Diffuse Lung Metastases in EGFR-Mutant Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Results
2.1. Patients
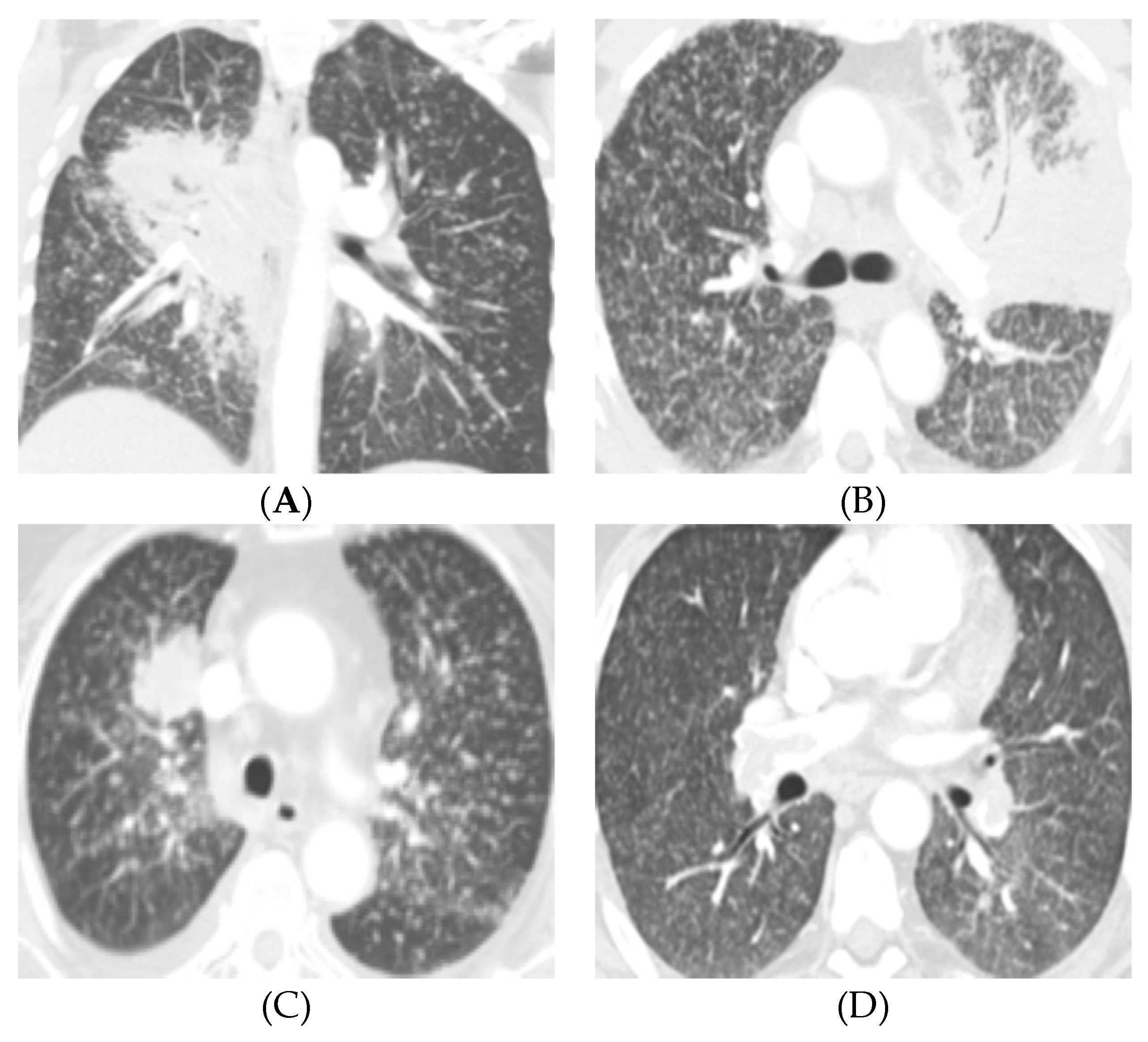
2.2. Diffuse Lung Metastases in EGFR-Mutant NSCLC
2.3. Multivariable Regression Model for Presence of Diffuse Lung Metastases in The Setting of EGFR-Mutation
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. Genetic Analysis
4.3. CT Imaging Protocol
4.4. Image Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.; Chirieac, L.R.; D’Amico, T.A.; DeCamp, M.M.; Dilling, T.J.; Dobelbower, M.; et al. Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. J. Natl. Compr. Canc. Netw. 2017, 15, 504–535. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Paez, J.G.; Jänne, P.A.; Lee, J.C.; Tracy, S.; Greulich, H.; Gabriel, S.; Herman, P.; Kaye, F.J.; Lindeman, N.; Boggon, T.J.; et al. EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non-Small-Cell Lung Cancer to Gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.-L.; Thongprasert, S.; Yang, C.-H.; Chu, D.-T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus Standard Chemotherapy as First-Line Treatment for European Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (EURTAC): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Sequist, L.V.; Yang, J.C.-H.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.-M.; Boyer, M.; et al. Phase III Study of Afatinib or Cisplatin plus Pemetrexed in Patients with Metastatic Lung Adenocarcinoma with EGFR Mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Lee, M.; Linke, R.; Rosell, R.; Corral, J.; et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. J. Clin. Oncol. 2018, 36, 2244–2250. [Google Scholar] [CrossRef]
- Kim, T.J.; Lee, C.-T.; Jheon, S.H.; Park, J.-S.; Chung, J.-H. Radiologic Characteristics of Surgically Resected Non-Small Cell Lung Cancer with ALK Rearrangement or EGFR Mutations. Ann. Thorac. Surg. 2016, 101, 473–480. [Google Scholar] [CrossRef]
- Yoon, H.J.; Sohn, I.; Cho, J.H.; Lee, H.Y.; Kim, J.-H.; Choi, Y.-L.; Kim, H.; Lee, G.; Lee, K.S.; Kim, J. Decoding Tumor Phenotypes for ALK, ROS1, and RET Fusions in Lung Adenocarcinoma Using a Radiomics Approach. Medicine 2015, 94, e1753. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Shan, F.; Yang, Y.; Shi, Y.; Zhang, Z. CT Characteristics of Non-Small Cell Lung Cancer with Epidermal Growth Factor Receptor Mutation: A Systematic Review and Meta-Analysis. BMC Med. Imaging 2017, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Halpenny, D.F.; Riely, G.J.; Hayes, S.; Yu, H.; Zheng, J.; Moskowitz, C.S.; Ginsberg, M.S. Are There Imaging Characteristics Associated with Lung Adenocarcinomas Harboring ALK Rearrangements? Lung Cancer 2014, 86, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Plodkowski, A.J.; Drilon, A.; Halpenny, D.F.; O’Driscoll, D.; Blair, D.; Litvak, A.M.; Zheng, J.; Moskowitz, C.S.; Ginsberg, M.S. From Genotype to Phenotype: Are There Imaging Characteristics Associated with Lung Adenocarcinomas Harboring RET and ROS1 Rearrangements? Lung Cancer 2015, 90, 321–325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mendoza, D.P.; Dagogo-Jack, I.; Chen, T.; Padole, A.; Shepard, J.-A.O.; Shaw, A.T.; Digumarthy, S.R. Imaging Characteristics of BRAF-Mutant Non-Small Cell Lung Cancer by Functional Class. Lung Cancer 2019, 129, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Kim, M.Y.; Park, S.; Lee, H.N.; Kim, H.J.; Lee, J.C.; Kim, S.-W.; Lee, D.H.; Choi, C.-M. Non-Small Cell Lung Cancer with Resistance to EGFR-TKI Therapy: CT Characteristics of T790M Mutation-Positive Cancer. Radiology 2018, 289, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Sakai, F.; Ishikawa, R.; Kimura, F.; Ishida, H.; Kobayashi, K. CT Features of Epidermal Growth Factor Receptor-Mutated Adenocarcinoma of the Lung: Comparison with Nonmutated Adenocarcinoma. J. Thorac. Oncol. 2016, 11, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Kim, Y.T.; Kang, C.H.; Zhao, B.; Tan, Y.; Schwartz, L.H.; Persigehl, T.; Jeon, Y.K.; Chung, D.H. Epidermal Growth Factor Receptor Mutation in Lung Adenocarcinomas: Relationship with CT Characteristics and Histologic Subtypes. Radiology 2013, 268, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kang, S.H.; Chung, H.W.; Lee, J.S.; Kim, S.J.; Yoo, K.H.; Lee, K.Y. Clinical Features of Lung Adenocarcinomas with Epidermal Growth Factor Receptor Mutations and Miliary Disseminated Carcinomatosis. Thorac. Cancer 2015, 6, 629–635. [Google Scholar] [CrossRef]
- Okuma, Y.; Kashima, J.; Watanabe, K.; Homma, S. Survival Analysis and Pathological Features of Advanced Non-Small Cell Lung Cancer with Miliary Pulmonary Metastases in Patients Harboring Epidermal Growth Factor Receptor Mutations. J. Cancer Res. Clin. Oncol. 2018, 144, 1601–1611. [Google Scholar] [CrossRef]
- Hsu, F.; Nichol, A.; Toriumi, T.; Caluwe, A.D. Miliary Metastases Are Associated with Epidermal Growth Factor Receptor Mutations in Non-Small Cell Lung Cancer: A Population-Based Study. Acta Oncol. 2017, 56, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Togashi, Y.; Masago, K.; Kubo, T.; Sakamori, Y.; Kim, Y.H.; Hatachi, Y.; Fukuhara, A.; Mio, T.; Togashi, K.; Mishima, M. Association of Diffuse, Random Pulmonary Metastases, Including Miliary Metastases, with Epidermal Growth Factor Receptor Mutations in Lung Adenocarcinoma. Cancer 2011, 117, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-G.; Hu, F.-C.; Chang, Y.-L.; Lee, Y.-C.; Yu, C.-J.; Chang, Y.-C.; Wu, J.-Y.; Shih, J.-Y.; Yang, P.