The Relationship between Imaging-Based Body Composition Analysis and the Systemic Inflammatory Response in Patients with Cancer: A Systematic Review
Abstract
:1. Introduction
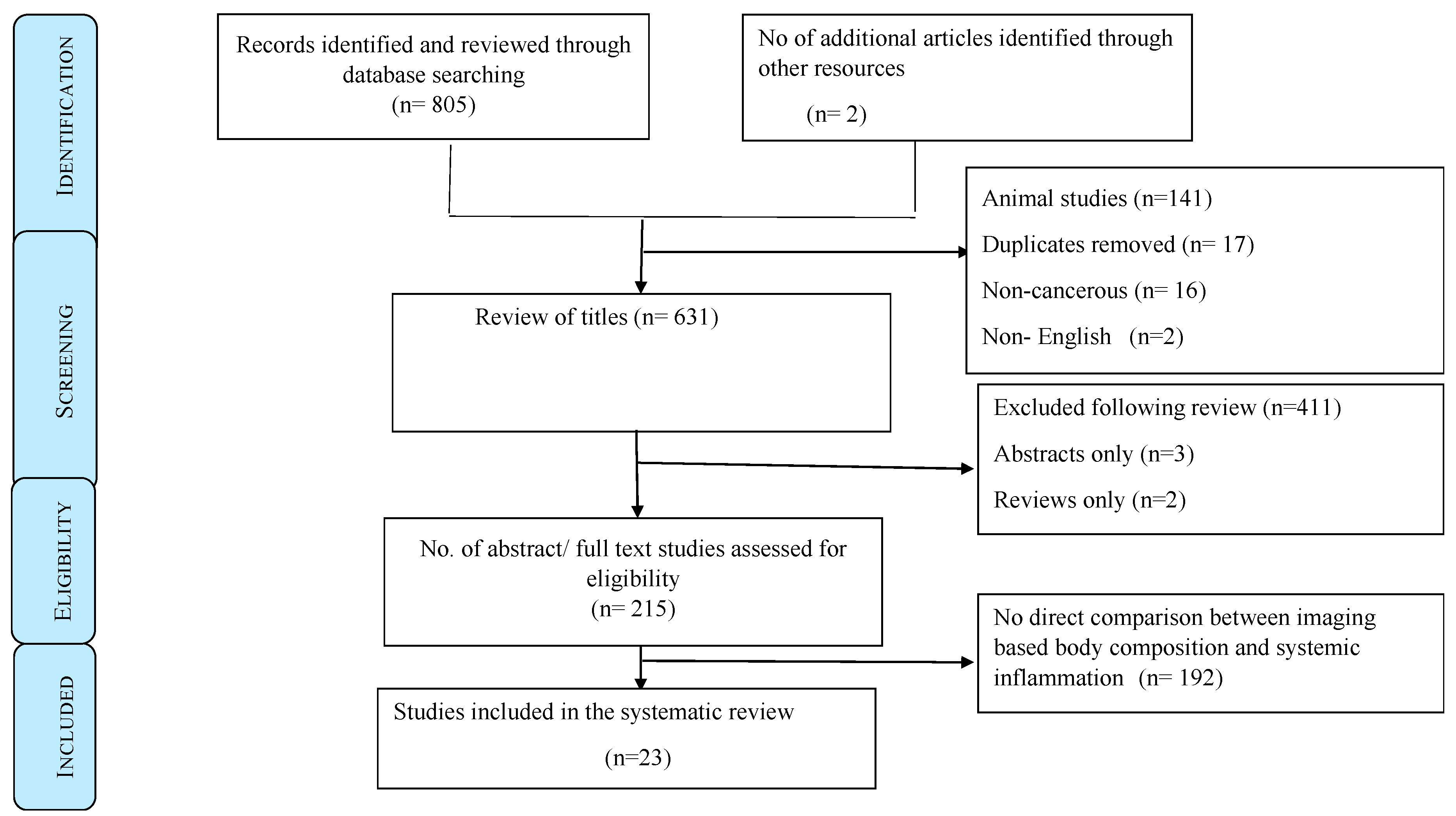
2. Patients and Methods
