Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child–Pugh A Liver Function: A Proof-Of-Concept Study
Abstract
1. Introduction
2. Methods
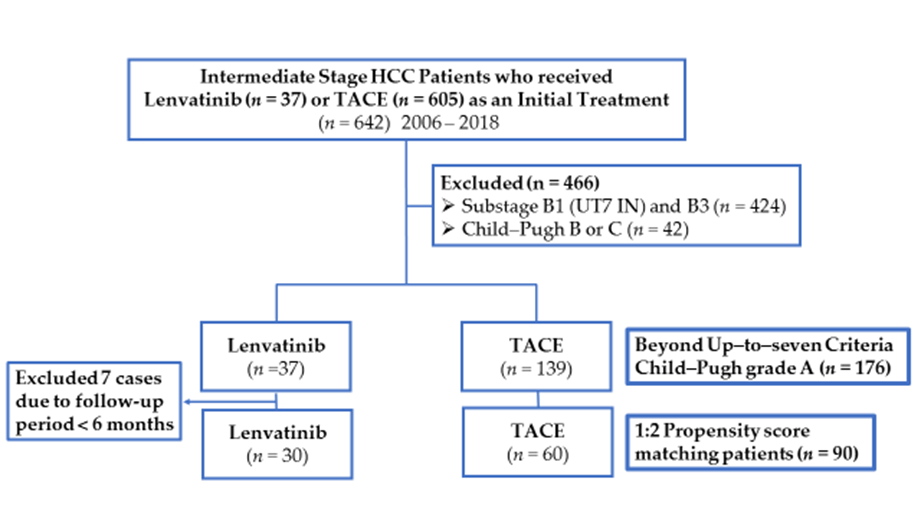
2.1. Patients
2.2. Treatment Protocol
2.3. Propensity Score Matching