-C. Frequent EGFR Mutations in Nonsmall Cell Lung Cancer Presenting with Miliary Intrapulmonary Carcinomatosis. Eur. Respir. J. 2013, 41, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Andreu, J.; Mauleón, S.; Pallisa, E.; Majó, J.; Martinez-Rodriguez, M.; Cáceres, J. Miliary Lung Disease Revisited. Curr. Probl. Diagn. Radiol. 2002, 31, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, M.; Karanth, S.; Ocazionez, D.; Estrada-Y-Martin, R.M.; Cherian, S.V. Clinical Characteristics and Etiologies of Miliary Nodules in the US: A Single-Center Study. Am. J. Med. 2019, 132, 767–769. [Google Scholar] [CrossRef]
- Touré, N.O.; Cissé, M.F.; Dia Kane, Y.; Diatta, A.; Bouker Bakioui, B.; Ndiaye, E.H.M.; Thiam, K.; Hane, A.A. Miliary tuberculosis: A report of 49 cases. Rev. Mal. Respir. 2011, 28, 312–316. [Google Scholar] [CrossRef]
- Leung, C.C.; Yu, I.T.S.; Chen, W. Silicosis. Lancet 2012, 379, 2008–2018. [Google Scholar] [CrossRef]
- Bui, P.V. Disseminated Histoplasmosis with Miliary Histoplasmosis, Neurohistoplasmosis, and Histoplasma Capsulatum Bacteremia in Probable Neurosarcoidosis. Case Rep. Med. 2018, 2018, 3162403. [Google Scholar] [CrossRef]
- Matsuura, S.; Mochizuka, Y.; Oishi, K.; Miyashita, K.; Naoi, H.; Mochizuki, E.; Mikura, S.; Tsukui, M.; Koshimizu, N.; Ohata, A.; et al. Sarcoidosis with Pancreatic Mass, Endobronchial Nodules, and Miliary Opacities in the Lung. Intern. Med. 2017, 56, 3083–3087. [Google Scholar] [CrossRef]
- Taki, M.; Ikegami, N.; Konishi, C.; Nakao, S.; Funazou, T.; Ariyasu, R.; Yoshida, M.; Nakagawa, K.; Morita, K.; Hee Hwang, M.; et al. Pulmonary Sarcoidosis Presenting with Miliary Opacities. Intern. Med. 2015, 54, 2483–2486. [Google Scholar] [CrossRef]
- Laack, E.; Simon, R.; Regier, M.; Andritzky, B.; Tennstedt, P.; Habermann, C.; Verth, C.Z.; Thöm, I.; Grob, T.; Sauter, G.; et al. Miliary Never-Smoking Adenocarcinoma of the Lung: Strong Association with Epidermal Growth Factor Receptor Exon 19 Deletion. J. Thorac. Oncol. 2011, 6, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Konishi, A.; Sasaki, H.; Takada, M.; Tanaka, H.; Okumura, M.; Kawahara, M.; Sugiura, H.; Kuwabara, Y.; Fukai, I.; et al. Epidermal Growth Factor Receptor Gene Mutation in Non-Small Cell Lung Cancer Using Highly Sensitive and Fast TaqMan PCR Assay. Lung Cancer 2005, 50, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Arcila, M.E.; Oxnard, G.R.; Nafa, K.; Riely, G.J.; Solomon, S.B.; Zakowski, M.F.; Kris, M.G.; Pao, W.; Miller, V.A.; Ladanyi, M. Rebiopsy of Lung Cancer Patients with Acquired Resistance to EGFR Inhibitors and Enhanced Detection of the T790M Mutation Using a Locked Nucleic Acid-Based Assay. Clin. Cancer Res. 2011, 17, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, L.; Jänne, P.A.; Makrigiorgos, G.M. Coamplification