Glypican 3-Targeted Therapy in Hepatocellular Carcinoma
Abstract
1. Introduction
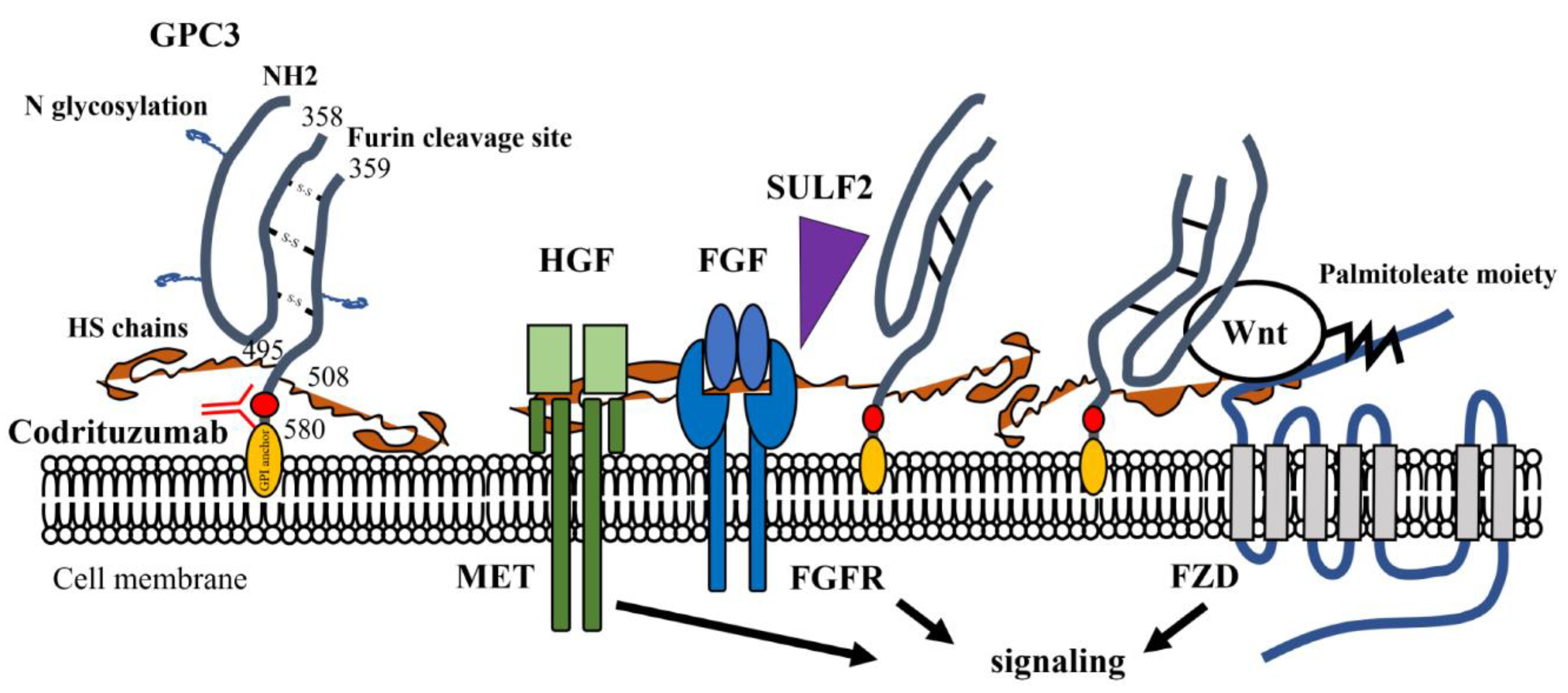
2. GPC3: Structure, Expression, and Functions
3. GPC3 and Tumor Progression
4. Clinical Trials of GPC3 Targeting Therapy
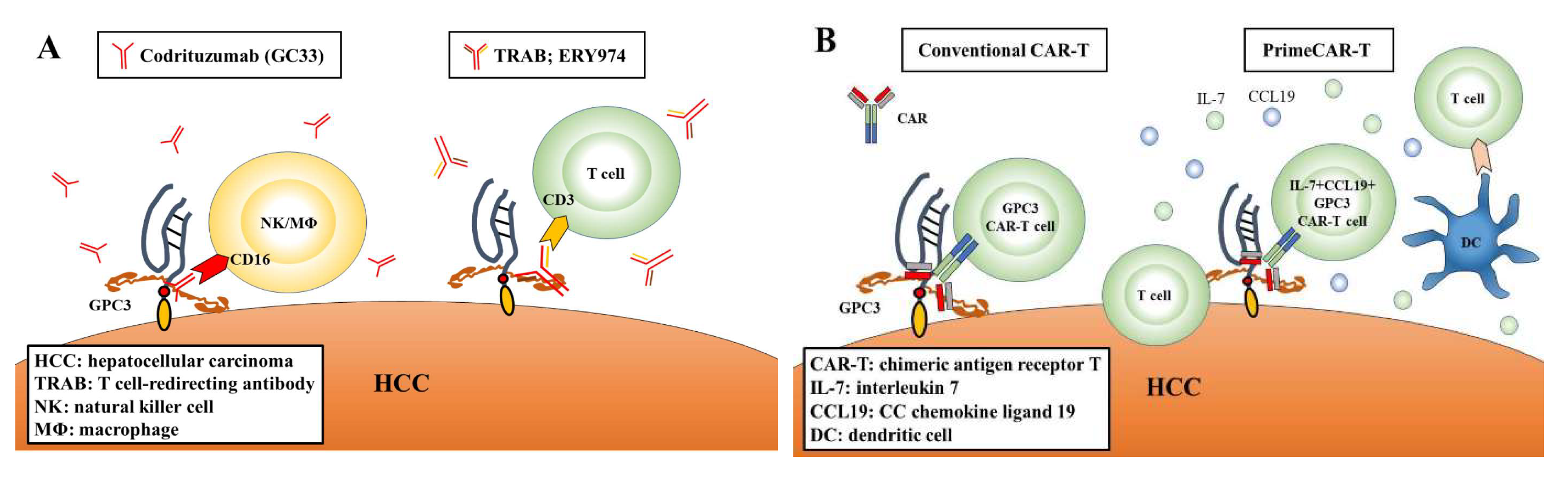
4.1. GPC3-Targeted Antibody Therapy
4.2. Vaccine Therapy
4.3. CAR-T Therapy
5. Detection of GPC3 In Vivo
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- London, W.T.; McGlynn, K.A. Liver cancer. In Cancer Epidemiology and Prevention, 3rd ed.; Oxford University Press: New York, NY, USA, 2006; pp. 763–786. [Google Scholar]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2016, 2, 16018. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Zhu, A.X.; Park, J.O.; Ryoo, B.Y.; Yen, C.J.; Poon, R.; Pastorelli, D.; Blanc, J.F.; Chung, H.C.; Baron, A.D.; Pfiffer, T.E.; et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015, 16, 859–870. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased a-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 2045, 1–15. [Google Scholar] [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef]
- Iwai, Y.; Terawaki, S.; Honjo, T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int. Immunol. 2005, 17, 133–144. [Google Scholar] [CrossRef]
- Ott, P.A.; Dotti, G.; Yee, C.; Goff, S.L. An Update on Adoptive T-Cell Therapy and Neoantigen Vaccines. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, e70–e78. [Google Scholar] [CrossRef] [PubMed]
- Filmus, J.; Church, J.G.; Buick, R.N. Isolation of a cDNA corresponding to a developmentally regulated transcript in rat intestine. Mol. Cell. Boil. 1988, 8, 4243–4249. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Choo, B.; Wong, Z.-M.; Filmus, J.; Buick, R.N. Expression of OCI-5/Glypican 3 during Intestinal Morphogenesis: Regulation by Cell Shape in Intestinal Epithelial Cells. Exp. Cell Res. 1997, 235, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Filmus, J.; Capurro, M. Glypican-3: A marker and a therapeutic target in hepatocellular carcinoma. FEBS J. 2013, 280, 2471–2476. [Google Scholar] [CrossRef] [PubMed]
- Filmus, J.; Capurro, M.; Rast, J. Glypicans. Genome Biol. 2008, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- De Cat, B.; Muyldermans, S.-Y.; Coomans, C.; Degeest, G.; Vanderschueren, B.; Creemers, J.; Biemar, F.; Peers, B.; David, G. Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J. Cell Biol. 2003, 163, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Capurro, M.; Wanless, I.R.; Sherman, M.; DeBoer, G.; Shi, W.; Miyoshi, E.; Filmus, J. Glypican-3: A novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003, 125, 89–97. [Google Scholar] [CrossRef]
- Hippo, Y. Identification of Soluble NH2-Terminal Fragment of Glypican-3 as a Serological Marker for Early-Stage Hepatocellular Carcinoma. Cancer Res. 2004, 64, 2418–2423. [Google Scholar] [CrossRef] [PubMed]
- Capurro, M.; Filmus, J. Glypican-3 as a serum marker for hepatocellular carcinoma. Cancer Res. 2005, 65, 372–373. [Google Scholar]
- Simpson, J.L.; Landey, S.; New, M.; German, J.A. A previously unrecognised X-linked syndrome of dysmorphia. Birth Defects Orig. Artic. Ser. 1975, 11, 18. [Google Scholar]
