Current Status of Gene Therapy in Hepatocellular Carcinoma
Abstract
1. Introduction
1.1. Hepatocellular Carcinoma (HCC)
1.2. Gene Therapy
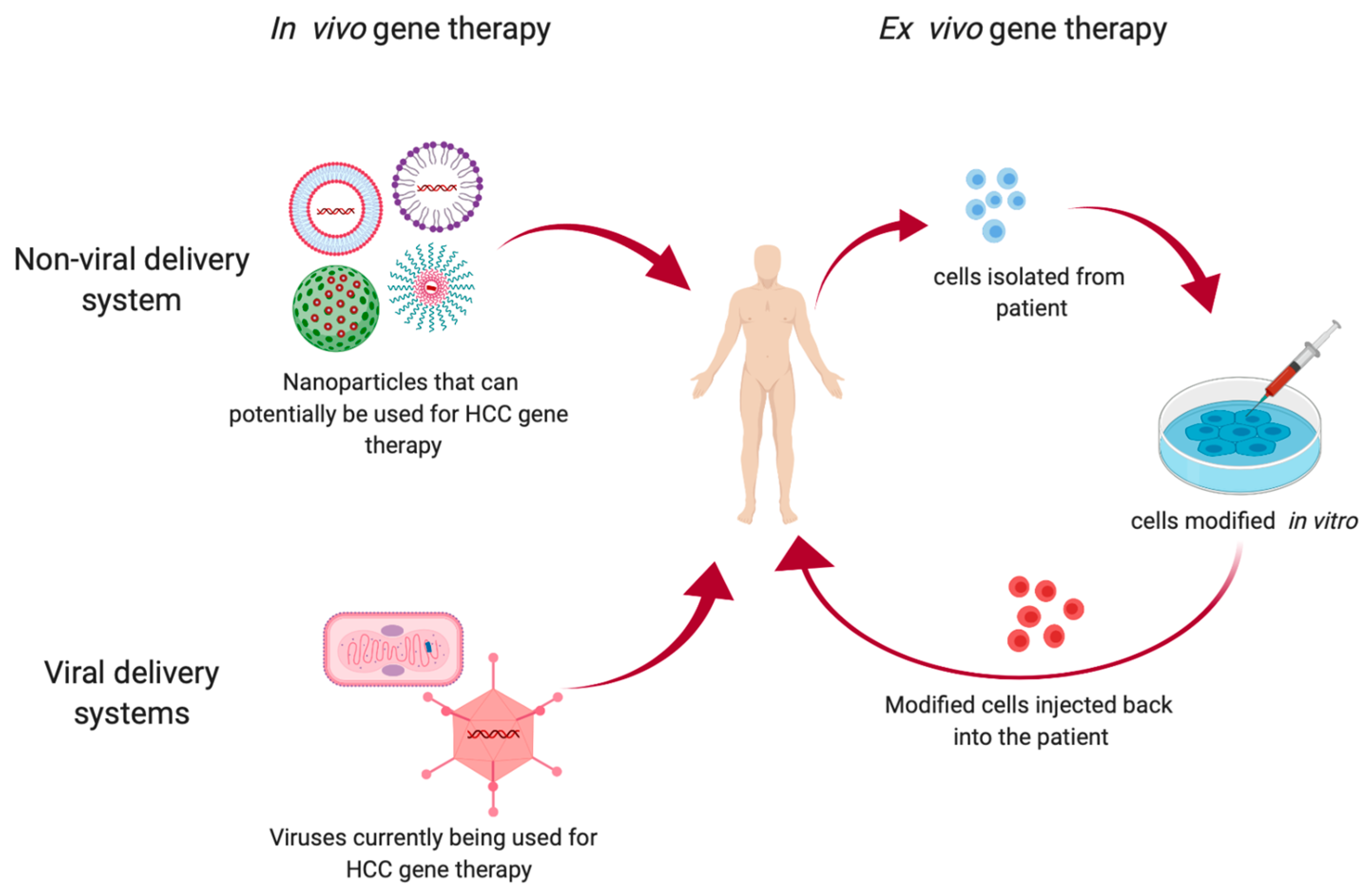
2. Types of Gene Therapy
2.1. Ex Vivo Gene Therapy
2.1.1. Glypican-3 (GPC-3)
2.1.2. Alpha Fetoprotein (AFP)
2.1.3. Cluster of Differentiation 147 (CD147)
2.1.4. Mucin-1 (Muc1)
2.1.5. Epithelial Cell Adhesion Molecule (EpCAM)
2.1.6. NY-ESO 1
2.2. In Vivo Gene Therapy
2.2.1. Non-Viral Delivery Systems
Nanoparticles (NPs)
Virus-Like Particles (VLPs)
2.2.2. Viral Delivery
Adeno and Adeno Associated Virus
Vaccinia Virus
Lentivirus
3. Targets of Gene Therapy
3.1. Suicide Genes
3.1.1. Herpes Simplex Virus Thymidine Kinase/Ganciclovir System
3.1.2. Cytosine Deaminase/5-Fluorocytosine System
3.1.3. Purine Nucleoside Phosphorylase/ Fludarabine Phosphate System
3.2. Oncogenes
3.2.1. Midkine
3.2.2. Yes-Associated Protein (YAP)
3.2.3. Survivin
3.2.4. B Cell-Specific Moloney Murine Leukemia Virus Integration Site 1 (Bmi-1)
3.2.5. Astrocyte Elevated Gene-1 (AEG-1)
3.2.6. Notch-1
3.3. Tumor Suppressor Genes
3.3.1. Long Non Coding and Micro RNAs
3.3.2. Potassium Voltage-gated Channel Subfamily Q Member 1 (KCNQ1)
3.3.3. Protocadherin9 (PCDH9)
3.3.4. Vacuolar Protein Sorting-Associated Protein 4A (Vps4A)
3.3.5. Catenin Alpha 3 (CTNNA3)
4. Conclusions
Funding
Conflicts of Interest
References
- Ghouri, A.Y.; Mian, I.; Rowe, J.H. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J. Carcinog. 2017, 16, 1. [Google Scholar] [PubMed]
- Rimassa, L.; Santoro, A. Sorafenib therapy in advanced hepatocellular carcinoma: The SHARP trial. Expert Rev. Anticancer Ther. 2009, 9, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Tisagenlecleucel (Kymriah) for ALL. Med. Lett. Drugs Ther. 2017, 59, 177–178.