Data Sources and Search Strategy
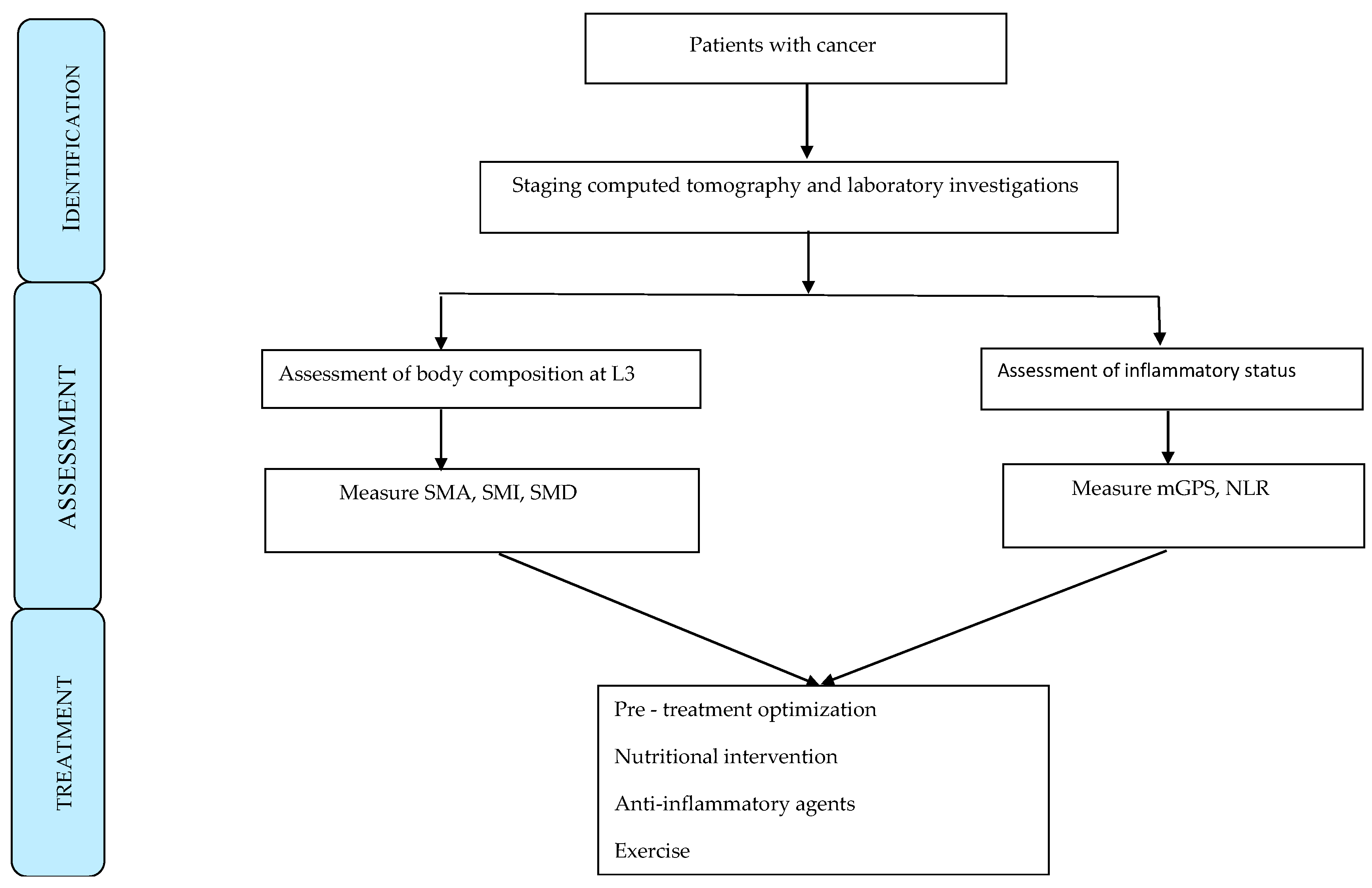
3. Results
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- World Health Organization. Cancer Statistics. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 12 September 2018).