- Golabi, M.; Rosen, L. A new X-linked mental retardation overgrowth syndrome? Am. J. Med. Genet. 1984, 17, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Behmel, A.; Rosenkranz, W. A new X-linked dysplasia gigantism syndrome: Identical with the Simpson dysplasia syndrome? Qual. Life Res. 1984, 67, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Pilia, G.; Hughes-Benzie, R.M.; MacKenzie, A.; Baybayan, P.; Chen, E.Y.; Huber, R.; Neri, G.; Cao, A.; Forabosco, A.; Schlessinger, D. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat. Genet. 1996, 12, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Vuillaume, M.-L.; Moizard, M.-P.; Baumer, A.; Cottereau, E.; Brioude, F.; Rauch, A.; Toutain, A. CUGC for Simpson-Golabi-Behmel syndrome (SGBS). Eur. J. Hum. Genet. 2019, 27, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, B.V.; Centeno, G.; Pascuccelli, H.; Ward, F.; Peters, M.G.; Filmus, J.; Puricelli, L.; Joffé, E.B.D.K. Expression pattern of glypican-3 (GPC3) during human embryonic and fetal development. Histol. Histopathol. 2008, 23, 1333–1340. [Google Scholar] [PubMed]
- Paine-Saunders, S.; Viviano, B.L.; Zupicich, J.; Skarnes, W.C.; Saunders, S. glypican-3 Controls Cellular Responses to Bmp4 in Limb Patterning and Skeletal Development. Dev. Boil. 2000, 225, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Capurro, M.I.; Xu, P.; Shi, W.; Li, F.; Jia, A.; Filmus, J. Glypican-3 Inhibits Hedgehog Signaling during Development by Competing with Patched for Hedgehog Binding. Dev. Cell 2008, 14, 700–711. [Google Scholar] [CrossRef]
- Midorikawa, Y.; Ishikawa, S.; Iwanari, H.; Imamura, T.; Sakamoto, H.; Miyazono, K.; Kodama, T.; Makuuchi, M.; Aburatani, H. Glypican-3, overexpressed in hepatocellular carcinoma, modulates FGF2 and BMP-7 signaling. Int. J. Cancer 2003, 103, 455–465. [Google Scholar] [CrossRef]
- Pellegrini, M.; Pilia, G.; Pantano, S.; Lucchini, F.; Uda, M.; Fumi, M.; Cao, A.; Schlessinger, D.; Forabosco, A. Gpc3 expression correlates with the phenotype of the Simpson-Golabi-Behmel syndrome. Dev. Dyn. 1998, 213, 431–439. [Google Scholar] [CrossRef]
- Hsu, H.C.; Cheng, W.; Lai, P.L. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: Biological significance and temporospatial distribution. Cancer Res. 1997, 57, 5179–5184. [Google Scholar]
- Huber, R.; Hansen, R.S.; Strazzullo, M.; Pengue, G.; Mazzarella, R.; D’Urso, M.; Schlessinger, D.; Pilia, G.; Gartler, S.M.; D’Esposito, M. DNA methylation in transcriptional repression of two differentially expressed X-linked genes, GPC3 and SYBL1. Proc. Natl. Acad. Sci. USA 1999, 96, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Boily, G.; Saikali, Z.; Sinnett, D. Methylation analysis of the glypican 3 gene in embryonal tumours. Br. J. Cancer 2004, 90, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-W.; Friess, H.; Wang, L.; Abou-Shady, M.; Zimmermann, A.; Lander, A.D.; Korc, M.; Kleeff, J.; Büchler, M.W. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut 2001, 48, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Xiong, L.W.; Pan, X.F.; Gen, J.F.; Bao, G.L.; Sha, H.F.; Feng, J.X.; Ji, C.Y.; Chen, M. Expression of GPC3 protein and its significance in lung squamous cell carcinoma. Med. Oncol. 