- Axicabtagene ciloleucel (Yescarta) for B-cell lymphoma. Med. Lett. Drugs Ther. 2018, 60, e122–e123.
- Ho, M.; Kim, H. Glypican-3: A new target for cancer immunotherapy. Eur. J. Cancer 2011, 47, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Capurro, M.; Wanless, I.R.; Sherman, M.; DeBoer, G.; Shi, W.; Miyoshi, E.; Filmus, J. Glypican-3: A novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003, 125, 89–97. [Google Scholar] [CrossRef]
- Johnson, P.J. The Role of Serum Alpha-Fetoprotein estimation in the Diagnosis and Management of Hepatocellular Carcinoma. Clin. Liver Dis. 2001, 5, 145–159. [Google Scholar] [CrossRef]
- Huang, Q.; Li, J.; Xing, J.; Li, W.; Li, H.; Ke, X.; Zhang, J.; Ren, T.; Shang, Y.; Yang, H.; et al. CD147 promotes reprogramming of glucose metabolism and cell proliferation in HCC cells by inhibiting the p53-dependent signaling pathway. J. Hepatol. 2014, 61, 859–866. [Google Scholar] [CrossRef]
- Lee, A.; Rode, A.; Nicoll, A.; Maczurek, A.E.; Lim, L.; Lim, S.; Angus, P.; Kronborg, I.; Arachchi, N.; Gorelik, A.; et al. Circulating CD147 predicts mortality in advanced hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2016, 31, 459–466. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; Dang, Y.Z.; Gao, H.X.; Jiang, J.L.; Chen, Z.N. HAb18G/CD147 promotes radioresistance in hepatocellular carcinoma cells: A potential role for integrin beta1 signaling. Mol. Cancer Ther. 2015, 14, 553–563. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Li, Q.; Wang, F.; Xie, F.; Zhai, R.; Guo, Y.; Chen, T.; Zhang, N.; Ni, W.; et al. Mucin1 promotes the migration and invasion of hepatocellular carcinoma cells via JNK-mediated phosphorylation of Smad2 at the C-terminal and linker regions. Oncotarget 2015, 6, 19264–19278. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, F.; Liu, G.; Yuan, H.; Chen, T.; Wang, J.; Xie, F.; Zhai, R.; Wang, F.; Guo, Y.; et al. Impact of Mucin1 knockdown on the phenotypic characteristics of the human hepatocellular carcinoma cell line SMMC-7721. Oncol. Rep. 2014, 31, 2811–2819. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Kuno, A.; Matsuda, A.; Tsujikawa, H.; Yamazaki, K.; Yasui, Y.; Tsuchiya, K.; Nakanishi, H.; Itakura, J.; Korenaga, M.; et al. Serum Wisteria Floribunda Agglutinin-Positive Sialylated Mucin 1 as a Marker of Progenitor/Biliary Features in Hepatocellular Carcinoma. Sci. Rep. 2017, 7, 244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhu, Y. The EpCAM overexpression is associated with clinicopathological significance and prognosis in hepatocellular carcinoma patients: A systematic review and meta-analysis. Int. J. Surg. 2018, 56, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Ji, J.; Budhu, A.; Forgues, M.; Yang, W.; Wang, H.Y.; Jia, H.; Ye, Q.; Wauthier, E.; Qin, L.-X.; et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 2009, 136, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Chen, H.S.; Luo, S.; Zhang, H.-H.; Fei, R.; Chen, H.-S.; Cai, J. Comparisons for detecting NY-ESO-1 mRNA expression levels in hepatocellular carcinoma tissues. Oncol. Rep. 2009, 21, 713–719. [Google Scholar]
- Xing, B.-C.; Yuan, Y.-H.; Hu, Z.-G.; Liu, Y.-X.; Han, Y.; Liu, N.; Chen, W.-F.; Wang, Y. Expression of NY-ESO-1/LAGE-1 genes in hepatocellular carcinoma and autologous humoral responses induced thereby. Zhonghua Yi Xue Za Zhi 2004, 84, 1980–1982. [Google Scholar]
- Xu, H.; Gu, N.; Liu, Z.B.; Zheng, M.; Xiong, F.; Wang, S.Y.; Lu, J. NY-ESO-1 expression in hepatocellular carcinoma: A potential new marker for early recurrence after surgery. Oncol. Lett. 2012, 3, 39–44. [Google Scholar] [CrossRef]
- Duan, F.; Lam, M.G.E.H. Delivery approaches of gene therapy in hepatocellular carcinoma. Anticancer Res. 2013, 33, 4711–4718. [Google Scholar]
- Tabernero, J.; Shapiro, G.I.; Lorusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M.; et al. First-in-Humans Trial of an RNA Interference Therapeutic Targeting VEGF and KSP in Cancer Patients with Liver Involvement. Cancer Discov. 2013, 3, 406–417. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [PubMed]
- Tezgel, Ö.; Szarpak-Jankowska, A.; Arnould, A.; Auzély-Velty, R.; Texier, I. Chitosan-lipid nanoparticles (CS-LNPs): Application to siRNA delivery. J. Colloid Interface Sci. 2018, 510, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, X.; He, S.; Miao, Y.; Wu, N.; Li, J.; Gan, Y. Sphk2 RNAi nanoparticles suppress tumor growth via downregulating cancer cell derived exosomal microRNA. J. Control. Release 2018, 286, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Huang, H.-L.; Li, J.; Qian, F.-C.; Li, L.-Q.; Niu, P.-P.; Dai, L.-C. Development of hybrid-type modified chitosan derivative nanoparticles for the intracellular delivery of midkine-siRNA in hepatocellular carcinoma cells. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 82–89. [Google Scholar] [CrossRef]