at Lower Denaturation Temperature-PCR Increases Mutation-Detection Selectivity of TaqMan-Based Real-Time PCR. Clin. Chem. 2009, 55, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.; Hawkins, N.; O’Grady, R.; Sheehan, C.; O’Connor, T.; Impey, H.; Roberts, N.; Fuery, C.; Todd, A. Restriction Endonuclease-Mediated Selective Polymerase Chain Reaction: A Novel Assay for the Detection of K-Ras Mutations in Clinical Samples. Am. J. Pathol. 1998, 153, 373–379. [Google Scholar] [CrossRef]
- Lim, C.; Tsao, M.S.; Le, L.W.; Shepherd, F.A.; Feld, R.; Burkes, R.L.; Liu, G.; Kamel-Reid, S.; Hwang, D.; Tanguay, J.; et al. Biomarker Testing and Time to Treatment Decision in Patients with Advanced Nonsmall-Cell Lung Cancer. Ann. Oncol. 2015, 26, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Cheema, P.K.; Menjak, I.B.; Winterton-Perks, Z.; Raphael, S.; Cheng, S.Y.; Verma, S.; Muinuddin, A.; Freedman, R.; Toor, N.; Perera, J.; et al. Impact of Reflex EGFR/ALK Testing on Time to Treatment of Patients With Advanced Nonsquamous Non–Small-Cell Lung Cancer. JOP 2016, 13, e130–e138. [Google Scholar] [CrossRef] [PubMed]
- Sequist, L.V.; Heist, R.S.; Shaw, A.T.; Fidias, P.; Rosovsky, R.; Temel, J.S.; Lennes, I.T.; Digumarthy, S.; Waltman, B.A.; Bast, E.; et al. Implementing Multiplexed Genotyping of Non-Small-Cell Lung Cancers into Routine Clinical Practice. Ann. Oncol. 2011, 22, 2616–2624. [Google Scholar] [CrossRef]
- Goldstraw, P.; Crowley, J.; Chansky, K.; Giroux, D.J.; Groome, P.A.; Rami-Porta, R.; Postmus, P.E.; Rusch, V.; Sobin, L.; International Association for the Study of Lung Cancer International Staging Committee. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the TNM Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J. Thorac. Oncol. 2007, 2, 706–714. [Google Scholar] [CrossRef]
| Characteristics | All (n = 217) | EGFR | p-Value * | |
|---|---|---|---|---|
| Mutant (n = 117) | Wild-type (n = 100) | |||
| Patients Characteristics | ||||
| Median (range) | ||||
| Age | 65 (26–90) | 63 (26–90) | 68 (42–84) | <0.01 |
| Gender | n (%) | |||
| Female | 123 (57%) | 81 (69%) | 42 (42%) | <0.01 |
| Male | 94 (43%) | 36 (31%) | 58 (58%) | |
| Race | ||||
| Caucasian | 185 (85%) | 94 (80%) | 91 (91%) | 0.03 |
| Asian | 19 (9%) | 12 (10%) | 7 (7%) | |
| African-American | 4 (2%) | 3 (3%) | 1 (1%) | |
| Hispanic | 3 (1%) | 3 (3%) | 0 (0%) | |
| Others/Unknown | 6 (3%) | 5 (4%) | 1 (1%) | |
| Smoking status | ||||
| Never | 88 (41%) | 72 (62%) | 16 (16%) | <0.01 |
| Ever | 129 (59%) | 45 (38%) | 84 (84%) | |
| Primary tumor features | ||||
| Size (mm) | 50 (10–134) | 47 (11–134) | 52 (10–115) | 0.84 |
| Primary lesion lobar zone | ||||
| Both | 51 (24%) | 29 (25%) | 22 (22%) | 0.18 |