2012, 29, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Ushiku, T.; Uozaki, H.; Shinozaki, A.; Ota, S.; Matsuzaka, K.; Nomura, S.; Kaminishi, M.; Aburatani, H.; Kodama, T.; Fukayama, M. Glypican 3-expressing gastric carcinoma: Distinct subgroup unifying hepatoid, clear-cell, and alpha-fetoprotein-producing gastric carcinomas. Cancer Sci. 2009, 100, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Maeda, D.; Ota, S.; Takazawa, Y.; Aburatani, H.; Nakagawa, S.; Yano, T.; Taketani, Y.; Kodama, T.; Fukayama, M. Glypican-3 expression in clear cell adenocarcinoma of the ovary. Mod. Pathol. 2009, 22, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Nakatsura, T.; Nishimura, Y. Usefulness of the novel oncofetal antigen glypican-3 for diagnosis of hepatocellular carcinoma and melanoma. BioDrugs 2005, 19, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.V.; Roberts, S.S.; Bender, J.G.; Shukla, N.; Wexler, L.H. Immunotherapeutic Targeting of GPC3 in Pediatric Solid Embryonal Tumors. Front. Oncol. 2019, 9, 108. [Google Scholar] [CrossRef]
- Capurro, M.I.; Xiang, Y.-Y.; Lobe, C.; Filmus, J. Glypican-3 Promotes the Growth of Hepatocellular Carcinoma by Stimulating Canonical Wnt Signaling. Cancer Res. 2005, 65, 6245–6254. [Google Scholar] [CrossRef]
- Capurro, M.; Martin, T.; Shi, W.; Filmus, J. Glypican-3 binds to Frizzled and plays a direct role in the stimulation of canonical Wnt signaling. J. Cell Sci. 2014, 127, 1565–1575. [Google Scholar] [CrossRef]
- Li, N.; Wei, L.; Liu, X.; Bai, H.; Ye, Y.; Li, D.; Li, N.; Baxa, U.; Wang, Q.; Lv, L.; et al. A Frizzled-Like Cysteine-Rich Domain in Glypican-3 Mediates Wnt Binding and Regulates Hepatocellular Carcinoma Tumor Growth in Mice. Hepatology 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, Y.; Zhang, Y.; Wang, L.; Chen, G. Glypican-3 promotes cell proliferation and tumorigenesis through up-regulation of β-catenin expression in lung squamous cell carcinoma. Biosci. Rep. 2019, 39, BSR20181147. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-P.; Sandhu, D.S.; Yu, C.; Han, T.; Moser, C.D.; Jackson, K.K.; Guerrero, R.B.; Aderca, I.; Isomoto, H.; Garrity-Park, M.M.; et al. Sulfatase 2 Up-Regulates Glypican 3, Promotes Fibroblast Growth Factor Signaling, and Decreases Survival in Hepatocellular Carcinoma. Hepatology 2008, 47, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Kim, H.; Ho, M. Human Monoclonal Antibody Targeting the Heparan Sulfate Chains of Glypican-3 Inhibits HGF-Mediated Migration and Motility of Hepatocellular Carcinoma Cells. PLoS ONE 2015, 10, e0137664. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, H.; Weng, H.; Zhang, X.; Li, P.; Fan, C.-L.; Li, B.; Dong, P.-L.; Li, L.; Dooley, S.; et al. Glypican-3 promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells through ERK signaling pathway. Int. J. Oncol. 2015, 46, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Tan, Y.; Yu, Y.; Hu, C.; Yang, W.; Fang, T.; Wang, C.; Li, T.; Wen, W. MXR7 facilitates liver cancer metastasis via epithelial-mesenchymal transition. Sci. China Life Sci. 2017, 60, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.P.; Oseini, A.M.; Moser, C.D.; Yu, C.; Elsawa, S.F.; Hu, C.; Nakamura, I.; Han, T.; Aderca, I.; Isomoto, H.; et al. The oncogenic effect of sulfatase 2 in human hepatocellular carcinoma is mediated in part by glypican 3-dependent Wnt activation. Hepatology 2010, 52, 1680–1689. [Google Scholar] [CrossRef]
- Zittermann, S.I.; Capurro, M.I.; Shi, W.; Filmus, J. Soluble glypican 3 inhibits the growth of hepatocellular carcinoma in vitro and in vivo. Int. J. Cancer 2010, 126, 1291–1301. [Google Scholar]
