Predicting Visual Acuity Deterioration and Radiation-Induced Toxicities after Brachytherapy for Choroidal Melanomas
Abstract
:1. Introduction
2. Methods and Materials
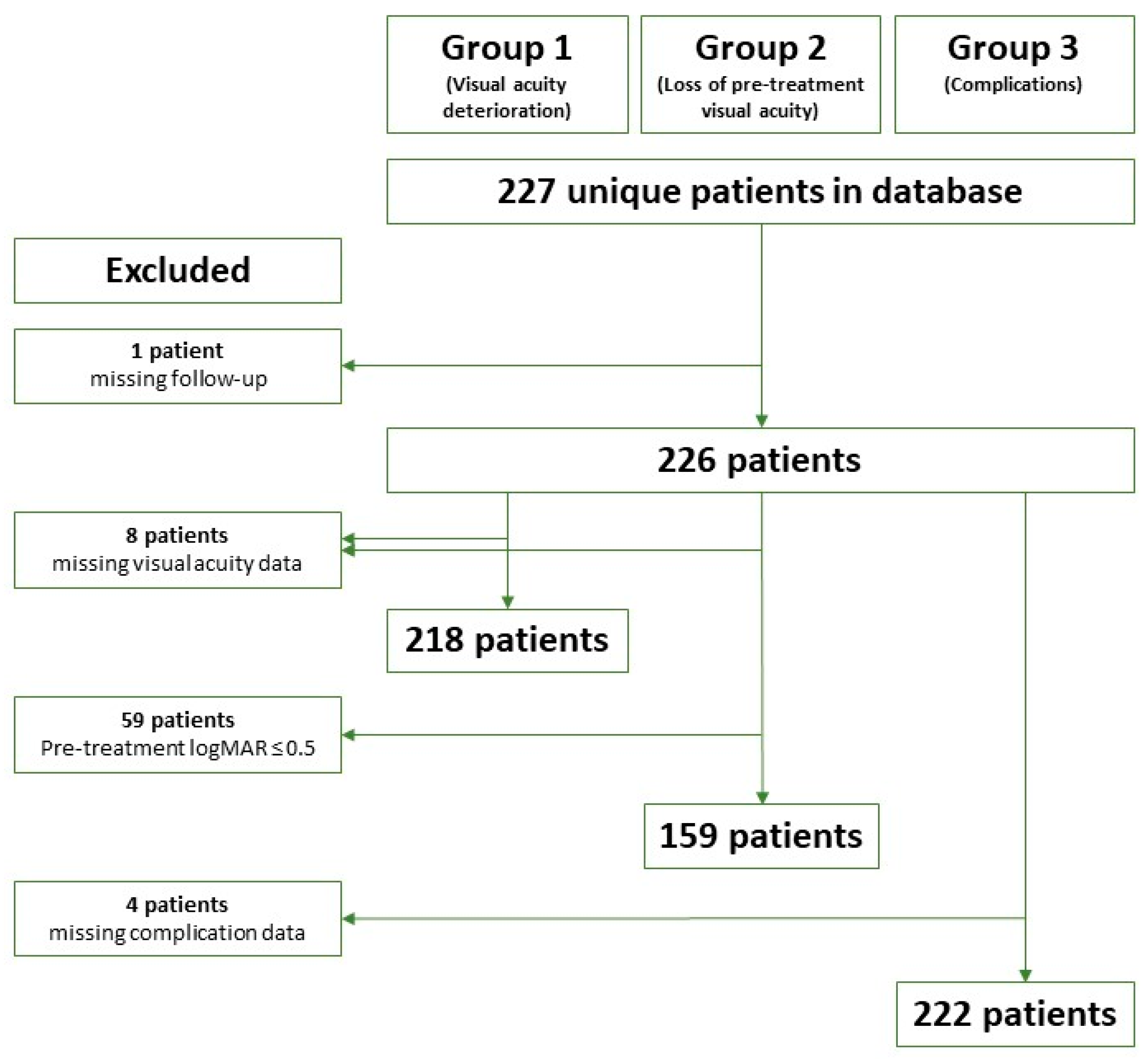
2.1. Patient Material
2.2. The Treatment Procedure
2.3. Regular Assessment
2.4. Dose Distribution Analysis
2.5. Definition of Outcomes
2.6. Data Analysis
3. Results
3.1. Visual Acuity Analysis
3.2. Late Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Damato, B.; Patel, I.; Campbell, I.R.; Mayles, H.M.; Errington, R.D. Local tumor control after 106Ru brachytherapy of choroidal melanoma. Int. J. Radiat. Oncol. 2005, 63, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Marconi, D.G.; De Castro, D.G.; Rebouças, L.M.; Bernardes Gil, G.O.; Fogaroli, R.C.; Conte Maia, M.A.; Gobo Silva, M.L.; Assis Pellizzon, A.C.; Motono Chojniak, M.M. Tumor control, eye preservation, and visual outcomes of ruthenium plaque brachytherapy for choroidal melanoma. Brachytherapy 2013, 12, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Espensen, C.A.; Appelt, A.L.; Fog, L.S.; Thariat, J.; Gothelf, A.B.; Aznar, M.C.; Kiilgaard, J.F. Tumour control probability after Ruthenium-106 brachytherapy for choroidal melanomas. Cancers 2019. under review. [Google Scholar]
- Eckert & Ziegler BEBIG GmbH Ophthalmic Brachytherapy. Available online: https://www.bebig.com/fileadmin/bebig_neu/user_uploads/Products/Ophthalmic_Brachytherapy/Ophthalmic_Brochure__Rev._06__English.pdf (accessed on 5 August 2019).
