Immunotherapies for the Treatment of Uveal Melanoma—History and Future
Abstract
1. Introduction
2. Dendritic-Cell Vaccination
3. Checkpoint Inhibitors
4. Bispecific Molecules
5. Adoptive T-Cell therapy
6. Other drugs on the horizon
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McLaughlin, C.C.; Wu, X.-C.; Jemal, A.; Martin, H.J.; Roche, L.M.; Chen, V.W. Incidence of noncutaneous melanomas in the U.S. Cancer 2005, 103, 1000–1007. [Google Scholar] [PubMed]
- Damato, B. Progress in the management of patients with uveal melanoma. The 2012 Ashton Lecture. Eye 2012, 26, 1157–1172. [Google Scholar] [PubMed]
- Van Raamsdonk, C.D.; Griewank, K.G.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.C.; Wackernagel, W.; Green, G.; Bouvier, N.; et al. Mutations in GNA11 in Uveal Melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [Google Scholar] [PubMed]
- Van Raamsdonk, C.D.; Bezrookove, V.; Green, G.; Bauer, J.; Gaugler, L.; O'Brien, J.M.; Simpson, E.M.; Barsh, G.S.; Bastian, B.C. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 2009, 457, 599–602. [Google Scholar] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar]
- Cancer Genome Atlas Network. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar]
- Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch. Ophthalmol. 2006, 124, 1684–1693. [Google Scholar]
- Schlereth, S.L.; Neuser, B.; Herwig, M.C.; Müller, A.M.; Koch, K.R.; Reitsamer, H.A.; Schrödl, F.; Cursiefen, C.; Heindl, L.M. Absence of lymphatic vessels in the developing human sclera. Exp. Eye Res. 2014, 125, 203–209. [Google Scholar]
- Schroedl, F.; Brehmer, A.; Neuhuber, W.L.; Kruse, F.E.; May, A.; Cursiefen, C.; Chang, C.-Y.; Green, C.R.; McGhee, C.N.J.; Sherwin, T. The Normal Human Choroid Is Endowed with a Significant Number of Lymphatic Vessel Endothelial Hyaluronate Receptor 1 (LYVE-1)-Positive Macrophages. Investig. Opthalmol. Vis. Sci. 2008, 49, 5222–5229. [Google Scholar]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.O.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent Mutation of BAP1 in Metastasizing Uveal Melanomas. Science 2010, 330, 1410–1413. [Google Scholar]
- Desjardins, L.; Levy-Gabriel, C.; Lumbroso-Lerouic, L.; Sastre, X.; Dendale, R.; Couturier, J.; Piperno-Neumann, S.; Dorval, T.; Mariani, P.; Salmon, R.; et al. Prognostic factors for malignant uveal melanoma. Retrospective study on 2,241 patients and recent contribution of monosomy-3 research. J. Français D’Ophtalmol. 2006, 29, 741–749. [Google Scholar]