2.4. Evaluation of Treatment Response
2.5. Efficacy Analysis
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
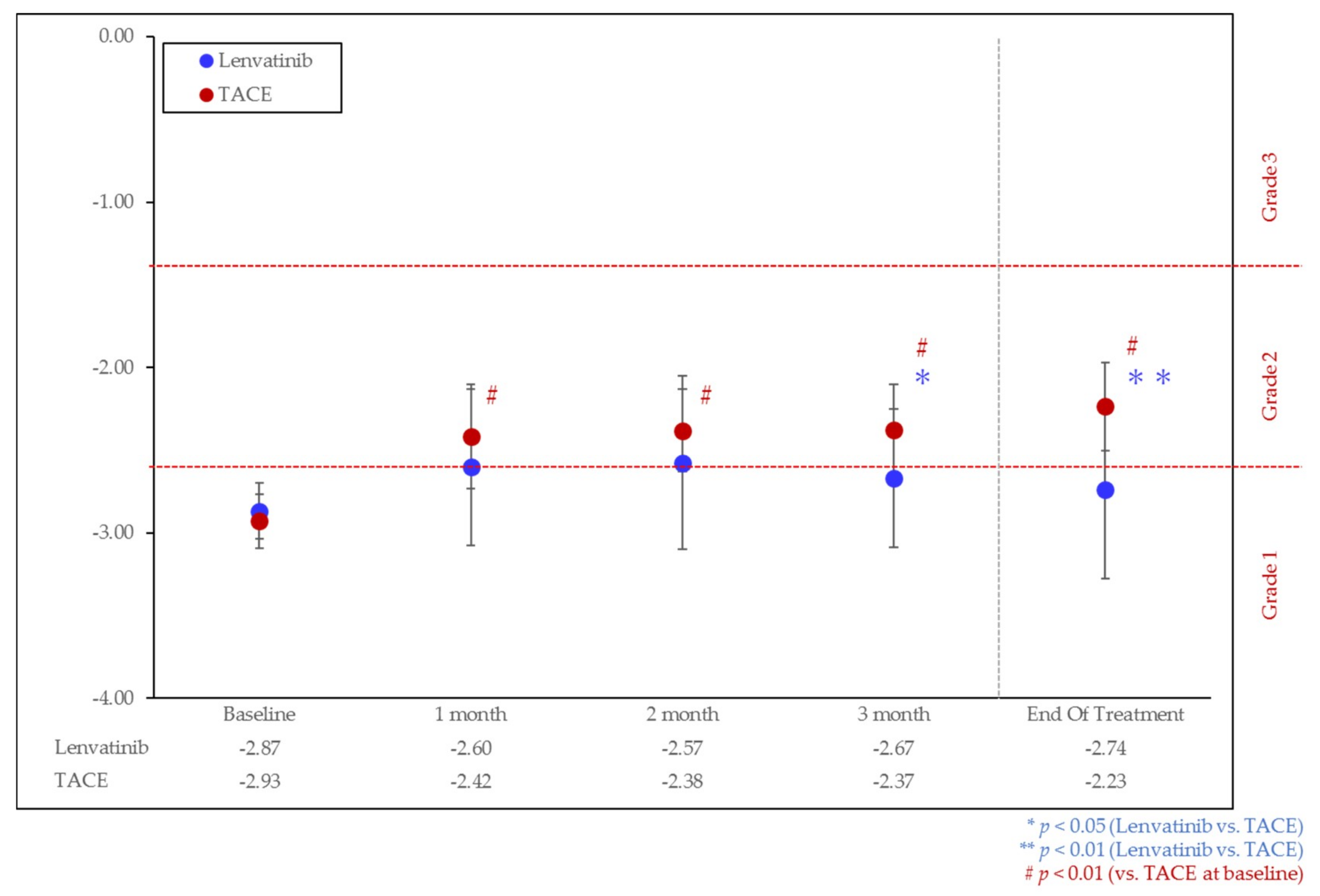
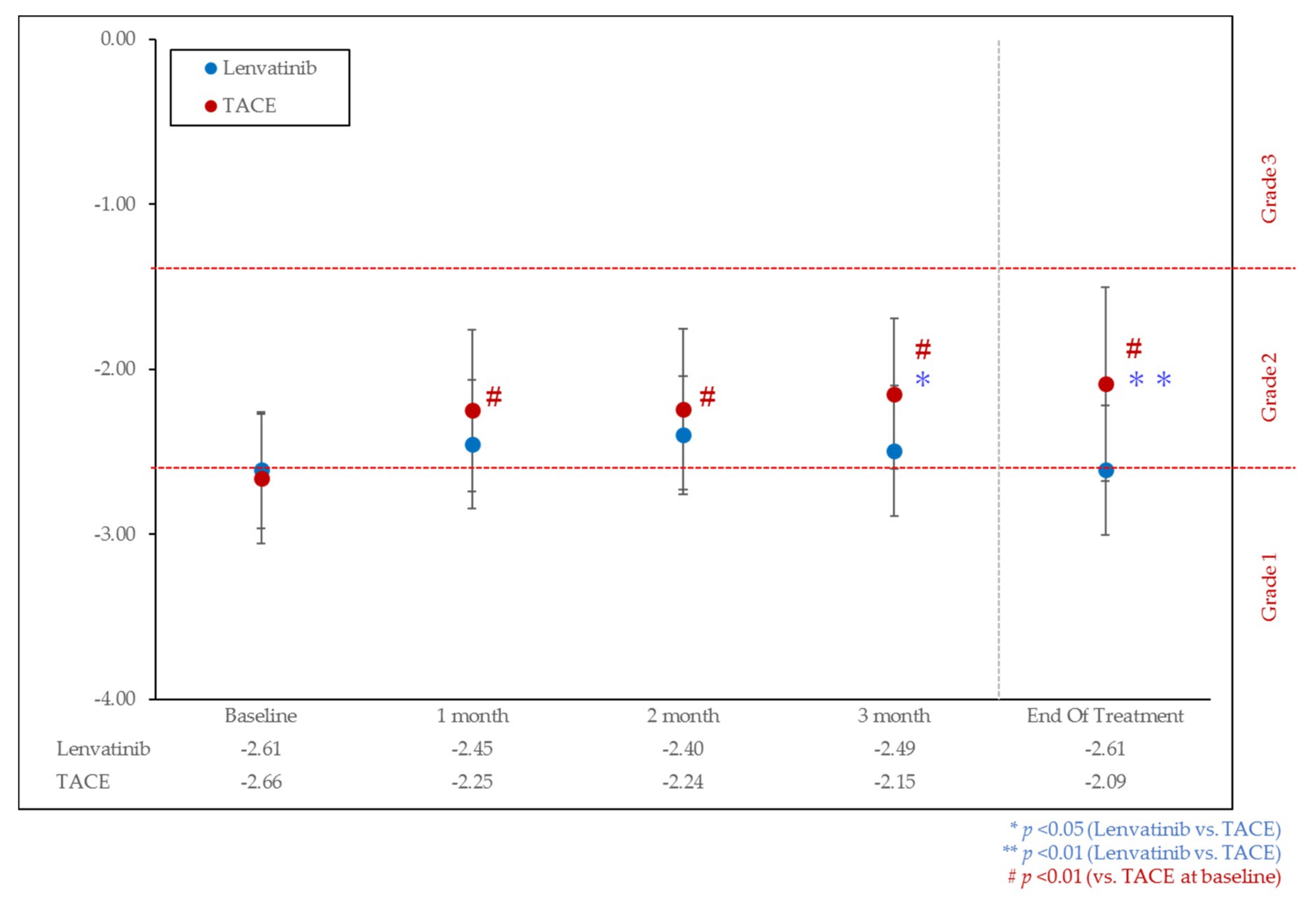
3.2. Change of Liver Function
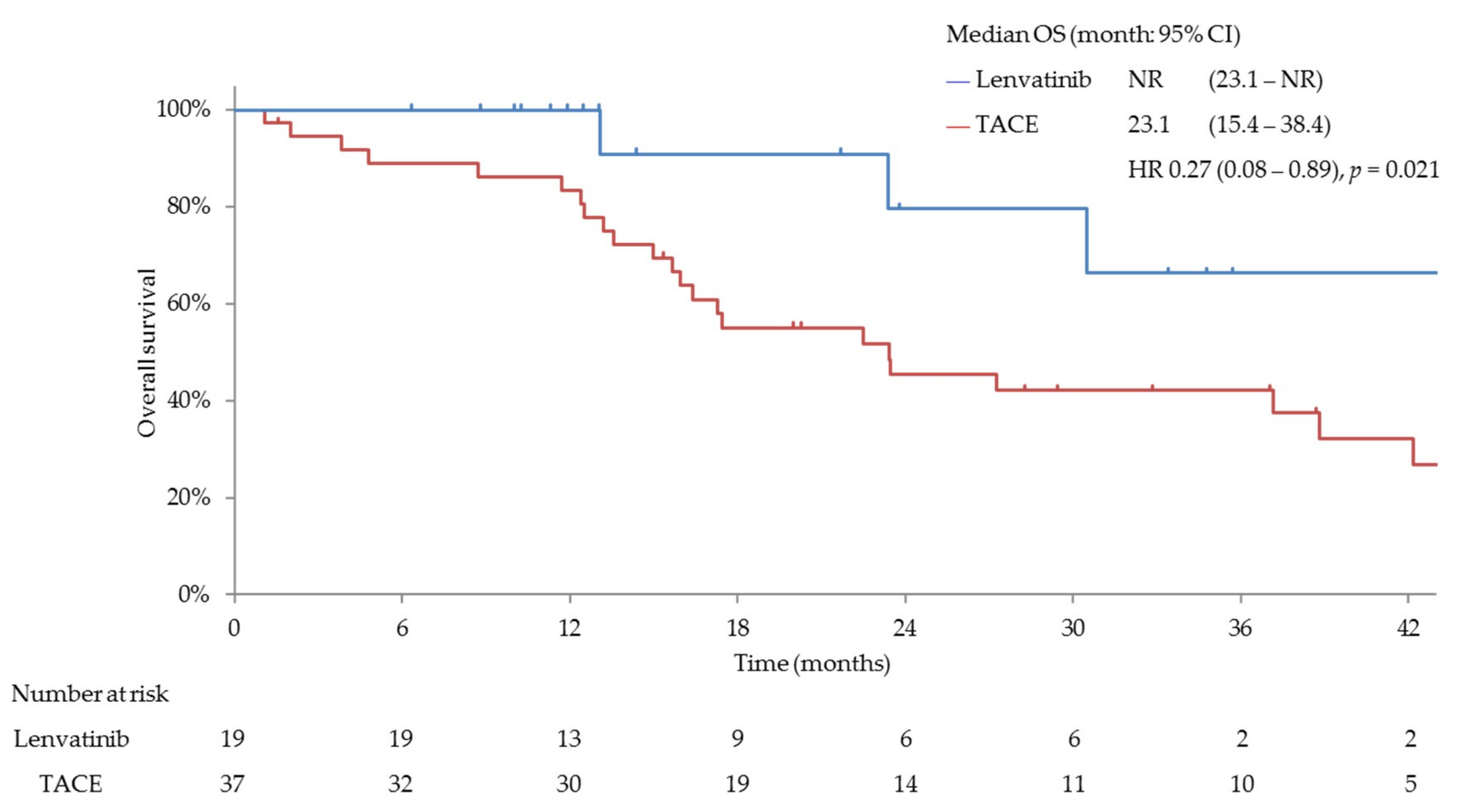