- Damato, B.; Patel, I.; Campbell, I.R.; Mayles, H.M.; Errington, R.D. Visual acuity after Ruthenium106 brachytherapy of choroidal melanomas. Int. J. Radiat. Oncol. 2005, 63, 392–400. [Google Scholar] [CrossRef] [PubMed]
- EyePhysicsPlaque Simulator User Guide. Available online: https://www.eyephysics.com/PS/Index.html (accessed on 6 August 2019).
- Finger, P.T.; Chin, K.J.; Yu, G.P.; Choroidal, P.; Study, M. Risk Factors for Radiation Maculopathy after Ophthalmic Plaque Radiation for Choroidal Melanoma. Am. J. Ophthalmol. 2010, 149, 608–615. [Google Scholar] [CrossRef]
- Danesh-Meyer, H.V. Radiation-induced optic neuropathy. J. Clin. Neurosci. 2008, 15, 95–100. [Google Scholar] [CrossRef]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Aziz, H.A.; Singh, N.; Bena, J.; Wilkinson, A.; Singh, A.D. Vision Loss Following Episcleral Brachytherapy for Uveal Melanoma. JAMA Ophthalmol. 2016, 134, 615–620. [Google Scholar] [CrossRef] [Green Version]
- Heilemann, G.; Fetty, L.; Blaickner, M.; Nesvacil, N.; Zehetmayer, M.; Georg, D.; Dunavoelgyi, R. Retina dose as a predictor for visual acuity loss in 106Ru eye plaque brachytherapy of uveal melanomas. Radiother. Oncol. 2018, 127, 379–384. [Google Scholar] [CrossRef]
- Isager, P.; Ehlers, N.; Urbak, S.F.; Overgaard, J. Visual outcome, local tumour control, and eye preservation after106Ru/Rh brachytherapy for choroidal melanoma. Acta Oncol. 2006, 45, 285–293. [Google Scholar] [CrossRef]
- Bergman, L.; Nilsson, B.; Lundell, G.; Lundell, M.; Seregard, S. Ruthenium brachytherapy for uveal melanoma, 1979-2003: Survival and functional outcomes in the Swedish population. Ophthalmology 2005, 112, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, L.; Pagliara, M.M.; Masciocchi, C.; Scupola, A.; Azario, L.; Grimaldi, G.; Autorino, R.; Gambacorta, M.A.; Laricchiuta, A.; Boldrini, L.; et al. Nomogram for predicting radiation maculopathy in patients treated with Ruthenium-106 plaque brachytherapy for uveal melanoma. J. Contemp. Brachyther. 