- Huang, K.-W.; Lai, Y.-T.; Chern, G.-J.; Huang, S.-F.; Tsai, C.-L.; Sung, Y.-C.; Chiang, C.-C.; Hwang, P.-B.; Ho, T.-L.; Huang, R.-L.; et al. Galactose Derivative-Modified Nanoparticles for Efficient siRNA Delivery to Hepatocellular Carcinoma. Biomacromolecules 2018, 19, 2330–2339. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lai, S.K. Engineering Well-Characterized PEG-Coated Nanoparticles for Elucidating Biological Barriers to Drug Delivery. Breast Cancer 2017, 1530, 125–137. [Google Scholar]
- Wang, D.; Chang, R.; Wang, G.; Hu, B.; Qiang, Y.; Chen, Z. Polo-like kinase 1-targeting Chitosan Nanoparticles suppress the progression of hepatocellular carcinoma. Anticancer Agents Med. Chem. 2017, 16, 1. [Google Scholar] [CrossRef]
- Cheng, X.; Lee, R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 2016, 99, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Fitamant, J.; Kottakis, F.; Benhamouche, S.; Tian, H.S.; Chuvin, N.; Parachoniak, C.A.; Nagle, J.M.; Perera, R.M.; Lapouge, M.; Deshpande, V.; et al. YAP inhibition restores hepatocyte differentiation in advanced HCC leading to tumor regression. Cell Rep. 2015, 10, 1692–1707. [Google Scholar] [CrossRef]
- Bogorad, R.L.; Yin, H.; Zeigerer, A.; Nonaka, H.; Ruda, V.M.; Zerial, M.; Anderson, D.G.; Koteliansky, V. Nanoparticle-formulated siRNA targeting integrins inhibits hepatocellular carcinoma progression in mice. Nat. Commun. 2014, 5, 3869. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Yu, B.; Wang, X.; Lu, Y.; Schmidt, C.R.; Lee, R.J.; Lee, L.J.; Jacob, S.T.; Ghoshal, K. Cationic Lipid Nanoparticles for Therapeutic Delivery of siRNA and miRNA to Murine Liver Tumor. Nanomedicine 2013, 9, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, S.; Tong, L.; Li, J.; Chen, F.; Han, Y.; Zhao, M.; Xiong, W. Superparamagnetic iron oxide nanoparticles mediated 131I-hVEGF siRNA inhibits hepatocellular carcinoma tumor growth in nude mice. BMC Cancer 2014, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, X.-L.; Zhang, J.-Z.; Mao, X.-H.; Xie, M.-W.; Cheng, Z.-L.; Lu, L.-J.; Duan, X.-H.; Zhang, L.-M.; Shen, J. Magnetic Cationic Amylose Nanoparticles Used to Deliver Survivin-Small Interfering RNA for Gene Therapy of Hepatocellular Carcinoma In Vitro. Nanomaterials 2017, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Kievit, F.M.; Sham, J.G.; Jeon, M.; Stephen, Z.R.; Bakthavatsalam, A.; Park, J.O.; Zhang, M. Iron-Oxide-Based Nanovector for Tumor Targeted siRNA Delivery in an Orthotopic Hepatocellular Carcinoma Xenograft Mouse Model. Small 2016, 12, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhang, R.; Zhang, J.; Hou, Y.; Chen, X.; Zhou, M.; Tian, X.; Hao, C.; Fan, K.; Yan, X. GRP78-targeted ferritin nanocaged ultra-high dose of doxorubicin for hepatocellular carcinoma therapy. Theranostics 2019, 9, 2167–2182. [Google Scholar] [CrossRef]
- Rajasekaran, D.; Srivastava, J.; Ebeid, K.; Gredler, R.; Akiel, M.; Jariwala, N.; Robertson, C.L.; Shen, X.-N.; Siddiq, A.; Fisher, P.B.; et al. Combination of nanoparticle-delivered siRNA for Astrocyte elevated gene-1 (AEG-1) and all-trans retinoic acid (ATRA): An effective therapeutic strategy for hepatocellular carcinoma (HCC). Bioconjug. Chem. 2015, 26, 1651–1661. [Google Scholar] [CrossRef]
- She, X.; Chen, L.; Yi, Z.; Li, C.; He, C.; Feng, C.; Wang, T.; Shigdar, S.; Duan, W.; Kong, L. Tailored Mesoporous Silica Nanoparticles for Controlled Drug Delivery: Platform Fabrication, Targeted Delivery, and Computational Design and Analysis. Mini Rev. Med. Chem. 2018, 18, 976–989. [Google Scholar] [CrossRef]
- Zheng, G.; Zhao, R.; Xu, A.; Shen, Z.; Chen, X.; Shao, J. Co-delivery of sorafenib and siVEGF based on mesoporous silica nanoparticles for ASGPR mediated targeted HCC therapy. Eur. J. Pharm. Sci. 2018, 111, 492–502. [Google Scholar] [CrossRef]
- Younis, M.A.; Khalil, I.A.; Elwakil, M.M.A.; Harashima, H. A Multifunctional Lipid-based Nanodevice for the Highly-specific Co-delivery of Sorafenib and Midkine siRNA to Hepatic Cancer Cells. Mol. Pharm. 2019, in press. [Google Scholar] [CrossRef]
- Shen, S.; Sun, C.-Y.; Du, X.-J.; Li, H.-J.; Liu, Y.; Xia, J.-X.; Zhu, Y.-H.; Wang, J. Co-delivery of platinum drug and siNotch1 with micelleplex for enhanced hepatocellular carcinoma therapy. Biomaterials 2015, 70, 71–83. [Google Scholar] [CrossRef]
- Yang, T.; Chen, Y.; Zhao, P.; Xue, H.; You, J.; Li, B.; Liu, Y.; He, C.; Zhang, X.; Fan, L.; et al. Enhancing the therapeutic effect via elimination of hepatocellular carcinoma stem cells using Bmi1 siRNA delivered by cationic cisplatin nanocapsules. Nanomedicine 2018, 14, 2009–2021. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.H.; Wang, M.L.; Chang, J.H.; Yarmishyn, A.A.; Nhi Nguyen, P.N.; Chen, W.; Chien, Y.; Huo, T.; Mou, C.-Y.; Chiou, S.-H. Dual Delivery of HNF4alpha and Cisplatin by Mesoporous Silica Nanoparticles Inhibits Cancer Pluripotency and Tumorigenicity in Hepatoma-Derived CD133-Expressing Stem Cells. ACS Appl. Mater. Interfaces 2019, 11, 19808–19818. [Google Scholar] [CrossRef] [PubMed]
