Novel Apoptosis-Inducing Agents for the Treatment of Cancer, a New Arsenal in the Toolbox
Abstract
1. Introduction
2. Extrinsic Pathway Targeting
2.1. TRAIL and Its Receptors
2.2. Clinical Development of Death Receptor (DR) Targeting Therapies
2.3. First Generation
2.4. Second Generation
2.5. Third Generation
2.6. Other Recent Developments
2.6.1. Mesenchymal Stem Cell-Mediated TRAIL Delivery
2.6.2. Nano Particle-Based Drug Delivery
2.6.3. CRISPR-Based TRAIL Therapy
3. Challenges and Strategies to Improve the Efficacy of DR-Targeted Therapy
3.1. Evaluation of Pharmacokinetic and Pharmacodynamic Characteristics of DR Targeting
3.2. Resistance Mechanisms to TRAIL-Induced Apoptosis
3.3. Combination Strategies
4. Intrinsic Pathway
4.1. Pro- and Anti-Apoptotic Regulators within the Pathway
4.2. B Cell Lymphoma-2 (BCL-2)
BCL-2 Inhibitors
4.3. Challenges and Strategies to Improve the Efficacy of BCL-2 Targeted Therapy
4.3.1. Toxicity of BCL-2 Inhibitors
4.3.2. Resistant Mechanisms
4.3.3. Combination Strategies
4.4. Inhibitor of Apoptosis Proteins (IAPs)
4.5. IAP Inhibitors
4.6. Challenges and Strategies to Improve the Efficacy of IAP Targeted Therapy
4.6.1. Toxicity of IAP Inhibitors
4.6.2. Resistant Mechanisms
4.6.3. Combination Strategy
4.7. Myeloid Leukemia Cell Differentiation Protein 1 (MCL1)
4.8. MCL1 Inhibitors
4.9. Challenges and Strategies to Improve the Efficacy of MCL1 Targeted Therapy
4.9.1. Toxicity of MCL1 Inhibitors
4.9.2. Resistant Mechanisms
4.9.3. Combination Strategy
5. Development of Biomarkers of Apoptosis
5.1. Biomarkers to Predict TRAIL Sensitivity
5.2. Biomarkers to Predict Sensitivity to Intrinsic Apoptosis-Targeted Agents
BH3 Profiling
5.3. Biomarkers for Pharmacodynamic and Downstream Effect
ELISA Based Protein Assay
6. Conclusions and Future Directions
Supplementary Materials
Funding
Conflicts of Interest
References
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Baehrecke, E.H. How death shapes life during development. Nat. Rev. Mol. Cell Biol. 2002, 3, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Reed, J.C. Apoptosis-based therapies. Nat. Rev. Drug Discov. 2002, 1, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Gonzalvez, F.; Ashkenazi, A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene 2010, 29, 4752–4765. [Google Scholar] [CrossRef] [PubMed]
- Galligan, L.; Longley, D.B.; McEwan, M.; Wilson, T.R.; McLaughlin, K.; Johnston, P.G. Chemotherapy and TRAIL-mediated colon cancer cell death: The roles of p53, TRAIL receptors, and c-FLIP. Mol. Cancer Ther. 2005, 4, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, T.; Mackey, M.R.; Perkins, G.; Ellisman, M.H.; Latterich, M.; Schneiter, R.; Green, D.R.; Newmeyer, D.D. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 2002, 111, 331–342. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Gong, J. Heat Shock Proteins Promote Cancer: It’s a Protection Racket. Trends Biochem. Sci. 2016, 41, 311–323. [Google Scholar] [CrossRef]
- Morishima, N.; Nakanishi, K.; Takenouchi, H.; Shibata, T.; Yasuhiko, Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 2002, 277, 34287–34294. [Google Scholar] [CrossRef]
- Iurlaro, R.; Munoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef]
- Jego, G.; Hazoume, A.; Seigneuric, R.; Garrido, C. Targeting heat shock proteins in cancer. Cancer Lett. 2013, 332, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Wiley, S.R.; Schooley, K.; Smolak, P.J.; Din, W.S.; Huang, C.P.; Nicholl, J.K.; Sutherland, G.R.; Smith, T.D.; Rauch, C.; Smith, C.A.; et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995, 3, 673–682. [Google Scholar] [CrossRef]
- Pennati, M.; Sbarra, S.; De Cesare, M.; Lopergolo, A.; Locatelli, S.L.; Campi, E.; Daidone, M.G.; Carlo-Stella, C.; Gianni, A.M.; Zaffaroni, N. YM155 sensitizes triple-negative breast cancer to membrane-bound TRAIL through p38 MAPK- and CHOP-mediated DR5 upregulation. Int. J. Cancer 2015, 136, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Hymowitz, S.G.; O’Connell, M.P.; Ultsch, M.H.; Hurst, A.; Totpal, K.; Ashkenazi, A.; de Vos, A.M.; Kelley, R.F. A unique zinc-binding site revealed by a high-resolution X-ray structure of homotrimeric Apo2L/TRAIL. Biochemistry 2000, 39, 633–640. [Google Scholar] [CrossRef]
- Degli-Esposti, M.A.; Dougall, W.C.; Smolak, P.J.; Waugh, J.Y.; Smith, C.A.; Goodwin, R.G. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 1997, 7, 813–820. [Google Scholar] [CrossRef]
- Degli-Esposti, M.A.; Smolak, P.J.; Walczak, H.; Waugh, J.; Huang, C.P.; DuBose, R.F.; Goodwin, R.G.; Smith, C.A. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J. Exp. Med. 1997, 186, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Pitti, R.M.; Marsters, S.A.; Ruppert, S.; Donahue, C.J.; Moore, A.; Ashkenazi, A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J. Biol. Chem. 1996, 271, 12687–12690. [Google Scholar] [CrossRef] [PubMed]
- Emery, J.G.; McDonnell, P.; Burke, M.B.; Deen, K.C.; Lyn, S.; Silverman, C.; Dul, E.; Appelbaum, E.R.; Eichman, C.; DiPrinzio, R.; et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J. Biol. Chem. 1998, 273, 14363–14367. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, M.; Ahmad, M.; Srinivasula, S.M.; Fernandes-Alnemri, T.; Cohen, G.M.; Alnemri, E.S. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J. Biol. Chem. 1997, 272, 25417–25420. [Google Scholar] [CrossRef] [PubMed]
- Marsters, S.A.; Sheridan, J.P.; Pitti, R.M.; Huang, A.; Skubatch, M.; Baldwin, D.; Yuan, J.; Gurney, A.; Goddard, A.D.; Godowski, P.; et al. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr. Biol. 1997, 7, 1003–1006. [Google Scholar] [CrossRef]
- Pan, G.; Ni, J.; Wei, Y.F.; Yu, G.; Gentz, R.; Dixit, V.M. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 1997, 277, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; O’Rourke, K.; Chinnaiyan, A.M.; Gentz, R.; Ebner, R.; Ni, J.; Dixit, V.M. The receptor for the cytotoxic ligand TRAIL. Science 1997, 276, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Ni, J.; Yu, G.; Wei, Y.F.; Dixit, V.M. TRUNDD, a new member of the TRAIL receptor family that antagonizes TRAIL signalling. FEBS Lett. 1998, 424, 41–45. [Google Scholar] [CrossRef]
- Schneider, P.; Bodmer, J.L.; Thome, M.; Hofmann, K.; Holler, N.; Tschopp, J. Characterization of two receptors for TRAIL. FEBS Lett. 1997, 416, 329–334. [Google Scholar] [CrossRef]
- Screaton, G.R.; Mongkolsapaya, J.; Xu, X.N.; Cowper, A.E.; McMichael, A.J.; Bell, J.I. TRICK2, a new alternatively spliced receptor that transduces the cytotoxic signal from TRAIL. Curr. Biol. 1997, 7, 693–696. [Google Scholar] [CrossRef]
- Sheridan, J.P.; Marsters, S.A.; Pitti, R.M.; Gurney, A.; Skubatch, M.; Baldwin, D.; Ramakrishnan, L.; Gray, C.L.; Baker, K.; Wood, W.I.; et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 1997, 277, 818–821. [Google Scholar] [CrossRef]
- Trauzold, A.; Wermann, H.; Arlt, A.; Schutze, S.; Schafer, H.; Oestern, S.; Roder, C.; Ungefroren, H.; Lampe, E.; Heinrich, M.; et al. CD95 and TRAIL receptor-mediated activation of protein kinase C and NF-kappaB contributes to apoptosis resistance in ductal pancreatic adenocarcinoma cells. Oncogene 2001, 20, 4258–4269. [Google Scholar] [CrossRef]
- Healy, S.; O’Leary, L.; Szegezdi, E. An added dimension to tumour TRAIL sensitivity. Oncoscience 2015, 2, 906–907. [Google Scholar] [CrossRef][Green Version]
- Riccioni, R.; Pasquini, L.; Mariani, G.; Saulle, E.; Rossini, A.; Diverio, D.; Pelosi, E.; Vitale, A.; Chierichini, A.; Cedrone, M.; et al. TRAIL decoy receptors mediate resistance of acute myeloid leukemia cells to TRAIL. Haematologica 2005, 90, 612–624. [Google Scholar]
- Van Dijk, M.; Halpin-McCormick, A.; Sessler, T.; Samali, A.; Szegezdi, E. Resistance to TRAIL in non-transformed cells is due to multiple redundant pathways. Cell Death Dis. 2013, 4, e702. [Google Scholar] [CrossRef]
- Jouan-Lanhouet, S.; Arshad, M.I.; Piquet-Pellorce, C.; Martin-Chouly, C.; Le Moigne-Muller, G.; Van Herreweghe, F.; Takahashi, N.; Sergent, O.; Lagadic-Gossmann, D.; Vandenabeele, P.; et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012, 19, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Kemp, T.J.; Kim, J.S.; Crist, S.A.; Griffith, T.S. Induction of necrotic tumor cell death by TRAIL/Apo-2L. Apoptosis 2003, 8, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Meurette, O.; Huc, L.; Rebillard, A.; Le Moigne, G.; Lagadic-Gossmann, D.; Dimanche-Boitrel, M.T. TRAIL (TNF-related apoptosis-inducing ligand) induces necrosis-like cell death in tumor cells at acidic extracellular pH. Ann. N. Y. Acad. Sci. 2005, 1056, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Meurette, O.; Rebillard, A.; Huc, L.; Le Moigne, G.; Merino, D.; Micheau, O.; Lagadic-Gossmann, D.; Dimanche-Boitrel, M.T. TRAIL induces receptor-interacting protein 1-dependent and caspase-dependent necrosis-like cell death under acidic extracellular conditions. Cancer Res. 2007, 67, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Azijli, K.; Weyhenmeyer, B.; Peters, G.J.; de Jong, S.; Kruyt, F.A. Non-canonical kinase signaling by the death ligand TRAIL in cancer cells: Discord in the death receptor family. Cell Death Differ. 2013, 20, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Screaton, G.; Xu, X.N. T cell life and death signalling via TNF-receptor family members. Curr. Opin. Immunol. 2000, 12, 316–322. [Google Scholar] [CrossRef]
- Falschlehner, C.; Schaefer, U.; Walczak, H. Following TRAIL’s path in the immune system. Immunology 2009, 127, 145–154. [Google Scholar] [CrossRef]
- Martinez-Lostao, L.; Marzo, I.; Anel, A.; Naval, J. Targeting the Apo2L/TRAIL system for the therapy of autoimmune diseases and cancer. Biochem. Pharmacol. 2012, 83, 1475–1483. [Google Scholar] [CrossRef]
- Cretney, E.; McQualter, J.L.; Kayagaki, N.; Yagita, H.; Bernard, C.C.; Grewal, I.S.; Ashkenazi, A.; Smyth, M.J. TNF-related apoptosis-inducing ligand (TRAIL)/Apo2L suppresses experimental autoimmune encephalomyelitis in mice. Immunol. Cell Biol. 2005, 83, 511–519. [Google Scholar] [CrossRef]
- Lamhamedi-Cherradi, S.E.; Zheng, S.J.; Maguschak, K.A.; Peschon, J.; Chen, Y.H. Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL-/-mice. Nat. Immunol. 2003, 4, 255–260. [Google Scholar] [CrossRef]
- Song, K.; Chen, Y.; Goke, R.; Wilmen, A.; Seidel, C.; Goke, A.; Hilliard, B.; Chen, Y. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression. J. Exp. Med. 2000, 191, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Cretney, E.; Takeda, K.; Yagita, H.; Glaccum, M.; Peschon, J.J.; Smyth, M.J. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J. Immunol. 2002, 168, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Frazzette, N.; Cruz, A.C.; Klebanoff, C.A.; Siegel, R.M. Beyond Cell Death: New Functions for TNF Family Cytokines in Autoimmunity and Tumor Immunotherapy. Trends Mol. Med. 2018, 24, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Pumphrey, J.G.; Lipkowitz, S. The TRAIL to targeted therapy of breast cancer. Adv. Cancer Res. 2009, 103, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Lemke, J.; von Karstedt, S.; Zinngrebe, J.; Walczak, H. Getting TRAIL back on track for cancer therapy. Cell Death Differ. 2014, 21, 1350–1364. [Google Scholar] [CrossRef] [PubMed]
- Grosse-Wilde, A.; Voloshanenko, O.; Bailey, S.L.; Longton, G.M.; Schaefer, U.; Csernok, A.I.; Schutz, G.; Greiner, E.F.; Kemp, C.J.; Walczak, H. TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development. J. Clin. Invest. 2008, 118, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.T.; Yue, P.; Wang, D.; Tong, J.S.; Chen, Z.G.; Khuri, F.R.; Sun, S.Y. Suppression of death receptor 5 enhances cancer cell invasion and metastasis through activation of caspase-8/TRAF2-mediated signaling. Oncotarget 2015, 6, 41324–41338. [Google Scholar] [CrossRef]
- Greer, Y.E.; Gilbert, S.F.; Gril, B.; Narwal, R.; Peacock Brooks, D.L.; Tice, D.A.; Steeg, P.S.; Lipkowitz, S. MEDI3039, a novel highly potent tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) receptor 2 agonist, causes regression of orthotopic tumors and inhibits outgrowth of metastatic triple-negative breast cancer. Breast Cancer Res. 2019, 21, 27. [Google Scholar] [CrossRef]
