Estrogens Counteract Platinum-Chemosensitivity by Modifying the Subcellular Localization of MDM4
Abstract
1. Introduction
2. Results
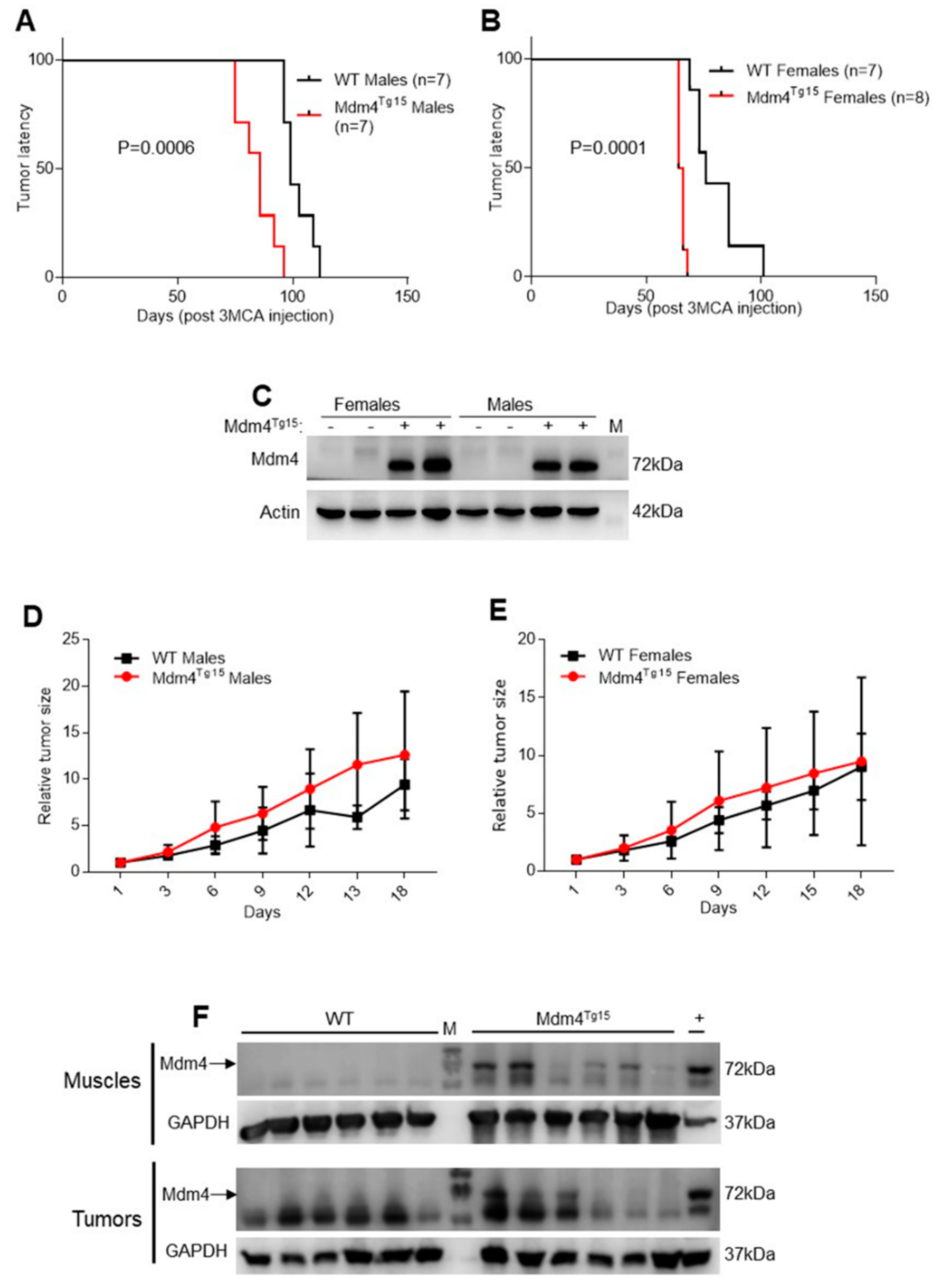
2.1. Enhanced Levels of MDM4 Accelerate DNA-Damage Induced Tumor Development in a Gender-Independent Manner
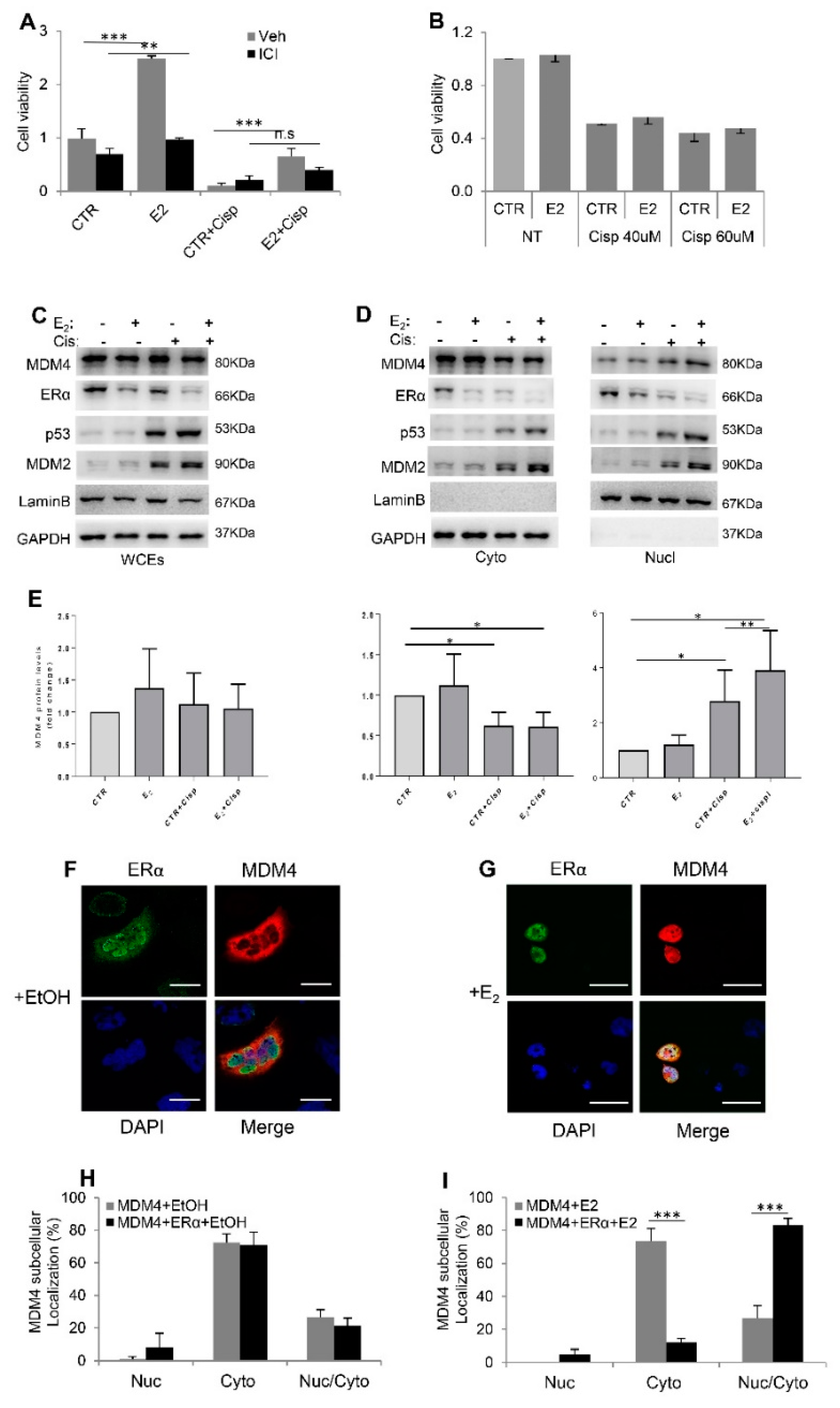
2.2. Cisplatin-Sensitivity Is Affected by Mdm4 in a Gender-Dependent Manner
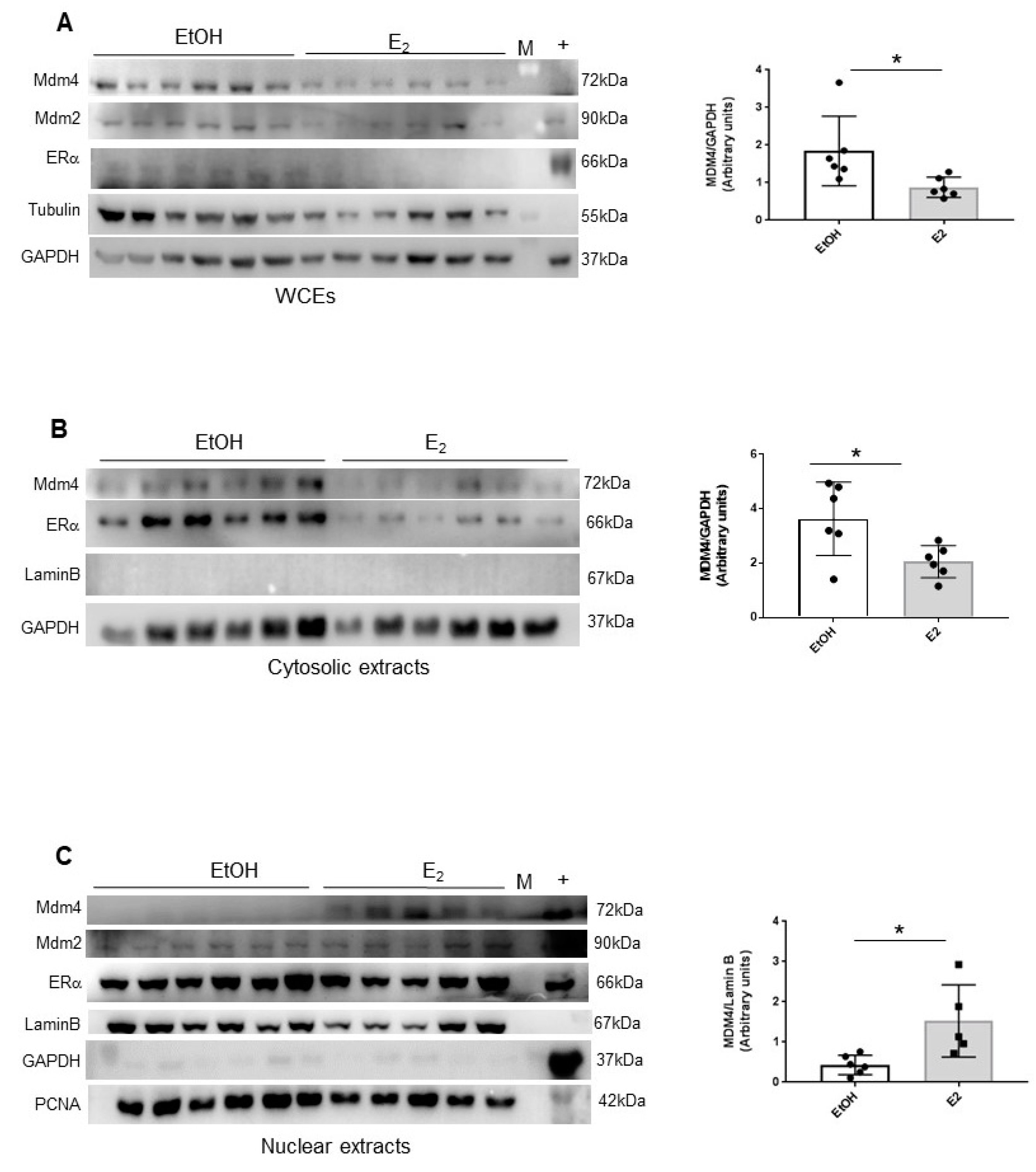
2.3. Estrogens Modulate Mdm4 Intracellular Localization
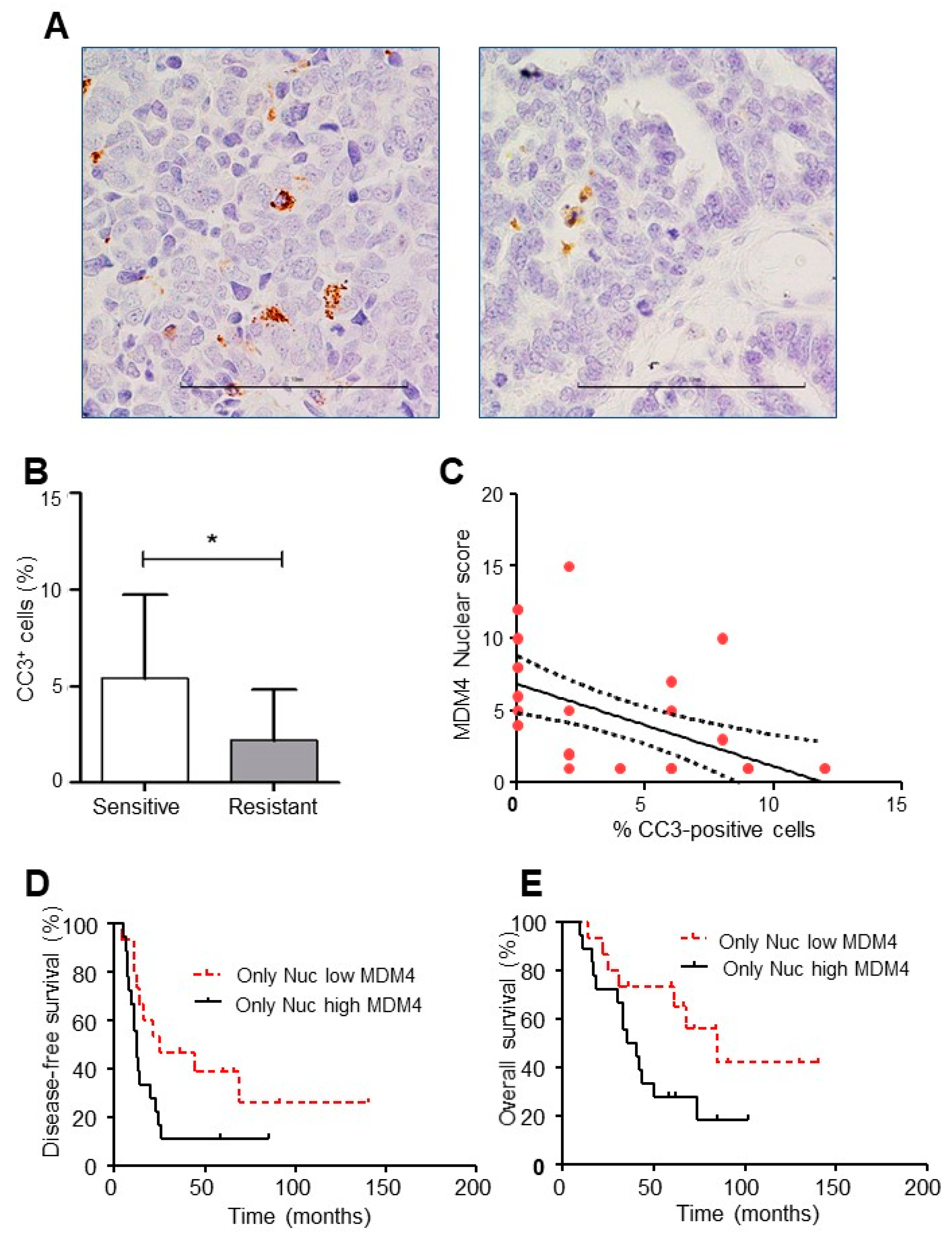
2.4. Mdm4 Intracellular Localization Correlates with Platinum Sensitivity in High-Grade Serous Ovarian Carcinoma
3. Discussion
4. Materials and Methods
4.1. Mouse Maintenance and Treatment
4.2. Cell Cultures and Treatments
4.3. Immunofluorescence
4.4. Protein Analysis
4.5. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.6. Patients
4.7. Immunohistochemistry
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosen, A.; Zeger, S.L. Precision medicine: Discovering clinically relevant and mechanistically anchored disease subgroups at scale. J. Clin. Investig. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ethier, J.L.; Desautels, D.N.; Amir, E.; MacKay, H. Is hormonal therapy effective in advanced endometrial cancer? A systematic review and meta-analysis. Gynecol Oncol. 2017, 147, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Phelps, M.; Darley, M.; Primrose, J.N.; Blaydes, J.P. p53-independent activation of the hdm2-P2 promoter through multiple transcription factor response elements results in elevated hdm2 expression in estrogen receptor alpha-positive breast cancer cells. Cancer Res. 2003, 63, 2616–2623. [Google Scholar] [PubMed]