- Ashley, C.E.; Carnes, E.C.; Phillips, G.K.; Durfee, P.N.; Buley, M.D.; Lino, C.A.; Padilla, D.P.; Phillips, B.; Carter, M.B.; Willman, C.L.; et al. Cell-Specific Delivery of Diverse Cargos by Bacteriophage MS2 Virus-Like Particles. ACS Nano 2011, 5, 5729–5745. [Google Scholar] [CrossRef]
- Chang, L.; Wang, G.; Jia, T.; Zhang, L.; Li, Y.; Han, Y.; Zhang, K.; Lin, G.; Zhang, R.; Li, J.; et al. Armored long non-coding RNA MEG3 targeting EGFR based on recombinant MS2 bacteriophage virus-like particles against hepatocellular carcinoma. Oncotarget 2016, 7, 23988–24004. [Google Scholar] [CrossRef]
- Wang, G.; Jia, T.; Xu, X.; Chang, L.; Zhang, R.; Fu, Y.; Li, Y.; Yang, X.; Zhang, K.; Lin, G.; et al. Novel miR-122 delivery system based on MS2 virus like particle surface displaying cell-penetrating peptide TAT for hepatocellular carcinoma. Oncotarget 2016, 7, 59402–59416. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Huang, P.-P.; Zhang, R.; Ma, B.-Y.; Zhou, X.-M.; Sun, Y.-F. Targeting adeno-associated virus and adenoviral gene therapy for hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 326–337. [Google Scholar] [CrossRef]
- Dong, J.; Li, W.; Dong, A.; Mao, S.; Shen, L.; Li, S.; Gong, X.; Wu, P. Gene therapy for unresectable hepatocellular carcinoma using recombinant human adenovirus type 5. Med. Oncol. 2014, 31, 95. [Google Scholar] [CrossRef] [PubMed]
- Breitbach, C.J.; Bell, J.C.; Hwang, T.-H.; Kirn, D.H.; Burke, J. The emerging therapeutic potential of the oncolytic immunotherapeutic Pexa-Vec (JX-594). Oncolytic Virother. 2015, 4, 25–31. [Google Scholar] [CrossRef]
- Park, B.-H.; Hwang, T.; Liu, T.-C.; Sze, D.Y.; Kim, J.-S.; Kwon, H.-C.; Oh, S.Y.; Han, S.-Y.; Yoon, J.-H.; Hong, S.-H.; et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: A phase I trial. Lancet Oncol. 2008, 9, 533–542. [Google Scholar] [CrossRef]
- Breitbach, C.J.; Moon, A.; Burke, J.; Hwang, T.-H.; Kirn, D.H. A Phase 2, Open-Label, Randomized Study of Pexa-Vec (JX-594) Administered by Intratumoral Injection in Patients with Unresectable Primary Hepatocellular Carcinoma. Methods Mol. Biol. 2015, 1317, 343–357. [Google Scholar] [PubMed]
- Bie, C.Q.; Liu, X.Y.; Cao, M.R.; Huang, Q.Y.; Tang, H.J.; Wang, M.; Cao, G.L.; Yi, T.Z.; Wu, S.L.; Xu, W.J.; et al. Lentivirus-mediated RNAi knockdown of insulin-like growth factor-1 receptor inhibits the growth and invasion of hepatocellular carcinoma via down-regulating midkine expression. Oncotarget 2016, 7, 79305–79318. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Cao, J.; Bai, W.H. Lentivirus-Mediated siRNA Targeting ER-alpha Inhibits Tumorigenesis and Induces Apoptosis in Hepatocarcinoma Cells. BioMed Res. Int. 2015, 2015, 490681. [Google Scholar]
- Kang, X.; Wang, F.; Lan, X.; Li, X.; Zheng, S.; Lv, Z.; Zhuang, Y.; Zhao, Y.; Zhou, S. Lentivirus-mediated shRNA Targeting CNN2 Inhibits Hepatocarcinoma in Vitro and in Vivo. Int. J. Med. Sci. 2018, 15, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wang, G.; Chen, G.; Zhu, M.; Lv, G. Lentivirus-mediated knockdown of P27RF-Rho inhibits hepatocellular carcinoma cell growth. Contemp. Onkol. 2017, 21, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.; Nakatani, T.; Masui, K.; Sakamoto, T.; Tominaga, K.; Yoshikawa, M.; Fukui, H.; Ikenaka, K.; Tsujii, T. Bystander effect caused by suicide gene expression indicates the feasibility of gene therapy for hepatocellular carcinoma. Hepatology 1995, 22, 1838–1846. [Google Scholar] [PubMed]
- Zhang, B.; Chen, M.; Zhang, Y.; Chen, W.; Zhang, L.; Chen, L. An ultrasonic nanobubble-mediated PNP/fludarabine suicide gene system: A new approach for the treatment of hepatocellular carcinoma. PLoS ONE 2018, 13, e0196686. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, P.; Li, L.; Zhang, Y.; Shao, Y.; Tang, L.; Tian, S. Effects of Cationic Microbubble Carrying CD/TK Double Suicide Gene and alphaVbeta3 Integrin Antibody in Human Hepatocellular Carcinoma HepG2 Cells. PLoS ONE 2016, 11, e0158592. [Google Scholar]
- Huang, H.-L.; Shen, J.-F.; Min, L.-S.; Ping, J.-L.; Lu, Y.-L.; Dai, L.-C. Inhibitory effect of midkine-binding peptide on tumor proliferation and migration. Int. J. Clin. Exp. Pathol. 2015, 8, 5387–5394. [Google Scholar]
- Sun, B.; Hu, C.; Yang, Z.; Zhang, X.; Zhao, L.; Xiong, J.; Ma, J.; Chen, L.; Qian, H.; Luo, X.; et al. Midkine promotes hepatocellular carcinoma metastasis by elevating anoikis resistance of circulating tumor cells. Oncotarget 2017, 8, 32523–32535. [Google Scholar] [CrossRef]
- Jia, H.-L.; Ye, Q.-H.; Qin, L.-X.; Budhu, A.; Forgues, M.; Chen, Y.; Liu, Y.-K.; Sun, H.-C.; Wang, L.; Lu, H.-Z.; et al. Gene Expression Profiling Reveals Potential Biomarkers of Human Hepatocellular Carcinoma. Clin. Cancer Res. 2007, 13, 1133–1139. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ma, Y.; Yang, L.; Wang, T.; Meng, X.; Zong, Z.; Sun, X.; Hua, X.; Li, H. Yes-associated protein (YAP) binds to HIF-1α and sustains HIF-1α protein stability to promote hepatocellular carcinoma cell glycolysis under hypoxic stress. J. Exp. Clin. Cancer Res. 2018, 37, 216. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Cai, Y.; Li, Y.; Li, Y.; Hu, N.; Ma, S.; Hu, S.; Zhu, P.; Wang, W.; Zhou, H. Yap promotes hepatocellular carcinoma metastasis and mobilization via governing cofilin/F-actin/lamellipodium axis by regulation of JNK/Bnip3/SERCA/CaMKII pathways. Redox Biol. 2018, 14, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Y.; Zhou, W.; Chen, T.; Wu, Q.; Chutturghoon, V.K.; Lin, B.; Geng, L.; Yang, Z.; Zhou, L.; et al. YAP promotes multi-drug resistance and inhibits autophagy-related cell death in hepatocellular carcinoma via the RAC1-ROS-mTOR pathway. Cancer Cell Int. 2019, 19, 179. [Google Scholar] [CrossRef] [PubMed]