- Rossini, A.; Giussani, M.; Giacomini, A.; Guarnotta, C.; Tagliabue, E.; Balsari, A. Surveillance of spontaneous breast cancer metastasis by TRAIL-expressing CD34(+) cells in a xenograft model. Breast Cancer Res. Treat. 2012, 136, 457–467. [Google Scholar] [CrossRef]
- Rahman, M.; Davis, S.R.; Pumphrey, J.G.; Bao, J.; Nau, M.M.; Meltzer, P.S.; Lipkowitz, S. TRAIL induces apoptosis in triple-negative breast cancer cells with a mesenchymal phenotype. Breast Cancer Res. Treat. 2009, 113, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Dufour, F.; Rattier, T.; Constantinescu, A.A.; Zischler, L.; Morle, A.; Ben Mabrouk, H.; Humblin, E.; Jacquemin, G.; Szegezdi, E.; Delacote, F.; et al. TRAIL receptor gene editing unveils TRAIL-R1 as a master player of apoptosis induced by TRAIL and ER stress. Oncotarget 2017, 8, 9974–9985. [Google Scholar] [CrossRef] [PubMed]
- Malin, D.; Chen, F.; Schiller, C.; Koblinski, J.; Cryns, V.L. Enhanced metastasis suppression by targeting TRAIL receptor 2 in a murine model of triple-negative breast cancer. Clin. Cancer Res. 2011, 17, 5005–5015. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.H.; Tsai, H.F.; Lin, L.L.; Hsieh, S.L.; Hsu, P.I.; Hsu, P.N. Enhanced proliferation and increased IFN-gamma production in T cells by signal transduced through TNF-related apoptosis-inducing ligand. J. Immunol. 2001, 167, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, H.; Heilmann, T.; Tower, R.J.; Hauser, C.; von Au, A.; El-Sheikh, D.; Campbell, G.M.; Alp, G.; Schewe, D.; Hubner, S.; et al. TRAIL-R2 promotes skeletal metastasis in a breast cancer xenograft mouse model. Oncotarget 2015, 6, 9502–9516. [Google Scholar] [CrossRef] [PubMed]
- Von Karstedt, S.; Conti, A.; Nobis, M.; Montinaro, A.; Hartwig, T.; Lemke, J.; Legler, K.; Annewanter, F.; Campbell, A.D.; Taraborrelli, L.; et al. Cancer cell-autonomous TRAIL-R signaling promotes KRAS-driven cancer progression, invasion, and metastasis. Cancer Cell 2015, 27, 561–573. [Google Scholar] [CrossRef]
- Lalaoui, N.; Silke, J. Jekyll & Hyde: The Other Life of the Death Ligand TRAIL. Mol. Cell 2017, 65, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.M.; Martin, S.J. Caspase-8 Acts in a Non-enzymatic Role as a Scaffold for Assembly of a Pro-inflammatory “FADDosome” Complex upon TRAIL Stimulation. Mol. Cell 2017, 65, 715–729.e5. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, T.; Montinaro, A.; von Karstedt, S.; Sevko, A.; Surinova, S.; Chakravarthy, A.; Taraborrelli, L.; Draber, P.; Lafont, E.; Arce Vargas, F.; et al. The TRAIL-Induced Cancer Secretome Promotes a Tumor-Supportive Immune Microenvironment via CCR2. Mol. Cell 2017, 65, 730–742.e5. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Dixit, V.M. Apoptosis control by death and decoy receptors. Curr. Opin. Cell Biol. 1999, 11, 255–260. [Google Scholar] [CrossRef]
- Walczak, H.; Miller, R.E.; Ariail, K.; Gliniak, B.; Griffith, T.S.; Kubin, M.; Chin, W.; Jones, J.; Woodward, A.; Le, T.; et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat. Med. 1999, 5, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Pai, R.C.; Fong, S.; Leung, S.; Lawrence, D.A.; Marsters, S.A.; Blackie, C.; Chang, L.; McMurtrey, A.E.; Hebert, A.; et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Invest. 1999, 104, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Vose, J.M.; Zelenetz, A.D.; Smith, M.R.; Burris, H.A.; Ansell, S.M.; Klein, J.; Halpern, W.; Miceli, R.; Kumm, E.; et al. A Phase 1b/2 trial of mapatumumab in patients with relapsed/refractory non-Hodgkin’s lymphoma. Br. J. Cancer 2010, 103, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Herbst, R.S.; Gordon, M.S.; Eckhardt, S.G.; Kurzrock, R.; Durbin, B.; Ing, J.; Tohnya, T.M.; Sager, J.; Ashkenazi, A.; et al. A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin. Cancer Res. 2010, 16, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Rocha Lima, C.M.; Bayraktar, S.; Flores, A.M.; MacIntyre, J.; Montero, A.; Baranda, J.C.; Wallmark, J.; Portera, C.; Raja, R.; Stern, H.; et al. Phase Ib study of drozitumab combined with first-line mFOLFOX6 plus bevacizumab in patients with metastatic colorectal cancer. Cancer Invest. 2012, 30, 727–731. [Google Scholar] [CrossRef]
- Herbst, R.S.; Kurzrock, R.; Hong, D.S.; Valdivieso, M.; Hsu, C.P.; Goyal, L.; Juan, G.; Hwang, Y.C.; Wong, S.; Hill, J.S.; et al. A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin. Cancer Res. 2010, 16, 5883–5891. [Google Scholar] [CrossRef] [PubMed]
- Kindler, H.L.; Richards, D.A.; Garbo, L.E.; Garon, E.B.; Stephenson, J.J., Jr.; Rocha-Lima, C.M.; Safran, H.; Chan, D.; Kocs, D.M.; Galimi, F.; et al. A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann. Oncol. 2012, 23, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.S.; Geller, J.I.; Baird, K.; Chou, A.J.; Galli, S.; Charles, A.; Amaoko, M.; Rhee, E.H.; Price, A.; Wexler, L.H.; et al. Phase I trial and pharmacokinetic study of lexatumumab in pediatric patients with solid tumors. J. Clin. Oncol. 2012, 30, 4141–4147. [Google Scholar] [CrossRef] [PubMed]
- Forero-Torres, A.; Varley, K.E.; Abramson, V.; Li, Y.; Vaklavas, C.; Lin, N.U.; Liu, M.C.; Rugo, H.S.; Nanda, R.; Stroniolo, A.M.; et al. TBCRC 019: Phase II trial of nab-PAC with/without the anti-death receptor 5 monoclonal antibody tigatuzumab in patients with triple negative breast cancer. Clin. Cancer Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; de Vries, E.G.; Infante, J.R.; Oldenhuis, C.N.; Gietema, J.A.; Yang, L.; Bilic, S.; Parker, K.; Goldbrunner, M.; Scott, J.W.; et al. Safety, pharmacokinetics, and pharmacodynamics of the DR5 antibody LBY135 alone and in combination with capecitabine in patients with advanced solid tumors. Invest. New Drugs 2014, 32, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Mark, Z.; Zatloukal, P.; Szima, B.; Albert, I.; Juhasz, E.; Pujol, J.L.; Kozielski, J.; Baker, N.; Smethurst, D.; et al. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J. Clin. Oncol. 2011, 29, 4442–4451. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Smit, E.; Khayat, D.; Besse, B.; Yang, X.; Hsu, C.P.; Reese, D.; Wiezorek, J.; Blackhall, F. Phase 1b study of dulanermin (recombinant human Apo2L/TRAIL) in combination with paclitaxel, carboplatin, and bevacizumab in patients with advanced non-squamous non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Cheah, C.Y.; Belada, D.; Fanale, M.A.; Janikova, A.; Czucman, M.S.; Flinn, I.W.; Kapp, A.V.; Ashkenazi, A.; Kelley, S.; Bray, G.L.; et al. Dulanermin with rituximab in patients with relapsed indolent B-cell lymphoma: An open-label phase 1b/2 randomised study. Lancet Haematol. 2015, 2, e166–e174. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, R.; Peach, M.; Huang, C.P.; Branstetter, D.; Novotny, W.; Herbst, R.S.; Eckhardt, S.G.; Holland, P.M. Evaluation of pharmacodynamic biomarkers in a Phase 1a trial of dulanermin (rhApo2L/TRAIL) in patients with advanced tumours. Br. J. Cancer 2011, 105, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, Z.A.; Messersmith, W.A.; Peddi, P.F.; Kapp, A.V.; Ashkenazi, A.; Royer-Joo, S.; Portera, C.C.; Kozloff, M.F. A phase 1B study of dulanermin in combination with modified FOLFOX6 plus bevacizumab in patients with metastatic colorectal cancer. Clin. Colorectal. Cancer 2013, 12, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Pukac, L.; Kanakaraj, P.; Humphreys, R.; Alderson, R.; Bloom, M.; Sung, C.; Riccobene, T.; Johnson, R.; Fiscella, M.; Mahoney, A.; et al. HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumour types in vitro and in vivo. Br. J. Cancer 2005, 92, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Tolcher, A.W.; Mita, M.; Meropol, N.J.; von Mehren, M.; Patnaik, A.; Padavic, K.; Hill, M.; Mays, T.; McCoy, T.; Fox, N.L.; et al. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J. Clin. Oncol. 2007, 25, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Hotte, S.J.; Hirte, H.W.; Chen, E.X.; Siu, L.L.; Le, L.H.; Corey, A.; Iacobucci, A.; MacLean, M.; Lo, L.; Fox, N.L.; et al. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin. Cancer Res. 2008, 14, 3450–3455. [Google Scholar] [CrossRef]
- Mom, C.H.; Verweij, J.; Oldenhuis, C.N.; Gietema, J.A.; Fox, N.L.; Miceli, R.; Eskens, F.A.; Loos, W.J.; de Vries, E.G.; Sleijfer, S. Mapatumumab, a fully human agonistic monoclonal antibody that targets TRAIL-R1, in combination with gemcitabine and cisplatin: A phase I study. Clin. Cancer Res. 2009, 15, 5584–5590. [Google Scholar] [CrossRef]
- Greco, F.A.; Bonomi, P.; Crawford, J.; Kelly, K.; Oh, Y.; Halpern, W.; Lo, L.; Gallant, G.; Klein, J. Phase 2 study of mapatumumab, a fully human agonistic monoclonal antibody which targets and activates the TRAIL receptor-1, in patients with advanced non-small cell lung cancer. Lung Cancer 2008, 61, 82–90. [Google Scholar] [CrossRef]
- Von Pawel, J.; Harvey, J.H.; Spigel, D.R.; Dediu, M.; Reck, M.; Cebotaru, C.L.; Humphreys, R.C.; Gribbin, M.J.; Fox, N.L.; Camidge, D.R. Phase II Trial of Mapatumumab, a Fully Human Agonist Monoclonal Antibody to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Receptor 1 (TRAIL-R1), in Combination With Paclitaxel and Carboplatin in Patients With Advanced Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2014, 15, 188–196.e2. [Google Scholar] [CrossRef] [PubMed]
- Trarbach, T.; Moehler, M.; Heinemann, V.; Kohne, C.H.; Przyborek, M.; Schulz, C.; Sneller, V.; Gallant, G.; Kanzler, S. Phase II trial of mapatumumab, a fully human agonistic monoclonal antibody that targets and activates the tumour necrosis factor apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with refractory colorectal cancer. Br. J. Cancer 2010, 102, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Ciuleanu, T.; Bazin, I.; Lungulescu, D.; Miron, L.; Bondarenko, I.; Deptala, A.; Rodriguez-Torres, M.; Giantonio, B.; Fox, N.L.; Wissel, P.; et al. A randomized, double-blind, placebo-controlled phase II study to assess the efficacy and safety of mapatumumab with sorafenib in patients with advanced hepatocellular carcinoma. Ann. Oncol. 2016, 27, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.; Totpal, K.; Lawrence, D.; Marsters, S.; Pitti, R.; Yee, S.; Ross, S.; Deforge, L.; Koeppen, H.; Sagolla, M.; et al. Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5. Cell Death Differ. 2008, 15, 751–761. [Google Scholar] [CrossRef]
- Kaplan-Lefko, P.J.; Graves, J.D.; Zoog, S.J.; Pan, Y.; Wall, J.; Branstetter, D.G.; Moriguchi, J.; Coxon, A.; Huard, J.N.; Xu, R.; et al. Conatumumab, a fully human agonist antibody to death receptor 5, induces apoptosis via caspase activation in multiple tumor types. Cancer Biol. Ther. 2010, 9, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wu, X.X.; Fiscella, M.; Shimada, O.; Humphreys, R.; Albert, V.; Kakehi, Y. Monoclonal antibody to tumor necrosis factor-related apoptosis-inducing ligand receptor 2 (TRAIL-R2) induces apoptosis in primary renal cell carcinoma cells in vitro and inhibits tumor growth in vivo. Int. J. Oncol. 2006, 28, 421–430. [Google Scholar] [CrossRef]
- Plummer, R.; Attard, G.; Pacey, S.; Li, L.; Razak, A.; Perrett, R.; Barrett, M.; Judson, I.; Kaye, S.; Fox, N.L.; et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin. Cancer Res. 2007, 13, 6187–6194. [Google Scholar] [CrossRef]
- Wakelee, H.A.; Patnaik, A.; Sikic, B.I.; Mita, M.; Fox, N.L.; Miceli, R.; Ullrich, S.J.; Fisher, G.A.; Tolcher, A.W. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann. Oncol. 2010, 21, 376–381. [Google Scholar] [CrossRef]
- Sikic, B.; Wakelee, H.; von Mehren, M.; Lewis, N.; Plummer, E.R.; Calvert, A.H.; Fox, N.L.; Kumm, E.; Jones, S.; Burris, H. A Phase 1b study to assess the safety of lexatumumab, a human monoclonal antibody that activates TRAIL-R2, in combination with gemcitabine, pemetrexed, doxorubicin or FOLFIRI. Mol. Target. Cancer Ther. 2007. [Google Scholar] [CrossRef]
- HGS-TR2J. Available online: https://adisinsight.springer.com/drugs/800021611 (accessed on 9 May 2019).