2017, 9, 540–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summanen, P.; Immonen, I.; Kivelä, T.; Tommila, P.; Heikkonen, J.; Tarkkanen, A. Radiation related complications after ruthenium plaque radiotherapy of uveal melanoma. Br. J. Ophthalmol. 1996, 80, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Naseripour, M.; Jaberi, R.; Sedaghat, A.; Azma, Z.; Nojomi, M.; Falavarjani, K.G.; Nazari, H. Ruthenium-106 brachytherapy for thick uveal melanoma: Reappraisal of apex and base dose radiation and dose rate. J. Contemp. Brachyther. 2016, 8, 66–73. [Google Scholar] [CrossRef]
- Finger, P.; Kurli, M. Laser photocoagulation for radiation retinopathy after ophthalmic plaque radiation therapy. Br. J. Ophthalmol. 2005, 89, 730–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouberol, F.; Roy, P.; Kodjikian, L.; Gérard, J.P.; Jean-Louis, B.; Grange, J.D. Survival, anatomic, and functional long-term results in choroidal and ciliary body melanoma after ruthenium brachytherapy (15 years’ experience with beta-rays). Am. J. Ophthalmol. 2004, 137, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Thariat, J.; Jacob, S.; Caujolle, J.P.; Maschi, C.; Baillif, S.; Angellier, G.; Mathis, T.; Rosier, L.; Carnicer, A.; Hérault, J.; et al. Cataract avoidance with proton therapy in ocular melanomas. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5378–5386. [Google Scholar] [CrossRef]
- Houston, S.K.; Boldt, H.C.; Markoe, A.M.; Murray, T.G. Brachytherapy for Choroidal Melanoma. In Retina; Elsevier: Amsterdam, The Netherlands, 2013; pp. 2275–2289. [Google Scholar]
- Fili, M.; Lundell, G.; Lundell, M.; Seregard, S. High dose rate and low dose rate ruthenium brachytherapy for uveal melanoma. No association with ocular outcome. Br. J. Ophthalmol. 2014, 98, 1349–1354. [Google Scholar] [CrossRef]
- Mossböck, G.; Rauscher, T.; Winkler, P.; Kapp, K.S.; Langmann, G. Impact of dose rate on clinical course in uveal melanoma after brachytherapy with ruthenium-106. Strahlenther. Onkol. 2007, 183, 571–575. [Google Scholar] [CrossRef]