- Su, C. Survivin in survival of hepatocellular carcinoma. Cancer Lett. 2016, 379, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Zhang, X.J.; Zhang, Z.; Zhang, A.H.; Wang, W.; Dong, J.H. Meta-Analysis: Prognostic Value of Survivin in Patients with Hepatocellular Carcinoma. PLoS ONE 2013, 8, e83350. [Google Scholar] [CrossRef]
- Xia, H.; Chen, J.; Shi, M.; Deivasigamani, A.; Ooi, L.L.P.; Hui, K.M. The over-expression of survivin enhances the chemotherapeutic efficacy of YM155 in human hepatocellular carcinoma. Oncotarget 2015, 6, 5990–6000. [Google Scholar] [CrossRef] [PubMed]
- Namgung, Y.; Kim, S.Y.; Kim, I. Down-regulation of Survivin by BIX-01294 Pretreatment Overcomes Resistance of Hepatocellular Carcinoma Cells to TRAIL. Anticancer Res. 2019, 39, 3571–3578. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Song, W.; Zhou, L.; Li, Q.; Tao, K.; Zhou, J.; Wang, X.; Zheng, Z.; You, N.; et al. Overexpression of Bmi-1 contributes to the invasion and metastasis of hepatocellular carcinoma by increasing the expression of matrix metalloproteinase (MMP)-2, MMP-9 and vascular endothelial growth factor via the PTEN/PI3K/Akt pathway. Int. J. Oncol. 2013, 43, 793–802. [Google Scholar] [CrossRef]
- Qi, S.; Li, B.; Yang, T.; Liu, Y.; Cao, S.; He, X.; Zhang, P.; Li, L.; Xu, C. Validation of Bmi1 as a Therapeutic Target of Hepatocellular Carcinoma in Mice. Int. J. Mol. Sci. 2014, 15, 20004–20021. [Google Scholar] [CrossRef]
- Yoo, B.K.; Emdad, L.; Lee, S.-G.; Su, Z.-Z.; Santhekadur, P.; Chen, D.; Gredler, R.; Fisher, P.B.; Sarkar, D. Astrocyte Elevated Gene-1 (AEG-1): A multifunctional regulator of normal and abnormal physiology. Pharmacol. Ther. 2011, 130, 1–8. [Google Scholar] [CrossRef]
- Robertson, C.L.; Srivastava, J.; Siddiq, A.; Gredler, R.; Emdad, L.; Rajasekaran, D.; Akiel, M.; Shen, X.-N.; Guo, C.; Giashuddin, S.; et al. Genetic deletion of AEG-1 prevents hepatocarcinogenesis. Cancer Res. 2014, 74, 6184–6193. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, N.; Rajasekaran, D.; Mendoza, R.G.; Shen, X.-N.; Siddiq, A.; Akiel, M.A.; Robertson, C.L.; Subler, M.A.; Windle, J.J.; Fisher, P.B.; et al. Oncogenic Role of SND1 in Development and Progression of Hepatocellular Carcinoma. Cancer Res. 2017, 77, 3306–3316. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Jiao, M.; Jing, L.; Wang, M.-C.; Sun, H.-F.; Li, Q.; Bai, Y.-Y.; Wei, Y.-C.; Nan, K.-J.; Guo, H. Prognostic value of Notch-1 expression in hepatocellular carcinoma: A meta-analysis. OncoTargets Ther. 2015, 8, 3105–3114. [Google Scholar] [CrossRef] [PubMed]
- Bouattour, M.; Raymond, E.; Qin, S.; Cheng, A.-L.; Stammberger, U.; Locatelli, G.; Faivre, S. Recent developments of c-Met as a therapeutic target in hepatocellular carcinoma. Hepatology 2018, 67, 1132–1149. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, J.; Zhen, C.; Yang, B.; Feng, L. Gankyrin as a potential target for tumor therapy: Evidence and perspectives. Am. J. Transl. Res. 2018, 10, 1949–1960. [Google Scholar] [PubMed]
- Wu, Y.; Meng, X.; Huang, C.; Li, J. Emerging role of silent information regulator 1 (SIRT1) in hepatocellular carcinoma: A potential therapeutic target. Tumor Boil. 2015, 36, 4063–4074. [Google Scholar] [CrossRef] [PubMed]
- Asghar, K.; Farooq, A.; Zulfiqar, B.; Rashid, M.U. Indoleamine 2,3-dioxygenase: As a potential prognostic marker and immunotherapeutic target for hepatocellular carcinoma. World J. Gastroenterol. 2017, 23, 2286–2293. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Cheng, T.-Y.; Chen, T.-Y.; Chang, K.-M.; Chuang, V.P.; Kao, K.-J. Plasmalemmal Vesicle Associated Protein (PLVAP) as a therapeutic target for treatment of hepatocellular carcinoma. BMC Cancer 2014, 14, 815. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Liu, L.; Wang, Q.; Li, S.; Chen, D.; Hu, Z.; Yu, T.; Ding, J.; Li, J.; et al. Long noncoding RNA TSLNC8 is a tumor suppressor that inactivates the interleukin-6/STAT3 signaling pathway. Hepatology 2018, 67, 171–187. [Google Scholar] [CrossRef]
- Pan, W.; Li, W.; Zhao, J.; Huang, Z.; Zhao, J.; Chen, S.; Wang, C.; Xue, Y.; Huang, F.; Fang, Q.; et al. lnc RNA—PDPK 2P promotes hepatocellular carcinoma progression through the PDK 1/AKT/Caspase 3 pathway. Mol. Oncol. 2019, in press. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, N.; Liu, W.; Liu, J.; Zhou, L.; Liu, Y.; Yang, M. The long noncoding RNA GAS8-AS1 suppresses hepatocarcinogenesis by epigenetically activating the tumor suppressor GAS8. J. Boil. Chem. 2018, 293, 17154–17165. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zheng, G.; Li, C.; Liu, C. Long noncoding RNA Fer1like family member 4 suppresses hepatocellular carcinoma cell proliferation by regulating PTEN in vitro and in vivo. Mol. Med. Rep. 2019, 19, 685–692. [Google Scholar] [PubMed]