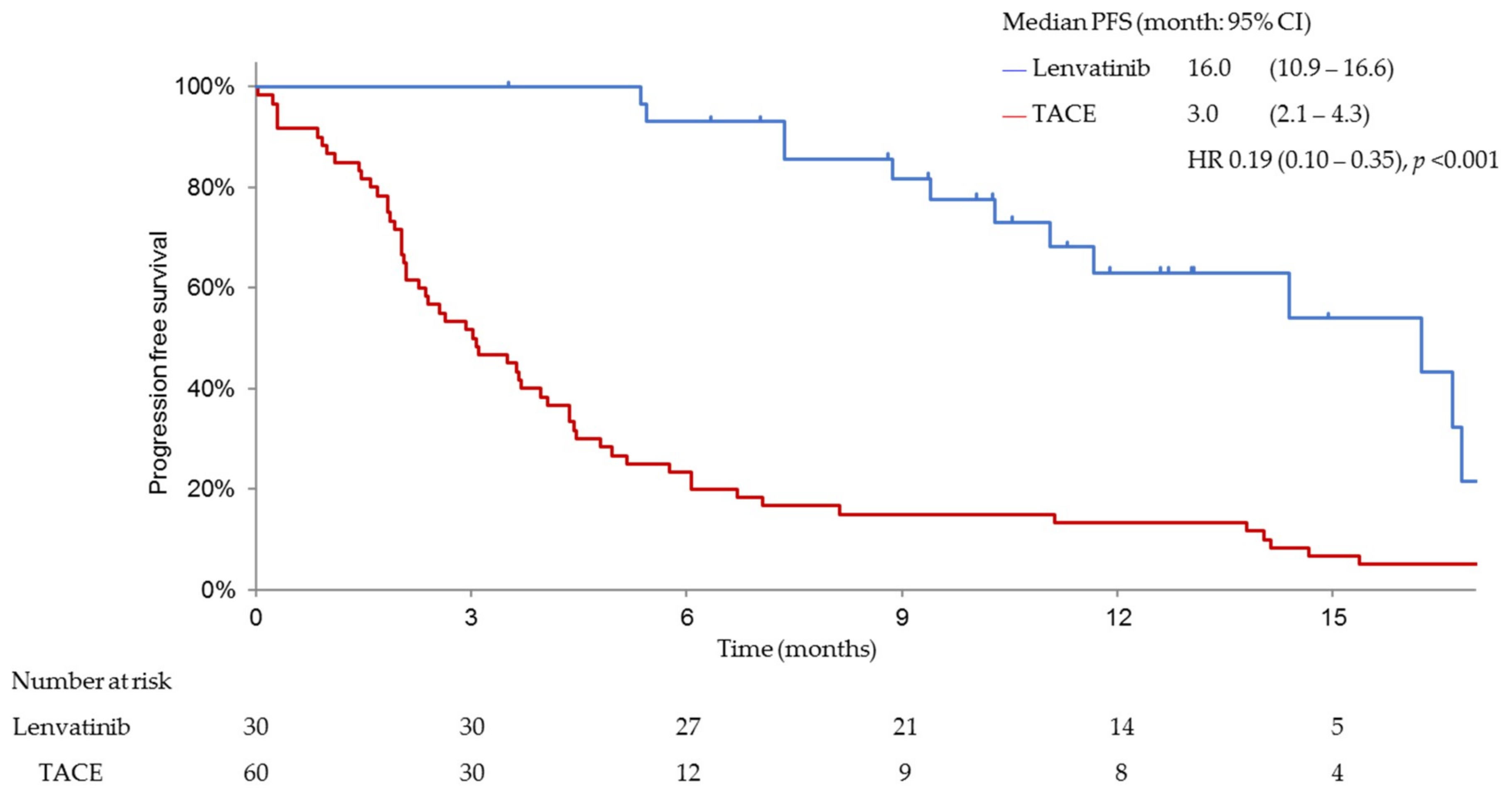
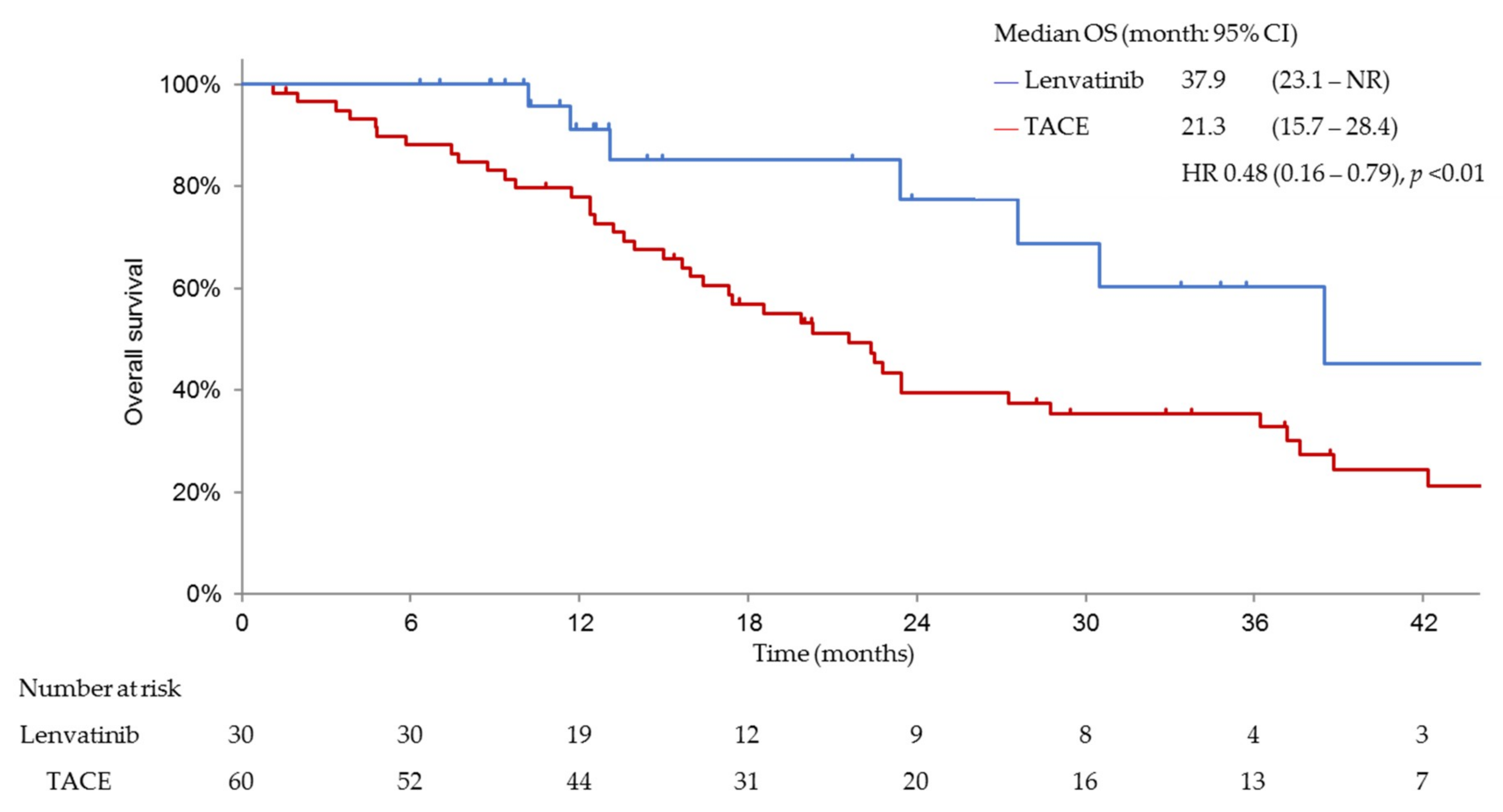
3.3. Efficacy and Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

| Response Category | Lenvatinib n = 19 (%) | TACE n = 37 (%) | p-Value Odds Ratio (95% CI) |
|---|---|---|---|
| ORR | 14 (73.7%) | 14 (37.8%) | p < 0.05 |
| 4.47 (1.19–19.51) | |||
| CBR (CR + PR + SD ≥ 24w) | 19 (100.0%) | 16 (43.2%) | |
| DCR | 19 (100.0%) | 22 (59.5%) | |
| CR | 1 | 4 | |
| PR | 13 | 10 | |
| SD | 5 | 8 | |
| Durable stable disease (SD ≥ 24w) | 5 | 2 | |
| PD | 0 | 13 | |
| NE | 0 | 2 |
References
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Balogh, J.; Victor, D., III; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- GLOBOCAN. 2018. Available online: http://gco.iarc.fr/today/fact-sheets-cancers (accessed on 1 January 2019).