- Humphreys, R.; Poortman, C.; McCormick, K.; Lincoln, C.; Halpern, W.; Moore, P.; Fikes, J.; Albert, V. HGS-TR2J, an agonistic, TRAIL receptor 2 monoclonal antibody, actively and rapidly stimulates the TRAIL receptor pathway that leads to significant inhibition of tumor growth in human tumor cell lines in vitro and in vivo. Proc. Amer. Assoc. Cancer Res. 2005, 46, 1430–1441. [Google Scholar]
- Yada, A.; Yazawa, M.; Ishida, S.; Yoshida, H.; Ichikawa, K.; Kurakata, S.; Fujiwara, K. A novel humanized anti-human death receptor 5 antibody CS-1008 induces apoptosis in tumor cells without toxicity in hepatocytes. Ann. Oncol. 2008, 19, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Forero-Torres, A.; Shah, J.; Wood, T.; Posey, J.; Carlisle, R.; Copigneaux, C.; Luo, F.R.; Wojtowicz-Praga, S.; Percent, I.; Saleh, M. Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5). Cancer Biother. Radiopharm. 2010, 25, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Krzakowski, M.; Chmielowska, E.; Sebastian, M.; Hadler, D.; Fox, T.; Wang, Q.; Greenberg, J.; Beckman, R.A.; von Pawel, J. A randomized, double-blind, placebo-controlled phase 2 study of tigatuzumab (CS-1008) in combination with carboplatin/paclitaxel in patients with chemotherapy-naive metastatic/unresectable non-small cell lung cancer. Lung Cancer 2013, 82, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Forero-Torres, A.; Infante, J.R.; Waterhouse, D.; Wong, L.; Vickers, S.; Arrowsmith, E.; He, A.R.; Hart, L.; Trent, D.; Wade, J.; et al. Phase 2, multicenter, open-label study of tigatuzumab (CS-1008), a humanized monoclonal antibody targeting death receptor 5, in combination with gemcitabine in chemotherapy-naive patients with unresectable or metastatic pancreatic cancer. Cancer Med. 2013, 2, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Kang, Y.K.; He, A.R.; Lim, H.Y.; Ryoo, B.Y.; Hung, C.H.; Sheen, I.S.; Izumi, N.; Austin, T.; Wang, Q.; et al. Safety and efficacy of tigatuzumab plus sorafenib as first-line therapy in subjects with advanced hepatocellular carcinoma: A phase 2 randomized study. J. Hepatol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.S.; Yang, B.; Yang, A.; Loeser, S.; Marsters, S.; Lawrence, D.; Li, Y.; Pitti, R.; Totpal, K.; Yee, S.; et al. An Fcgamma receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell 2011, 19, 101–113. [Google Scholar] [CrossRef]
- Stadel, D.; Mohr, A.; Ref, C.; MacFarlane, M.; Zhou, S.; Humphreys, R.; Bachem, M.; Cohen, G.; Moller, P.; Zwacka, R.M.; et al. TRAIL-induced apoptosis is preferentially mediated via TRAIL receptor 1 in pancreatic carcinoma cells and profoundly enhanced by XIAP inhibitors. Clin. Cancer Res. 2010, 16, 5734–5749. [Google Scholar] [CrossRef]
- Weiner, L.M.; Surana, R.; Wang, S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 2010, 10, 317–327. [Google Scholar] [CrossRef]
- Morgan-Lappe, S.E. ABBV-621: A best-in-class TRAIL-receptor agonist fusion protein that enhances optimal clustering for the treatment of solid and hematologic tumors. Proc. AACR Annu. Meet. 2017, 77. [Google Scholar] [CrossRef]
- Gieffers, C.; Kluge, M.; Merz, C.; Sykora, J.; Thiemann, M.; Schaal, R.; Fischer, C.; Branschadel, M.; Abhari, B.A.; Hohenberger, P.; et al. APG350 induces superior clustering of TRAIL receptors and shows therapeutic antitumor efficacy independent of cross-linking via Fcgamma receptors. Mol. Cancer Ther. 2013, 12, 2735–2747. [Google Scholar] [CrossRef]
- Legler, K.; Hauser, C.; Egberts, J.H.; Willms, A.; Heneweer, C.; Boretius, S.; Rocken, C.; Gluer, C.C.; Becker, T.; Kluge, M.; et al. The novel TRAIL-receptor agonist APG350 exerts superior therapeutic activity in pancreatic cancer cells. Cell Death Dis. 2018, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Brunker, P.; Wartha, K.; Friess, T.; Grau-Richards, S.; Waldhauer, I.; Koller, C.F.; Weiser, B.; Majety, M.; Runza, V.; Niu, H.; et al. RG7386, a Novel Tetravalent FAP-DR5 Antibody, Effectively Triggers FAP-Dependent, Avidity-Driven DR5 Hyperclustering and Tumor Cell Apoptosis. Mol. Cancer Ther. 2016, 15, 946–957. [Google Scholar] [CrossRef]
- Friess, T.; Broeske, A.M.; Lechner, S.; Abraham, E.; Hoelzlwimmer, G.; Sade, H.; Bruenker, P.; Krieter, O. Preclinical pharmacodynamic biomarker and combination strategy of RG7386, a novel FAP-DR5 bispecific antibody for targeting solid tumors. Mol. Cancer. Ther. 2015, 14. [Google Scholar] [CrossRef]
- Bendell, J.; Blay, J.Y.; Cassier, P.; Bauer, T.; Terret, C.; Mueller, C.; Morel, A.; Chesne, E.; Xu, Z.X.; Tessier, J.; et al. Abstract A092: Phase 1 trial of RO6874813, a novel bispecific FAP-DR5 antibody, in patients with solid tumors. Mol. Cancer Ther. 2018, 17. [Google Scholar] [CrossRef]
- RG7386/RO6874813. Available online: https://adisinsight.springer.com/drugs/800043294 (accessed on 9 May 2019).
- Papadopoulos, K.P.; Isaacs, R.; Bilic, S.; Kentsch, K.; Huet, H.A.; Hofmann, M.; Rasco, D.; Kundamal, N.; Tang, Z.; Cooksey, J.; et al. Unexpected hepatotoxicity in a phase I study of TAS266, a novel tetravalent agonistic Nanobody((R)) targeting the DR5 receptor. Cancer Chemother. Pharmacol. 2015, 75, 887–895. [Google Scholar] [CrossRef]
- Kolluri, K.K.; Alifrangis, C.; Kumar, N.; Ishii, Y.; Price, S.; Michaut, M.; Williams, S.; Barthorpe, S.; Lightfoot, H.; Busacca, S.; et al. Loss of functional BAP1 augments sensitivity to TRAIL in cancer cells. Elife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Swers, J.S.; Grinberg, L.; Wang, L.; Feng, H.; Lekstrom, K.; Carrasco, R.; Xiao, Z.; Inigo, I.; Leow, C.C.; Wu, H.; et al. Multivalent scaffold proteins as superagonists of TRAIL receptor 2-induced apoptosis. Mol. Cancer Ther. 2013, 12, 1235–1244. [Google Scholar] [CrossRef]
- Xie, B.; Tomaszewski, M.R.; Neves, A.A.; Ros, S.; Hu, D.E.; McGuire, S.; Mullins, S.R.; Tice, D.; Sainson, R.C.A.; Bohndiek, S.E.; et al. Optoacoustic Detection of Early Therapy-Induced Tumor Cell Death Using a Targeted Imaging Agent. Clin. Cancer Res. 2017, 23, 6893–6903. [Google Scholar] [CrossRef]
- Leahy, D.J.; Hendrickson, W.A.; Aukhil, I.; Erickson, H.P. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Science 1992, 258, 987–991. [Google Scholar] [CrossRef]
- Greer, Y.E.; Tice, D.; Lipkowitz, S. MEDI3039, a novel highly potent tumor necrosis factor (TNF)-related apoptosis inducing ligand (TRAIL) receptor agonist, induces apoptotic cell death in breast cancer cells. Cancer Res. 2016, 76. [Google Scholar] [CrossRef]
- De Jong, R.N.; Beurskens, F.J.; Verploegen, S.; Strumane, K.; van Kampen, M.D.; Voorhorst, M.; Horstman, W.; Engelberts, P.J.; Oostindie, S.C.; Wang, G.; et al. A Novel Platform for the Potentiation of Therapeutic Antibodies Based on Antigen-Dependent Formation of IgG Hexamers at the Cell Surface. PLoS Biol. 2016, 14, e1002344. [Google Scholar] [CrossRef] [PubMed]
- Van der Horst, H.J.; Overdijk, M.B.; Breij, E.C.W.; Chamuleau, M.; Lokhorst, H.M.; Mutis, T. Potent Ex Vivo Anti-Tumor Activity in Relapsed Refractory Multiple Myeloma Using Novel DR5-Specific Antibodies with Enhanced Capacity to Form Hexamers upon Target Binding. Blood 2017, 130, 1835. [Google Scholar]
- Leng, Y.; Qiu, L.; Hou, J.; Zhao, Y.; Zhang, X.; Yang, S.; Xi, H.; Huang, Z.; Pan, L.; Chen, W. Phase II open-label study of recombinant circularly permuted TRAIL as a single-agent treatment for relapsed or refractory multiple myeloma. Chin. J. Cancer 2016, 35, 86. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Qiu, L.; Zhao, Y.; Zhang, X.; Liu, Y.; Wang, Z.; Zhou, F.; Leng, Y.; Yang, S.; Xi, H.; et al. A Phase1b Dose Escalation Study of Recombinant Circularly Permuted TRAIL in Patients With Relapsed or Refractory Multiple Myeloma. Am. J. Clin. Oncol. 2018, 41, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Hou, J.; Zhao, Y.; Ke, X.; Wang, Z.; Qiu, L.; Xi, H.; Wang, F.; Wei, N.; Liu, Y.; et al. A multicenter, open-label phase II study of recombinant CPT (Circularly Permuted TRAIL) plus thalidomide in patients with relapsed and refractory multiple myeloma. Am. J. Hematol. 2014, 89, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Hou, J.; Jin, J.; Zhang, M.; Ke, X.; Jiang, B.; Pan, L.; Yang, L.; Zhou, F.; Wang, J.; et al. Circularly permuted TRAIL plus thalidomide and dexamethasone versus thalidomide and dexamethasone for relapsed/refractory multiple myeloma: A phase 2 study. Cancer Chemother. Pharmacol. 2017, 79, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E.; Krigsfeld, G.; Mayes, P.A.; Patel, L.; Dicker, D.T.; Patel, A.S.; Dolloff, N.G.; Messaris, E.; Scata, K.A.; Wang, W.; et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci. Transl. Med. 2013, 5, 171ra117. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E.; Krigsfeld, G.; Patel, L.; Mayes, P.A.; Dicker, D.T.; Wu, G.S.; El-Deiry, W.S. Identification of TRAIL-inducing compounds highlights small molecule ONC201/TIC10 as a unique anti-cancer agent that activates the TRAIL pathway. Mol. Cancer 2015, 14, 99. [Google Scholar] [CrossRef]
- Jacob, N.T.; Lockner, J.W.; Kravchenko, V.V.; Janda, K.D. Pharmacophore reassignment for induction of the immunosurveillance cytokine TRAIL. Angew. Chem. Int. Ed. Engl. 2014, 53, 6628–6631. [Google Scholar] [CrossRef]
- Allen, J.E.; Prabhu, V.V.; Talekar, M.; van den Heuvel, A.P.; Lim, B.; Dicker, D.T.; Fritz, J.L.; Beck, A.; El-Deiry, W.S. Genetic and Pharmacological Screens Converge in Identifying FLIP, BCL2, and IAP Proteins as Key Regulators of Sensitivity to the TRAIL-Inducing Anticancer Agent ONC201/TIC10. Cancer Res. 2015, 75, 1668–1674. [Google Scholar] [CrossRef]
- Talekar, M.K.; Allen, J.E.; Dicker, D.T.; El-Deiry, W.S. ONC201 Induces Cell Death in Pediatric non-Hodgkin’s Lymphoma Cells. Cell Cycle 2015. [Google Scholar] [CrossRef] [PubMed]
- Greer, Y.E.; Porat-Shliom, N.; Nagashima, K.; Stuelten, C.; Crooks, D.; Koparde, V.N.; Gilbert, S.F.; Islam, C.; Ubaldini, A.; Ji, Y.; et al. ONC201 kills breast cancer cells in vitro by targeting mitochondria. Oncotarget 2018, 9, 18454–18479. [Google Scholar] [CrossRef] [PubMed]
- Kline, C.L.; Van den Heuvel, A.P.; Allen, J.E.; Prabhu, V.V.; Dicker, D.T.; El-Deiry, W.S. ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2alpha kinases. Sci. Signal. 2016, 9, ra18. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, J.; Kojima, K.; Chachad, D.; Ruvolo, P.; Ruvolo, V.; Jacamo, R.O.; Borthakur, G.; Mu, H.; Zeng, Z.; Tabe, Y.; et al. ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Sci. Signal. 2016, 9, ra17. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Kline, C.L.; Zhou, L.; Campbell, K.S.; MacFarlane, A.W.; Olszanski, A.J.; Cai, K.Q.; Hensley, H.H.; Ross, E.A.; Ralff, M.D.; et al. Dose intensification of TRAIL-inducing ONC201 inhibits metastasis and promotes intratumoral NK cell recruitment. J. Clin. Invest. 2018, 128, 2325–2338. [Google Scholar] [CrossRef] [PubMed]
- Endo Greer, Y.; Lipkowitz, S. ONC201: Stressing tumors to death. Sci. Signal. 2016, 9, fs1. [Google Scholar] [CrossRef] [PubMed]
- Greer, Y.E.; Lipkowitz, S. TIC10/ONC201: A bend in the road to clinical development. Oncoscience 2015, 2, 75–76. [Google Scholar] [CrossRef]
- Stein, M.N.; Bertino, J.R.; Kaufman, H.L.; Mayer, T.; Moss, R.; Silk, A.; Chan, N.; Malhotra, J.; Rodriguez, L.; Aisner, J.; et al. First-in-Human Clinical Trial of Oral ONC201 in Patients with Refractory Solid Tumors. Clin. Cancer Res. 2017, 23, 4163–4169. [Google Scholar] [CrossRef]
- Arrillaga-Romany, I.; Chi, A.S.; Allen, J.E.; Oster, W.; Wen, P.Y.; Batchelor, T.T. A phase 2 study of the first imipridone ONC201, a selective DRD2 antagonist for oncology, administered every three weeks in recurrent glioblastoma. Oncotarget 2017, 8, 79298–79304. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Yu, H.; Wei, S.; Williams, N.; Holmes, D.L.; Halfmann, R.; Naidoo, J.; Wang, L.; Li, L.; et al. Small-molecule activation of the TRAIL receptor DR5 in human cancer cells. Nat. Chem. Biol. 2013, 9, 84–89. [Google Scholar] [CrossRef]
- Ramdasi, S.; Sarang, S.; Viswanathan, C. Potential of Mesenchymal Stem Cell based application in Cancer. Int. J. Hematol. Oncol. Stem Cell Res. 2015, 9, 95–103. [Google Scholar] [PubMed]
- Kim, N.; Cho, S.G. Clinical applications of mesenchymal stem cells. Korean J. Intern. Med. 2013, 28, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Grisendi, G.; Spano, C.; D’Souza, N.; Rasini, V.; Veronesi, E.; Prapa, M.; Petrachi, T.; Piccinno, S.; Rossignoli, F.; Burns, J.S.; et al. Mesenchymal progenitors expressing TRAIL induce apoptosis in sarcomas. Stem Cells 2015, 33, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Peng, R.; Leng, W.; Jia, R.; Zeng, X.; Yang, X.; Fan, M. TRAIL-expressing gingival-derived mesenchymal stem cells inhibit tumorigenesis of tongue squamous cell carcinoma. J. Dent. Res. 2015, 94, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Bagci-Onder, T.; Du, W.; Figueiredo, J.L.; Martinez-Quintanilla, J.; Shah, K. Targeting breast to brain metastatic tumours with death receptor ligand expressing therapeutic stem cells. Brain 2015, 138, 1710–1721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tang, X.J.; Lu, J.T.; Tu, H.J.; Huang, K.M.; Fu, R.; Cao, G.; Huang, M.; Cheng, L.H.; Dai, L.J.; Zhang, L. TRAIL-engineered bone marrow-derived mesenchymal stem cells: TRAIL expression and cytotoxic effects on C6 glioma cells. Anticancer Res. 2014, 34, 729–734. [Google Scholar] [PubMed]