- Saad, A.; Liet, B.; Joucla, G.; Santarelli, X.; Charpentier, J.; Claverol, S.; Grosset, C.F.; Trézéguet, V. Role of Glycanation and Convertase Maturation of Soluble Glypican-3 in Inhibiting Proliferation of Hepatocellular Carcinoma Cells. Biochemistry 2018, 57, 1201–1211. [Google Scholar] [CrossRef]
- Metz, C.; Brunner, G.; Choi-Muira, N.; Nguyen, H.; Gabrilove, J.; Caras, I.; Altszuler, N.; Rifkin, D.; Wilson, E.; Davitz, M. Release of GPI-anchored membrane proteins by a cell-associated GPI-specific phospholipase D. EMBO J. 1994, 13, 1741–1751. [Google Scholar] [CrossRef]
- Traister, A.; Shi, W.; Filmus, J. Mammalian Notum induces the release of glypicans and other GPI-anchored proteins from the cell surface. Biochem. J. 2008, 410, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Kakugawa, S.; Langton, P.F.; Zebisch, M.; Howell, S.A.; Chang, T.H.; Liu, Y.; Feizi, T.; Bineva, G.; O’Reilly, N.; Snijders, A.P.; et al. Notum deacylates Wnt proteins to suppress signalling activity. Nature 2015, 519, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Montani, F.; Bianchi, F. Circulating Cancer Biomarkers: The Macro-revolution of the Micro-RNA. EBioMedicine 2016, 5, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Bolha, L.; Ravnik-Glavač, M.; Glavač, D. Long Noncoding RNAs as Biomarkers in Cancer. Dis. Markers. 2017, 2017, 7243968. [Google Scholar] [CrossRef]
- Tian, Z.; Jiang, H.; Liu, Y.; Huang, Y.; Xiong, X.; Wu, H.; Dai, X. MicroRNA-133b inhibits hepatocellular carcinoma cell progression by targeting Sirt1. Exp. Cell Res. 2016, 343, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-T.; Yuan, J.-H.; Zhu, T.-T.; Li, Y.-Y.; Cheng, X.-Y. Long noncoding RNA glypican 3 (GPC3) antisense transcript 1 promotes hepatocellular carcinoma progression via epigenetically activating GPC3. FEBS J. 2016, 283, 3739–3754. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Ning, X.; Deng, Z.; Liu, M.; Zhou, B.; Chen, X.; Huang, S.; Xu, Y.; Chen, Z.; Luo, R. Propofol-induced miR-219-5p inhibits growth and invasion of hepatocellular carcinoma through suppression of GPC3-mediated Wnt/β-catenin signalling activation. J. Cell. Biochem. 2019, 120, 16934–16945. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xian, J.; Zang, J.; Xiao, L.; Li, Y.; Sha, M.; Shen, M. Long non-coding RNA FENDRR inhibits proliferation and invasion of hepatocellular carcinoma by down-regulating glypican-3 expression. Biochem. Biophys. Res. Commun. 2019, 509, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Zhang, S.; An, J.; Zhang, J.; Huang, J.; Jin, Y. HOXA-AS2 Promotes Proliferation and Induces Epithelial-Mesenchymal Transition via the miR-520c-3p/GPC3 Axis in Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2018, 50, 2124–2138. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, T.; Sugimoto, M.; Kinoshita, Y.; Miyazaki, Y.; Nakano, K.; Tsunoda, H.; Sugo, I.; Ohizumi, I.; Aburatani, H.; Hamakubo, T.; et al. Anti-Glypican 3 Antibody as a Potential Antitumor Agent for Human Liver Cancer. Cancer Res. 2008, 68, 9832–9838. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Orita, T.; Nezu, J.; Yoshino, T.; Ohizumi, I.; Sugimoto, M.; Furugaki, K.; Kinoshita, Y.; Ishiguro, T.; Hamakubo, T.; et al. Anti-glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2009, 378, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Gold, P.J.; El-Khoueiry, A.B.; Abrams, T.A.; Morikawa, H.; Ohishi, N.; Ohtomo, T.; Philip, P.A. First-in-Man Phase I Study of GC33, a Novel Recombinant Humanized Antibody Against Glypican-3, in Patients with Advanced Hepatocellular Carcinoma. Clin. Cancer Res. 2013, 19, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Ohkawa, S.; Okusaka, T.; Mitsunaga, S.; Kobayashi, S.; Morizane, C.; Suzuki, I.; Yamamoto, S.; Furuse, J. Japanese phase I study of GC33, a humanized antibody against glypican-3 for advanced hepatocellular carcinoma. Cancer Sci. 2014, 105, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Puig, O.; Daniele, B.; Kudo, M.; Merle, P.; Park, J.W.; Ross, P.; Peron, J.M.; Ebert, O.; Chan, S.; et al. Randomized phase II placebo controlled study of codrituzumab in previously treated patients with advanced hepatocellular carcinoma. J. Hepatol. 2016, 65, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, Y.-C.; Reis, B.; Belousov, A.; Jukofsky, L.; Rossin, C.; Muehlig, A.; Xu, C.; Essioux, L.; Ohtomo, T.; et al. Combining expression of GPC3 in tumors and CD16 on NK cells from peripheral blood to identify patients responding to codrituzumab. Oncotarget 2018, 9, 10436–10444. [Google Scholar] [CrossRef] [PubMed]
- Shiraiwa, H.; Narita, A.; Kamata-Sakurai, M.; Ishiguro, T.; Sano, Y.; Hironiwa, N.; Tsushima, T.; Segawa, H.; Tsunenari, T.; Ikeda, Y.; et al. Engineering a bispecific antibody with a common light chain: Identification and optimization of an anti-CD3 epsilon and anti-GPC3 bispecific antibody, ERY974. Methods 2019, 154, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, T.; Sano, Y.; Komatsu, S.I.; Kamata-Sakurai, M.; Kaneko, A.; Kinoshita, Y.; Shiraiwa, H.; Azuma, Y.; Tsunenari, T.; Kayukawa, Y.; et al. An anti-glypican 3/CD3 bispecific T cell-redirecting antibody for treatment of solid tumors. Sci. Transl. Med. 2017, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Kinoshita, Y.; Adachi, K.; Narita, Y.; Amano, J.; Kato, A.; Watanabe, T.; Kayukawa, Y.; Miyazaki, Y.; Ohtomo, T. Abstract 2747: Anti-glypican-3 monoclonal antibody (codrituzumab/GC33/RO5137382) treatment enhances tumor infiltration of PD-L1-positive macrophages, and combination therapy with anti-PD-L1 monoclonal antibody promotes antitumor effects. Immunology 2018, 78, 2747. [Google Scholar]
- Cheng, A.L.; Yen, C.J.; Okusaka, T.; Ikeda, M.; Hsu, C.H.; Wu, S.Y.; Morizane, C.; Hashimoto, Y.; Ueshima, K.; Ohtomo, T.; et al. A phase I, open-label, multi-center, dose-escalation study of codrituzumab, an anti-glypican-3 monoclonal antibody, in combination with atezolizumab in patients with locally advanced or metastatic hepatocellular carcinoma. Ann Oncol. 2018, 29, viii205–viii270. [Google Scholar] [CrossRef]
- Sawada, Y.; Yoshikawa, T.; Nobuoka, D.; Shirakawa, H.; Kuronuma, T.; Motomura, Y.; Mizuno, S.; Ishii, H.; Nakachi, K.; Konishi, M.; et al. Phase I Trial of a Glypican-3-Derived Peptide Vaccine for Advanced Hepatocellular Carcinoma: Immunologic Evidence and Potential for Improving Overall Survival. Clin. Cancer Res. 2012, 18, 3686–3696. [Google Scholar] [CrossRef]
- Sawada, Y.; Yoshikawa, T.; Ofuji, K.; Yoshimura, M.; Tsuchiya, N.; Takahashi, M.; Nobuoka, D.; Gotohda, N.; Takahashi, S.; Kato, Y.; et al. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. OncoImmunology 2016, 5, e1129483. [Google Scholar] [CrossRef] [PubMed]