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Reig, M.; Sherman, M. Evidence-Based Diagnosis, Staging, and Treatment of Patients with Hepatocellular Carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef] [PubMed]
- Bolondi, L.; Burroughs, A.; Dufour, J.F.; Galle, P.R.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Sangro, B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: Proposal for a subclassification to facilitate treatment decisions. Semin. Liver Dis. 2012, 32, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Arizumi, T.; Ueshima, K.; Sakurai, T.; Kitano, M.; Nishida, N. Subclassification of BCLC B Stage Hepatocellular Carcinoma and Treatment Strategies: Proposal of Modified Bolondi’s Subclassification (Kinki Criteria). Dig. Dis. 2015, 33, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Lenvatinib may drastically change the treatment landscape of hepatocellular carcinoma. Liver Cancer 2018, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Arizumi, T.; Minami, T.; Chishina, H.; Kono, M.; Takita, M.; Yada, N.; Hagiwara, S.; Minami, Y.; Ida, H.; Ueshima, K.; et al. Time to Transcatheter Arterial Chemoembolization Refractoriness in Patients with Hepatocellular Carcinoma in Kinki Criteria Stages B1 and B2. Dig. Dis. 2017, 35, 589–597. [Google Scholar] [CrossRef]
- Matsui, J.; Funahashi, Y.; Uenaka, T.; Watanabe, T.; Tsuruoka, A.; Asada, M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin. Cancer Res. 2008, 14, 5459–5465. [Google Scholar] [CrossRef]
- Matsui, J.; Yamamoto, Y.; Funahashi, Y.; Tsuruoka, A.; Watanabe, T.; Wakabayashi, T.; Uenaka, T.; Asada, M. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int. J. Cancer 2008, 122, 664–671. [Google Scholar] [CrossRef]
- Yamada, K.; Yamamoto, N.; Yamada, Y.; Nokihara, H.; Fujiwara, Y.; Hirata, T.; Koizumi, F.; Nishio, K.; Koyama, N.; Tamura, T. Phase I dose-escalation study and biomarker analysis of E7080 in patients with advanced solid tumors. Clin. Cancer Res. 2011, 17, 2528–2537. [Google Scholar] [CrossRef]
- Boss, D.S.; Glen, H.; Beijnen, J.H.; Keesen, M.; Morrison, R.; Tait, B.; Copalu, W.; Mazur, A.; Wanders, J.; O’Brien, J.P.; et al. A phase I study of E7080, a multitargeted tyrosine kinase inhibitor, in patients with advanced solid tumours. Br. J. Cancer 2012, 106, 1598–1604. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Yamashita, T.; Kudo, M.; Ikeda, K.; Izumi, N.; Tateishi, R.; Ikeda, M.; Aikata, H.; Kawaguchi, Y.; Wada, Y.; Numata, K.; et al. REFLECT-A phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: Ananalysis of Japanesesubset. J. Gastroenterol. 2019, 54, 558–570. [Google Scholar]
- Kudo, M. Extremely High Objective Response Rate of Lenvatinib: Its Clinical Relevance and Changing the Treatment Paradigm in Hepatocellular Carcinoma. Liver Cancer 2018, 7, 215–224. [Google Scholar] [CrossRef]
- Kudo, M. Lenvatinib in Advanced Hepatocellular Carcinoma. Liver Cancer 2017, 6, 253–263. [Google Scholar] [CrossRef]
- Ogasawara, S.; Chiba, T.; Ooka, Y.; Kanogawa, N.; Motoyama, T.; Suzuki, E.; Tawada, A.; Kanai, F.; Yoshikawa, M.; Yokosuka, O. Efficacy of Sorafenib in Intermediate-Stage Hepatocellular Carcinoma Patients Refractory to Transarterial Chemoembolization. Oncology 2014, 87, 330–341. [Google Scholar] [CrossRef]
- Arizumi, T.; Ueshima, K.; Minami, T.; Kono, M.; Chishina, H.; Takita, M.; Kitai, S.; Inoue, T.; Yada, N.; Hagiwara, S.; et al. Effectiveness of Sorafenib in patients with transcatheter arterial chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer 2015, 4, 253–262. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Kudo, M.; Hirooka, M.; Koizumi, Y.; Hiasa, Y.; Tajiri, K.; Toyoda, H.; Tada, T.; Ochi, H.; et al. Hepatic Function during Repeated TACE Procedures and Prognosis after Introducing Sorafenib in Patients with Unresectable Hepatocellular Carcinoma: Multicenter Analysis. Dig. Dis. 2017, 35, 602–610. [Google Scholar] [CrossRef]
- Arizumi, T.; Minami, T.; Chishina, H.; Kono, M.; Takita, M.; Yada, N.; Hagiwara, S.; Minami, Y.; Ida, H.; Ueshima, K.; et al. Impact of Tumor Factors on Survival in Patients with Hepatocellular Carcinoma Classified Based on Kinki Criteria Stage B2. Dig. Dis. 2017, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Trevisani, F.; Abou-Alfa, G.K.; Rimassa, L. Hepatocellular Carcinoma: Therapeutic Guidelines and Medical Treatment. Liver Cancer 2017, 6, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Kudo, M.; Kawazoe, S.; Osaki, Y.; Ikeda, M.; Okusaka, T.; Tamai, T.; Suzuki, T.; Hisai, T.; Hayato, S.; et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J. Gastroenterol. 2017, 52, 512–519. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Protocol Development. Cancer Therapy Evaluation Program. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 21 March 2017).