- Bornfeld, N.; Prescher, G.; Becher, R.; Hirche, H.; Jöckel, K.-H.; Horsthemke, B. Prognostic implications of monosomy 3 in uveal melanoma. Lancet 1996, 347, 1222–1225. [Google Scholar]
- Gragoudas, E.S.; Egan, K.M.; Seddon, J.M.; Glynn, R.J.; Walsh, S.M.; Finn, S.M.; Munzenrider, J.E.; Spar, M.D. Survival of patients with metastases from uveal melanoma. Ophthalmology 1991, 98, 383–389. [Google Scholar] [PubMed]
- Diener-West, M.; Reynolds, S.M.; Agugliaro, D.J.; Caldwell, R.; Cumming, K.; Earle, J.D.; Hawkins, B.S.; Hayman, J.A.; Jaiyesimi, I.; Jampol, L.M.; et al. Development of Metastatic Disease after Enrollment in the COMS Trials for Treatment of Choroidal Melanoma. Arch. Ophthalmol. 2005, 123, 1639–1643. [Google Scholar] [PubMed]
- Eskelin, S.; Pyrhönen, S.; Hahka-Kemppinen, M.; Tuomaala, S.; Kivelä, T. A prognostic model and staging for metastatic uveal melanoma. Cancer 2003, 97, 465–475. [Google Scholar] [PubMed]
- Pons, F.; Plana, M.; Caminal, J.M.; Pera, J.; Fernandes, I.; Perez, J.; Garcia-Del-Muro, X.; Marcoval, J.; Penin, R.; Fabra, A.; et al. Metastatic uveal melanoma: Is there a role for conventional chemotherapy?—A single center study based on 58 patients. Melanoma Res. 2011, 21, 217–222. [Google Scholar] [PubMed]
- Olofsson, R.; Ny, L.; Eilard, M.S.; Rizell, M.; Cahlin, C.; Stierner, U.; Lönn, U.; Hansson, J.; Ljuslinder, I.; Lundgren, L.; et al. Isolated hepatic perfusion as a treatment for uveal melanoma liver metastases (the SCANDIUM trial): Study protocol for a randomized controlled trial. Trials 2014, 15, 317. [Google Scholar] [PubMed]
- De Vries, T.J.; Trancikova, D.; Ruiter, D.J.; van Muijen, G.N. High expression of immunotherapy candidate proteins gp100, MART-1, tyrosinase and TRP-1 in uveal melanoma. Br. J. Cancer 1998, 78, 1156–1161. [Google Scholar]
- Steuhl, K.-P.; Rohrbach, J.M.; Knorr, M.; Thiel, H.-J. Significance, Specificity, and Ultrastructural Localization of HMB-45 Antigen in Pigmented Ocular Tumors. Ophthalmology 1993, 100, 208–215. [Google Scholar]
- Meecham, W.J.; Char, D.H.; Kaleta-Michaels, S. Infiltrating Lymphocytes and Antigen Expression in Uveal Melanoma. Ophthalmic Res. 1992, 24, 20–26. [Google Scholar]
- De Waard-Siebinga, I.; Hilders, C.G.; Hansen, B.E.; van Delft, J.L.; Jager, M.J. HLA expression and tumor-infiltrating immune cells in uveal melanoma. Graefes Arch. Clin. Exp. Ophthalmol. 1996, 234, 34–42. [Google Scholar] [PubMed]
- Polak, M.E.; Borthwick, N.J.; Johnson, P.; Hungerford, J.L.; Higgins, B.; Di Palma, S.; Jager, M.J.; Cree, I.A. Presence and phenotype of dendritic cells in uveal melanoma. Br. J. Ophthalmol. 2007, 91, 971–976. [Google Scholar] [PubMed]