| Central | 91 (42%) | 54 (46%) | 37 (37%) | |
| Peripheral | 75 (35%) | 34 (29%) | 41 (41%) | |
| Solid | ||||
| No | 21 (10%) | 13 (11%) | 8 (8%) | 0.5 |
| Yes | 196 (90%) | 104 (89%) | 92 (92%) | |
| Air bronchograms | ||||
| No | 156 (72%) | 84 (72%) | 72 (72%) | > 0.99 |
| Yes | 61 (28%) | 33 (28%) | 28 (28%) | |
| Cavity | ||||
| No | 198 (91%) | 111 (95%) | 87 (87%) | 0.05 |
| Yes | 19 (9%) | 6 (5%) | 13 (13%) | |
| Tumor Calcification | ||||
| No | 211 (97%) | 112 (96%) | 99 (99%) | 0.22 |
| Yes | 6 (3%) | 5 (4%) | 1 (1%) | |
| Nodal disease | ||||
| Negative | 23 (11%) | 19 (16%) | 4 (4%) | <0.01 |
| Positive | 194 (89%) | 98 (84%) | 96 (96%) | |
| Metastatic sites | ||||
| Intrathoracic | ||||
| Absent | 33 (15%) | 21 (18%) | 12 (12%) | 0.26 |
| Present | 184 (85%) | 96 (82%) | 88 (88%) | |
| Pleura | ||||
| No | 100 (46%) | 70 (60%) | 30 (30%) | <0.01 |
| Yes | 117 (54%) | 47 (40%) | 70 (70%) | |
| Lung | ||||
| No | 67 (31%) | 34 (29%) | 33 (33%) | 0.56 |
| Yes | 150 (69%) | 83 (71%) | 67 (67%) | |
| Diffuse lung | ||||
| No | 193 (89%) | 96 (82%) | 97 (97%) | <0.01 |
| Yes | 24 (11%) | 21 (18%) | 3 (3%) | |
| Extrathoracic | ||||
| Absent | 63 (29%) | 33 (28%) | 30 (30%) | 0.88 |
| Present | 154 (71%) | 84 (72%) | 70 (70%) | |
| Bone | ||||
| No | 133 (61%) | 68 (58%) | 65 (65%) | 0.33 |
| Yes | 84 (39%) | 49 (42%) | 35 (35%) | |
| Brain | ||||
| No | 140 (65%) | 70 (60%) | 70 (70%) | 0.15 |
| Yes | 77 (35%) | 47 (40%) | 30 (30%) | |
| Adrenal | ||||
| No | 170 (78%) | 101 (86%) | 69 (69%) | <0.01 |
| Yes | 47 (22%) | 16 (14%) | 31 (31%) | |
| Soft tissue | ||||
| No | 179 (82%) | 92 (79%) | 87 (87%) | 0.11 |
| Yes | 38 (18%) | 25 (21%) | 13 (13%) | |
| Characteristics | All (n = 117) | Diffuse Lung Metastases | p-Value * | |
|---|---|---|---|---|
| Yes (n = 21) | No (n = 96) | |||
| Patients characteristics | ||||
| Median (range) | ||||
| Age | 63 (26–90) | 58 (37–82) | 64 (26–90) | 0.16 |
| Size (mm) | 47 (11–134) | 47 (17–99) | 47 (11–134) | 0.42 |
| Age | n (%) | n (%) | ||
| <63 | 57 (49%) | 14 (67%) | 43 (45%) | 0.09 |
| ≥63 | 60 (51%) | 7 (33%) | 53 (55%) | |
| Gender | ||||
| Female | 81 (69%) | 11 (52%) | 70 (73%) | 0.07 |
| Male | 36 (31%) | 10 (48%) | 26 (27%) | |
| Ethnicity | ||||
| Caucasian | 94 (80%) | 15 (71%) | 79 (82%) | 0.36 |
| Asian | 12 (10%) | 3 (14%) | 9 (9%) | |
| African American | 3 (3%) | 1 (5%) | 2 (2%) | |
| Hispanic | 3 (3%) | 0 (0%) | 3 (3%) | |
| Others/Unknown | 5 (4%) | 2 (10%) | 3 (3%) | |
| Smoking status | ||||
| Never | 72 (62%) | 16 (76%) | 56 (58%) | 0.15 |
| Ever | 45 (38%) | 5 (24%) | 40 (42%) | |
| EGFR subtype | ||||
| Exon 19 | 61 (52%) | 12 (57%) | 49 (51%) | 0.9 |
| Exon 21 | 33 (28%) | 5 (24%) | 28 (29%) | |
| Exon 18 | 13 (11%) | 1 (5%) | 12 (13%) | |