- Bond, G.L.; Hu, W.; Bond, E.E.; Robins, H.; Lutzker, S.G.; Arva, N.C.; Bargonetti, J.; Bartel, F.; Taubert, H.; Wuerl, P.; et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 2004, 119, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Bond, G.L.; Hirshfield, K.M.; Kirchhoff, T.; Alexe, G.; Bond, E.E.; Robins, H.; Bartel, F.; Taubert, H.; Wuerl, P.; Hait, W.; et al. MDM2 SNP309 accelerates tumor formation in a gender-specific and hormone-dependent manner. Cancer Res. 2006, 66, 5104–5110. [Google Scholar] [CrossRef]
- Ueda, M.; Yamamoto, M.; Nunobiki, O.; Toji, E.; Sato, N.; Izuma, S.; Okamoto, Y.; Torii, K.; Noda, S. Murine double-minute 2 homolog single nucleotide polymorphism 309 and the risk of gynecologic cancer. Hum. Cell 2009, 22, 49–54. [Google Scholar] [CrossRef]
- Garcia-Closas, M.; Couch, F.J.; Lindstrom, S.; Michailidou, K.; Schmidt, M.K.; Brook, M.N.; Orr, N.; Rhie, S.K.; Riboli, E.; Feigelson, H.S.; et al. Genome-wide association studies identify four ER negative-specific breast cancer risk loci. Nat. Genet. 2013, 45, 392–398. [Google Scholar] [CrossRef]
- Eeles, R.A.; Olama, A.A.; Benlloch, S.; Saunders, E.J.; Leongamornlert, D.A.; Tymrakiewicz, M.; Ghoussaini, M.; Luccarini, C.; Dennis, J.; Jugurnauth-Little, S.; et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat. Genet. 2013, 45, 385–391. [Google Scholar] [CrossRef]
- Wasylishen, A.R.; Lozano, G. Attenuating the p53 Pathway in Human Cancers: Many Means to the Same End. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef]
- Wynendaele, J.; Bohnke, A.; Leucci, E.; Nielsen, S.J.; Lambertz, I.; Hammer, S.; Sbrzesny, N.; Kubitza, D.; Wolf, A.; Gradhand, E.; et al. An illegitimate microRNA target site within the 3’ UTR of MDM4 affects ovarian cancer progression and chemosensitivity. Cancer Res. 2010, 70, 9641–9649. [Google Scholar] [CrossRef] [PubMed]
- Stegeman, S.; Moya, L.; Selth, L.A.; Spurdle, A.B.; Clements, J.A.; Batra, J. A genetic variant of MDM4 influences regulation by multiple microRNAs in prostate cancer. Endocr. Relat. Cancer 2015, 22, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.L.; Kuchenbaecker, K.B.; Michailidou, K.; Beesley, J.; Kar, S.; Lindstrom, S.; Hui, S.; Lemacon, A.; Soucy, P.; Dennis, J.; et al. Identification of ten variants associated with risk of estrogen-receptor-negative breast cancer. Nat. Genet. 