- Gross, S.; Erdmann, M.; Haendle, I.; Voland, S.; Berger, T.; Schultz, E.; Strasser, E.; Dankerl, P.; Janka, R.; Schliep, S.; et al. Twelve-year survival and immune correlates in dendritic cell–vaccinated melanoma patients. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Bol, K.F.; Mensink, H.W.; Aarntzen, E.H.; Schreibelt, G.; Keunen, J.E.; Coulie, P.G.; De Klein, A.; Punt, C.J.; Paridaens, D.; Figdor, C.G.; et al. Long Overall Survival after Dendritic Cell Vaccination in Metastatic Uveal Melanoma Patients. Am. J. Ophthalmol. 2014, 158, 939–947. [Google Scholar] [PubMed]
- Gajewski, T.F. Failure at the Effector Phase: Immune Barriers at the Level of the Melanoma Tumor Microenvironment. Clin. Cancer Res. 2007, 13, 5256–5261. [Google Scholar] [PubMed]
- Bol, K.F.; Bosch, T.V.D.; Schreibelt, G.; Mensink, H.W.; Keunen, J.E.; Kiliç, E.; Japing, W.J.; Geul, K.W.; Westdorp, H.; Boudewijns, S.; et al. Adjuvant Dendritic Cell Vaccination in High-Risk Uveal Melanoma. Ophthalmology 2016, 123, 2265–2267. [Google Scholar] [PubMed]
- Schuler-Thurner, B.; Bartz-Schmidt, K.U.; Bornfeld, N.; Cursiefen, C.; Fuisting, B.; Grisanti, S.; Heindl, L.M.; Holbach, L.; Keserü, M.; Knorr, H.; et al. Immunotherapy of uveal melanoma: Vaccination against cancer. Multicenter adjuvant phase 3 vaccination study using dendritic cells laden with tumor RNA for large newly diagnosed uveal melanoma. Ophthalmologe 2015, 112, 1017–1021. [Google Scholar]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar]
- Zimmer, L.; Vaubel, J.; Mohr, P.; Hauschild, A.; Utikal, J.; Šimon, J.; Garbe, C.; Herbst, R.; Enk, A.; Kämpgen, E.; et al. Phase II DeCOG-Study of Ipilimumab in Pretreated and Treatment-Naïve Patients with Metastatic Uveal Melanoma. PLoS ONE 2015, 10, e0118564. [Google Scholar]
- Heppt, M.V.; Heinzerling, L.; Kähler, K.C.; Forschner, A.; Kirchberger, M.C.; Loquai, C.; Meissner, M.; Meier, F.; Terheyden, P.; Schell, B.; et al. Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur. J. Cancer 2017, 82, 56–65. [Google Scholar] [PubMed]
- Algazi, A.P.; Tsai, K.K.; Shoushtari, A.N.; Munhoz, R.R.; Eroglu, Z.; Piulats, J.M.; Ott, P.A.; Johnson, D.B.; Hwang, J.; Daud, A.I.; et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016, 122, 3344–3353. [Google Scholar] [PubMed]
- Rodriguez, J.M.P.; Merino, L.D.L.C.; Espinosa, E.; Carrión, L.A.; Algarra, S.M.; López-Castro, R.; García, M.T.C.; Abreu, D.R.; Iriarte, A.J.R.; Jaime, A.B. Phase II multicenter, single arm, open label study of Nivolumab in combination with Ipilimumab in untreated patients with metastatic uveal melanoma. Ann. Oncol. 2018, 29, viii442–viii466. [Google Scholar]