- Deutz, N.E.; Ashurst, I.; Ballesteros, M.D.; Bear, D.E.; Cruz-Jentoft, A.J.; Genton, L.; Landi, F.; Laviano, A.; Norman, K.; Prado, C.M. The Underappreciated Role of Low Muscle Mass in the Management of Malnutrition. J. Am. Med. Dir. Assoc. 2019, 20, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsiopoulos, N.; Baumgartner, R.N.; Heymsfield, S.B.; Lyons, W.; Gallagher, D.; Ross, R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 1998, 85, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St-Onge, M.P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Bredella, M.A.; Ghomi, R.H.; Thomas, B.J.; Torriani, M.; Brick, D.J.; Gerweck, A.V.; Misra, M.; Klibanski, A.; Miller, K.K. Comparison of DXA and CT in the assessment of body composition in premenopausal women with obesity and anorexia nervosa. Obesity 2010, 18, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Leinhard, O.D. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Birdsell, L.A.; Baracos, V.E. The emerging role of computerized tomography in assessing cancer cachexia. Curr. Opin. Support. Palliat. Care 2009, 3, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Engelke, K.; Museyko, O.; Wang, L.; Laredo, J.D. Quantitative analysis of skeletal muscle by computed tomography imaging—State of the art. J. Orthop. Transl. 2019, 15, 91–103. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [Green Version]
- Douglas, E.; McMillan, D.C. Towards a simple objective framework for the investigation and treatment of cancer cachexia: The Glasgow Prognostic Score. Cancer Treat. Rev. 2014, 40, 685–691. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Epidemiology 2007, 18, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Ellegård, L.H.; Åhlén, M.; Körner, U.; Lundholm, K.G.; Plank, L.D.; Bosaeus, I.G. Bioelectric impedance spectroscopy underestimates fat-free mass compared to dual energy X-ray absorptiometry in incurable cancer patients. Eur. J. Clin. Nutr. 2009, 63, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Wallengren, O.; Iresjö, B.M.; Lundholm, K.; Bosaeus, I. Loss of muscle mass in the end of life in patients with advanced cancer. Support. Care Cancer 2015, 23, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Chambard, L.; Girard, N.; Ollier, E.; Rousseau, J.C.; Duboeuf, F.; Carlier, M.C.; Brevet, M.; Szulc, P.; Pialat, J.B.; Wegrzyn, J.; et al. Bone, muscle, and metabolic parameters predict survival in patients with synchronous bone metastases from lung cancers. Bone 2018, 108, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Rollins, K.E.; Tewari, N.; Ackner, A.; Awwad, A.; Madhusudan, S.; Macdonald, I.A.; Fearon, K.C.; Lobo, D.N. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin. Nutr. 2016, 35, 1103–1109. [Google Scholar] [CrossRef]
- van Dijk, D.P.; Bakens, M.J.; Coolsen, M.M.; Rensen, S.S.; van Dam, R.M.; Bours, M.J.; Weijenberg, M.P.; Dejong, C.H.; Olde Damink, S.W. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 317–326. [Google Scholar] [CrossRef]
- Malietzis, G.; Currie, A.C.; Athanasiou, T.; Johns, N.; Anyamene, N.; Glynne-Jones, R.; Kennedy, R.H.; Fearon, K.C.H.; Jenkins, J.T. Influence of body composition profile on outcomes following colorectal cancer surgery. Br. J. Surg. 2016, 103, 572–580. [Google Scholar] [CrossRef]
- Feliciano, E.M.C.; Kroenke, C.H.; Meyerhardt, J.A.; Prado, C.M.; Bradshaw, P.T.; Kwan, M.L.; Xiao, J.; Alexeeff, S.; Corley, D.; Weltzien, E.; et al. Association of Systemic Inflammation and Sarcopenia With Survival in Nonmetastatic Colorectal Cancer: Results From the C SCANS Study. JAMA Oncol. 2017, 3, e172319. [Google Scholar] [CrossRef]