- Yan, S.; Tang, Z.; Chen, K.; Liu, Y.; Yu, G.; Chen, Q.; Dang, H.; Chen, F.; Ling, J.; Zhu, L.; et al. Long noncoding RNA MIR31HG inhibits hepatocellular carcinoma proliferation and metastasis by sponging microRNA-575 to modulate ST7L expression. J. Exp. Clin. Cancer Res. 2018, 37, 214. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ye, H.; Huang, G.; Luo, F.; Liu, Y.; Liu, Y.; Yang, X.; Shen, J.; Liu, Q.; Zhang, J. Long noncoding RNA GAS5 suppresses the migration and invasion of hepatocellular carcinoma cells via miR-21. Tumor Biol. 2016, 37, 2691–2702. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-G.; Wang, T.; Shi, M.; Zhai, B. Long noncoding RNA EPB41L4A-AS2 inhibits hepatocellular carcinoma development by sponging miR-301a-5p and targeting FOXL1. J. Exp. Clin. Cancer Res. 2019, 38, 153. [Google Scholar] [CrossRef]
- Liu, F.; Yuan, J.-H.; Huang, J.-F.; Yang, F.; Wang, T.-T.; Ma, J.-Z.; Zhang, L.; Zhou, C.-C.; Wang, F.; Yu, J.; et al. Long noncoding RNA FTX inhibits hepatocellular carcinoma proliferation and metastasis by binding MCM2 and miR-374a. Oncogene 2016, 35, 5422–5434. [Google Scholar] [CrossRef] [PubMed]
- Chen, B. A novel long noncoding RNA lncWDR26 suppresses the growth and metastasis of hepatocellular carcinoma cells through interaction with SIX3. Am. J. Cancer Res. 2018, 8, 688–698. [Google Scholar]
- Sui, J.; Yang, X.; Qi, W.; Guo, K.; Gao, Z.; Wang, L.; Sun, D. Long Non-Coding RNA Linc-USP16 Functions As a Tumour Suppressor in Hepatocellular Carcinoma by Regulating PTEN Expression. Cell. Physiol. Biochem. 2017, 44, 1188–1198. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Y.; Huan, L.; Xu, L.; Shen, M.; Huang, S.; Liang, L. LncRNA MIR22HG inhibits growth, migration and invasion through regulating the miR-10a-5p/NCOR2 axis in hepatocellular carcinoma cells. Cancer Sci. 2019, 110, 973–984. [Google Scholar] [CrossRef]
- Wang, T.-H.; Lin, Y.-S.; Chen, Y.; Yeh, C.-T.; Huang, Y.-L.; Hsieh, T.-H.; Shieh, T.-M.; Hsueh, C.; Chen, T.-C. Long non-coding RNA AOC4P suppresses hepatocellular carcinoma metastasis by enhancing vimentin degradation and inhibiting epithelial-mesenchymal transition. Oncotarget 2015, 6, 23342–23357. [Google Scholar] [CrossRef]
- Chen, S.; Yang, C.; Sun, C.; Sun, Y.; Yang, Z.; Cheng, S.; Zhuge, B. miR-21-5p Suppressed the Sensitivity of Hepatocellular Carcinoma Cells to Cisplatin by Targeting FASLG. DNA Cell Boil. 2019, 38, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhou, J.; Jian, Y.; Xiu, Z.; Xiang, L.; Yang, D.; Zeng, W. MicroRNA-214 suppresses cell proliferation and migration and cell metabolism by targeting PDK2 and PHF6 in hepatocellular carcinoma. Cell Boil. Int. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zou, H.; Luo, L.; Wang, X.; Wang, G. MicroRNA-9 exerts antitumor effects on hepatocellular carcinoma progression by targeting HMGA2. FEBS Open Bio 2019. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.P.; Wang, H.X.; Tong, D.M.; Li, Y.; Huang, L.H.; Wang, C. miRNA-370 acts as a tumor suppressor via the downregulation of PIM1 in hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1254–1263. [Google Scholar] [PubMed]
- Song, W.-H.; Feng, X.-J.; Gong, S.-J.; Chen, J.-M.; Wang, S.-M.; Xing, D.-J.; Zhu, M.-H.; Zhang, S.-H.; Xu, A.-M. microRNA-622 acts as a tumor suppressor in hepatocellular carcinoma. Cancer Boil. Ther. 2015, 16, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Fan, W.; Guo, W.; Zhang, Y.; Wang, L.; Guo, L.; Duan, X.; Wei, J.; Xu, G. miR-1247-5p functions as a tumor suppressor in human hepatocellular carcinoma by targeting Wnt3. Oncol. Rep. 2017, 38, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Pang, X.; Zhang, Q.; Zhang, Y.; Niu, J. miR-154 targeting ZEB2 in hepatocellular carcinoma functions as a potential tumor suppressor. Oncol. Rep. 2015, 34, 3272–3279. [Google Scholar]
- Yu, S.; Jing, L.; Yin, X.-R.; Wang, M.-C.; Chen, Y.-M.; Guo, Y.; Nan, K.-J.; Han, L.-L. MiR-195 suppresses the metastasis and epithelial–mesenchymal transition of hepatocellular carcinoma by inhibiting YAP. Oncotarget 2017, 8, 99757–99771. [Google Scholar] [CrossRef][Green Version]
- Yan, J.-J.; Chang, Y.; Zhang, Y.-N.; Lin, J.-S.; He, X.-X.; Huang, H.-J. miR-195 inhibits cell proliferation via targeting AEG-1 in hepatocellular carcinoma. Oncol. Lett. 2017, 13, 3118–3126. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, K.S.; Bae, H.J.; Eun, J.W.; Shen, Q.; Park, S.J.; Shin, W.C.; Yang, H.D.; Park, M.; Park, W.S.; et al. MicroRNA-31 functions as a tumor suppressor by regulating cell cycle and epithelial-mesenchymal transition regulatory proteins in liver cancer. Oncotarget 2015, 6, 8089–8102. [Google Scholar] [CrossRef]