- Fountain, E.; Bassett, R.L.; Cain, S.; Posada, L.; Gombos, D.S.; Hwu, P.; Bedikian, A.; Patel, S.P. Adjuvant Ipilimumab in High-Risk Uveal Melanoma. Cancers 2019, 11, 152. [Google Scholar]
- Buder-Bakhaya, K.; Hassel, J.C. Biomarkers for Clinical Benefit of Immune Checkpoint Inhibitor Treatment—A Review from the Melanoma Perspective and Beyond. Front. Immunol. 2018, 9, 1474. [Google Scholar] [PubMed]
- Weide, B.; Martens, A.; Hassel, J.C.; Berking, C.; Postow, M.A.; Bisschop, K.; Simeone, E.; Mangana, J.; Schilling, B.; Di Giacomo, A.-M.; et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin. Cancer Res. 2016, 22, 5487–5496. [Google Scholar] [PubMed]
- Abdel-Rahman, M.H.; Akbani, R.; Ally, A.; Auman, J.T.; Babur, O.; Balasundaram, M.; Balu, S.; Benz, C.; Beroukhim, R.; Birol, I.; et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell 2017, 32, 204–220. [Google Scholar]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar]
- Johansson, P.A.; Stark, A.; Palmer, J.M.; Bigby, K.; Brooks, K.; Rolfe, O.; Pritchard, A.L.; Whitehead, K.; Warrier, S.; Glasson, W.; et al. Prolonged stable disease in a uveal melanoma patient with germline MBD4 nonsense mutation treated with pembrolizumab and ipilimumab. Immunogenetics 2019, 71, 433–436. [Google Scholar]
- Rodrigues, M.; Mobuchon, L.; Houy, A.; Fievet, A.; Gardrat, S.; Barnhill, R.L.; Popova, T.; Servois, V.; Rampanou, A.; Mouton, A.; et al. Outlier response to anti-PD1 in uveal melanoma reveals germline MBD4 mutations in hypermutated tumors. Nat. Commun. 2018, 9, 1866. [Google Scholar]
- Stauner, C.T.; Drexler, K.; Berneburg, M.; Haferkamp, S. Complete response to pembrolizumab after initial progress in a patient with metastatic uveal melanoma. J. Dtsch. Dermatol. Ges. 2018, 16, 1376–1378. [Google Scholar] [PubMed]
- Krishna, Y.; McCarthy, C.; Kalirai, H.; Coupland, S.E. Inflammatory cell infiltrates in advanced metastatic uveal melanoma. Hum. Pathol. 2017, 66, 159–166. [Google Scholar] [PubMed]
- Liddy, N.; Bossi, G.; Adams, K.J.; Lissina, A.; Mahon, T.M.; Hassan, N.J.; Gavarret, J.; Bianchi, F.C.; Pumphrey, N.J.; Ladell, K.; et al. Monoclonal TCR-redirected tumor cell killing. Nat. Med. 2012, 18, 980–987. [Google Scholar] [PubMed]
- Sato, T.; Nathan, P.D.; Hernandez-Aya, L.F.; Sacco, J.J.; Orloff, M.M.; Truscello, J.; McAlpine, C.; Hulstine, A.-M.; Lanasa, M.C.; Coughlin, C.M.; et al. Intra-patient escalation dosing strategy with IMCgp100 results in mitigation of T-cell based toxicity and preliminary efficacy in advanced uveal melanoma. J. Clin. Oncol. 2017, 35, 9531. [Google Scholar]