- Takiar, V.; Voong, K.R.; Gombos, D.S.; Mourtada, F.; Rechner, L.A.; Lawyer, A.A.; Morrison, W.H.; Garden, A.S.; Beadle, B.M. A choice of radionuclide: Comparative outcomes and toxicity of ruthenium-106 and iodine-125 in the definitive treatment of uveal melanoma. Pract. Radiat. Oncol. 2015, 5, e169–e176. [Google Scholar] [CrossRef]
- Browne, A.W.; Dandapani, S.V.; Jennelle, R.; Stevanovic, M.; Lee, T.C.; Murphree, A.L.; Kampp, T.D.; Astrahan, M.A.; Kim, J.W.; Berry, J.L. Outcomes of medium choroidal melanomas treated with ruthenium brachytherapy guided by three-dimensional pretreatment modeling. Brachytherapy 2015, 14, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Miguel, D.; Frutos-Baraja, J.M.; López-Lara, F.; Saornil, M.A.; García-Álvarez, C.; Alonso, P.; Diezhandino, P. Radiobiological doses, tumor, and treatment features influence on local control, enucleation rates, and survival after epiescleral brachytherapy. A 20-year retrospective analysis from a single-institution: Part I. J. Contemp. Brachyther. 2018, 10, 337–346. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Wang, Y.; Zhou, X.; Xu, H.; Huang, C. An improved variable selection procedure for adaptive Lasso in high-dimensional survival analysis. Lifetime Data Anal. 2019, 25, 569–585. [Google Scholar] [CrossRef] [PubMed]
- Stöckel, E.; Eichmann, M.; Flühs, D.; Sommer, H.; Biewald, E.; Bornfeld, N.; Spaan, B.; Sauerwein, W. Dose Distributions and Treatment Margins in Ocular Brachytherapy with 106Ru Eye Plaques. Ocul. Oncol. Pathol. 2017, 4, 122–128. [Google Scholar] [PubMed]
- Zaragoza, F.J.; Eichmann, M.; Flühs, D.; Sauerwein, W.; Brualla, L. Monte Carlo Estimation of Absorbed Dose Distributions Obtained from Heterogeneous 106Ru Eye Plaques. Ocul. Oncol. Pathol. 2017, 3, 204–209. [Google Scholar] [CrossRef] [PubMed]
| Late Complication | Clinical Findings | Important Examination |
|---|---|---|
| Visual acuity deterioration | Increase of minimum 0.3 logMAR from the pre-treatment measure | Snellen’s chart (converted to logMAR for statistical purposes) |