2017, 49, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Pant, V.; Zhang, Y.; Aryal, N.K.; You, M.J.; Kusewitt, D.; Lozano, G. The p53 inhibitor Mdm4 cooperates with multiple genetic lesions in tumourigenesis. J. Pathol. 2017, 241, 501–510. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, L.; Qiu, W.; Deng, T.; Zhang, Y.; Bergholz, J.; Xiao, Z.X. MDMX exerts its oncogenic activity via suppression of retinoblastoma protein. Oncogene 2015, 34, 5560–5569. [Google Scholar] [CrossRef]
- Chen, S.H.; Forrester, W.; Lahav, G. Schedule-dependent interaction between anticancer treatments. Science 2016, 351, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Wang, J.Y.; Liu, Z.Y.; Ma, X.M.; Wang, X.W.; Jin, H.; Zhang, X.P.; Fu, D.; Hou, L.J.; Lu, Y.C. Ubiquitin-specific protease 2a stabilizes MDM4 and facilitates the p53-mediated intrinsic apoptotic pathway in glioblastoma. Carcinogenesis 2014, 35, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Pieroni, L.; Monteleone, V.; Luca, R.; Fici, L.; Luca, E.; Urbani, A.; Xiong, S.; Soddu, S.; Masetti, R.; et al. MDM4/HIPK2/p53 cytoplasmic assembly uncovers coordinated repression of molecules with anti-apoptotic activity during early DNA damage response. Oncogene 2016, 35, 228–240. [Google Scholar] [CrossRef]
- Mancini, F.; Di Conza, G.; Pellegrino, M.; Rinaldo, C.; Prodosmo, A.; Giglio, S.; D’Agnano, I.; Florenzano, F.; Felicioni, L.; Buttitta, F.; et al. MDM4 (MDMX) localizes at the mitochondria and facilitates the p53-mediated intrinsic-apoptotic pathway. EMBO J. 2009, 28, 1926–1939. [Google Scholar] [CrossRef]
- Kon, N.; Wang, D.; Li, T.; Jiang, L.; Qiang, L.; Gu, W. Inhibition of Mdmx (Mdm4) in vivo induces anti-obesity effects. Oncotarget 2018, 9, 7282–7297. [Google Scholar] [CrossRef]