- Middleton, M.R.; Steven, N.M.; Evans, T.J.; Infante, J.R.; Sznol, M.; Mulatero, C.; Hamid, O.; Shoushtari, A.N.; Shingler, W.; Johnson, A.; et al. Safety, pharmacokinetics and efficacy of IMCgp100, a first-in-class soluble TCR-antiCD3 bispecific t cell redirector with solid tumour activity: Results from the FIH study in melanoma. J. Clin. Oncol. 2016, 34, 3016. [Google Scholar]
- Sato, T.; Nathan, P.D.; Hernandez-Aya, L.; Sacco, J.J.; Orloff, M.M.; Visich, J.; Little, N.; Hulstine, A.-M.; Coughlin, C.M.; Carvajal, R.D. Redirected T cell lysis in patients with metastatic uveal melanoma with gp100-directed TCR IMCgp100: Overall survival findings. J. Clin. Oncol. 2018, 36, 9521. [Google Scholar]
- Carvajal, R.D.; Sacco, J.; Nathan, P.; Orloff, M.; Little, N.; McAlpine, C.; Krige, D.; Hassan, N.; Hulstine, A.M.; Coughlin, C.; et al. Safety, efficacy and biology of the gp100 TCR-based bispecific T cell redirector IMCgp100 in advanced uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3622. [Google Scholar]
- Immunocore. Pipeline. Available online: https://www.immunocore.com/pipeline (accessed on 15 January 2019).
- Yang, H.; Buisson, S.; Bossi, G.; Wallace, Z.; Hancock, G.; So, C.; Ashfield, R.; Vuidepot, A.; Mahon, T.; Molloy, P.; et al. Elimination of Latently HIV-infected Cells from Antiretroviral Therapy-suppressed Subjects by Engineered Immune-mobilizing T-cell Receptors. Mol. Ther. 2016, 24, 1913–1925. [Google Scholar]
- Tran, E.; Turcotte, S.; Gros, A.; Robbins, P.F.; Lu, Y.C.; Dudley, M.E.; Wunderlich, J.R.; Somerville, R.P.; Hogan, K.; Hinrichs, C.S.; et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014, 344, 641–645. [Google Scholar]
- Stevanović, S.; Draper, L.M.; Langhan, M.M.; Campbell, T.E.; Kwong, M.L.; Wunderlich, J.R.; Dudley, M.E.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; et al. Complete Regression of Metastatic Cervical Cancer after Treatment with Human Papillomavirus—Targeted Tumor-Infiltrating T Cells. J. Clin. Oncol. 2015, 33, 1543–1550. [Google Scholar]
- Chandran, S.S.; Somerville, R.P.T.; Yang, J.C.; Sherry, R.M.; Klebanoff, C.A.; Goff, S.L.; Wunderlich, J.R.; Danforth, D.N.; Zlott, D.; Paria, B.C.; et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: A single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 792–802. [Google Scholar] [PubMed]