| Maculopathy | Micro aneurysms, ischemia, oedema, and/or atrophy in the macular region [7] | Ophthalmoscopy, retinography, OCT and/or fluorescein angiography. |
| Optic neuropathy | Swelling, ischemia, atrophy and/or pallor occurring optic disc [8] | Ophthalmoscopy, retinography and/or OCT |
| Ocular hypertension | Intraocular pressure ≥21 mm Hg (at least three months post-treatment) | Tonometry |
| Vascular obliteration | Narrow and obliterated blood vessels on the retina | Ophthalmoscopy |
| Cataract | Lens opacities along with gradually deterioration of the visual acuity | Slit lamp examination or ophthalmoscopy |
| Retinal detachment | Fluttering membrane | Ophthalmoscopy |
| Patient Characteristics | Value (Median (IQR)) | |
|---|---|---|
| Age (years) | 62 (53–69) | |
| Gender male/female | 118/108 | |
| Eye left/right | 117/109 | |
| Follow-up (years) | 5 (95% CI: 4.5–6.0) | |
| Pre-treatment VA (logMAR) | 0.3 (0.0–0.6) | |
| Pre-treatment VA ≤0.5 logMAR (y/n) | 165/61 | |
| Last VA (logMAR) | 0.9 (0.3–3.0) (NA = 4) | |
| Last VA ≤0.5 logMAR (y/n) | 82/140 (NA = 4) | |
| Tumour characteristics | ||
| Largest basal dimension (mm) | 11.4 (9.0–13.3) | |
| Height (mm) | 3.9 (2.8–5.8) | |
| Optic disc–tumour distance (mm) | 2.4 (0.4–4.9) | |
| Macula–tumour distance (mm) | 2.5 (0.1–5.0) | |
| Treatment characteristics | ||
| Treatment (time hours) | 120 (74–191) | |
| Plaque type CCA/CCB/CCC/COB | 53/101/12/60 | |
| Late complication | Number (%) | 5-year probability of freedom from toxicity (95% CI) |
| Loss of pre-treatment visual acuity | 101 (66) | 29 (22–38) |
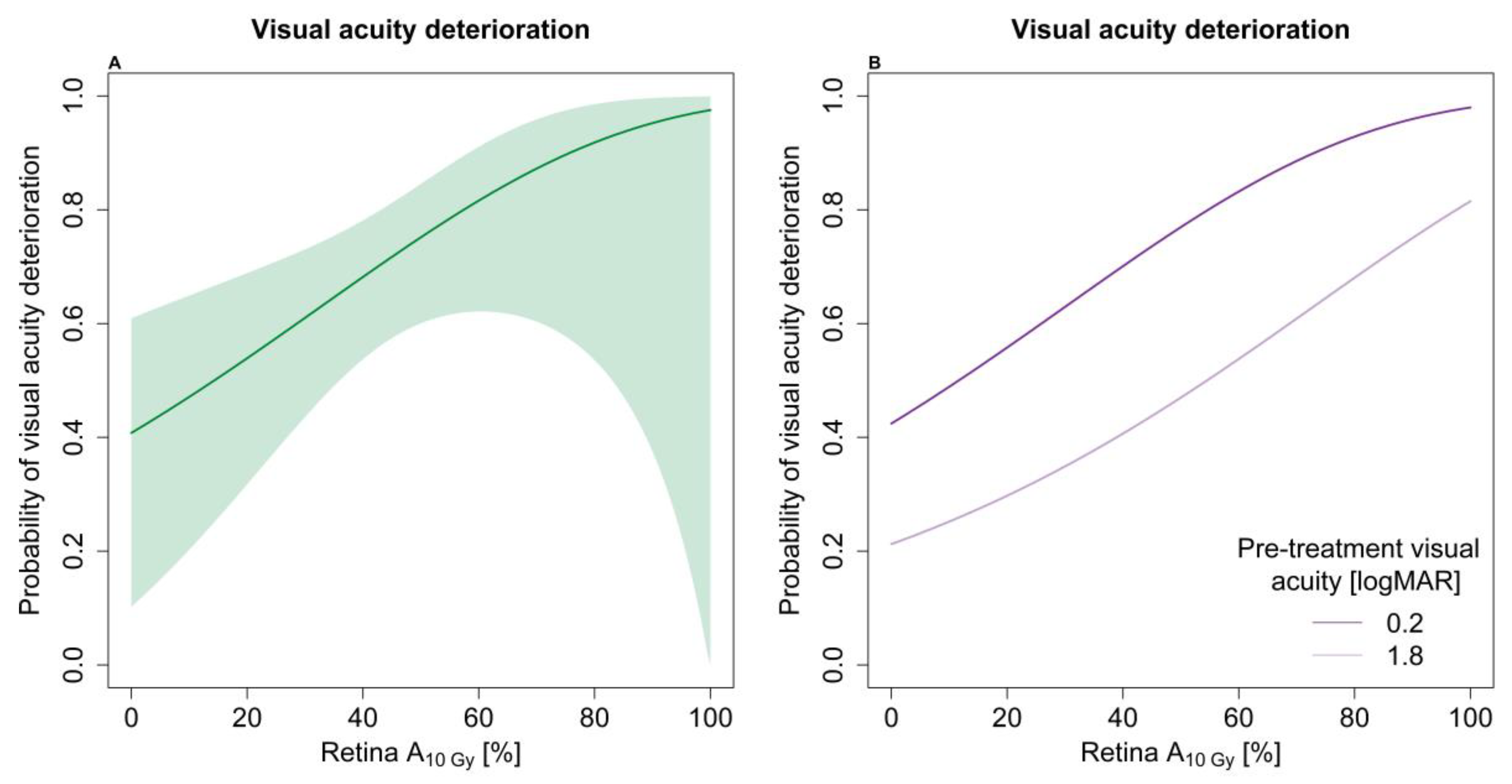
| Visual acuity deterioration | 136 (62) | 35 (29–43) |
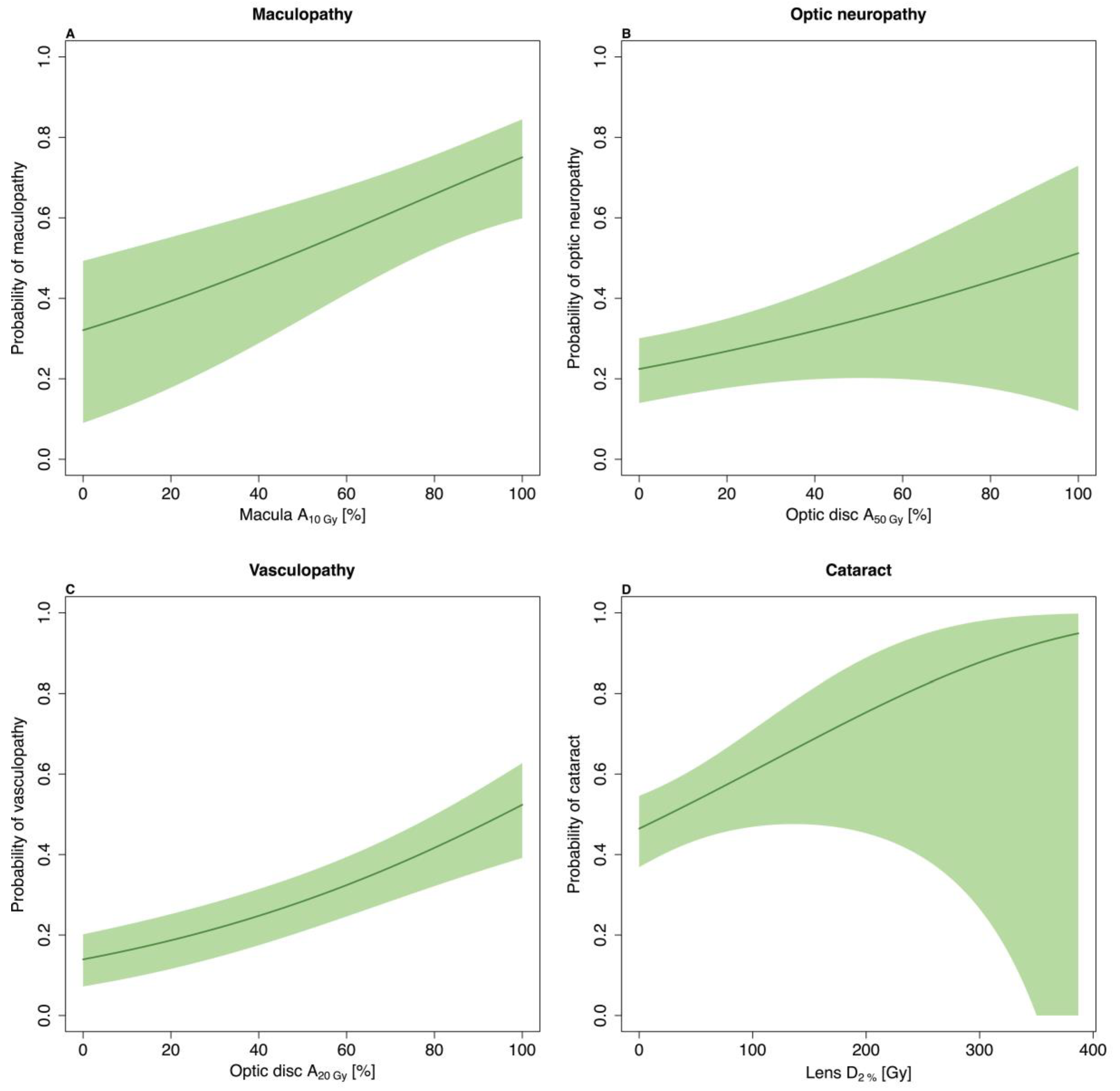
| Maculopathy | 64 (29) | 45 (36–56) |
| Optic neuropathy | 62 (28) | 68 (62–76) |
| Ocular hypertension | 26 (12) | 87 (82–92) |
| Vascular obliteration | 63 (28) | 70 (63–77) |
| Cataract | 103 (46) | 52 (45–61) |
| Retinal detachment | 15 (7) | 94 (91–97) |
| Visual Acuity Deterioration | Hazard Ratio (95% CI) |
|---|---|
| Optic disc-tumour distance + | 0.91 (0.85–0.97) |