| Exon 20 | 10 (9%) | 3 (14%) | 7 (7%) | |
| Primary tumor features | ||||
| Size >30 mm | ||||
| No | 22 (19%) | 3 (14%) | 19 (20%) | 0.76 |
| Yes | 95 (81%) | 18 (86%) | 77 (80%) | |
| Location | ||||
| Both | 29 (25%) | 9 (43%) | 20 (21%) | 0.11 |
| Central | 54 (46%) | 8 (38%) | 46 (48%) | |
| Peripheral | 34 (29%) | 4 (19%) | 30 (31%) | |
| Solid | ||||
| No | 13 (11%) | 3 (14%) | 10 (10%) | 0.7 |
| Yes | 104 (89%) | 18 (86%) | 86 (90%) | |
| Air bronchograms | ||||
| No | 84 (72%) | 15 (71%) | 69 (72%) | >0.99 |
| Yes | 33 (28%) | 6 (29%) | 27 (28%) | |
| Cavity | ||||
| No | 111 (95%) | 19 (90%) | 92 (96%) | 0.29 |
| Yes | 6 (5%) | 2 (10%) | 4 (4%) | |
| Calcification | ||||
| No | 112 (96%) | 21 (100%) | 91 (95%) | 0.58 |
| Yes | 5 (4%) | 0 (0%) | 5 (5%) | |
| Nodal disease | ||||
| Negative | 19 (16%) | 1 (5%) | 18 (19%) | 0.19 |
| Positive | 98 (84%) | 20 (95%) | 78 (81%) | |
| Metastases sites | ||||
| Pleural | ||||
| No | 70 (60%) | 11 (52%) | 59 (61%) | 0.47 |
| Yes | 47 (40%) | 10 (48%) | 37 (39%) | |
| Extra-thoracic | ||||
| Absent | 33 (28%) | 6 (29%) | 27 (28%) | >0.99 |
| Present | 84 (72%) | 15 (71%) | 69 (72%) | |
| Bone | ||||
| No | 68 (58%) | 12 (57%) | 56 (58%) | >0.99 |
| Yes | 49 (42%) | 9 (43%) | 40 (42%) | |
| Brain | ||||
| No | 70 (60%) | 11 (52%) | 59 (61%) | 0.47 |
| Yes | 47 (40%) | 10 (48%) | 37 (39%) | |
| Adrenal | ||||
| No | 101 (86%) | 17 (81%) | 84 (88%) | 0.48 |
| Yes | 16 (14%) | 4 (19%) | 12 (12%) | |
| Soft tissue | ||||
| No | 92 (79%) | 17 (81%) | 75 (78%) | >0.99 |
| Yes | 25 (21%) | 4 (19%) | 21 (22%) | |
| Other lung metastasis >6 mm | ||||
| No | 49 (42%) | 16 (76%) | 33 (34%) | <0.01 |
| Yes | 68 (58%) | 5 (24%) | 63 (66%) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Digumarthy, S.R.; Mendoza, D.P.; Padole, A.; Chen, T.; Peterson, P.G.; Piotrowska, Z.; Sequist, L.V. Diffuse Lung Metastases in EGFR-Mutant Non-Small Cell Lung Cancer. Cancers 2019, 11, 1360. https://doi.org/10.3390/cancers11091360
Digumarthy SR, Mendoza DP, Padole A, Chen T, Peterson PG, Piotrowska Z, Sequist LV. Diffuse Lung Metastases in EGFR-Mutant Non-Small Cell Lung Cancer. Cancers. 2019; 11(9):1360. https://doi.org/10.3390/cancers11091360
Chicago/Turabian StyleDigumarthy, Subba R., Dexter P. Mendoza, Atul Padole, Tianqi Chen, P. Gabriel Peterson, Zofia Piotrowska, and Lecia V. Sequist. 2019. "Diffuse Lung Metastases in EGFR-Mutant Non-Small Cell Lung Cancer" Cancers 11, no. 9: 1360. https://doi.org/10.3390/cancers11091360
APA StyleDigumarthy, S. R., Mendoza, D. P., Padole, A., Chen, T., Peterson, P. G., Piotrowska, Z., & Sequist, L. V. (2019). Diffuse Lung Metastases in EGFR-Mutant Non-Small Cell Lung Cancer. Cancers, 11(9), 1360. https://doi.org/10.3390/cancers11091360