- van Dijk, D.P.; Krill, M.; Farshidfar, F.; Li, T.; Rensen, S.S.; Olde Damink, S.W.; Dixon, E.; Sutherland, F.R.; Ball, C.G.; Mazurak, V.C.; et al. Host phenotype is associated with reduced survival independent of tumour biology in patients with colorectal liver metastases. J. Cachexia Sarcopenia Muscle 2018, 10, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Caan, B.J.; Cespedes Feliciano, E.M.; Meyerhardt, J.A.; Kroenke, C.H.; Baracos, V.E.; Weltzien, E.; Kwan, M.L. The association of medical and demographic characteristics with sarcopenia and low muscle radiodensity in patients with nonmetastatic colorectal cancer. Am. J. Clin. Nutr. 2019, 109, 615–625. [Google Scholar] [CrossRef]
- Richards, C.H.; Roxburgh, C.S.; MacMillan, M.T.; Isswiasi, S.; Robertson, E.G.; Guthrie, G.K.; Horgan, P.G.; McMillan, D.C. The relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancer. PLoS ONE 2012, 7, e41883. [Google Scholar] [CrossRef] [PubMed]
- McSorley, S.T.; Black, D.H.; Horgan, P.G.; McMillan, D.C. The relationship between tumour stage, systemic inflammation, body composition and survival in patients with colorectal cancer. Clin. Nutr. 2018, 37, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.D.; Almasaudi, A.S.; Dieu, L.B.; Horgan, P.G.; McSorley, S.T.; McMillan, D.C. The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Kiyotoki, T.; Nakamura, K.; Haraga, J.; Omichi, C.; Ida, N.; Saijo, M.; Nishida, T.; Kusumoto, T.; Masuyama, H. Sarcopenia Is an Important Prognostic Factor in Patients With Cervical Cancer Undergoing Concurrent Chemoradiotherapy. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2018, 28, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Sueda, T.; Takahasi, H.; Nishimura, J.; Hata, T.; Matsuda, C.; Mizushima, T.; Doki, Y.; Mori, M. Impact of Low Muscularity and Myosteatosis on Long-term Outcome After Curative Colorectal Cancer Surgery: A Propensity Score-Matched Analysis. Dis. Colon Rectum 2018, 61, 364–374. [Google Scholar] [CrossRef]
- Zhuang, C.L.; Huang, D.D.; Pang, W.Y.; Zhou, C.J.; Wang, S.L.; Lou, N.; Ma, L.L.; Yu, Z.; Shen, X. Sarcopenia is an Independent Predictor of Severe Postoperative Complications and Long-Term Survival After Radical Gastrectomy for Gastric Cancer: Analysis from a Large-Scale Cohort. Medicine 2016, 95, e3164. [Google Scholar] [CrossRef]
- Huang, D.D.; Zhou, C.J.; Wang, S.L.; Mao, S.T.; Zhou, X.Y.; Lou, N.; Zhang, Z.; Yu, Z.; Shen, X.; Zhuang, C.L. Impact of different sarcopenia stages on the postoperative outcomes after radical gastrectomy for gastric cancer. Surgery 2017, 161, 680–693. [Google Scholar] [CrossRef]
- Reisinger, K.W.; Derikx, J.P.; van Vugt, J.L.; Von Meyenfeldt, M.F.; Hulsewé, K.W.; Damink, S.W.O.; Stoot, J.H.; Poeze, M. Sarcopenia is associated with an increased inflammatory response to surgery in colorectal cancer. Clin. Nutr. 2016, 35, 924–927. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, Y.S.; Seo, J.Y.; Park, I.; Ahn, H.K.; Jeong, Y.M.; Kim, J.H.; Kim, N. The Relationship between Sarcopenia and Systemic Inflammatory Response for Cancer Cachexia in Small Cell Lung Cancer. PLoS ONE 2016, 11, 572–580. [Google Scholar] [CrossRef]
- Serra, M.C.; Ryan, A.S.; Ortmeyer, H.K.; Addison, O.; Goldberg, A.P. Resistance training reduces inflammation and fatigue and improves physical function in older breast cancer survivors. Menopause 2018, 25, 211–216. [Google Scholar] [CrossRef]