- Redjal, N.; Zhu, Y.; Shah, K. Combination of systemic chemotherapy with local stem cell delivered S-TRAIL in resected brain tumors. Stem Cells 2015, 33, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wu, X.; Mao, Y.; Bao, W.; Gao, L.; Zhou, P.; Xie, R.; Zhou, L.; Zhu, J. Dual-targeted antitumor effects against brainstem glioma by intravenous delivery of tumor necrosis factor-related, apoptosis-inducing, ligand-engineered human mesenchymal stem cells. Neurosurgery 2009, 65, 610–624, discussion 624. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, P.P.G.; Gaglione, S.; Sewastianik, T.; Carrasco, R.D.; Langer, R.; Mitchell, M.J. Nanoparticles for Immune Cytokine TRAIL-Based Cancer Therapy. ACS Nano 2018, 12, 912–931. [Google Scholar] [CrossRef]
- Naoum, G.E.; Tawadros, F.; Farooqi, A.A.; Qureshi, M.Z.; Tabassum, S.; Buchsbaum, D.J.; Arafat, W. Role of nanotechnology and gene delivery systems in TRAIL-based therapies. Ecancermedicalscience 2016, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, S.; Li, M.; Wang, A.; Zhou, Y.; Li, P.; Wang, Y. Nanocarriers for TRAIL delivery: Driving TRAIL back on track for cancer therapy. Nanoscale 2017, 9, 13879–13904. [Google Scholar] [CrossRef] [PubMed]
- Reinshagen, C.; Bhere, D.; Choi, S.H.; Hutten, S.; Nesterenko, I.; Wakimoto, H.; Le Roux, E.; Rizvi, A.; Du, W.; Minicucci, C.; et al. CRISPR-enhanced engineering of therapy-sensitive cancer cells for self-targeting of primary and metastatic tumors. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Murakami, H.; Ohtsu, A.; Fuse, N.; Yoshino, T.; Yamamoto, N.; Boku, N.; Onozawa, Y.; Hsu, C.P.; Gorski, K.S.; et al. Phase 1 study of conatumumab, a pro-apoptotic death receptor 5 agonist antibody, in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2011, 68, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.H.; Cummings, J.; Dean, E.; Greystoke, A.; Hou, J.M.; Backen, A.; Ranson, M.; Dive, C. Biomarkers of apoptosis. Br. J. Cancer 2008, 99, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.R.; Cui, Y.; Fang, J.Y. Biological functions of cytokeratin 18 in cancer. Mol. Cancer Res. 2012, 10, 485–493. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Fakih, M.; Schwartzberg, L.; Cohn, A.L.; Yee, L.; Dreisbach, L.; Kozloff, M.F.; Hei, Y.J.; Galimi, F.; Pan, Y.; et al. TRAIL receptor agonist conatumumab with modified FOLFOX6 plus bevacizumab for first-line treatment of metastatic colorectal cancer: A randomized phase 1b/2 trial. Cancer 2013, 119, 4290–4298. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Hodgkinson, C.; Odedra, R.; Sini, P.; Heaton, S.P.; Mundt, K.E.; Ward, T.H.; Wilkinson, R.W.; Growcott, J.; Hughes, A.; et al. Preclinical evaluation of M30 and M65 ELISAs as biomarkers of drug induced tumor cell death and antitumor activity. Mol. Cancer Ther. 2008, 7, 455–463. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B. TRAIL resistance of breast cancer cells is associated with constitutive endocytosis of death receptors 4 and 5. Mol. Cancer Res. 2008, 6, 1861–1871. [Google Scholar] [CrossRef]
- Mazurek, N.; Byrd, J.C.; Sun, Y.; Hafley, M.; Ramirez, K.; Burks, J.; Bresalier, R.S. Cell-surface galectin-3 confers resistance to TRAIL by impeding trafficking of death receptors in metastatic colon adenocarcinoma cells. Cell Death Differ. 2012, 19, 523–533. [Google Scholar] [CrossRef]
- Lu, T.; Shao, N.; Ji, C. Targeting microRNAs to modulate TRAIL-induced apoptosis of cancer cells. Cancer Gene Ther. 2013, 20, 33–37. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, Q.; Tan, S. Targeting miRNAs associated with surface expression of death receptors to modulate TRAIL resistance in breast cancer. Cancer Lett. 2016, 383, 154–160. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, L.; van der Sloot, A.M.; Reis, C.R.; Deegan, S.; Ryan, A.E.; Dhami, S.P.; Murillo, L.S.; Cool, R.H.; Correa de Sampaio, P.; Thompson, K.; et al. Decoy receptors block TRAIL sensitivity at a supracellular level: The role of stromal cells in controlling tumour TRAIL sensitivity. Oncogene 2016, 35, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.L.; Gaudet, S.; Albeck, J.G.; Burke, J.M.; Sorger, P.K. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature 2009, 459, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Flusberg, D.A.; Sorger, P.K. Modulating cell-to-cell variability and sensitivity to death ligands by co-drugging. Phys. Biol. 2013, 10, 035002. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat. Rev. Cancer 2002, 2, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Endo, S.; Saito, R.; Hirose, M.; Hirai, S.; Suzuki, H.; Yamato, K.; Hyodo, I. The sirtuin inhibitor tenovin-6 upregulates death receptor 5 and enhances cytotoxic effects of 5-fluorouracil and oxaliplatin in colon cancer cells. Oncol. Res. 2013, 21, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Finnberg, N.K.; Gokare, P.; Navaraj, A.; Lang Kuhs, K.A.; Cerniglia, G.; Yagita, H.; Takeda, K.; Motoyama, N.; El-Deiry, W.S. Agonists of the TRAIL Death Receptor DR5 Sensitize Intestinal Stem Cells to Chemotherapy-Induced Cell Death and Trigger Gastrointestinal Toxicity. Cancer Res. 2016, 76, 700–712. [Google Scholar] [CrossRef]
- Rajeshkumar, N.V.; Rasheed, Z.A.; Garcia-Garcia, E.; Lopez-Rios, F.; Fujiwara, K.; Matsui, W.H.; Hidalgo, M. A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model. Mol. Cancer Ther. 2010, 9, 2582–2592. [Google Scholar] [CrossRef]
- DeRosier, L.C.; Huang, Z.Q.; Sellers, J.C.; Buchsbaum, D.J.; Vickers, S.M. Treatment with gemcitabine and TRA-8 anti-death receptor-5 mAb reduces pancreatic adenocarcinoma cell viability in vitro and growth in vivo. J. Gastrointest. Surg. 2006, 10, 1291–1300, discussion 1300. [Google Scholar] [CrossRef]
- DeRosier, L.C.; Buchsbaum, D.J.; Oliver, P.G.; Huang, Z.Q.; Sellers, J.C.; Grizzle, W.E.; Wang, W.; Zhou, T.; Zinn, K.R.; Long, J.W.; et al. Combination treatment with TRA-8 anti death receptor 5 antibody and CPT-11 induces tumor regression in an orthotopic model of pancreatic cancer. Clin. Cancer Res. 2007, 13, 5535s–5543s. [Google Scholar] [CrossRef]
- Buchsbaum, D.J.; Zhou, T.; Grizzle, W.E.; Oliver, P.G.; Hammond, C.J.; Zhang, S.; Carpenter, M.; LoBuglio, A.F. Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clin. Cancer Res. 2003, 9, 3731–3741. [Google Scholar] [PubMed]
- Holland, P.M. Death receptor agonist therapies for cancer, which is the right TRAIL? Cytokine Growth Factor Rev. 2014, 25, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Finlay, D.; Vamos, M.; Gonzalez-Lopez, M.; Ardecky, R.J.; Ganji, S.R.; Yuan, H.; Su, Y.; Cooley, T.R.; Hauser, C.T.; Welsh, K.; et al. Small-molecule IAP antagonists sensitize cancer cells to TRAIL-induced apoptosis: Roles of XIAP and cIAPs. Mol. Cancer Ther. 2014, 13, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Allensworth, J.L.; Sauer, S.J.; Lyerly, H.K.; Morse, M.A.; Devi, G.R. Smac mimetic Birinapant induces apoptosis and enhances TRAIL potency in inflammatory breast cancer cells in an IAP-dependent and TNF-alpha-independent mechanism. Breast Cancer Res. Treat. 2013, 137, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, G.; Zhao, Y.; Liu, G.; Wang, Y.; Ma, X.; Li, D.; Wu, Y.; Lu, J. Smac mimetic SM-164 potentiates APO2L/TRAIL-and doxorubicin-mediated anticancer activity in human hepatocellular carcinoma cells. PLoS ONE 2012, 7, e51461. [Google Scholar] [CrossRef] [PubMed]
- Cristofanon, S.; Abhari, B.A.; Krueger, M.; Tchoghandjian, A.; Momma, S.; Calaminus, C.; Vucic, D.; Pichler, B.J.; Fulda, S. Identification of RIP1 as a critical mediator of Smac mimetic-mediated sensitization of glioblastoma cells for Drozitumab-induced apoptosis. Cell Death Dis. 2015, 6, e1724. [Google Scholar] [CrossRef] [PubMed]
- Roesler, S.; Eckhardt, I.; Wolf, S.; Fulda, S. Cooperative TRAIL production mediates IFNalpha/Smac mimetic-induced cell death in TNFalpha-resistant solid cancer cells. Oncotarget 2016, 7, 3709–3725. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perimenis, P.; Galaris, A.; Voulgari, A.; Prassa, M.; Pintzas, A. IAP antagonists Birinapant and AT-406 efficiently synergise with either TRAIL, BRAF, or BCL-2 inhibitors to sensitise BRAFV600E colorectal tumour cells to apoptosis. BMC Cancer 2016, 16, 624. [Google Scholar] [CrossRef] [PubMed]
- Garimella, S.V.; Gehlhaus, K.; Dine, J.L.; Pitt, J.J.; Grandin, M.; Chakka, S.; Nau, M.M.; Caplen, N.J.; Lipkowitz, S. Identification of novel molecular regulators of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in breast cancer cells by RNAi screening. Breast Cancer Res. 2014, 16, R41. [Google Scholar] [CrossRef]
- Cristofanon, S.; Fulda, S. ABT-737 promotes tBid mitochondrial accumulation to enhance TRAIL-induced apoptosis in glioblastoma cells. Cell Death Dis. 2012, 3, e432. [Google Scholar] [CrossRef]
- Teraishi, F.; Kagawa, S.; Watanabe, T.; Tango, Y.; Kawashima, T.; Umeoka, T.; Nisizaki, M.; Tanaka, N.; Fujiwara, T. ZD1839 (Gefitinib, ‘Iressa’), an epidermal growth factor receptor-tyrosine kinase inhibitor, enhances the anti-cancer effects of TRAIL in human esophageal squamous cell carcinoma. FEBS Lett. 2005, 579, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Cuello, M.; Ettenberg, S.A.; Clark, A.S.; Keane, M.M.; Posner, R.H.; Nau, M.M.; Dennis, P.A.; Lipkowitz, S. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res. 2001, 61, 4892–4900. [Google Scholar] [PubMed]
- Venza, I.; Visalli, M.; Oteri, R.; Teti, D.; Venza, M. Class I-specific histone deacetylase inhibitor MS-275 overrides TRAIL-resistance in melanoma cells by downregulating c-FLIP. Int. Immunopharmacol. 2014, 21, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Jazirehi, A.R.; Kurdistani, S.K.; Economou, J.S. Histone deacetylase inhibitor sensitizes apoptosis-resistant melanomas to cytotoxic human T lymphocytes through regulation of TRAIL/DR5 pathway. J. Immunol. 2014, 192, 3981–3989. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Histone deacetylase (HDAC) inhibitors and regulation of TRAIL-induced apoptosis. Exp. Cell Res. 2012, 318, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Garimella, S.V.; Rocca, A.; Lipkowitz, S. WEE1 inhibition sensitizes basal breast cancer cells to TRAIL-induced apoptosis. Mol. Cancer Res. 2012, 10, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Lemke, J.; von Karstedt, S.; Abd El Hay, M.; Conti, A.; Arce, F.; Montinaro, A.; Papenfuss, K.; El-Bahrawy, M.A.; Walczak, H. Selective CDK9 inhibition overcomes TRAIL resistance by concomitant suppression of cFlip and Mcl-1. Cell Death Differ. 2014, 21, 491–502. [Google Scholar] [CrossRef]
- Chen, J.J.; Chou, C.W.; Chang, Y.F.; Chen, C.C. Proteasome inhibitors enhance TRAIL-induced apoptosis through the intronic regulation of DR5: Involvement of NF-kappa B and reactive oxygen species-mediated p53 activation. J. Immunol. 2008, 180, 8030–8039. [Google Scholar] [CrossRef]
- Shankar, S.; Singh, T.R.; Srivastava, R.K. Ionizing radiation enhances the therapeutic potential of TRAIL in prostate cancer in vitro and in vivo: Intracellular mechanisms. Prostate 2004, 61, 35–49. [Google Scholar] [CrossRef]
- Shankar, S.; Singh, T.R.; Chen, X.; Thakkar, H.; Firnin, J.; Srivastava, R.K. The sequential treatment with ionizing radiation followed by TRAIL/Apo-2L reduces tumor growth and induces apoptosis of breast tumor xenografts in nude mice. Int. J. Oncol. 2004, 24, 1133–1140. [Google Scholar] [CrossRef]
- Marini, P.; Schmid, A.; Jendrossek, V.; Faltin, H.; Daniel, P.T.; Budach, W.; Belka, C. Irradiation specifically sensitises solid tumour cell lines to TRAIL mediated apoptosis. BMC Cancer 2005, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiyan, A.M.; Prasad, U.; Shankar, S.; Hamstra, D.A.; Shanaiah, M.; Chenevert, T.L.; Ross, B.D.; Rehemtulla, A. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. PNAS 2000, 97, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Hamasu, T.; Inanami, O.; Asanuma, T.; Kuwabara, M. Enhanced induction of apoptosis by combined treatment of human carcinoma cells with X rays and death receptor agonists. J. Radiat. Res. 2005, 46, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Maduro, J.H.; de Vries, E.G.; Meersma, G.J.; Hougardy, B.M.; van der Zee, A.G.; de Jong, S. Targeting pro-apoptotic trail receptors sensitizes HeLa cervical cancer cells to irradiation-induced apoptosis. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, G.A.; Tsamis, K.I.; Vartholomatos, E.; Peponi, E.; Tzima, E.; Tasiou, I.; Lykoudis, E.; Tsekeris, P.; Kyritsis, A.P. Combination treatment of TRAIL, DFMO and radiation for malignant glioma cells. J. Neuro-Oncol. 2015, 123, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Kondo, T.; Kanamori, M.; Tabuchi, Y.; Ogawa, R.; Zhao, Q.L.; Ahmed, K.; Yasuda, T.; Seki, S.; Suzuki, K.; et al. Ionizing radiation enhances tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through up-regulations of death receptor 4 (DR4) and death receptor 5 (DR5) in human osteosarcoma cells. J. Orthop. Res. 2010, 28, 739–745. [Google Scholar] [CrossRef]