- Nowicki, T.S.; Berent-Maoz, B.; Cheung-Lau, G.C.; Huang, R.R.; Wang, X.; Tsoi, J.; Kaplan-Lefko, P.J.; Cabrera, P.; Tran, J.; Pang, J.; et al. A Pilot Trial of the Combination of Transgenic NY-ESO-1–reactive Adoptive Cellular Therapy with Dendritic Cell Vaccination with or without Ipilimumab. Clin. Cancer Res. 2018, 25, 2096–2108. [Google Scholar] [PubMed]
- Sadelain, M.; Brentjens, R.; Rivière, I. The basic principles of chimeric antigen receptor (CAR) design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [PubMed]
- Forsberg, E.M.; Lindberg, M.F.; Jespersen, H.; Alsén, S.; Bagge, R.O.; Donia, M.; Svane, I.M.; Nilsson, O.; Ny, L.; Nilsson, L.M.; et al. HER2 CAR-T Cells Eradicate Uveal Melanoma and T-cell Therapy–Resistant Human Melanoma in IL2 Transgenic NOD/SCID IL2 Receptor Knockout Mice. Cancer Res. 2019, 79, 899–904. [Google Scholar]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [PubMed]
- Chauvin, J.-M.; Pagliano, O.; Fourcade, J.; Sun, Z.; Wang, H.; Sander, C.; Kirkwood, J.M.; Chen, T.H.; Maurer, M.; Korman, A.J.; et al. TIGIT and PD-1 impair tumor antigen–specific CD8+ T cells in melanoma patients. J. Clin. Investig. 2015, 125, 2046–2058. [Google Scholar]
- Ascierto, P.A.; Melero, I.; Bhatia, S.; Bono, P.; Sanborn, R.E.; Lipson, E.J.; Callahan, M.K.; Gajewski, T.; Gomez-Roca, C.A.; Hodi, F.S.; et al. Initial efficacy of anti-lymphocyte activation gene-3 (anti–LAG-3; BMS-986016) in combination with nivolumab (nivo) in pts with melanoma (MEL) previously treated with anti–PD-1/PD-L1 therapy. J. Clin. Oncol. 2017, 35, 9520. [Google Scholar]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.-P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar]
- Rosenberg, S.A.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; Hughes, M.S.; Phan, G.Q.; Citrin, D.E.; Restifo, N.P.; Robbins, P.F.; Wunderlich, J.R.; et al. Durable Complete Responses in Heavily Pretreated Patients with Metastatic Melanoma Using T-Cell Transfer Immunotherapy. Clin. Cancer Res. 2011, 17, 4550–4557. [Google Scholar]
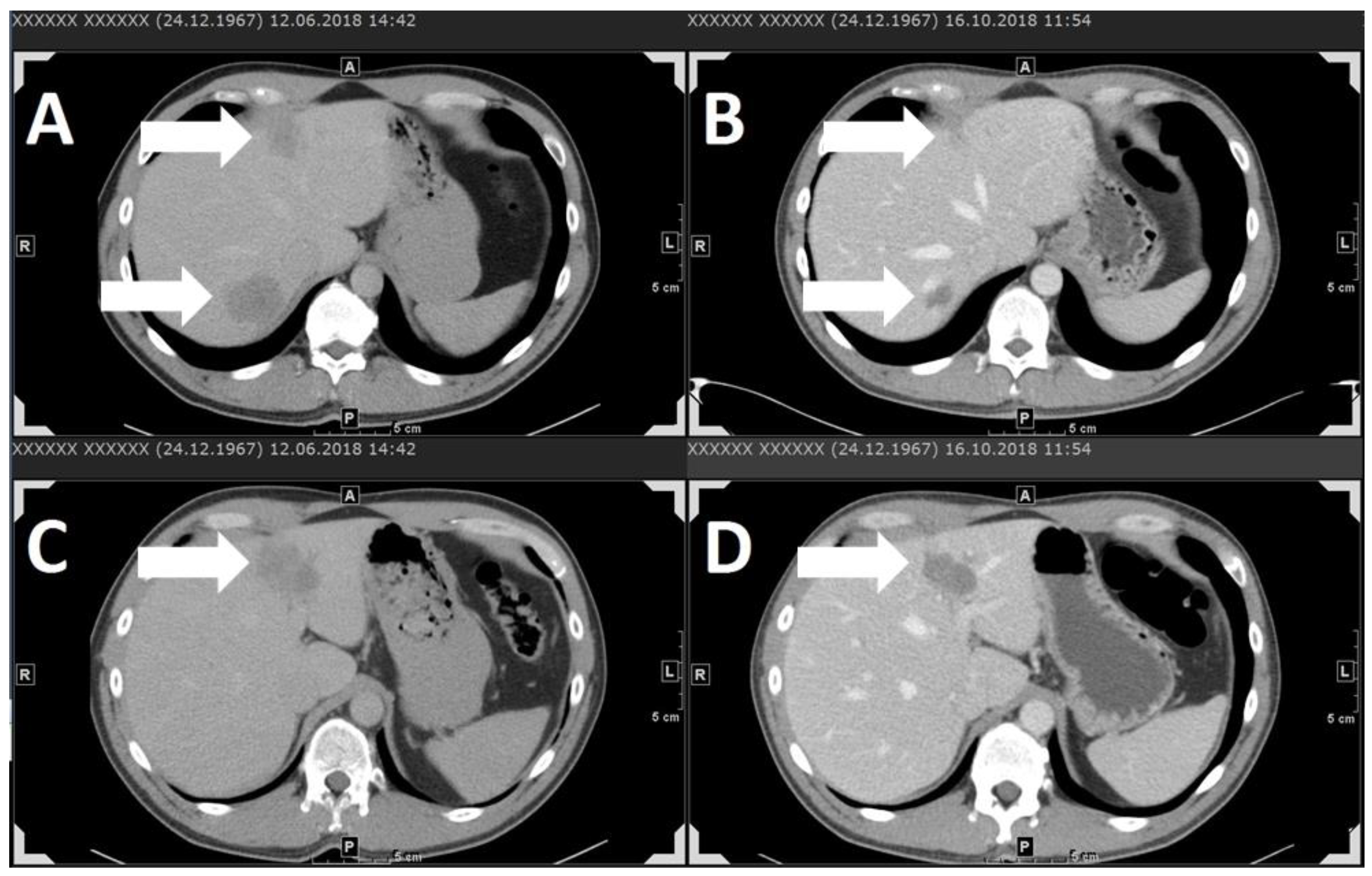
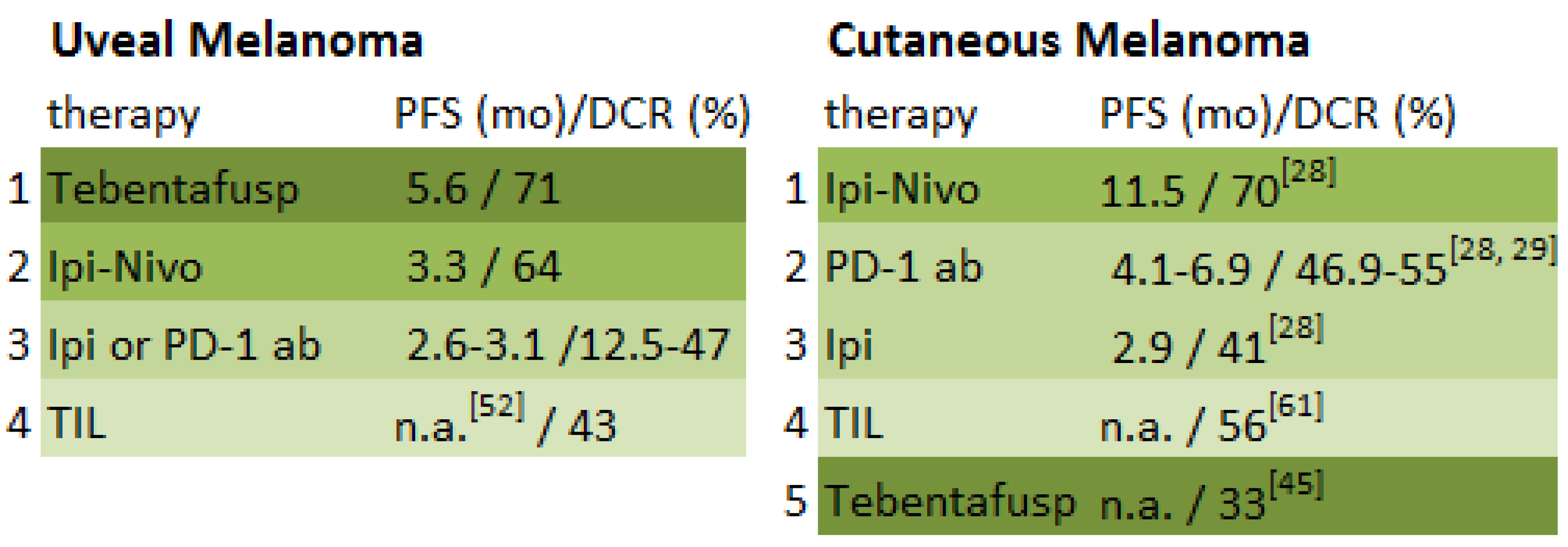
| Study | Study Design | Number of Patients | Elevated LDH at Baseline | Therapy | Best Response | Overall Response Rate (CR + PR) | Median Duration of Response (mo) | Disease-Control Rate (CR + PR + SD) | Durable Disease-Control Rate (CR + PR + SD ≥ 6 mo) | Median PFS/DFS (mo) | AEs Grade ≥3 % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [24] | case series, stage-IV UM | 14 | 21% | dendritic-cell vaccination | SD | 0% | n.a. | 71% | 21% | n.a. | 0 |
| [26] | adjuvant, open-label, phase-II study, UM | 23 | n.a. | adjuvant dendritic-cell vaccination | n.a. | n.a. | n.a. | n.a. | n.a. | 34.5 | 0 |