- Itoh, S.; Shirabe, K.; Matsumoto, Y.; Yoshiya, S.; Muto, J.; Harimoto, N.; Yamashita, Y.I.; Ikegami, T.; Yoshizumi, T.; Nishie, A.; et al. Effect of body composition on outcomes after hepatic resection for hepatocellular carcinoma. Ann. Surg. Oncol. 2014, 21, 3063–3068. [Google Scholar] [CrossRef] [PubMed]
- Srdic, D.; Plestina, S.; Sverko-Peternac, A.; Nikolac, N.; Simundic, A.M.; Samarzija, M. Cancer cachexia, sarcopenia and biochemical markers in patients with advanced non-small cell lung cancer-chemotherapy toxicity and prognostic value. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2016, 24, 4495–4502. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, Y.; Toiyama, Y.; Yamamoto, A.; Shigemori, T.; Kitamura, A.; Ichikawa, T.; Ide, S.; Kitajima, T.; Fujikawa, H.; Yasuda, H.; et al. Close Relationship Between Immunological/Inflammatory Markers and Myopenia and Myosteatosis in Patients With Colorectal Cancer: A Propensity Score Matching Analysis. J. Parent. Enter. Nutr. 2018, 43, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.; Parnofiello, A.; Vitale, M.G.; Cortiula, F.; Gerratana, L.; Fanotto, V.; Lisanti, C.; Pelizzari, G.; Ongaro, E.; Bartoletti, M.; et al. The IMPACT study: Early loss of skeletal muscle mass in advanced pancreatic cancer patients. J. Cachexia Sarcopenia Muscle 2019, 10, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Irving, B.A.; Weltman, J.Y.; Brock, D.W.; Davis, C.K.; Gaesser, G.A.; Weltman, A. NIH ImageJ and Slice-O-Matic computed tomography imaging software to quantify soft tissue. Obesity 2007, 15, 370–376. [Google Scholar] [CrossRef]
- van Vugt, J.L.; Levolger, S.; Gharbharan, A.; Koek, M.; Niessen, W.J.; Burger, J.W.; Willemsen, S.P.; de Bruin, R.W.; IJzermans, J.N. A comparative study of software programmes for cross-sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J. Cachexia Sarcopenia Muscle 2017, 8, 285–297. [Google Scholar] [CrossRef]
- Teigen, L.M.; Kuchnia, A.J.; Nagel, E.; Deuth, C.; Vock, D.M.; Mulasi, U.; Earthman, C.P. Impact of Software Selection and ImageJ Tutorial Corrigendum on Skeletal Muscle Measures at the Third Lumbar Vertebra on Computed Tomography Scans in Clinical Populations. J. Parenter. Enter. Nutr. 2018, 42, 933–941. [Google Scholar] [CrossRef]
- Dolan, R.D.; McSorley, S.T.; Horgan, P.G.; Laird, B.; McMillan, D.C. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Crit. Rev. Oncol./Hematol. 2017, 116, 134–146. [Google Scholar] [CrossRef]
- Dolan, R.D.; Lim, J.; McSorley, S.T.; Horgan, P.G.; McMillan, D.C. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: Systematic review and meta-analysis. Sci. Rep. 2017, 7, 16717. [Google Scholar] [CrossRef]
- Baracos, V.E. Psoas as a sentinel muscle for sarcopenia: A flawed premise. J. Cachexia Sarcopenia Muscle 2017, 8, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Icard, P.; Iannelli, A.; Lincet, H.; Alifano, M. Sarcopenia in resected non-small cell lung cancer: let’s move to patient-directed strategies. J. Thorac. Dis. 2018, 10, S3138–S3142. [Google Scholar] [CrossRef] [PubMed]
- Hervochon, R.; Bobbio, A.; Guinet, C.; Mansuet-Lupo, A.; Rabbat, A.; Régnard, J.F.; Roche, N.; Damotte, D.; Iannelli, A.; Alifano, M. Body Mass Index and Total Psoas Area Affect Outcomes in Patients Undergoing Pneumonectomy for Cancer. Ann. Thorac. Surg. 2017, 103, 287–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okugawa, Y.; Toiyama, Y.; Yamamoto, A.; Shigemori, T.; Kitamura, A.; Ichikawa, T.; Ide, S.; Kitajima, T.; Fujikawa, H.; Yasuda, H.; et al. Relationship Between Immunological/Inflammatory Markers and Myopenia and Myosteatosis in Patients With Colorectal Cancer: A Propensity Score Matching Analysis. J. Parent. Enter. Nutr. 2019, 43, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.J.; Morrison, D.S.; Talwar, D.; Balmer, S.M.; O’reilly, D.S.J.; Foulis, A.K.; Horgan, P.G.; McMillan, D.C. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: A Glasgow Inflammation Outcome Study. Br. J. Cancer 2011, 104, 726–734. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, N. Terminology in cancer cachexia: Importance and status. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 220–225. [Google Scholar] [CrossRef]
- Zimmers, T.A.; Fishel, M.L.; Bonetto, A. STAT3 in the systemic inflammation of cancer cachexia. Semin. Cell Dev. Biol. 2016, 54, 28–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, J.J.; McMillan, D.C.; Laird, B.J. Targeting IL-1alpha in cancer cachexia: A narrative review. Curr. Opin. Support. Palliat. Care 2018, 12, 453–459. [Google Scholar] [PubMed]
- Guthrie, G.J.; Charles, K.A.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol./Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- Izano, M.; Wei, E.K.; Tai, C.; Swede, H.; Gregorich, S.; Harris, T.B.; Klepin, H.; Satterfield, S.; Murphy, R.; Newman, A.B.; et al. Chronic inflammation and risk of colorectal and other obesity-related cancers: The health, aging and body composition study. Int. J. Cancer 2016, 138, 1118–1128. [Google Scholar] [CrossRef]
- Demb, J.; Wei, E.K.; Izano, M.; Kritchevsky, S.; Swede, H.; Newman, A.B.; Shlipak, M.; Akinyemiju, T.; Gregorich, S.; Braithwaite, D. Chronic inflammation and risk of lung cancer in older adults in the health, aging and body composition cohort study. J. Geriatr. Oncol. 2019, 10, 265–271. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Baracos, V.E.; Mazurak, V.C.; Bhullar, A.S. Cancer cachexia is defined by an ongoing loss of skeletal muscle mass. Ann. Palliat. Med. 2018, 8, 3–12. [Google Scholar] [CrossRef]
- da Silva, G.A.; Wiegert, E.V.M.; Calixto-Lima, L.; Oliveira, L.C. Clinical utility of the modified Glasgow Prognostic Score to classify cachexia in patients with advanced cancer in palliative care. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Laird, B.J.; Kaasa, S.; McMillan, D.C.; Fallon, M.T.; Hjermstad, M.J.; Fayers, P.; Klepstad, P. Prognostic factors in patients with advanced cancer: A comparison of clinicopathological factors and the development of an inflammation-based prognostic system. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 5456–5464. [Google Scholar] [CrossRef]
- Simmons, C.; McMillan, D.C.; Tuck, S.; Graham, C.; McKeown, A.; Bennett, M.; O’Neill, C.; Wilcock, A.; Usborne, C.; Fearon, K.C.; et al. “How Long Have I Got?”—A Prospective Cohort Study Comparing Validated Prognostic Factors for Use in Patients with Advanced Cancer. Oncol. Theoncol. 2019, 4. [Google Scholar] [CrossRef]
- Solheim, T.S.; Fearon, K.C.; Blum, D.; Kaasa, S. Non-steroidal anti-inflammatory treatment in cancer cachexia: A systematic literature review. Acta Oncol. 2013, 52, 6–17. [Google Scholar] [CrossRef]
- Solheim, T.S.; Laird, B.J.; Balstad, T.R.; Bye, A.; Stene, G.; Baracos, V.; Strasser, F.; Griffiths, G.; Maddocks, M.; Fallon, M.; et al. Cancer cachexia: Rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support. Palliat. Care 2018, 8, 258–265. [Google Scholar] [CrossRef]
- Miller, J.; Skipworth, R.J. Novel molecular targets of muscle wasting in cancer patients. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 196–204. [Google Scholar] [CrossRef]