- Hu, X.; Pang, P.; Zhou, B.; Li, D.; Mao, J.; Shan, H. miR-30e acts as a tumor suppressor in hepatocellular carcinoma partly via JAK1/STAT3 pathway. Oncol. Rep. 2017, 38, 393–401. [Google Scholar]
- Quan, H.; Li, B.; Yang, J. MicroRNA-504 functions as a tumor suppressor in hepatocellular carcinoma through inhibiting Frizzled-7-mediated-Wnt/beta-catenin signaling. Biomed. Pharmacother. 2018, 107, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhang, M.; Liu, W. Hypermethylated KCNQ1 acts as a tumor suppressor in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2018, 503, 3100–3107. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhu, P.; Zhang, X.; Zhang, L.; Chen, X.; Lu, F.; Yu, Z.; Liu, S. PCDH9 acts as a tumor suppressor inducing tumor cell arrest at G0/G1 phase and is frequently methylated in hepatocellular carcinoma. Mol. Med. Rep. 2017, 16, 4475–4482. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-X.; Lv, L.-H.; Wan, Y.-L.; Cao, Y.; Li, G.-L.; Lin, H.-M.; Zhou, R.; Shang, C.-Z.; Cao, J.; He, H.; et al. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology 2015, 61, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Lv, L.; Wei, J.; Lei, X.; Lin, H.; Li, G.; Cao, J.; Xie, J.; Yang, W.; Wu, S.; et al. Vps4A mediates the localization and exosome release of beta-catenin to inhibit epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2019, 457, 47–59. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Li, T.; Guan, L.; Liu, F.-E.; Chen, X.-M.; Zhao, J.; Lin, S.; Liu, Z.-Z.; Zhang, H.-Q. CTNNA3 is a tumor suppressor in hepatocellular carcinomas and is inhibited by miR-425. Oncotarget 2016, 7, 8078–8089. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Qin, S.-K. Features and treatment options of Chinese hepatocellular carcinoma. Chin. Clin. Oncol. 2013, 2, 38. [Google Scholar]
| Study Identifier | Biological Used | Study Status | Delivery Route | Clinical Trial Phase | Adjuvant Therapy Used | Country | Patients Enrolled |
|---|---|---|---|---|---|---|---|
| NCT03146234 | GPC-3 CAR-T | Recruiting | Intravenous | N/A | - | China | HCC patients with relapsed or refractory cancer |
| NCT02905188 | GPC-3 CAR-T | Recruiting | Intravenous | Phase 1 | - | USA | HCC patients with unresectable, recurrent and/or metastatic cancer |
| NCT03084380 | GPC-3 CAR-T | Not yet recruiting | Intravenous | Phase 1/2 | TACE | China | HCC patients who cannot receive TACE with sorafenib treatment |
| NCT03884751 | GPC-3 CAR-T | Not yet recruiting | Intravenous | Phase 1 | - | China | Advanced HCC patients with no effective treatment |
| NCT03980288 | GPC-3 CAR-T | Not yet recruiting | Intravenous | Phase 1 | - | China | Advanced HCC patients intolerant to standard treatment |
| NCT02723942 | GPC-3 CAR-T | Completed | N/A | Phase 1/2 | - | China | HCC patients with non-diffuse HCC without extrahepatic metastasis or portal vein vascular invasion. |
| NCT02395250 | GPC-3 CAR-T | Completed | N/A | Phase 1 | - | China | HCC patients with relapsed or refractory cancer |
| NCT03198546 | GPC-3 CAR-T | Recruiting | N/A | Phase 1 | - | China | Advanced HCC patients |
| NCT02715362 | GPC-3 CAR-T | Unknown | Transcatheter Arterial Infusion | Phase 1/2 | - | China | HCC patients with unresectable advanced cancer |
| NCT03130712 | GPC-3 CAR-T | Unknown | Intratumoral | Phase 1/2 | - | China | Advanced HCC patients with persistent cancer after standard chemotherapy or surgery |
| NCT03993743 | CD147-CAR-T | Active, not Recruiting | Hepatic Artery Infusion | Phase 1 | - | China | Advanced HCC patients who have failed first and second lines of HCC treatment |
| NCT03349255 | ET1402L1-CAR-T | Terminated | Intravenous | Phase 1 | - | China | HCC patients with at least one measurable tumor |
| NCT02587689 | Anti-MUC1 CAR-T | Unknown | N/A | Phase 1 | - | China | HCC patients with relapsed or refractory cancer |
| NCT03013712 | EpCAM specific CAR-T | Recruiting | Vascular intervention | Phase 1 | - | China | HCC patients with relapsed or refractory cancer |
| Nanodrug Type | Classification/Examples | Characteristics | Mechanism of Action | Gene(s) Targeted |
|---|---|---|---|---|
| Lipid nanoparticles | Micelles | Lipid monolayer enclosing a hydrophobic core. | The therapeutic agent is entrapped within the hydrophobic or aqueous core. Once drugs reach the cell membrane, they are taken up by the cell through endocytosis. As pH in the endosome decreases, the therapeutic agent is released. In case of siRNA, it incorporates into the RISC complex and cleaves target mRNA. | YAP, Integrin, miR-122, Bmi1 |
| Liposomes | Lipid bilayer membrane surrounding an aqueous core. | |||
| Dendrimers | PAMAM | Dendrimers are nanoparticles with repetitive branching. PAMAM dendrimers have repetitive branches of amidoamine radiating from a central core of ethylenediamine. | The structure of PAMAM contains cavities within the assembled molecule that can be exploited to carry therapeutic agents. They also have positively charged primary amines on their surface that allow binding of nucleic acids. Dendrimers that are chemically modified to be recognized by membrane proteins are internalized by endocytosis after interaction with surface protein. Otherwise they are internalized by non-specific endocytosis | siAEG-1 |