- Mancini, F.; Teveroni, E.; Di Conza, G.; Monteleone, V.; Arisi, I.; Pellegrino, M.; Buttarelli, M.; Pieroni, L.; D’Onofrio, M.; Urbani, A.; et al. MDM4 actively restrains cytoplasmic mTORC1 by sensing nutrient availability. Mol. Cancer 2017, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fatah, T.M.; Powe, D.G.; Agboola, J.; Adamowicz-Brice, M.; Blamey, R.W.; Lopez-Garcia, M.A.; Green, A.R.; Reis-Filho, J.S.; Ellis, I.O. The biological, clinical and prognostic implications of p53 transcriptional pathways in breast cancers. J. Pathol. 2010, 220, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Pant, V.; Suh, Y.A.; Van Pelt, C.S.; Wang, Y.; Valentin-Vega, Y.A.; Post, S.M.; Lozano, G. Spontaneous tumorigenesis in mice overexpressing the p53-negative regulator Mdm4. Cancer Res. 2010, 70, 7148–7154. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cao, I.; Garcia-Cao, M.; Martin-Caballero, J.; Criado, L.M.; Klatt, P.; Flores, J.M.; Weill, J.C.; Blasco, M.A.; Serrano, M. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 2002, 21, 6225–6235. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Di Conza, G.; Monti, O.; Macchiarulo, A.; Pellicciari, R.; Pontecorvi, A.; Moretti, F. Puzzling over MDM4-p53 network. Int. J. Biochem. Cell Biol. 2010, 42, 1080–1083. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Regunath, K.; Jacq, X.; Prives, C. Cisplatin causes cell death via TAB1 regulation of p53/MDM2/MDMX circuitry. Genes Dev. 2013, 27, 1739–1751. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, E.S.; Askautrud, H.A.; Kees, T.; Park, J.H.; Plaks, V.; Ewald, A.J.; Fein, M.; Rasch, M.G.; Tan, Y.X.; Qiu, J.; et al. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell 2012, 21, 488–503. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Zaitchouk, T.; Vacher, M.; Duthu, A.; Canivet, M.; Choisy-Rossi, C.; Nieruchalski, M.; May, E. Tissue and cell-specific expression of the p53-target genes: Bax, fas, mdm2 and waf1/p21, before and following ionising irradiation in mice. Oncogene 2000, 19, 649–660. [Google Scholar] [CrossRef]