| mGPS | Biochemical Markers | Cachexia Stage | |
|---|---|---|---|
| CRP (mg/L) | Albumin (g/L) | ||
| 0 | <10 | ≥35 | No cachexia |
| 0 | <10 | <35 | Undernourished |
| 1 | >10 | ≥35 | Pre-cachexia |
| 2 | >10 | <35 | Refractory cachexia |
| Authors (Year) | Reported STROBE Checklist Points | Type of Study | n (F/M) | Country | Cancer Studied | Cancer Stage | Level of Analysis | Systemic Inflammation | Comments |
|---|---|---|---|---|---|---|---|---|---|
| DEXA | |||||||||
| Ellegård et al., 2009 [14] | 20 | Prospective cross-sectional | 132 (46/86) | Sweden & New Zealand | Gastrointestinal | Advanced inoperable | Whole body | CRP, Albumin | Low SMI directly associated with elevated CRP and low albumin (p < 0.05). |
| Wallengren et al., 2014 [15] | 19 | Prospective longitudinal | 471 (212/259) | Sweden | Gastrointestinal, pancreatic-biliary | Advanced inoperable | Whole body | CRP, Albumin | Low SMI directly associated with elevated CRP (p < 0.001). |
| Chambard et al., 2018 [16] | 20 | Prospective cross-sectional | 64 (16/48) | France | Non-small cell Lung | Advanced inoperable | Whole body | CRP, Albumin, WCC | Low SMI directly associated with elevated CRP (p < 0.05) & WCC (p < 0.001). |
| CT | |||||||||
| Richards et al., 2012 [23] | 20 | Prospective cross-sectional | 174 (79/95) | United Kingdom | Colo-rectal | Primary operable | L3 | CRP, Albumin, mGPS, NLR | Low SMI (34%) directly associated with elevated mGPS (32%) (p < 0.001) |
| Itoh et al., 2013 [33] | 19 | Retrospective cross-sectional | 190 (44/146) | Japan | Hepatocellular | Primary operable | L3 | Albumin | Low visceral fat area associated with sarcopenia (p < 0.001) and low albumin (p < 0.005) |
| Reisinger et al., 2016 [30] | 17 | Prospective cross-sectional | 87 (31/56) | Netherlands | Colo-rectal | Primary operable | L3 | CRP, mGPS | Low SMI associated with elevated CRP (p = 0.05). |
| Rollins et al., 2016 [17] | 18 | Retrospective cross-sectional | 229 (105/124) | United Kingdom | Pancreatic-biliary | Advanced inoperable | L3 | CRP, Albumin, mGPS, NLR | Low SMI and SMD associated with elevated CRP (p < 0.05), low albumin (p < 0.001) and elevated NLR (p < 0.01). |
| Malietz et al., 2016 [19] | 19 | Prospective longitudinal | 763 (306/457) | United Kingdom | Colo-rectal | Primary operable | L3 | Albumin, NLR | Low SMI (65%) and low SMD (84%) associated with NLR > 3 (61% & 57%) (p < 0.001) and low albumin (28% each) (p = 0.01). |
| Kim et al., 2016 [31] | 20 | Retrospective cross-sectional | 186 (30/156) | South Korea | Small cell lung | Primary operable | L3 | CRP, Albumin, mGPS, NLR | Low SMI associated with elevated CRP (p < 0.05), low albumin (p < 0.05) and elevated NLR (p < 0.01). |
| Zhuang et al., 2016 [28] | 19 | Retrospective cross-sectional | 937 (207/730) | China | Gastric | Primary operable | L3 | Albumin | Low SMI associated with low albumin (p < 0.001). |
| Huang, et al., 2016 [29] | 20 | Prospective cross-sectional | 470 (364/106) | China | Gastric | Primary operable | L3 | Albumin | Low SMI associated with low albumin (p < 0.001). |
| Van Di Jik et al., 2017 [18] | 19 | Prospective cross-sectional | 186 (84/102) | Netherlands | Pancreatic | Both operable and inoperable | L3 | CRP, Albumin, mGPS | Low SMD associated with low albumin (p < 0.01) |
| Feliciano et al., 2017 (C-SCANS study) [20] | 20 | Retrospective longitudinal | 2470 (1219/1251) | United States, Canada | Colo-rectal | Primary operable | L3 | CRP, Albumin, NLR, IL-6 | Low SMI associated with elevated CRP (p < 0.05), low albumin (p < 0.01) and elevated IL-6 (p < 0.05) |