- Marini, P.; Budach, W.; Niyazi, M.; Junginger, D.; Stickl, S.; Jendrossek, V.; Belka, C. Combination of the pro-apoptotic TRAIL-receptor antibody mapatumumab with ionizing radiation strongly increases long-term tumor control under ambient and hypoxic conditions. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 198–202. [Google Scholar] [CrossRef]
- Rezacova, M.; Vavrova, J.; Vokurkova, D. Ionizing radiation sensitizes leukemic MOLT-4 cells to TRAIL-induced apoptosis. Acta Med. 2008, 51, 101–105. [Google Scholar]
- Di Pietro, R.; Secchiero, P.; Rana, R.; Gibellini, D.; Visani, G.; Bemis, K.; Zamai, L.; Miscia, S.; Zauli, G. Ionizing radiation sensitizes erythroleukemic cells but not normal erythroblasts to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)--mediated cytotoxicity by selective up-regulation of TRAIL-R1. Blood 2001, 97, 2596–2603. [Google Scholar] [CrossRef]
- Nagane, M.; Cavenee, W.K.; Shiokawa, Y. Synergistic cytotoxicity through the activation of multiple apoptosis pathways in human glioma cells induced by combined treatment with ionizing radiation and tumor necrosis factor-related apoptosis-inducing ligand. J. Neurosurg. 2007, 106, 407–416. [Google Scholar] [CrossRef]
- Uckun, F.M.; Myers, D.E.; Ma, H.; Rose, R.; Qazi, S. Low Dose Total Body Irradiation Combined With Recombinant CD19-Ligand x Soluble TRAIL Fusion Protein is Highly Effective Against Radiation-Resistant B-Precursor Acute Lymphoblastic Leukemia in Mice. EBioMedicine 2015, 2, 306–316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lomonosova, E.; Chinnadurai, G. BH3-only proteins in apoptosis and beyond: An overview. Oncogene 2008, 27, S2–S19. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.H.; Plenchette, S.; Kern, C.J.; Mahoney, D.J.; Korneluk, R.G. The RING domain of cIAP1 mediates the degradation of RING-bearing inhibitor of apoptosis proteins by distinct pathways. Mol. Biol. Cell 2008, 19, 2729–2740. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000, 102, 33–42. [Google Scholar] [CrossRef]
- LaCasse, E.; Baird, S.; Korneluk, R.; MacKenzie, A. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene 1999, 17. [Google Scholar] [CrossRef] [PubMed]
- Smolewski, P.; Robak, T. Inhibitors of apoptosis proteins (IAPs) as potential molecular targets for therapy of hematological malignancies. Curr. Mol. Med. 2011, 11, 633–649. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, P.; Huang, J.; Ge, S.; Lu, J.; Qian, G. Sp1 and Sp3 regulate basal transcription of the survivin gene. Biochem. Biophys. Res. Commun. 2007, 356, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, W.H.; Biade, S.; Zilfou, J.T.; Chen, J.; Murphy, M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J. Biol. Chem. 2002, 277, 3247–3257. [Google Scholar] [CrossRef]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. New Eng. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef]
- Seymour, J.F.; Ma, S.; Brander, D.M.; Choi, M.Y.; Barrientos, J.; Davids, M.S.; Anderson, M.A.; Beaven, A.W.; Rosen, S.T.; Tam, C.S.; et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: A phase 1b study. Lancet Oncol. 2017, 18, 230–240. [Google Scholar] [CrossRef]
- Montero, J.; Stephansky, J.; Cai, T.; Griffin, G.K.; Cabal-Hierro, L.; Togami, K.; Hogdal, L.J.; Galinsky, I.; Morgan, E.A.; Aster, J.C.; et al. Blastic Plasmacytoid Dendritic Cell Neoplasm Is Dependent on BCL2 and Sensitive to Venetoclax. Cancer Discov. 2017, 7, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Moretti, L.; Mitchell, L.R.; Jung, D.K.; Lu, B. Combined Bcl-2/mammalian target of rapamycin inhibition leads to enhanced radiosensitization via induction of apoptosis and autophagy in non-small cell lung tumor xenograft model. Clin. Cancer Res. 2009, 15, 6096–6105. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.H.; Jiang, X.M.; Guo, X.; Fong, C.M.; Chen, X.; Lu, J.J. Characterization of osimertinib (AZD9291)-resistant non-small cell lung cancer NCI-H1975/OSIR cell line. Oncotarget 2016, 7, 81598–81610. [Google Scholar] [CrossRef] [PubMed]
- Tutusaus, A.; Stefanovic, M.; Boix, L.; Cucarull, B.; Zamora, A.; Blasco, L.; de Frutos, P.G.; Reig, M.; Fernandez-Checa, J.C.; Mari, M.; et al. Antiapoptotic BCL-2 proteins determine sorafenib/regorafenib resistance and BH3-mimetic efficacy in hepatocellular carcinoma. Oncotarget 2018, 9, 16701–16717. [Google Scholar] [CrossRef] [PubMed]
- Frederick, D.T.; Salas Fragomeni, R.A.; Schalck, A.; Ferreiro-Neira, I.; Hoff, T.; Cooper, Z.A.; Haq, R.; Panka, D.J.; Kwong, L.N.; Davies, M.A.; et al. Clinical profiling of BCL-2 family members in the setting of BRAF inhibition offers a rationale for targeting de novo resistance using BH3 mimetics. PLoS ONE 2014, 9, e101286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, A.; Strickland, A.S.; Roboz, G.J.; Hou, J.Z.; Fiedler, W.; Lin, T.L.; Walter, R.B.; Enjeti, A.; Chyla, B.; Popovic, R.; et al. Paper: Phase 1/2 Study of Venetoclax with Low-Dose Cytarabine in Treatment-Naive, Elderly Patients with Acute Myeloid Leukemia Unfit for Intensive Chemotherapy: 1-Year Outcomes. ASH 59th Annu. Meet. Expos. Proc. 2018, 130, 890. [Google Scholar]
- Elledge, R.M.; Green, S.; Howes, L.; Clark, G.M.; Berardo, M.; Allred, D.C.; Pugh, R.; Ciocca, D.; Ravdin, P.; O’Sullivan, J.; et al. bcl-2, p53, and response to tamoxifen in estrogen receptor-positive metastatic breast cancer: A Southwest Oncology Group study. J. Clin. Oncol. 1997, 15, 1916–1922. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00445198 (accessed on 9 May 2019).
- ClinicalTrials.gov. 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT02143401 (accessed on 9 May 2019).
- ClinicalTrials.gov. 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT01989585 (accessed on 9 May 2019).
- Yip, K.W.; Mocanu, J.D.; Au, P.Y.; Sleep, G.T.; Huang, D.; Busson, P.; Yeh, W.C.; Gilbert, R.; O’Sullivan, B.; Gullane, P.; et al. Combination bcl-2 antisense and radiation therapy for nasopharyngeal cancer. Clin. Cancer Res. 2005, 11, 8131–8144. [Google Scholar] [CrossRef]
- Mu, Z.; Hachem, P.; Pollack, A. Antisense Bcl-2 sensitizes prostate cancer cells to radiation. Prostate 2005, 65, 331–340. [Google Scholar] [CrossRef][Green Version]
- Zerp, S.F.; Stoter, R.; Kuipers, G.; Yang, D.; Lippman, M.E.; van Blitterswijk, W.J.; Bartelink, H.; Rooswinkel, R.; Lafleur, V.; Verheij, M. AT-101, a small molecule inhibitor of anti-apoptotic Bcl-2 family members, activates the SAPK/JNK pathway and enhances radiation-induced apoptosis. Radiat. Oncol. 2009, 4, 47. [Google Scholar] [CrossRef]
- Schimmer, A.D.; Raza, A.; Carter, T.H.; Claxton, D.; Erba, H.; DeAngelo, D.J.; Tallman, M.S.; Goard, C.; Borthakur, G. A multicenter phase I/II study of obatoclax mesylate administered as a 3- or 24-hour infusion in older patients with previously untreated acute myeloid leukemia. PLoS ONE 2014, 9, e108694. [Google Scholar] [CrossRef] [PubMed]
- Goy, A.; Hernandez-Ilzaliturri, F.J.; Kahl, B.; Ford, P.; Protomastro, E.; Berger, M. A phase I/II study of the pan Bcl-2 inhibitor obatoclax mesylate plus bortezomib for relapsed or refractory mantle cell lymphoma. Leuk. Lymphoma 2014, 55, 2761–2768. [Google Scholar] [CrossRef] [PubMed]
- McGregor, N.; Patel, L.; Craig, M.; Weidner, S.; Wang, S.; Pienta, K.J. AT-101 (R-(-)-gossypol acetic acid) enhances the effectiveness of androgen deprivation therapy in the VCaP prostate cancer model. J. Cell Biochem. 2010, 110, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Antonietti, P.; Gessler, F.; Dussmann, H.; Reimertz, C.; Mittelbronn, M.; Prehn, J.H.; Kogel, D. AT-101 simultaneously triggers apoptosis and a cytoprotective type of autophagy irrespective of expression levels and the subcellular localization of Bcl-xL and Bcl-2 in MCF7 cells. Biochim. Biophys. Acta 2016, 1863, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Mani, J.; Vallo, S.; Rakel, S.; Antonietti, P.; Gessler, F.; Blaheta, R.; Bartsch, G.; Michaelis, M.; Cinatl, J.; Haferkamp, A.; et al. Chemoresistance is associated with increased cytoprotective autophagy and diminished apoptosis in bladder cancer cells treated with the BH3 mimetic (-)-Gossypol (AT-101). BMC Cancer 2015, 15, 224. [Google Scholar] [CrossRef] [PubMed]
- Van Poznak, C.; Seidman, A.D.; Reidenberg, M.M.; Moasser, M.M.; Sklarin, N.; Van Zee, K.; Borgen, P.; Gollub, M.; Bacotti, D.; Yao, T.J.; et al. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: A phase I/II clinical trial. Breast Cancer Res. Treat. 2001, 66, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Bushunow, P.; Reidenberg, M.M.; Wasenko, J.; Winfield, J.; Lorenzo, B.; Lemke, S.; Himpler, B.; Corona, R.; Coyle, T. Gossypol treatment of recurrent adult malignant gliomas. J. Neurooncol. 1999, 43, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Casara, P.; Davidson, J.; Claperon, A.; Le Toumelin-Braizat, G.; Vogler, M.; Bruno, A.; Chanrion, M.; Lysiak-Auvity, G.; Le Diguarher, T.; Starck, J.B.; et al. S55746 is a novel orally active BCL-2 selective and potent inhibitor that impairs hematological tumor growt. Oncotarget 2018, 9, 20075–20088. [Google Scholar] [CrossRef]
- Debrincat, M.A.; Pleines, I.; Lebois, M.; Lane, R.M.; Holmes, M.L.; Corbin, J.; Vandenberg, C.J.; Alexander, W.S.; Ng, A.P.; Strasser, A.; et al. BCL-2 is dispensable for thrombopoiesis and platelet survival. Cell Death Dis. 2015, 6, e1721. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Vogler, M.; Hamali, H.A.; Sun, X.M.; Bampton, E.T.; Dinsdale, D.; Snowden, R.T.; Dyer, M.J.; Goodall, A.H.; Cohen, G.M. BCL2/BCL-X(L) inhibition induces apoptosis, disrupts cellular calcium homeostasis, and prevents platelet activation. Blood 2011, 117, 7145–7154. [Google Scholar] [CrossRef] [PubMed]
- Leverson, J.D.; Phillips, D.C.; Mitten, M.J.; Boghaert, E.R.; Diaz, D.; Tahir, S.K.; Belmont, L.D.; Nimmer, P.; Xiao, Y.; Ma, X.M.; et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci. Transl. Med. 2015, 7, 279ra240. [Google Scholar] [CrossRef] [PubMed]
- King, K.; Cidlowski, J. Cell cycle regulation and apoptosis. Annu. Rev. Physiol. 1998, 60, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Reyna, D.E.; Garner, T.P.; Lopez, A.; Kopp, F.; Choudhary, G.S.; Sridharan, A.; Narayanagari, S.R.; Mitchell, K.; Dong, B.; Bartholdy, B.A.; et al. Direct activation of BAX by BTSA1 overcomes apoptosis resistance in acute myeloid leukemia. Cancer Cell 2017, 32, 490–505. [Google Scholar] [CrossRef] [PubMed]
- Garner, T.P.; Reyna, D.E.; Priyadarshi, A.; Chen, H.C.; Li, S.; Wu, Y.; Ganesan, Y.T.; Malashkevich, V.N.; Almo, S.S.; Cheng, E.H.; et al. An Autoinhibited Dimeric Form of BAX Regulates the BAX Activation Pathway. Mol. Cell 2016, 63, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.T.; Lin, X.; Faivre, E.J.; Yang, Z.; Huang, X.; Wilcox, D.M.; Bellin, R.J.; Jin, S.; Tahir, S.K.; Mitten, M.; et al. Vulnerability of Small-Cell Lung Cancer to Apoptosis Induced by the Combination of BET Bromodomain Proteins and BCL2 Inhibitors. Mol. Cancer Ther. 2017, 16, 1511–1520. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Cheng, K.A.; Hata, A.N.; Faber, A.C.; Ebi, H.; Coffee, E.M.; Greninger, P.; Brown, R.D.; Godfrey, J.T.; Cohoon, T.J.; et al. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell 2013, 23, 121–128. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02079740 (accessed on 9 May 2019).
- Laurent-Puig, P.; Cayre, A.; Manceau, G.; Buc, E.; Bachet, J.B.; Lecomte, T.; Rougier, P.; Lievre, A.; Landi, B.; Boige, V.; et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J. Clin. Oncol. 2009, 27, 5924–5930. [Google Scholar] [CrossRef]
- Vucic, D. Targeting IAP (inhibitor of apoptosis) proteins for therapeutic intervention in tumors. Curr. Cancer Drug Targets 2008, 8, 110–117. [Google Scholar] [CrossRef]
- Varfolomeev, E.; Moradi, E.; Dynek, J.N.; Zha, J.; Fedorova, A.V.; Deshayes, K.; Fairbrother, W.J.; Newton, K.; Le Couter, J.; Vucic, D. Characterization of ML-IAP protein stability and physiological role in vivo. Biochem. J. 2012, 447, 427–436. [Google Scholar] [CrossRef][Green Version]
- Varfolomeev, E.; Vucic, D. Inhibitor of apoptosis proteins: Fascinating biology leads to attractive tumor therapeutic targets. Future Oncol. 2011, 7, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Varfolomeev, E.; Goncharov, T.; Fedorova, A.V.; Dynek, J.N.; Zobel, K.; Deshayes, K.; Fairbrother, W.J.; Vucic, D. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J. Biol. Chem. 2008, 283, 24295–24299. [Google Scholar] [CrossRef] [PubMed]
- Varfolomeev, E.; Blankenship, J.W.; Wayson, S.M.; Fedorova, A.V.; Kayagaki, N.; Garg, P.; Zobel, K.; Dynek, J.N.; Elliott, L.O.; Wallweber, H.J.; et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 2007, 131, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, Z.; Anghel, A.; Desai, A.; Ayala, R.; Luo, T.; Safran, B.; Fejzo, M.; Hecht, J.; Slamon, D.; Finn, R. Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin. Cancer Res. 2010, 16, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Eckelman, B.P.; Salvesen, G.S.; Scott, F.L. Human inhibitor of apoptosis proteins: Why XIAP is the black sheep of the family. EMBO Rep. 2006, 7, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Design of small-molecule Smac mimetics as IAP antagonists. Curr. Top. Microbiol. Immunol. 2011, 348, 89–113. [Google Scholar] [CrossRef]
- Clinical Trials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01955434 (accessed on 9 May 2019).