| Pre-treatment VA (1 logMAR increase) | 0.59 (0.44–0.80) |
| Retina A10Gy * | 1.22 (1.03–1.44) |
| Macula A20Gy * | 1.04 (0.98–1.10) |
| Macula A80Gy * | 0.92 (0.62–1.38) |
| Loss of pre-treatment visual acuity | No variables selected |
| Maculopathy | |
| Optic disc–tumour distance + | 0.87 (0.79–0.96) |
| Macula A10Gy * | 1.15 (1.05–1.26) |
| Optic neuropathy | |
| Optic disc–tumour distance + | 0.75 (0.63–0.89) |
| Optic disc A50Gy * | 1.11 (1.02–1.22) |
| Optic disc A20Gy * | 1.08 (0.98–1.18) |
| Ocular hypertension (post-treatment) | No variables selected |
| Vascular obliteration | |
| Optic disc A20Gy * | 1.17 (1.11–1.25) |
| Cataract | |
| Age at treatment (10 years increase) | 1.38 (1.17–1.62) |
| Largest base dimension + | 1.08 (1.01–1.16) |
| Lens D2% (10 Gy increase) | 1.04 (1.01–1.07) |
| Retinal detachment (post-treatment) | No variables selected |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espensen, C.A.; Appelt, A.L.; Fog, L.S.; Gothelf, A.B.; Thariat, J.; Kiilgaard, J.F. Predicting Visual Acuity Deterioration and Radiation-Induced Toxicities after Brachytherapy for Choroidal Melanomas. Cancers 2019, 11, 1124. https://doi.org/10.3390/cancers11081124
Espensen CA, Appelt AL, Fog LS, Gothelf AB, Thariat J, Kiilgaard JF. Predicting Visual Acuity Deterioration and Radiation-Induced Toxicities after Brachytherapy for Choroidal Melanomas. Cancers. 2019; 11(8):1124. https://doi.org/10.3390/cancers11081124
Chicago/Turabian StyleEspensen, Charlotte A., Ane L. Appelt, Lotte S. Fog, Anita B. Gothelf, Juliette Thariat, and Jens F. Kiilgaard. 2019. "Predicting Visual Acuity Deterioration and Radiation-Induced Toxicities after Brachytherapy for Choroidal Melanomas" Cancers 11, no. 8: 1124. https://doi.org/10.3390/cancers11081124
APA StyleEspensen, C. A., Appelt, A. L., Fog, L. S., Gothelf, A. B., Thariat, J., & Kiilgaard, J. F. (2019). Predicting Visual Acuity Deterioration and Radiation-Induced Toxicities after Brachytherapy for Choroidal Melanomas. Cancers, 11(8), 1124. https://doi.org/10.3390/cancers11081124