| Srdic et al., 2017 [34] | 20 | Prospective cross-sectional | 100 (33/67) | Croatia | Non-small cell lung | Advanced inoperable | L3 | CRP, albumin, mGPS | Low SMI (15% loss of skeletal muscle mass) associated with low albumin (p < 0.01) |
| Kiyotoki et al., 2017 [26] | 20 | Retrospective cross-sectional | 60 All females | Japan | Cervical | Primary operable | L3 | CRP, Albumin | Low SMI associated with low albumin (p < 0.01). |
| Serra et al., 2017 [32] | 16 | Prospective cross-sectional | 11 All females | United States | Breast | Primary operable | L4-L5 | CRP, Albumin | Significant improvement in muscle strength with resistance training with reduction in inflammatory mediators including CRP. |
| McSorley et al., 2017 [24] | 20 | Retrospective cross-sectional | 322 (148/174) | United Kingdom | Colo-rectal | Primary operable | L3 | CRP, Albumin, mGPS, NLR | Low SMI (47%) and SMD (58%) associated with elevated mGPS (23%) and NLR > 3 (44%) (p < 0.01). |
| Van DiJik et al., 2018 [21] | 20 | Prospective cross-sectional | 97 (30/67) | Canada | Colo-rectal | Primary & metastatic both operable | L3 | CRP, Albumin | Low SMI (65%) associated with elevated CRP > 5 mg/dL (74%) (p < 0.05). |
| Okugawa et al., 2018 [35] | 20 | Prospective cross-sectional | 308 (125/183) | Japan | Colo-rectal | Primary operable | L3 | CRP, Albumin, NLR, PLR | Low SMI and SMD associated with elevated CRP (p < 0.0001) and low albumin (p < 0.05). |
| Dolan et al., 2018 [25] | 19 | Retrospective cross-sectional | 650 (296/354) | United Kingdom | Colo-rectal | Primary operable | L3 | CRP, Albumin, mGPS, NLR | Low SMI (44%) and SMD (60%) associated with elevated mGPS (23%) (p < 0.001) and NLR > 3 (43%) (p < 0.05). |
| Sueda et al., 2018 [27] | 20 | Retrospective cross-sectional | 211 (77/134) | Japan | Colo-rectal | Primary operable | L3 | Albumin, NLR | Low SMI (48%) and SMD (49%) associated with NLR > 3 (41%) with (p < 0.05) and p < 0.01 respectively. |
| Basile et al., 2019 [36] | 20 | Retrospective longitudinal | 94 (42/52) | Italy | Pancreatic | Advanced inoperable | L3 | CRP, Albumin, NLR | Low SMI & SMD associated with NLR > 5(p < 0.001). |
| Xiao et al., 2019 [22] | 20 | Retrospective cross-sectional | 3262 (1628/1624) | United States | Colo-rectal | Primary Operable | L3 | CRP, Albumin, NLR | Low SMI & SMD associated with raised NLR ≥ 5 (p < 0.001). |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbass, T.; Dolan, R.D.; Laird, B.J.; McMillan, D.C. The Relationship between Imaging-Based Body Composition Analysis and the Systemic Inflammatory Response in Patients with Cancer: A Systematic Review. Cancers 2019, 11, 1304. https://doi.org/10.3390/cancers11091304
Abbass T, Dolan RD, Laird BJ, McMillan DC. The Relationship between Imaging-Based Body Composition Analysis and the Systemic Inflammatory Response in Patients with Cancer: A Systematic Review. Cancers. 2019; 11(9):1304. https://doi.org/10.3390/cancers11091304
Chicago/Turabian StyleAbbass, Tanvir, Ross D Dolan, Barry J Laird, and Donald C McMillan. 2019. "The Relationship between Imaging-Based Body Composition Analysis and the Systemic Inflammatory Response in Patients with Cancer: A Systematic Review" Cancers 11, no. 9: 1304. https://doi.org/10.3390/cancers11091304
APA StyleAbbass, T., Dolan, R. D., Laird, B. J., & McMillan, D. C. (2019). The Relationship between Imaging-Based Body Composition Analysis and the Systemic Inflammatory Response in Patients with Cancer: A Systematic Review. Cancers, 11(9), 1304. https://doi.org/10.3390/cancers11091304