| Polysaccharides | Chitosan | Polysaccharide made up of units of beta (1→4) linked glucosamine and N-acetyl glucosamine | Therapeutic nucleic acids can be adsorbed onto chitosan nanoparticles, complexed with chitosan or enclosed within the chitosan nanoparticles. Cellular uptake is through non-specific endocytosis or receptor-mediated endocytosis. | Sphk2, Midkine, PLK1 |
| Iron oxide | _ | Made up of γ-Fe2O3 and/or Fe3O4 particles | Iron oxide nanoparticles can be produced using many methods including coprecipitation of ferric and ferrous ions. They are usually coated with polymers and negatively charged nucleic acids are bound to their surface. | VEGF |
| Mesoporous silica nanoparticles | _ | Silica nanoparticles with pores of 2–50 nm | Surfactants are mixed with silica precursors to form silica structures around the surfactant. The surfactant is then removed to produce MSN. Therapeutic agents can be loaded into these pores and delivered into cells. MSNs can be modified to target certain cells. They are internalized through receptor-mediated or specific endocytosis and released into the cytoplasm. | VEGF, siNotch1 |
| Study Identifier | Biological Used | Study Status | Delivery Route | Clinical Trial Phase | Adjuvant Therapy Used | Country | Patients Enrolled |
|---|---|---|---|---|---|---|---|
| NCT01869088 | rhAdV type-5 | Unknown | Arterial injection | Phase 3 | TACE | China | Advanced HCC patients who cannot undergo surgery or local ablative therapy |
| NCT03790059 | rhAdV type-5 | Recruiting | Intraoperative injection | N/A | RFA | China | Patients with single HCC of diameter less than 3 cm |
| NCT03780049 | rhAdV type-5 | Recruiting | Hepatic artery infusion | Phase 3 | HAIC | China | HCC patients with unresectable tumors |
| NCT00669136 | AFP AdV | Terminated (Poor accrual) | Intramuscular | Phase 1 | - | USA | HCC patients with locoregionally pre-treated cancer |
| NCT02202564 | ADV-TK | Completed | Intraperitoneal | Phase 2 | Liver Transplant | China | HCC patients who can undergo liver transplantation |
| NCT00300521 | ADV-TK | Completed | N/A | Phase 2 | Liver Transplant | China | Intermediate or advanced HCC patients who can undergo liver transplantation |
| NCT00844623 | TK99UN (AdV with HsV-TK) | Completed | Intratumoral | Phase 1 | N/A | Spain | HCC patients who cannot undergo curative therapy |
| NCT03313596 | ADV-Tk | Recruiting | Intraperitoneal | Phase 3 | Liver Transplant | China | Advanced HCC patients who can undergo liver transplantation |
| NCT02561546 | p53 | Unknown | Arterial injection | Phase 2 | TACE | China | HCC patients with unresectable cancer |
| NCT02509169 | P53 | Unknown | N/A | Phase 2 | TAE | China | HCC patients with unresectable cancer |
| NCT00003147 | Ad5CMV-p53 gene | Terminated (Administratively complete) | Percutaneous | Phase 1 | - | USA | HCC patients with unresectable cancer |
| NCT02418988 | rAd-p53 | Unknown | Arterial injection | Phase 2 | TACE | china | HCC patients with unresectable cancer |
| Study Identifier | Biological Used | Study Status | Delivery Route | Clinical Trial Phase | Adjuvant | Country | Patients Enrolled |
|---|---|---|---|---|---|---|---|
| NCT00629759 | JX-594 (Pexa-Vec) | completed | Transdermal injection | Phase 1 | - | Korea | HCC patients resistant to standard treatment |
| NCT01636284 | JX-594 (Pexa-Vec) | completed | Intravenous | Phase 2a | - | US and Korea | Advanced HCC patients who have not been treated with. sorafenib |
| NCT01171651 | JX-594 (Pexa-Vec) | completed | Intravenous and intratumoral | Phase 2 | sorafenib | Korea | HCC patients with unresectable cancer |
| NCT01387555 | JX-594 (Pexa-Vec) | completed | N/A | Phase 2b | - | US | Advanced HCC patients intolerant to sorafenib |
| NCT00554372 | JX-594 (Pexa-Vec) | completed | Intratumoral | Phase 2 | - | US | HCC patients with unresectable cancer |
| NCT02562755 | JX-594 (Pexa-Vec) | Recruiting | Intratumoral | Phase 3 | sorafenib | US | Advanced HCC without prior systemic therapy |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reghupaty, S.C.; Sarkar, D. Current Status of Gene Therapy in Hepatocellular Carcinoma. Cancers 2019, 11, 1265. https://doi.org/10.3390/cancers11091265
Reghupaty SC, Sarkar D. Current Status of Gene Therapy in Hepatocellular Carcinoma. Cancers. 2019; 11(9):1265. https://doi.org/10.3390/cancers11091265
Chicago/Turabian StyleReghupaty, Saranya Chidambaranathan, and Devanand Sarkar. 2019. "Current Status of Gene Therapy in Hepatocellular Carcinoma" Cancers 11, no. 9: 1265. https://doi.org/10.3390/cancers11091265
APA StyleReghupaty, S. C., & Sarkar, D. (2019). Current Status of Gene Therapy in Hepatocellular Carcinoma. Cancers, 11(9), 1265. https://doi.org/10.3390/cancers11091265