- Grupp, S.A.; Maude, S.L.; Rives, S.; Baruchel, A.; Boyer, M.W.; Bittencourt, H.; Bader, P.; Büchner, J.; Laetsch, T.W.; Stefanski, H.; et al. Updated Analysis of the Efficacy and Safety of Tisagenlecleucel in Pediatric and Young Adult Patients with Relapsed/Refractory (r/r) Acute Lymphobastic Leukemia. Blood 2018, 132, 895. [Google Scholar]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Shi, D.; Gao, H.; Qi, X.; Jiang, H.; Zhang, Y.; Chi, J.; Ruan, H.; Wang, H.; Ru, Q.C.; et al. A phase I study of anti-GPC3 chimeric antigen receptor modified T cells (GPC3 CAR-T) in Chinese patients with refractory of relapsed GPC3+ hepatocellular carcinoma (r/r GPC3+ HCC) (NCT02395250). J. Clin. Oncol. 2017. [Google Scholar] [CrossRef]
- Guo, X.; Jiang, H.; Shi, B.; Zhou, M.; Zhang, H.; Shi, Z.; Du, G.; Luo, H.; Wu, X.; Wang, Y.; et al. Disruption of PD-1 Enhanced the Anti-tumor Activity of Chimeric Antigen Receptor T Cells Against Hepatocellular Carcinoma. Front. Pharmacol. 2018, 9, 1118. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Luo, H.; Shi, B.; Di, S.; Sun, R.; Su, J.; Liu, Y.; Li, H.; Jiang, H.; Li, Z. Combined Antitumor Effects of Sorafenib and GPC3-CAR T Cells in Mouse Models of Hepatocellular Carcinoma. Mol. Ther. 2019, 27, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Kano, Y.; Nagai, T.; Okuyama, N.; Sakoda, Y.; Tamada, K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 2018, 36, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Haruyama, Y.; Yorita, K.; Yamaguchi, T.; Kitajima, S.; Amano, J.; Ohtomo, T.; Ohno, A.; Kondo, K.; Kataoka, H. High preoperative levels of serum glypican-3 containing N-terminal subunit are associated with poor prognosis in patients with hepatocellular carcinoma after partial hepatectomy. Int. J. Cancer 2015, 137, 1643–1651. [Google Scholar] [CrossRef]
- Ofuji, K.; Saito, K.; Suzuki, S.; Shimomura, M.; Shirakawa, H.; Nobuoka, D.; Sawada, Y.; Yoshimura, M.; Tsuchiya, N.; Takahashi, M.; et al. Perioperative plasma glypican-3 level may enable prediction of the risk of recurrence after surgery in patients with stage I hepatocellular carcinoma. Oncotarget 2017, 8, 37835–37844. [Google Scholar] [CrossRef]
- Lacin, S.; Abdel-Wahab, R.; Hassan, M.; Shalaby, A.S.; Amin, H.M.; Wolff, R.A.; Yao, J.C.; Mistry, A.; Feng, J.D.; Ohtomo, T.; et al. Evaluating clinical and prognostic implications of Glypican 3 in hepatocellular carcinoma. J. Clin. Oncol. 2016, 34, e15619. [Google Scholar] [CrossRef]
- Kawaida, M.; Yamazaki, K.; Tsujikawa, H.; Fukuma, M.; Abe, Y.; Kitago, M.; Shinoda, M.; Kitagawa, Y.; Sakamoto, M. Diffuse and canalicular patterns of glypican-3 expression reflect malignancy of hepatocellular carcinoma. Pathol. Int. 2019, 69, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Haruyama, Y.; Kataoka, H. Glypican-3 is a prognostic factor and an immunotherapeutic target in hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.; Ma, H.; Song, X.; He, L.; Ye, X.; Li, X. Overexpression of glypican-3 is a predictor of poor prognosis in hepatocellular carcinoma: An updated meta-analysis. Medicine 2018, 97, e11130. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, T.; Jin, B.; Li, W.; Wang, Z.; Zhang, H.; Song, Y.; Li, N. Diagnosis accuracy of serum glypican-3 level in patients with hepatocellular carcinoma: A systematic review with meta-analysis. Int. J. Boil. Markers 2018, 33, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Su, C.; Sun, L.; Gao, Y.; Li, Y. Performance of Serum Glypican 3 in Diagnosis of Hepatocellular Carcinoma: A meta-analysis. Ann. Hepatol. 2019, 18, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Anatelli, F.; Zhai, Q.J.; Adley, B.; Chuang, S.-T.; Yang, X.J. Glypican-3 as a useful diagnostic marker that distinguishes hepatocellular carcinoma from benign hepatocellular mass lesions. Arch. Pathol. Lab. Med. 2008, 132, 1723–1728. [Google Scholar] [PubMed]
- Wang, Z.; Han, Y.J.; Huang, S.; Wang, M.; Zhou, W.L.; Li, H.S.; Wang, Q.S.; Wu, H.B. Imaging the expression of glypican-3 in hepatocellular carcinoma by PET. Amino Acids. 2018, 50, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Gu, J.; Hu, K.; Huang, S.; Conti, P.S.; Wu, H.; Chen, K. Radiofluorinated GPC3-Binding Peptides for PET Imaging of Hepatocellular Carcinoma. Mol. Imaging Boil. 2019. [Google Scholar] [CrossRef] [PubMed]
- Carrasquillo, J.A.; O’Donoghue, J.A.; Beylergil, V.; Ruan, S.; Pandit-Taskar, N.; Larson, S.M.; Smith-Jones, P.M.; Lyashchenko, S.K.; Ohishi, N.; Ohtomo, T.; et al. I-124 codrituzumab imaging and biodistribution in patients with hepatocellular carcinoma. EJNMMI Res. 2018, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiao, X.; Li, X.; Xu, Y.; Ma, L.; Guo, L.; Yan, C.; Wu, Y. Detecting GPC3-Expressing Hepatocellular Carcinoma with L5 Peptide-Guided Pretargeting Approach: In Vitro and In Vivo MR Imaging Experiments. Contrast Media Mol. Imaging 2018, 2018, 9169072. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, Z.; Dong, L.; Zhou, H.; Yang, S.; Wu, W.; Lin, J. A GPC3-specific aptamer-mediated magnetic resonance probe for hepatocellular carcinoma. Int. J. Nanomed. 2018, 13, 4433–4443. [Google Scholar] [CrossRef] [PubMed]
| Study Title | Phase | Trial No. | Conditions | Outcomes | Reference |
| Molecular targeting therapy | |||||
| A phase I study of GC33 in advanced or metastatic liver cancer (hepatocellular carcinoma) | I | NCT 00746317 | Advanced or metastatic HCC | GC33 was well tolerated. GPC3 expression in HCC may be associated with the clinical benefit to GC33. | Zhu et al. 2013 [63] |
| Phase I study of GC33 in patients with advanced HCC | I | JapicCTI 101255 | Japanese patients with advanced HCC | GC33 was well tolerated. The correlation between antitumor activity and GPC3 expression was not clear. | Ikeda et al. 2014 [64] |
| A study of RO5137382 (GC33) in patients with advanced or metastatic HCC | II | NCT 01507168 | Patients with advanced HCC who had failed prior systemic therapy | Codrituzumab did not show any clinical benefit. A high dose of codrituzumab or a high GPC3 level or its mediator CD16 may improve outcome. | Abou-Alfa et al. 2016 [65] |
| Vaccines therapy | |||||
| Phase I clinical study of glypican-3 peptide vaccine in patients with advanced HCC | I | UMIN 000001395 | Advanced HCC patients | GPC3 vaccination was well tolerated. Peptide-specific CTL frequency may be a predictive marker of OS. | Sawada et al. 2012 [71] |