- West, A.C.; Martin, B.P.; Andrews, D.A.; Hogg, S.J.; Banerjee, A.; Grigoriadis, G.; Johnstone, R.W.; Shortt, J. The SMAC mimetic, LCL-161, reduces survival in aggressive MYC-driven lymphoma while promoting susceptibility to endotoxic shock. Oncogenesis 2016, 5, e216. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Carter, Z.B.; Kantarjian, H.M.; Cortes, J.E.; Kadia, T.M.; Garcia-Manero, G.; DiNardo, C.D.; Bose, P.; Pierce, S.; Zhou, L.; et al. Results for Phase II Clinical Trial of LCL161, a SMAC Mimetic, in Patients with Primary Myelofibrosis (PMF), Post-Polycythemia Vera Myelofibrosis (post-PV MF) or Post-Essential Thrombocytosis Myelofibrosis (post-ET MF). Blood 2016, 128, 3105. [Google Scholar]
- Infante, J.; Dees, E.; Olszanski, A.; Dhuria, S.; Sen, S.; Cameron, S.; Cohen, R. Phase I Dose-Escalation Study of LCL161, an Oral Inhibitor of Apoptosis Proteins Inhibitor, in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2014. [Google Scholar] [CrossRef]
- Dienstmann, R.; Vidal, L.; Dees, E.C.; Chia, S.; Mayer, E.L.; Porter, D.; Baney, T.; Dhuria, S.; Sen, S.K.; Firestone, B.; et al. A phase Ib study of LCL161, an oral inhibitor of apoptosis (IAP) antagonist, in combination with weekly paclitaxel in patients with advanced solid tumors. Cancer Res. 2012, 72. [Google Scholar] [CrossRef]
- Gerges, S.; Rohde, K.; Fulda, S. Cotreatment with Smac mimetics and demethylating agents induces both apoptotic and necroptotic cell death pathways in acute lymphoblastic leukemia cells. Cancer Lett. 2016, 375, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Eytan, D.F.; Snow, G.E.; Carlson, S.; Derakhshan, A.; Saleh, A.; Schiltz, S.; Cheng, H.; Mohan, S.; Cornelius, S.; Coupar, J.; et al. SMAC Mimetic Birinapant plus Radiation Eradicates Human Head and Neck Cancers with Genomic Amplifications of Cell Death Genes FADD and BIRC2. Cancer Res. 2016, 76, 5442–5454. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.Z.; Mak, P.Y.; Mak, D.H.; Shi, Y.; Qiu, Y.; Bogenberger, J.M.; Mu, H.; Tibes, R.; Yao, H.; Coombes, K.R.; et al. Synergistic targeting of AML stem/progenitor cells with IAP antagonist birinapant and demethylating agents. J. Natl. Cancer Inst. 2014, 106, 440. [Google Scholar] [CrossRef] [PubMed]
- Hehlgans, S.; Oppermann, J.; Reichert, S.; Fulda, S.; Rodel, C.; Rodel, F. The SMAC mimetic BV6 sensitizes colorectal cancer cells to ionizing radiation by interfering with DNA repair processes and enhancing apoptosis. Radiat. Oncol. 2015, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Noonan, A.M.; Bunch, K.P.; Chen, J.Q.; Herrmann, M.A.; Lee, J.M.; Kohn, E.C.; O’Sullivan, C.C.; Jordan, E.; Houston, N.; Takebe, N.; et al. Pharmacodynamic markers and clinical results from the phase 2 study of the SMAC mimetic birinapant in women with relapsed platinum-resistant or -refractory epithelial ovarian cancer. Cancer 2016, 122, 588–597. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https:// clinicaltrials.gov/ct2/show/ NCT02587962 (accessed on 9 May 2019).
- Ward, G.A.; Lewis, E.J.; Ahn, J.S.; Johnson, C.N.; Lyons, J.F.; Martins, V.; Munck, J.M.; Rich, S.J.; Smyth, T.; Thompson, N.T.; et al. ASTX660, a Novel Non-peptidomimetic Antagonist of cIAP1/2 and XIAP, Potently Induces TNFalpha-Dependent Apoptosis in Cancer Cell Lines and Inhibits Tumor Growth. Mol. Cancer Ther. 2018, 17, 1381–1391. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02503423 (accessed on 9 May 2019).
- Tolcher, A.W.; Bendell, J.C.; Papadopoulos, K.P.; Burris, H.A.; Patnaik, A.; Fairbrother, W.J.; Wong, H.; Budha, N.; Darbonne, W.C.; Peale, F.; et al. A Phase I Dose-Escalation Study Evaluating the Safety Tolerability and Pharmacokinetics of CUDC-427, a Potent, Oral, Monovalent IAP Antagonist, in Patients with Refractory Solid Tumors. Clin. Cancer Res. 2016, 22, 4567–4573. [Google Scholar] [CrossRef]
- Carter, B.Z.; Mak, D.H.; Morris, S.J.; Borthakur, G.; Estey, E.; Byrd, A.L.; Konopleva, M.; Kantarjian, H.; Andreeff, M. XIAP antisense oligonucleotide (AEG35156) achieves target knockdown and induces apoptosis preferentially in CD34+38- cells in a phase 1/2 study of patients with relapsed/refractory AML. Apoptosis 2011, 16, 67–74. [Google Scholar] [CrossRef]
- Schimmer, A.D.; Estey, E.H.; Borthakur, G.; Carter, B.Z.; Schiller, G.J.; Tallman, M.S.; Altman, J.K.; Karp, J.E.; Kassis, J.; Hedley, D.W.; et al. Phase I/II trial of AEG35156 X-linked inhibitor of apoptosis protein antisense oligonucleotide combined with idarubicin and cytarabine in patients with relapsed or primary refractory acute myeloid leukemia. J. Clin. Oncol. 2009, 27, 4741–4746. [Google Scholar] [CrossRef]
- Ngeow, J.; Tan, I.B.; Choo, S.P. Targeted therapies in the treatment of gastric cancer. Asia Pac. J. Clin. Oncol. 2011, 7, 224–235. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Lopez-Jimenez, J.; Smith, S.E.; Steinberg, J.; Keating, A.; Sasse, C.; Jie, F.; Thyss, A. A multicenter phase II study of sepantronium bromide (YM155) plus rituximab in patients with relapsed aggressive B-cell Non-Hodgkin lymphoma. Leuk. Lymphoma 2016, 57, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Natale, R.; Blackhall, F.; Kowalski, D.; Ramlau, R.; Bepler, G.; Grossi, F.; Lerchenmuller, C.; Pinder-Schenck, M.; Mezger, J.; Danson, S.; et al. Evaluation of antitumor activity using change in tumor size of the survivin antisense oligonucleotide LY2181308 in combination with docetaxel for second-line treatment of patients with non-small-cell lung cancer: A randomized open-label phase II study. J. Thorac. Oncol. 2014, 9, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Wiechno, P.; Somer, B.G.; Mellado, B.; Chlosta, P.L.; Cervera Grau, J.M.; Castellano, D.; Reuter, C.; Stockle, M.; Kamradt, J.; Pikiel, J.; et al. A randomised phase 2 study combining LY2181308 sodium (survivin antisense oligonucleotide) with first-line docetaxel/prednisone in patients with castration-resistant prostate cancer. Eur. Urol. 2014, 65, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Fenstermaker, R.A.; Ciesielski, M.J.; Qiu, J.; Yang, N.; Frank, C.L.; Lee, K.P.; Mechtler, L.R.; Belal, A.; Ahluwalia, M.S.; Hutson, A.D. Clinical study of a survivin long peptide vaccine (SurVaxM) in patients with recurrent malignant glioma. Cancer Immun. Immunother. 2016, 65, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, N.J.; Bjoern, J.; Met, O.; Svane, I.M.; Andersen, M.H. Therapeutic Vaccination against A Modified Minimal Survivin Epitope Induces Functional CD4 T Cells That Recognize Survivin-Expressing Cells. Scand. J. Immunol. 2016, 84, 191–193. [Google Scholar] [CrossRef]
- Berinstein, N.L.; Van Der Jagt, R.H.; Cheung, M.C.; Buckstein, R.; Karkada, M.; Quinton, T.; MacDonald, L.; Stanford, M.; Nigam, R.; Mansour, M. A phase 2 clinical trial testing DPX-Survivac and metronomic low dose cyclophosphamide as immunotherapy for patients with recurrent diffuse large b-cell lymphoma. J. Clin. Oncol. 2017, 34, e14578. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Schilder, R.J.; Martin, L.P.; Levin, M.; Graham, M.A.; Weng, D.E.; Adjei, A.A. A Phase I Study of the SMAC-Mimetic Birinapant in Adults with Refractory Solid Tumors or Lymphoma. Mol. Cancer Ther. 2015, 14, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Ghislat, G.; Luo, S.; Renna, M.; Siddiqi, F.; Rubinsztein, D.C. XIAP and cIAP1 amplifications induce Beclin 1-dependent autophagy through NFkappaB activation. Hum. Mol. Gen. 2015, 24, 2899–2913. [Google Scholar] [CrossRef]
- Pinzon-Ortiz, M.; Hastings, W.; Longmire, T.; Shaw, P.; Rong, X.; Murakami, M.; Lee, B.H.; Dranoff, D.; Maclsaac, K.; Cao, A.Z. Abstract 2343: The immune modulatory roles of IAP inhibitor, LCL161, and its connection to immune-checkpoint molecules. Cancer Res. 2016. [Google Scholar] [CrossRef]
- Willis, S.N.; Chen, L.; Dewson, G.; Wei, A.; Naik, E.; Fletcher, J.I.; Adams, J.M.; Huang, D.C. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005, 19, 1294–1305. [Google Scholar] [CrossRef]
- Abulwerdi, F.; Liao, C.; Liu, M.; Azmi, A.S.; Aboukameel, A.; Mady, A.S.; Gulappa, T.; Cierpicki, T.; Owens, S.; Zhang, T.; et al. A novel small-molecule inhibitor of mcl-1 blocks pancreatic cancer growth in vitro and in vivo. Mol. Cancer Ther. 2014, 13, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Leverson, J.D.; Zhang, H.; Chen, J.; Tahir, S.K.; Phillips, D.C.; Xue, J.; Nimmer, P.; Jin, S.; Smith, M.; Xiao, Y.; et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis. 2015, 6, e1590. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Yacoub, A.; Hossein, H.; Martin, A.P.; Bareford, M.D.; Eulitt, P.; Yang, C.; Nephew, K.P.; Dent, P. Inhibition of MCL-1 in breast cancer cells promotes cell death in vitro and in vivo. Cancer Biol. Ther. 2010, 10, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Balko, J.M.; Giltnane, J.M.; Wang, K.; Schwarz, L.J.; Young, C.D.; Cook, R.S.; Owens, P.; Sanders, M.E.; Kuba, M.G.; Sanchez, V.; et al. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov. 2014, 4, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; He, X.; Xia, W.; Hsu, J.M.; Chen, C.T.; Li, L.Y.; Lee, D.F.; Yang, J.Y.; Xie, X.; Liu, J.C.; et al. Myeloid cell leukemia-1 inversely correlates with glycogen synthase kinase-3beta activity and associates with poor prognosis in human breast cancer. Cancer Res. 2007, 67, 4564–4571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gojo, I.; Fenton, R.G. Myeloid cell factor-1 is a critical survival factor for multiple myeloma. Blood 2002, 99, 1885–1893. [Google Scholar] [CrossRef]
- Edupuganti, R.; Taliaferro, J.M.; Wang, Q.; Xie, X.; Cho, E.J.; Vidhu, F.; Ren, P.; Anslyn, E.V.; Bartholomeusz, C.; Dalby, K.N. Discovery of a potent inhibitor of MELK that inhibits expression of the anti-apoptotic protein Mcl-1 and TNBC cell growth. Bioorgan. Med. Chem. 2017, 25, 2609–2616. [Google Scholar] [CrossRef] [PubMed]
- Caenepeel, S.R.; Belmontes, B.; Sun, J.; Coxon, A.; Moody, G.; Hughes, P.E. Abstract 2027: Preclinical evaluation of AMG 176, a novel, potent and selective Mcl-1 inhibitor with robust anti-tumor activity in Mcl-1 dependent cancer models. Cancer Res. 2017, 77. [Google Scholar] [CrossRef]
- Ramsey, H.E.; Fischer, M.A.; Lee, T.; Gorska, A.E.; Arrate, M.P.; Fuller, L.; Boyd, K.L.; Strickland, S.A.; Sensintaffar, J.; Hogdal, L.J.; et al. A Novel MCL1 Inhibitor Combined with Venetoclax Rescues Venetoclax-Resistant Acute Myelogenous Leukemia. Cancer Discov. 2018, 8, 1566–1581. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT02675452 (accessed on 9 May 2019).
- ClinicalTrials.gov. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT02675452 (accessed on 9 May 2019).