- Fei, P.; Bernhard, E.J.; El-Deiry, W.S. Tissue-specific induction of p53 targets in vivo. Cancer Res. 2002, 62, 7316–7327. [Google Scholar]
- Migliorini, D.; Danovi, D.; Colombo, E.; Carbone, R.; Pelicci, P.G.; Marine, J.C. Hdmx recruitment into the nucleus by Hdm2 is essential for its ability to regulate p53 stability and transactivation. J. Biol. Chem. 2002, 277, 7318–7323. [Google Scholar] [CrossRef]
- Di Conza, G.; Mancini, F.; Buttarelli, M.; Pontecorvi, A.; Trimarchi, F.; Moretti, F. MDM4 enhances p53 stability by promoting an active conformation of the protein upon DNA damage. Cell Cycle 2012, 11, 749–760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, C.; Chen, L.; Chen, J. DNA damage induces MDMX nuclear translocation by p53-dependent and -independent mechanisms. Mol. Cell. Biol. 2002, 22, 7562–7571. [Google Scholar] [CrossRef] [PubMed]
- LeBron, C.; Chen, L.; Gilkes, D.M.; Chen, J. Regulation of MDMX nuclear import and degradation by Chk2 and 14-3-3. EMBO J. 2006, 25, 1196–1206. [Google Scholar] [CrossRef]
- Gembarska, A.; Luciani, F.; Fedele, C.; Russell, E.A.; Dewaele, M.; Villar, S.; Zwolinska, A.; Haupt, S.; de Lange, J.; Yip, D.; et al. MDM4 is a key therapeutic target in cutaneous melanoma. Nat. Med. 2012, 18, 1239–1247. [Google Scholar] [CrossRef]
- Kawashima, I.; Seiki, K.; Sakabe, K.; Ihara, S.; Akatsuka, A.; Katsumata, Y. Localization of estrogen receptors and estrogen receptor-mRNA in female mouse thymus. Thymus 1992, 20, 115–121. [Google Scholar] [PubMed]
- Mungenast, F.; Thalhammer, T. Estrogen biosynthesis and action in ovarian cancer. Front. Endocrinol. (Lausanne) 2014, 5, 192. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wu, X.; Hillier, S.G.; Fegan, K.S.; Critchley, H.O.; Mason, J.I.; Sarvi, S.; Harlow, C.R. Local estrogen metabolism in epithelial ovarian cancer suggests novel targets for therapy. J. Steroid Biochem. Mol. Biol. 2015, 150, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Colombo, P.E.; Fabbro, M.; Theillet, C.; Bibeau, F.; Rouanet, P.; Ray-Coquard, I. Sensitivity and resistance to treatment in the primary management of epithelial ovarian cancer. Crit. Rev. Oncol. Hematol. 2014, 89, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Sieh, W.; Kobel, M.; Longacre, T.A.; Bowtell, D.D.; deFazio, A.; Goodman, M.T.; Hogdall, E.; Deen, S.; Wentzensen, N.; Moysich, K.B.; et al. Hormone-receptor expression and ovarian cancer survival: An Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013, 14, 853–862. [Google Scholar] [CrossRef]
- Konings, G.; Brentjens, L.; Delvoux, B.; Linnanen, T.; Cornel, K.; Koskimies, P.; Bongers, M.; Kruitwagen, R.; Xanthoulea, S.; Romano, A. Intracrine Regulation of Estrogen and Other Sex Steroid Levels in Endometrium and Non-gynecological Tissues; Pathology, Physiology, and Drug Discovery. Front. Pharm. 2018, 9, 940. [Google Scholar] [CrossRef]
- Kirilovas, D.; Schedvins, K.; Naessen, T.; Von Schoultz, B.; Carlstrom, K. Conversion of circulating estrone sulfate to 17beta-estradiol by ovarian tumor tissue: A possible mechanism behind elevated circulating concentrations of 17beta-estradiol in postmenopausal women with ovarian tumors. Gynecol. Endocrinol. 2007, 23, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Saito, H.; Sekizawa, A.; Shimizu, Y.; Akamatsu, T.; Kushima, M.; Yanaihara, T.; Okai, T.; Farina, A. Steroid sulfatase expression in ovarian clear cell adenocarcinoma: Immunohistochemical study. Gynecol. Oncol. 2001, 82, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Chura, J.C.; Blomquist, C.H.; Ryu, H.S.; Argenta, P.A. Estrone sulfatase activity in patients with advanced ovarian cancer. Gynecol. Oncol. 2009, 112, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Christophorou, M.A.; Ringshausen, I.; Finch, A.J.; Swigart, L.B.; Evan, G.I. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature 2006, 443, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.A.; Jiang, D.; Mello, S.S.; Johnson, T.M.; Jarvis, L.A.; Kozak, M.M.; Kenzelmann Broz, D.; Basak, S.; Park, E.J.; McLaughlin, M.E.; et al. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell 2011, 145, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Raphael, J.; Gandhi, S.; Li, N.; Lu, F.I.; Trudeau, M. The role of quantitative estrogen receptor status in predicting tumor response at surgery in breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2017, 164, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Swetzig, W.M.; Wang, J.; Das, G.M. Estrogen receptor alpha (ERalpha/ESR1) mediates the p53-independent overexpression of MDM4/MDMX and MDM2 in human breast cancer. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.; Alhaiek, A.A.; Amadi, S.; Qattan, A.T.; Crawford, M.; Radulovic, M.; Godovac-Zimmermann, J. Systematic nucleo-cytoplasmic trafficking of proteins following exposure of MCF7 breast cancer cells to estradiol. J. Proteome Res. 2014, 13, 1112–1127. [Google Scholar] [CrossRef]
- Teveroni, E.; Pellegrino, M.; Sacconi, S.; Calandra, P.; Cascino, I.; Farioli-Vecchioli, S.; Puma, A.; Garibaldi, M.; Morosetti, R.; Tasca, G.; et al. Estrogens enhance myoblast differentiation in facioscapulohumeral muscular dystrophy by antagonizing DUX4 activity. J. Clin. Investig. 2017, 127, 1531–1545. [Google Scholar] [CrossRef]
- Mahmoodzadeh, S.; Haase, H.; Sporbert, A.; Rharass, T.; Panakova, D.; Morano, I. Nuclear translocation of the cardiac L-type calcium channel C-terminus is regulated by sex and 17beta-estradiol. J. Mol. Cell. Cardiol. 2016, 97, 226–234. [Google Scholar] [CrossRef]