- Kudo, M.; Ueshima, K.; Yokosuka, O.; Ogasawara, S.; Obi, S.; Izumi, N.; Aikata, H.; Nagano, H.; Hatano, E.; Sasaki, Y.; et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): A randomised, open label, phase 3 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 424–432. [Google Scholar] [CrossRef]
- Kudo, M.; Matsui, O.; Izumi, N.; Iijima, H.; Kadoya, M.; Imai, Y.; Okusaka, T.; Miyayama, S.; Tsuchiya, K.; Ueshima, K.; et al. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer 2014, 3, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Mamdani, M.M. A comparison of propensity score methods: A case-study estimating the effectiveness of post-AMI statin use. Stat Med. 2006, 25, 2084–2106. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Arizumi, T.; Ueshima, K.; Iwanishi, M.; Minami, T.; Chishina, H.; Kono, M.; Takita, M.; Kitai, S.; Inoue, T.; Yada, N.; et al. Validation of a Modified Substaging System (Kinki Criteria) for Patients with Intermediate-Stage Hepatocellular Carcinoma. Oncology 2015, 89 (Suppl. S2), 47–52. [Google Scholar] [CrossRef]
- Peck-Radosavljevic, M.; Kudo, M.; Raoul, J.L.; Lee, H.C.; Decaens, T.; Heo, J.; Lin, S.M.; Shan, H.; Yang, Y.; Bayh, I.; et al. Outcomes of patients (pts) with hepatocellular carcinoma (HCC) treated with transarterial chemoembolization (TACE): Global OPTIMIS final analysis. J. Clin. Oncol. 2018, 36, 4018. [Google Scholar] [CrossRef]
- Yasui, Y.; Tsuchiya, K.; Kurosaki, M.; Takeguchi, T.; Takeguchi, Y.; Okada, M.; Wang, W.; Kubota, Y.; Goto, T.; Komiyama, Y.; et al. Up-to-seven criteria as a useful predictor for tumor downstaging to within Milan criteria and Child-Pugh grade deterioration after initial conventional transarterial chemoembolization. Hepatol. Res. 2018, 48, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Ohkawa, K.; Miyazaki, M.; Sakakibara, M.; Imanaka, K.; Tamura, T.; Sueyoshi, H.; Takada, R.; Fukutake, N.; Uehara, H.; et al. Subclassification of patients with intermediate-stage (Barcelona Clinic Liver Cancer stage-B) hepatocellular carcinoma using the up-to-seven criteria and serum tumor markers. Hepatol. Int. 2017, 11, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Takayasu, K.; Arii, S.; Kudo, M.; Ichida, T.; Matsui, O.; Izumi, N.; Matsuyama, Y.; Sakamoto, M.; Nakashima, O.; Ku, Y.; et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J. Hepatol. 2012, 56, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Terashima, T.; Yamashita, T.; Arai, K.; Kawaguchi, K.; Kitamura, K.; Yamashita, T.; Sakai, Y.; Mizukoshi, E.; Honda, M.; Kaneko, S. Beneficial Effect of Maintaining Hepatic Reserve during Chemotherapy on the Outcomes of Patients with Hepatocellular Carcinoma. Liver Cancer 2017, 6, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, K.; Nishida, N.; Hagiwara, S.; Aoki, T.; Minami, T.; Chishina, H.; Takita, M.; Minami, Y.; Ida, H.; Takenaka, M.; et al. Impact of baseline ALBI grade on the outcomes of hepatocellular carcinoma patients treated with lenvatinib: A multicenter study. Cancers 2019, 11, 952. [Google Scholar] [CrossRef]
- Li, X.; Feng, G.S.; Zheng, C.S.; Zhuo, C.K.; Liu, X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J. Gastroenterol. 2004, 10, 2878–2882. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Wang, B.; Xu, H.; Gao, Z.Q.; Ning, H.F.; Sun, Y.Q.; Cao, G.W. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008, 49, 523–529. [Google Scholar] [CrossRef]
- Sergio, A.; Cristofori, C.; Cardin, R.; Pivetta, G.; Ragazzi, R.; Baldan, A.; Girardi, L.; Cillo, U.; Burra, P.; Giacomin, A.; et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): The role of angiogenesis and invasiveness. Am. J. Gastroenterol. 2008, 103, 914–921. [Google Scholar] [CrossRef]