- Hird AW, S.J.; Adam, A.; Belmonte, M.A.; Gangl, E.; Gibbons, F.; Hargreaves, D.; Johannes, J.W.; Kazmirski, S.L.; Kettle, J.G.; Kurtz, S.E.; et al. Abstract DDT01-02: AZD5991: A potent and selective macrocyclic inhibitor of Mcl-1 for treatment of hematologic cancers. Cancer Res. 2017, 77. [Google Scholar] [CrossRef]
- Kotschy, A.; Szlavik, Z.; Murray, J.; Davidson, J.; Maragno, A.L.; Le Toumelin-Braizat, G.; Chanrion, M.; Kelly, G.L.; Gong, J.N.; Moujalled, D.M.; et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 2016, 538, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Dettman, E.J.; Warner, S.L.; Doykan, C.E.; Arn, M.; Blake, N.; Bearss, D.; Cardone, M.; Smith, B.D. Mitochondrial profiling in AML patients treated with an Alvocidib containing regimen reveals MCL1 dependency in responder bone marrow. In Proceedings of the 106th Annual Meeting of the American Association for Cancer Research, Philadelphia, PA, USA, 18–22 April 2015. [Google Scholar]
- Whatcott, C. The MCL-1 targeting effect of alvocidib potentiates the activity of cytarabine and mitoxantrone in a time-sequential regimen in AML. In Proceedings of the SOHO 2015 Annual Meeting, Houston, TX, USA, 16 September 2015. [Google Scholar]
- Ploner, C.; Kofler, R.; Villunger, A. Noxa: At the tip of the balance between life and death. Oncogene 2008, 27, S84–S92. [Google Scholar] [CrossRef] [PubMed]
- Torres-Adorno, A.M.; Lee, J.; Kogawa, T.; Ordentlich, P.; Tripathy, D.; Lim, B.; Ueno, N.T. Histone Deacetylase Inhibitor Enhances the Efficacy of MEK Inhibitor through NOXA-Mediated MCL1 Degradation in Triple-Negative and Inflammatory Breast Cancer. Clin. Cancer Res. 2017, 23, 4780–4792. [Google Scholar] [CrossRef] [PubMed]
- Moujalled, D.M.; Pomilio, G.; Ghiurau, C.; Ivey, A.; Salmon, J.; Rijal, S.; Macraild, S.; Zhang, L.; Teh, T.C.; Tiong, I.S.; et al. Combining BH3-mimetics to target both BCL-2 and MCL1 has potent activity in pre-clinical models of acute myeloid leukemia. Leukemia 2018. [Google Scholar] [CrossRef] [PubMed]
- Siroy, A.; Abdul-Karim, F.W.; Miedler, J.; Fong, N.; Fu, P.; Gilmore, H.; Baar, J. MUC1 is expressed at high frequency in early-stage basal-like triple-negative breast cancer. Hum. Pathol. 2013, 44, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Goode, G.; Gunda, V.; Chaika, N.V.; Purohit, V.; Yu, F.; Singh, P.K. MUC1 facilitates metabolomic reprogramming in triple-negative breast cancer. PLoS ONE 2017, 12, e0176820. [Google Scholar] [CrossRef]
- Rajabi, H.; Hiraki, M.; Tagde, A.; Alam, M.; Bouillez, A.; Christensen, C.L.; Samur, M.; Wong, K.K.; Kufe, D. MUC1-C activates EZH2 expression and function in human cancer cells. Sci. Rep. 2017, 7, 7481. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, M.; Suzuki, Y.; Alam, M.; Hinohara, K.; Hasegawa, M.; Jin, C.; Kharbanda, S.; Kufe, D. MUC1-C Stabilizes MCL-1 in the Oxidative Stress Response of Triple-Negative Breast Cancer Cells to BCL-2 Inhibitors. Sci. Rep. 2016, 6, 26643. [Google Scholar] [CrossRef]
- Dyer, M.J.; MacFarlane, M.; Cohen, G.M. Barriers to effective TRAIL-targeted therapy of malignancy. J. Clin. Oncol. 2007, 25, 4505–4506. [Google Scholar] [CrossRef]
- Koschny, R.; Walczak, H.; Ganten, T.M. The promise of TRAIL--potential and risks of a novel anticancer therapy. J. Mol. Med. (Berl) 2007, 85, 923–935. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, X.; Xu, T.; Kong, Q.; Zhang, Y.; Shen, Y.; Wei, Y.; Wang, G.; Chang, K.J. Overcoming resistance to TRAIL-induced apoptosis in solid tumor cells by simultaneously targeting death receptors, c-FLIP and IAPs. Int. J. Oncol. 2016, 49, 153–163. [Google Scholar] [CrossRef]
- Herbst, R.S.; Eckhardt, S.G.; Kurzrock, R.; Ebbinghaus, S.; O’Dwyer, P.J.; Gordon, M.S.; Novotny, W.; Goldwasser, M.A.; Tohnya, T.M.; Lum, B.L.; et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J. Clin. Oncol. 2010, 28, 2839–2846. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Brown, R.E.; Buryanek, J.; Trent, J.; Ashkenazi, A.; Herbst, R.; Kurzrock, R. Targeting the apoptotic pathway in chondrosarcoma using recombinant human Apo2L/TRAIL (dulanermin), a dual proapoptotic receptor (DR4/DR5) agonist. Mol. Cancer Ther. 2012, 11, 2541–2546. [Google Scholar] [CrossRef]
- Wang, S.; El-Deiry, W.S. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 2003, 22, 8628–8633. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.M.; Ettenberg, S.A.; Nau, M.M.; Russell, E.K.; Lipkowitz, S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 1999, 59, 734–741. [Google Scholar] [PubMed]
- Twomey, J.D.; Kim, S.R.; Zhao, L.; Bozza, W.P.; Zhang, B. Spatial dynamics of TRAIL death receptors in cancer cells. Drug Resist. Updat. 2015, 19, 13–21. [Google Scholar] [CrossRef]
- Wagner, K.W.; Punnoose, E.A.; Januario, T.; Lawrence, D.A.; Pitti, R.M.; Lancaster, K.; Lee, D.; von Goetz, M.; Yee, S.F.; Totpal, K.; et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat. Med. 2007, 13, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Dufour, F.; Rattier, T.; Shirley, S.; Picarda, G.; Constantinescu, A.A.; Morle, A.; Zakaria, A.B.; Marcion, G.; Causse, S.; Szegezdi, E.; et al. N-glycosylation of mouse TRAIL-R and human TRAIL-R1 enhances TRAIL-induced death. Cell Death Differ. 2017, 24, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Micheau, O. Regulation of TNF-Related Apoptosis-Inducing Ligand Signaling by Glycosylation. Int. J. Mol. Sci. 2018, 19, 715. [Google Scholar] [CrossRef] [PubMed]
- Merino, D.; Lalaoui, N.; Morizot, A.; Schneider, P.; Solary, E.; Micheau, O. Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol. Cell Biol. 2006, 26, 7046–7055. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, A.; Farshdousti Hagh, M.; Alivand, M.R.; Akbari, A.A.M.; Shams Asenjan, K.; Saraei, R.; Solali, S. Down-regulation of intracellular anti-apoptotic proteins, particularly c-FLIP by therapeutic agents; the novel view to overcome resistance to TRAIL. J. Cell Physiol. 2018, 233, 6470–6485. [Google Scholar] [CrossRef] [PubMed]
- Safa, A.R. c-FLIP, a master anti-apoptotic regulator. Exp. Oncol. 2012, 34, 176–184. [Google Scholar] [PubMed]
- Shirley, S.; Micheau, O. Targeting c-FLIP in cancer. Cancer Lett. 2013, 332, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Debatin, K.M. Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res. 2004, 64, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Guseva, N.V.; Rokhlin, O.W.; Taghiyev, A.F.; Cohen, M.B. Unique resistance of breast carcinoma cell line T47D to TRAIL but not anti-Fas is linked to p43cFLIP(L). Breast Cancer Res. Treat. 2008, 107, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Yerbes, R.; Lopez-Rivas, A. Flavopiridol induces cellular FLICE-inhibitory protein degradation by the proteasome and promotes TRAIL-induced early signaling and apoptosis in breast tumor cells. Cancer Res. 2006, 66, 8858–8869. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Marsters, S.; Ye, X.; Luis, E.; Gonzalez, L.; Ashkenazi, A. E-cadherin couples death receptors to the cytoskeleton to regulate apoptosis. Mol. Cell 2014, 54, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Raulf, N.; El-Attar, R.; Kulms, D.; Lecis, D.; Delia, D.; Walczak, H.; Papenfuss, K.; Odell, E.; Tavassoli, M. Differential response of head and neck cancer cell lines to TRAIL or Smac mimetics is associated with the cellular levels and activity of caspase-8 and caspase-10. Br. J. Cancer 2014, 111, 1955–1964. [Google Scholar] [CrossRef]
- Polanski, R.; Vincent, J.; Polanska, U.M.; Petreus, T.; Tang, E.K. Caspase-8 activation by TRAIL monotherapy predicts responses to IAPi and TRAIL combination treatment in breast cancer cell lines. Cell Death Dis. 2015, 6, e1893. [Google Scholar] [CrossRef][Green Version]
- Chen, J.J.; Knudsen, S.; Mazin, W.; Dahlgaard, J.; Zhang, B. A 71-gene signature of TRAIL sensitivity in cancer cells. Mol. Cancer Ther. 2012, 11, 34–44. [Google Scholar] [CrossRef]
- Mauro-Lizcano, M.; Lopez-Rivas, A. Glutamine metabolism regulates FLIP expression and sensitivity to TRAIL in triple-negative breast cancer cells. Cell Death Dis. 2018, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, E.; Malin, D.; Good, D.M.; Cryns, V.L. Methionine Deprivation Induces a Targetable Vulnerability in Triple-Negative Breast Cancer Cells by Enhancing TRAIL Receptor-2 Expression. Clin. Cancer Res. 2015, 21, 2780–2791. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, E.; Malin, D.; Rajanala, H.; Cryns, V.L. Metformin sensitizes triple-negative breast cancer to proapoptotic TRAIL receptor agonists by suppressing XIAP expression. Breast Cancer Res. Treat. 2017, 163, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Jurado, S.; Diaz-Colunga, J.; das Neves, R.P.; Martinez-Lorente, A.; Almazan, F.; Guantes, R.; Iborra, F.J. Mitochondrial levels determine variability in cell death by modulating apoptotic gene expression. Nat. Commun. 2018, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Croce, C.M.; Reed, J.C. Finally, An Apoptosis-Targeting Therapeutic for Cancer. Cancer Res 2016, 76, 5914–5920. [Google Scholar] [CrossRef] [PubMed]
- Del Gaizo Moore, V.; Letai, A. BH3 profiling—Measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett. 2013, 332, 202–205. [Google Scholar] [CrossRef]
- Butterworth, M.; Pettitt, A.; Varadarajan, S.; Cohen, G.M. BH3 profiling and a toolkit of BH3-mimetic drugs predict anti-apoptotic dependence of cancer cells. Br. J. Cancer 2016, 114, 638–641. [Google Scholar] [CrossRef]
- Bhola, P.D.; Mar, B.G.; Lindsley, R.C.; Ryan, J.A.; Hogdal, L.J.; Vo, T.T.; DeAngelo, D.J.; Galinsky, I.; Ebert, B.L.; Letai, A. Functionally identifiable apoptosis-insensitive subpopulations determine chemoresistance in acute myeloid leukemia. J. Clin. Invest. 2016, 126, 3827–3836. [Google Scholar] [CrossRef]
- Ishizawa, J.; Kojima, K.; McQueen, T.; Ruvolo, V.; Chachad, D.; Nogueras-Gonzalez, G.M.; Huang, X.; Pierceall, W.E.; Dettman, E.J.; Cardone, M.H.; et al. Mitochondrial Profiling of Acute Myeloid Leukemia in the Assessment of Response to Apoptosis Modulating Drugs. PLoS ONE 2015, 10, e0138377. [Google Scholar] [CrossRef]
- Pierceall, W.E.; Kornblau, S.M.; Carlson, N.E.; Huang, X.; Blake, N.; Lena, R.; Elashoff, M.; Konopleva, M.; Cardone, M.H.; Andreeff, M. BH3 profiling discriminates response to cytarabine-based treatment of acute myelogenous leukemia. Mol. Cancer Ther. 2013, 12, 2940–2949. [Google Scholar] [CrossRef]