- Lu, M.; Muers, M.R.; Lu, X. Introducing STRaNDs: Shuttling transcriptional regulators that are non-DNA binding. Nat. Rev. Mol. Cell. Biol. 2016, 17, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Zannoni, G.F.; Prisco, M.G.; Vellone, V.G.; De Stefano, I.; Scambia, G.; Gallo, D. Changes in the expression of oestrogen receptors and E-cadherin as molecular markers of progression from normal epithelium to invasive cancer in elderly patients with vulvar squamous cell carcinoma. Histopathology 2011, 58, 265–275. [Google Scholar] [CrossRef] [PubMed]


| Mdm4Tg | WT | |||
|---|---|---|---|---|
| P53 Status | ♀ | ♂ | ♀ | ♂ |
| WT | 3 # | 2 | 3 | 2 |
| Mut | 4 | 3 | 4 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucà, R.; di Blasio, G.; Gallo, D.; Monteleone, V.; Manni, I.; Fici, L.; Buttarelli, M.; Ciolli, G.; Pellegrino, M.; Teveroni, E.; et al. Estrogens Counteract Platinum-Chemosensitivity by Modifying the Subcellular Localization of MDM4. Cancers 2019, 11, 1349. https://doi.org/10.3390/cancers11091349
Lucà R, di Blasio G, Gallo D, Monteleone V, Manni I, Fici L, Buttarelli M, Ciolli G, Pellegrino M, Teveroni E, et al. Estrogens Counteract Platinum-Chemosensitivity by Modifying the Subcellular Localization of MDM4. Cancers. 2019; 11(9):1349. https://doi.org/10.3390/cancers11091349
Chicago/Turabian StyleLucà, Rossella, Giorgia di Blasio, Daniela Gallo, Valentina Monteleone, Isabella Manni, Laura Fici, Marianna Buttarelli, Germana Ciolli, Marsha Pellegrino, Emanuela Teveroni, and et al. 2019. "Estrogens Counteract Platinum-Chemosensitivity by Modifying the Subcellular Localization of MDM4" Cancers 11, no. 9: 1349. https://doi.org/10.3390/cancers11091349
APA StyleLucà, R., di Blasio, G., Gallo, D., Monteleone, V., Manni, I., Fici, L., Buttarelli, M., Ciolli, G., Pellegrino, M., Teveroni, E., Maiullari, S., Ciucci, A., Apollo, A., Mancini, F., Gentileschi, M. P., Zannoni, G. F., Pontecorvi, A., Scambia, G., & Moretti, F. (2019). Estrogens Counteract Platinum-Chemosensitivity by Modifying the Subcellular Localization of MDM4. Cancers, 11(9), 1349. https://doi.org/10.3390/cancers11091349