- Jiang, H.; Meng, Q.; Tan, H.; Pan, S.; Sun, B.; Xu, R.; Sun, X. Antiangiogenic therapy enhances the efficacy of transcatheter arterial embolization for hepatocellular carcinomas. Int. J. Cancer 2007, 121, 416–424. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Kano, M.R.; Komuta, Y.; Iwata, C.; Oka, M.; Shirai, Y.T.; Morishita, Y.; Ouchi, Y.; Kataoka, K.; Miyazono, K. Comparison of the effects of the kinase inhibitors imatinib, sorafenib, and transforming growth factor-beta receptor inhibitor on extravasation of nanoparticles from neovasculature. Cancer Sci. 2009, 100, 173–180. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| Lenvatinib n = 37 (%) | TACE n = 139 (%) | p Value | Lenvatinib n = 30 (%) | TACE n = 60 (%) | p Value | |
| Age, mean | 68.6 | 71.9 | 0.173 | 68.2 | 72.4 | 0.272 |
| Gender, male | 30 (81.1%) | 106 (76.3%) | 0.661 | 24 (80.0%) | 42 (70.0%) | 0.449 |
| HCV positive | 15 (40.5%) | 84 (60.4%) | 0.040 | 12 (40.0%) | 29 (48.3%) | 0.506 |
| HBV positive | 8 (21.6%) | 12 (8.6%) | 0.039 | 7 (23.3%) | 10 (16.7%) | 0.430 |
| Alcohol abuse | 6 (16.2%) | 27 (19.4%) | 0.814 | 3 (10.0%) | 10 (16.7%) | 0.532 |
| Size of intrahepatic lesion, >30 mm | 26 (70.3%) | 81 (58.3%) | 0.255 | 20 (66.7%) | 39 (65.0%) | 1.000 |
| Number of intrahepatic lesion, >5 | 17 (45.9%) | 70 (50.4%) | 0.713 | 14 (46.7%) | 35 (45.0%) | 0.371 |
| EHS positive | 0 (0%) | 0 (0%) | 1.000 | 0 (0%) | 0 (0%) | 1.000 |
| MVI positive | 0 (0%) | 0 (0%) | 1.000 | 0 (0%) | 0 (0%) | 1.000 |
| Child–Pugh score 5A | 25 (67.6%) | 91 (65.5%) | 0.848 | 20 (66.7%) | 37 (61.7%) | 0.817 |
| Child–Pugh score 6A | 12 (32.4%) | 48 (34.5%) | 0.848 | 10 (33.3%) | 23 (38.3%) | 0.817 |
| Child–Pugh score ≥ 7 | 0 (0%) | 0 (0%) | 1.000 | 0 (0%) | 0 (0%) | 1.000 |
| ALBI grade 1 | 22 (59.5%) | 66 (47.5%) | 0.267 | 19 (63.3%) | 37 (61.7%) | 0.880 |
| Albumin, median (g/dL) | 4.0 | 3.8 | 0.092 | 4.0 | 3.9 | 0.881 |
| Total bilirubin, median (mg/dL) | 0.7 | 0.7 | 0.098 | 0.7 | 0.7 | 0.293 |
| Baseline AFP ≥ 200 ng/mL | 18 (48.6%) | 97 (69.8%) | 0.020 | 15 (50.0%) | 28 (46.7%) | 0.825 |
| Baseline AFP, median (ng/mL) | 101 | 28 | 0.049 | 103 | 107 | 0.355 |
| Response Category | Lenvatinib n = 30 (%) | TACE n = 60 (%) | p-Value Odds Ratio (95% CI) |
|---|---|---|---|
| ORR | 22 (73.3%) | 20 (33.3%) | p < 0.001 |
| 5.39 (1.90–16.67) | |||
| CBR(CR + PR + SD ≥ 24w) | 29 (96.7%) | 22 (36.7%) | p < 0.001 |
| 48.1 (7.01–2073.85) | |||
| DCR | 30 (100.0%) | 32 (53.3%) | |
| CR | 2 | 4 | |
| PR | 20 | 16 | |
| SD | 8 | 12 | |
| Durable stable disease (SD ≥ 24w) | 7 | 2 | |
| PD | 0 | 26 | |
| NE | 0 | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudo, M.; Ueshima, K.; Chan, S.; Minami, T.; Chishina, H.; Aoki, T.; Takita, M.; Hagiwara, S.; Minami, Y.; Ida, H.; et al. Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child–Pugh A Liver Function: A Proof-Of-Concept Study. Cancers 2019, 11, 1084. https://doi.org/10.3390/cancers11081084
Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, Takita M, Hagiwara S, Minami Y, Ida H, et al. Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child–Pugh A Liver Function: A Proof-Of-Concept Study. Cancers. 2019; 11(8):1084. https://doi.org/10.3390/cancers11081084
Chicago/Turabian StyleKudo, Masatoshi, Kazuomi Ueshima, Stephan Chan, Tomohiro Minami, Hirokazu Chishina, Tomoko Aoki, Masahiro Takita, Satoru Hagiwara, Yasunori Minami, Hiroshi Ida, and et al. 2019. "Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child–Pugh A Liver Function: A Proof-Of-Concept Study" Cancers 11, no. 8: 1084. https://doi.org/10.3390/cancers11081084
APA StyleKudo, M., Ueshima, K., Chan, S., Minami, T., Chishina, H., Aoki, T., Takita, M., Hagiwara, S., Minami, Y., Ida, H., Takenaka, M., Sakurai, T., Watanabe, T., Morita, M., Ogawa, C., Wada, Y., Ikeda, M., Ishii, H., Izumi, N., & Nishida, N. (2019). Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child–Pugh A Liver Function: A Proof-Of-Concept Study. Cancers, 11(8), 1084. https://doi.org/10.3390/cancers11081084