- Flanagan, L.; Meyer, M.; Fay, J.; Curry, S.; Bacon, O.; Duessmann, H.; John, K.; Boland, K.C.; McNamara, D.A.; Kay, E.W.; et al. Low levels of Caspase-3 predict favourable response to 5FU-based chemotherapy in advanced colorectal cancer: Caspase-3 inhibition as a therapeutic approach. Cell Death Dis. 2016, 7, e2087. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.L.; Cawthorne, C.; Zhou, C.; Hodgkinson, C.L.; Walker, M.J.; Trapani, F.; Kadirvel, M.; Brown, G.; Dawson, M.J.; MacFarlane, M.; et al. A caspase-3 ‘death-switch’ in colorectal cancer cells for induced and synchronous tumor apoptosis in vitro and in vivo facilitates the development of minimally invasive cell death biomarkers. Cell Death Dis. 2013, 4, e613. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Jaganathan, S.; Stephen, L.; Hollingshead, M.G.; Layhee, A.; Damour, E.; Govindharajulu, J.P.; Donohue, J.; Esposito, D.; Mapes, J.P.; et al. Effect of a Smac Mimetic (TL32711, Birinapant) on the Apoptotic Program and Apoptosis Biomarkers Examined with Validated Multiplex Immunoassays Fit for Clinical Use. Clin. Cancer Res. 2016, 22, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Schinkothe, T.; Thiruchittampalam, D.; Schmidt, A.; Bomholt, N.; Staib, P. The AutoDiSC: Development and validation of a novel chemotherapy sensitivity and resistance assay. Anticancer Res. 2013, 33, 2491–2498. [Google Scholar] [PubMed]
- Choe, S.C.; Hamacher-Brady, A.; Brady, N.R. Autophagy capacity and sub-mitochondrial heterogeneity shape Bnip3-induced mitophagy regulation of apoptosis. Cell Commun. Signal. CCS 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
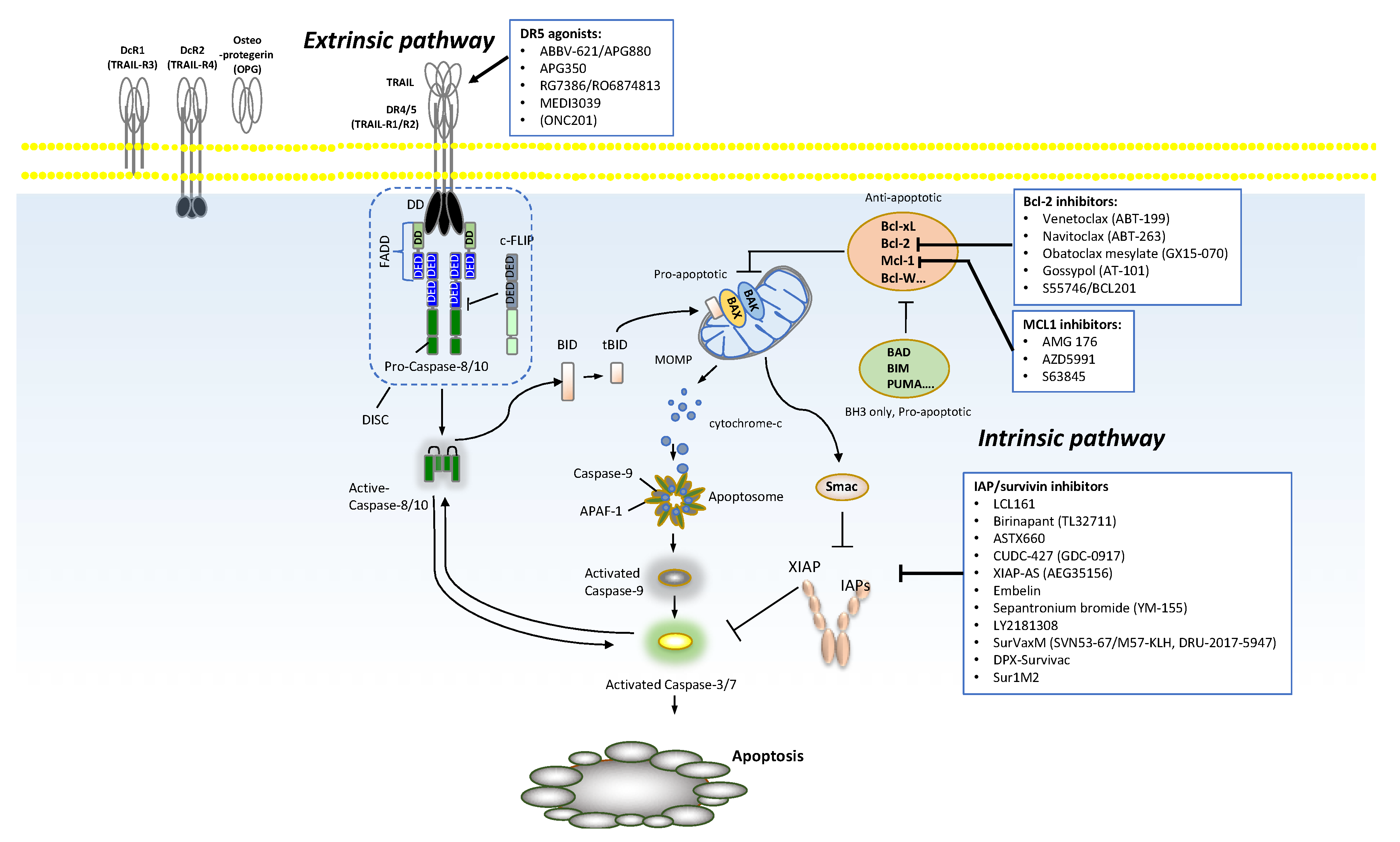
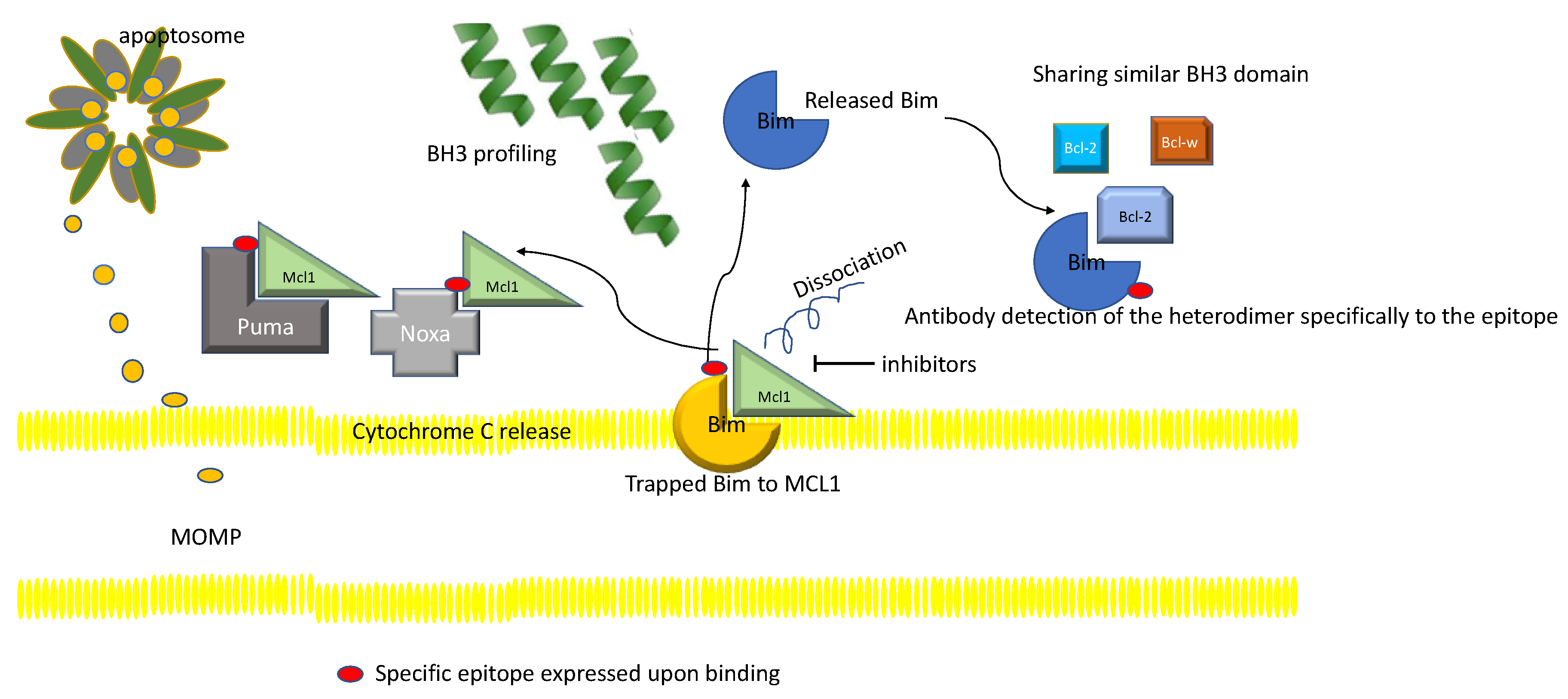
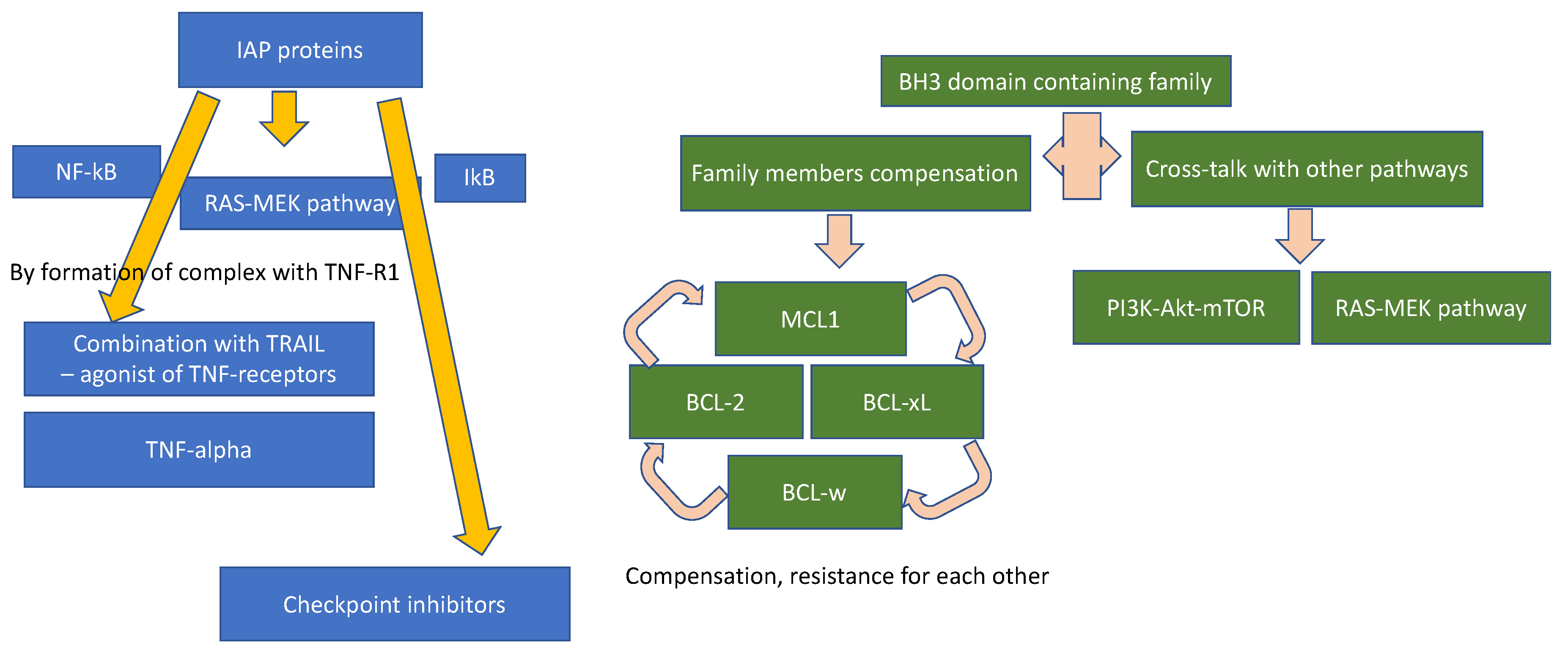

| Agents | Molecule Type and Target | Clinical Trial Phase | Current Status | Combinatorial Agents | Target Disease | Clinical Trial Number |
|---|---|---|---|---|---|---|
| ABBV-621/ | fusion protein monomer | I | recruiting | venetoclax (DLBCL, AML only) | previously treated solid tumors and hematologic malignancies | NCT03082209 |
| APG880 | DR 4/5 | |||||
| APG350 | fusion protein monomer | Preclinical yet | Single agent | solid tumors (colon, pancreatic cancer) in mouse xenograft model | ||
| DR 4/5 | ||||||
| RG7386/ | bispecific antibody | I | completed | Single agent | locally advanced or metastatic solid tumors | NCT02558140 |
| RO6874813 | DR5 | |||||
| TAS266 | tetravalent nanobody | I | terminated | Single agent | solid tumors | NCT01529307 |
| DR5 | ||||||
| MEDI3039 | multivalent scaffold protein superagonist | Preclinical yet | Single agent | solid tumors (breast, colon) in mouse xenograft model | ||
| DR5 | ||||||
| HexaBody®-DR5/DR5 (GEN1029) | DR5 | I/II | recruiting | Single agent | solid tumors | NCT03576131 |
| CPT | circularly permuted TRAIL | Ia | completed | Single agent | lung cancer, colon cancer, lymphoma, multiple myeloma, etc. | ChiCTR-ONRC-12002084 (http://www.chictr.org.cn/) |
| Ib | completed | Single agent | relapsed or refractory multiple myeloma | ChiCTR-TNRC-12001896 | ||
| II | completed | Single agent | relapsed or refractory multiple myeloma | ChiCTR-ONC-12002065 | ||
| IIa | completed | Single agent | multiple malignant solid tumors | ChiCTR-ONRC-12002086 | ||
| II | completed | + thalidomide | relapsed or refractory multiple myeloma | ChiCTR-ONC-12002066 | ||
| II | recruiting | CPT + thalidomide and dexamethasone (TD) or TD alone | relapsed or refractory multiple myeloma | ChiCTR-TRC-11001625 | ||
| III | recruiting | CPT +/− TD | relapsed or refractory multiple myeloma | ChiCTR-IPR-15006024 | ||
| ONC201 | small molecule compound | I | recruiting | Single agent | recurrent H3 K27M-mutant glioma | NCT03416530 |
| I | recruiting | Single agent | solid tumors | NCT02324621 | ||
| I | recruiting | Single agent | solid tumors, multiple myeloma | NCT02609230 | ||
| I | suspended | Single agent | solid tumors | NCT02250781 | ||
| I/II | recruiting | Single agent | multiple myeloma | NCT02863991 | ||
| I/II | recruiting | ixazomib, dexamethasone | multiple myeloma | NCT03492138 | ||
| I/II | recruiting | +/− cytarabine | relapsed/refractory acute leukemia and high-risk myelodysplastic syndromes | NCT02392572 | ||
| I/II | recruiting | Single agent | relapsed/refractory non-Hodgkin’s lymphoma (NHL) | NCT02420795 | ||
| I/II | withdrawn | N/A | glioblastoma | NCT02038699 | ||
| II | recruiting | Single agent | recurrent/refractory metastatic breast cancer and advanced endometrial carcinoma | NCT03394027 | ||
| II | recruiting | Single agent | recurrent or metastatic endometrial cancer | NCT03099499 | ||
| II | recruiting | Single agent | recurrent or metastatic type II endometrial cancer endometrial cancer | NCT03485729 | ||
| II | recruiting | Single agent | neuroendocrine tumors | NCT03034200 | ||
| II | recruiting | Single agent | recurrent glioblastoma and H3 K27M-mutant glioma | NCT02525692 | ||
| II | recruiting | Single agent | recurrent H3 K27M-mutant glioma | NCT03295396 |
| Agents and Ongoing Trials | Target Disease | Phase of the Trial | Combinatorial Agents | Supportive Pre-Clinical Data/Mechanism of Action | Serial NCT Number |
|---|---|---|---|---|---|
| Venetoclax (BCL2 inhibitor without BCL XL activity) | |||||
| A Study of Venetoclax and Dexamethasone compared with Pomalidomide and Dexamethasone in subjects with relapsed or refractory Multiple Myeloma | Multiple myeloma | III | Pomalidomide Dexamethasone | Baseline multiple myeloma therapy Bcl-2 one of the critical mechanisms of resistance | NCT03539744 |
| Duvelisib and Venetoclax in Relapsed or Refractory CLL or SLL | Chronic/small lymphocytic leukemia | I/II | Duvelisib | Pre-clinical study showed the combination with duvelisib, a PI3K-δ and PI3K-γ inhibitor reversing the resistance to Bcl2 inhibition (which one to pick) | NCT03534323 |
| Venetoclax in treating participants with recurrent or refractory Mature T-Cell Lymphoma | T cell lymphoma | II | Single agent | Bcl-2 overexpression as a resistance mechanism to conventional therapy in mantle cell lymphma | NCT03534180 |
| Venetoclax, Lenalidomide and Rituximab in patients with previously untreated Mantle Cell Lymphoma | Mantle cell lymphoma | I | Lenalidomide and rituximab | Combination of venetoclax to preclude resistance to standard of care, as first line therapy | NCT03523975 |
| Venetoclax and Ibrutinib in treating in participants with Chronic Lymphocytic Leukemia and Ibrutinib resistance mutations | Chronic lymphocytic leukemia | II | ibrutinib | Overcome the resistant to ibrutinib by combination with venetoclax | NCT03513562 |
| Venetoclax, Lenalidomide and Rituximab in patients with relapsed/refractory Mantle Cell Lymphoma | Mantle cell lymphoma | I/II | Lenalidomide and rituximab | Combination of venetoclax to overcome resistance to standard therapy | NCT03505944 |
| Venetoclax and Vincristine Liposomal in treating patients with relapsed or refractory T-cell or B-cell Acute Lymphoblastic Leukemia | Acute lymphoblastic leukemia | Ib/II | Liposomal vincristine | Addition of venetoclax to the previously shown efficacy of vincristine to induce Philadelphia chromosome negative ALL | NCT03504644 |
| Study of Venetoclax, a BCL2 antagonist, for patients with Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN) | BPDCN | I | Single agent | BPDCN depends on BCL2 thus sensitive to venetoclax [203] | NCT03485547 |
| A Study of Venetoclax and Dinaciclib (MK7965) in patients with relapsed/refractory Acute Myeloid Leukemia | Acute myeloid leukemia | II | Dinaciclib | Pre-clinical study showed that CDK9 inhibition mediates venetoclax sensitization | NCT03484520 |
| Study of Venetoclax with the mIDH1 Inhibitor Ivosidenib (AG120) in IDH1-mutated hematologic malignancies | Hematological malignancies with IDH1 mutations | Phase I with dose expansion | Ivosidenib (inhibitor of mutated IDH1) | Preclinical study showing hematological malignancies with mutated IDH1 | NCT03471260 |
| Navitoclax (BCL2, BCL-Xl, BCLw) | |||||
| Navitoclax and Vistusertib in treating patients with relapsed Small Cell Lung Cancer and other solid tumors | Small cell lung cancer, other metastatic solid tumors | I/II | vistusertib | Apoptosis and mTOR inhibition synergistically inhibit the growth of lung cancer (however, the preclinical study was more in NSCLC) [204] | NCT03366103 |
| Osimertinib and Navitoclax in treating patients with EGFR- positive previously treated advanced or metastatic Non-Small Cell Lung Cancer | Non-small cell lung cancer | Ib | Osimertinib (T790M cell sensitive EGFR inhibitor) | Bcl-2 inhibitor navitoclax induce tumor growth inhibition in osimertinib resistant NSCLC [205] | NCT02520778 |
| Navitoclax and Sorafenib Tosylate in treating patients with relapsed or refractory solid tumors | Metastatic solid tumors | I | Sorafenib | BCL-2 proteins determine sorafenib/regorafenib resistance and BH3-mimetic efficacy in hepatocellular carcinoma [206] | NCT02143401 |
| Trametinib and Navitoclax in treating patients with advanced or metastatic solid tumors | Metastatic solid tumors | Ib/II | Trametinib | BCL-2 confers de novo resistance in BRCA mutated solid cancer [207] | NCT02079740 |
| Dabrafenib, Trametinib, and Navitoclax in treating patients with BRAF mutant Melanoma or solid tumors | Solid tumors | I/II | Dabrafenib, trametinib | Combination of RAF/MEK pathway inhibitor along with BCL2 inhibitor, often noted as a resistance mechanism in BRAF mutant solid tumors | NCT01989585 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, B.; Greer, Y.; Lipkowitz, S.; Takebe, N. Novel Apoptosis-Inducing Agents for the Treatment of Cancer, a New Arsenal in the Toolbox. Cancers 2019, 11, 1087. https://doi.org/10.3390/cancers11081087
Lim B, Greer Y, Lipkowitz S, Takebe N. Novel Apoptosis-Inducing Agents for the Treatment of Cancer, a New Arsenal in the Toolbox. Cancers. 2019; 11(8):1087. https://doi.org/10.3390/cancers11081087
Chicago/Turabian StyleLim, Bora, Yoshimi Greer, Stanley Lipkowitz, and Naoko Takebe. 2019. "Novel Apoptosis-Inducing Agents for the Treatment of Cancer, a New Arsenal in the Toolbox" Cancers 11, no. 8: 1087. https://doi.org/10.3390/cancers11081087
APA StyleLim, B., Greer, Y., Lipkowitz, S., & Takebe, N. (2019). Novel Apoptosis-Inducing Agents for the Treatment of Cancer, a New Arsenal in the Toolbox. Cancers, 11(8), 1087. https://doi.org/10.3390/cancers11081087