| [30] | multicentre, phase-II, stage-IV UM | 53 | 38% | ipi 3 mg/kg Q3W | SD | 0% | n.a. | 47% | 21% | 2.8 | 36%, one death |
| [31] | retrospective, multicentre, stage-IV UM | pembro: 54 | 57% | pembro 2 mg/kg Q3W | PR | 4.7% | n.a. | 22.7% | n.a. | 3.1 | 7% |
| nivo: 32 | 53% | nivo 3 mg/kg Q2W | PR | 4.7% | n.a. | 18.7% | n.a. | 2.8 | 13%, one death | ||
| ipi + PD-1 ab: 15 total: 86 | 47% | ipi 3 mg/kg + nivo 1 mg/kg Q3W, followed by nivo 3 mg/kg Q2W ipi 1 mg/kg + pembro 2 mg/kg Q3W, followed by pembro 2 mg/kg Q3W ipi 1 mg/kg + nivo 3 mg/kg Q3W, followed by nivo 3 mg/kg Q2W | PR | 16.7% | n.a. | 33.4% | n.a. | 2.8 | 13% | ||
| [32] | retrospective, multicenter, stage-IV UM | pembro: 38 nivo: 16 atezo: 2 total: 56 | 71% | pembro 2 mg/kg Q3W 10 mg/kg Q2W 10 mg/kg Q3W Unknown Q3W nivo 1 mg/kg Q2W 2 mg/kg Q2W 3 mg/kg Q2W 10 mg/kg Q2W atezo 10 mg/kg Q2W 15 mg/kg Q2W | PR | 3.6% | n.a. | 12.5% | 8.9% | 2.6 | 13% |
| Case series HD, 2019 (unpublished) | retrospective, monocentre, stage-IV UM | pembro: 12 nivo: 1 ipi + nivo: 7 total: 20 | 55% | pembro 2 mg/kg Q3W nivo 3 mg/kg Q2W ipi 3 mg/kg + nivo 1 mg/kg Q3W, followed by nivo 3 mg/kg Q2W | PR | 11.8% | 2.85 | 29.4% | 5.9% | 2.75 | 30% |
| [33] | prospective, phase-II, multicentre, open-label, single-arm, stage-IV UM | 50 | 32% | ipi 3 mg/kg + nivo 1 mg/kg Q3W, followed by nivo 3 mg/kg Q2W | PR | 12% | n.a. | 64% | n.a. | 3.3 | 54%, one death |
| [46,47] | phase-I study, prospective, stage-IV UM | 19 | 73% | tebentafusp (IMCgp100) | PR | 11% | 7.1 | 71% | 41% | 5.6 | 79% |
| [52] | interim analysis of a monocentre, two-stage, single-arm, phase-II study, stage-IV UM | 21 | 52% | adoptive T-cell therapy | CR | 35% | 4+ | 43% | 10% | n.a. | 100%, one death |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schank, T.E.; Hassel, J.C. Immunotherapies for the Treatment of Uveal Melanoma—History and Future. Cancers 2019, 11, 1048. https://doi.org/10.3390/cancers11081048
Schank TE, Hassel JC. Immunotherapies for the Treatment of Uveal Melanoma—History and Future. Cancers. 2019; 11(8):1048. https://doi.org/10.3390/cancers11081048
Chicago/Turabian StyleSchank, Timo E., and Jessica C. Hassel. 2019. "Immunotherapies for the Treatment of Uveal Melanoma—History and Future" Cancers 11, no. 8: 1048. https://doi.org/10.3390/cancers11081048
APA StyleSchank, T. E., & Hassel, J. C. (2019). Immunotherapies for the Treatment of Uveal Melanoma—History and Future. Cancers, 11(8), 1048. https://doi.org/10.3390/cancers11081048