| A phase II study of GPC3 peptide vaccine as adjuvant treatment for HCC after surgical resection or radiofrequency ablation (RFS) | II | UMIN 000002614 | Patients with initial HCC who had undergone surgery or radiofrequency ablation | GPC3 vaccination did not have longer RFS or OS. Vaccination in patients with GPC3-positive tumors improved 1-y recurrence rates. | Sawada et al. 2016 [72] |
| Ongoing GPC3-Targeted Antibody Therapy Trials | Phase | Trial No. | Conditions | Status | Country |
| A study of ERY974 in patient with advanced solid tumors | I | NCT 02748837 | GPC3-positive advanced solid tumors | Active, not recruiting | United States |
| A phase I study of codrituzumab, in combination with atezolizumab in patients with HCC | I | JapicCTI 163325 | Locally advanced or metastatic HCC in which GPC3 is expressed by IHC | Active, not recruiting | Japan, Taiwan |
| Study Title | Phase | Trial No. | Conditions | Status | Country |
| Anti-GPC3 CAR-T for treating patients with advanced HCC | I | NCT 02395250 | Non-diffuse HCC with the presence of extrahepatic metastasis or portal vein vascular invasion. GPC3 is expressed by IHC. | Completed | China |
| CAR-T cell immunotherapy for HCC targeting GPC3 | I & II | NCT 02723942 | Non-diffuse HCC, no extrahepatic metastasis or portal vein vascular invasion. GPC3 high expression HCC. | Completed | China |
| CAR-GPC3 T cells in patients with refractory HCC | — | NCT 03146234 | Relapsed or refractory HCC. GPC3 is expressed by IHC. | Recruiting | China |
| Glypican 3-specific CAR expressing T cells for HCC (GLYCAR) | I | NCT 02905188 | Unresectable, recurrent, and/or metastatic HCC. GPC3-positive HCC. | Recruiting | United States |
| GPC3-T2-CAR-T Cells for Immunotherapy of Cancer With GPC3 Expression | I | NCT 03198546 | Advanced HCC that expresses GPC3 protein. | Recruiting | China |
| Anti-GPC3 CAR-T for treating GPC3-positive advanced HCC | I & II | NCT 03084380 | GPC3 is expressed by IHC. Patients with no ability to receive TACE combined with sorafenib. | Not yet recruiting | China |
| CAR-T cells targeting GPC3 | I | NCT 03884751 | Advanced HCC that is not suitable for surgery or local treatment, with no effective treatment after standard systemic therapies. GPC3 is expressed by IHC. | Not yet recruiting | China |
| 4th generation CAR-T cells targeting GPC3 | I | NCT 03980288 | Advanced HCC that is not suitable for surgery or local treatment, with no effective treatment after standard systemic therapies. GPC3 is expressed by IHC. | Not yet recruiting | China |
| Clinical study of redirected autologous T cells with a CAR in patients with malignant tumors | — | NCT 03302403 | HCC that cannot be eradicated by resection or ablation. GPC3 is expressed by IHC. Other malignancies. | Not yet recruiting | China |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishida, T.; Kataoka, H. Glypican 3-Targeted Therapy in Hepatocellular Carcinoma. Cancers 2019, 11, 1339. https://doi.org/10.3390/cancers11091339
Nishida T, Kataoka H. Glypican 3-Targeted Therapy in Hepatocellular Carcinoma. Cancers. 2019; 11(9):1339. https://doi.org/10.3390/cancers11091339
Chicago/Turabian StyleNishida, Takahiro, and Hiroaki Kataoka. 2019. "Glypican 3-Targeted Therapy in Hepatocellular Carcinoma" Cancers 11, no. 9: 1339. https://doi.org/10.3390/cancers11091339
APA StyleNishida, T., & Kataoka, H. (2019). Glypican 3-Targeted Therapy in Hepatocellular Carcinoma. Cancers, 11(9), 1339. https://doi.org/10.3390/cancers11091339





