1.00 | |||
| Normal | 17 | 16 | 33 | |
| High (>11mg/dL) | 2 | 3 | 5 | |
| Unknown | 7 | 5 | 12 | |
| Lytic bone lesion | 0.20 | |||
| 0 | 4 | 8 | 12 | |
| 1–3 | 6 | 7 | 13 | |
| >3 | 16 | 9 | 25 | |
| ISS stage | 0.48 | |||
| I | 10 | 6 | 16 | |
| II | 10 | 10 | 20 | |
| III | 5 | 8 | 13 | |
| Unknown | 1 | 0 | 1 | |
| R-ISS stage | 0.036 | |||
| I | 8 | 1 | 9 | |
| II | 13 | 12 | 25 | |
| III | 1 | 4 | 5 | |
| Unknown | 4 | 7 | 11 | |
| Induction regimen | 0.56 | |||
| VAD | 9 | 11 | 20 | |
| Novel agent-based | 17 | 13 | 30 | |
| Response before ASCT | 0.11 | |||
| sCR | 3 | 2 | 5 | |
| CR | 5 | 1 | 6 | |
| VGPR | 7 | 4 | 11 | |
| PR | 11 | 13 | 24 | |
| SD | 0 | 4 | 4 | |
| Response after ASCT | 0.36 | |||
| sCR | 9 | 5 | 14 | |
| CR | 5 | 2 | 7 | |
| VGPR | 5 | 9 | 14 | |
| PR | 7 | 6 | 13 | |
| SD | 0 | 2 | 2 |
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (≥65 yrs) | 1.160 (0.467–2.880) | 0.75 | - | - |
| Gender (Male) | 2.130 (1.036–4.381) | 0.04 | 1.401 (0.668–2.936) | 0.37 |
| ISS (stage III) | 1.636 (0.811–3.299) | 0.17 | - | - |
| Induction regimen (novel agent-based) | 1.019 (0.523–1.986) | 0.96 | - | - |
| Response after ASCT (non-CR) | 4.312 (2.000–9.295) | 0.00019 | 4.284 (1.868–9.826) | 0.00059 |
| Ig recovery (not all) | 2.533 (1.261–5.087) | 0.009 | 2.804 (1.334–5.896) | 0.0065 |
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (≥65 yrs) | 0.354 (0.0466–2.684) | 0.31 | - | - |
| Gender (Male) | 1.409 (0.541–3.668) | 0.48 | - | - |
| ISS (stage III) | 1.231 (0.454–3.340) | 0.68 | - | - |
| Induction regimen (novel agent-based) | 0.358 (0.115–1.114) | 0.076 | - | - |
| Response after ASCT (non-CR) | 7.595 (1.727–33.4) | 0.0073 | 8.245 (1.528–44.47) | 0.014 |
| Ig recovery (not all) | 29.46 (3.815–227.6) | 0.0012 | 36.55 (3.942–338.8) | 0.0015 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozaki, S.; Harada, T.; Yagi, H.; Sekimoto, E.; Shibata, H.; Shigekiyo, T.; Fujii, S.; Nakamura, S.; Miki, H.; Kagawa, K.; et al. Polyclonal Immunoglobulin Recovery after Autologous Stem Cell Transplantation Is an Independent Prognostic Factor for Survival Outcome in Patients with Multiple Myeloma. Cancers 2020, 12, 12. https://doi.org/10.3390/cancers12010012
Ozaki S, Harada T, Yagi H, Sekimoto E, Shibata H, Shigekiyo T, Fujii S, Nakamura S, Miki H, Kagawa K, et al. Polyclonal Immunoglobulin Recovery after Autologous Stem Cell Transplantation Is an Independent Prognostic Factor for Survival Outcome in Patients with Multiple Myeloma. Cancers. 2020; 12(1):12. https://doi.org/10.3390/cancers12010012
Chicago/Turabian StyleOzaki, Shuji, Takeshi Harada, Hikaru Yagi, Etsuko Sekimoto, Hironobu Shibata, Toshio Shigekiyo, Shiro Fujii, Shingen Nakamura, Hirokazu Miki, Kumiko Kagawa, and et al. 2020. "Polyclonal Immunoglobulin Recovery after Autologous Stem Cell Transplantation Is an Independent Prognostic Factor for Survival Outcome in Patients with Multiple Myeloma" Cancers 12, no. 1: 12. https://doi.org/10.3390/cancers12010012
APA StyleOzaki, S., Harada, T., Yagi, H., Sekimoto, E., Shibata, H., Shigekiyo, T., Fujii, S., Nakamura, S., Miki, H., Kagawa, K., & Abe, M. (2020). Polyclonal Immunoglobulin Recovery after Autologous Stem Cell Transplantation Is an Independent Prognostic Factor for Survival Outcome in Patients with Multiple Myeloma. Cancers, 12(1), 12. https://doi.org/10.3390/cancers12010012





