Circulating Tumor Cells in Right- and Left-Sided Colorectal Cancer
Abstract
1. Introduction
2. Results
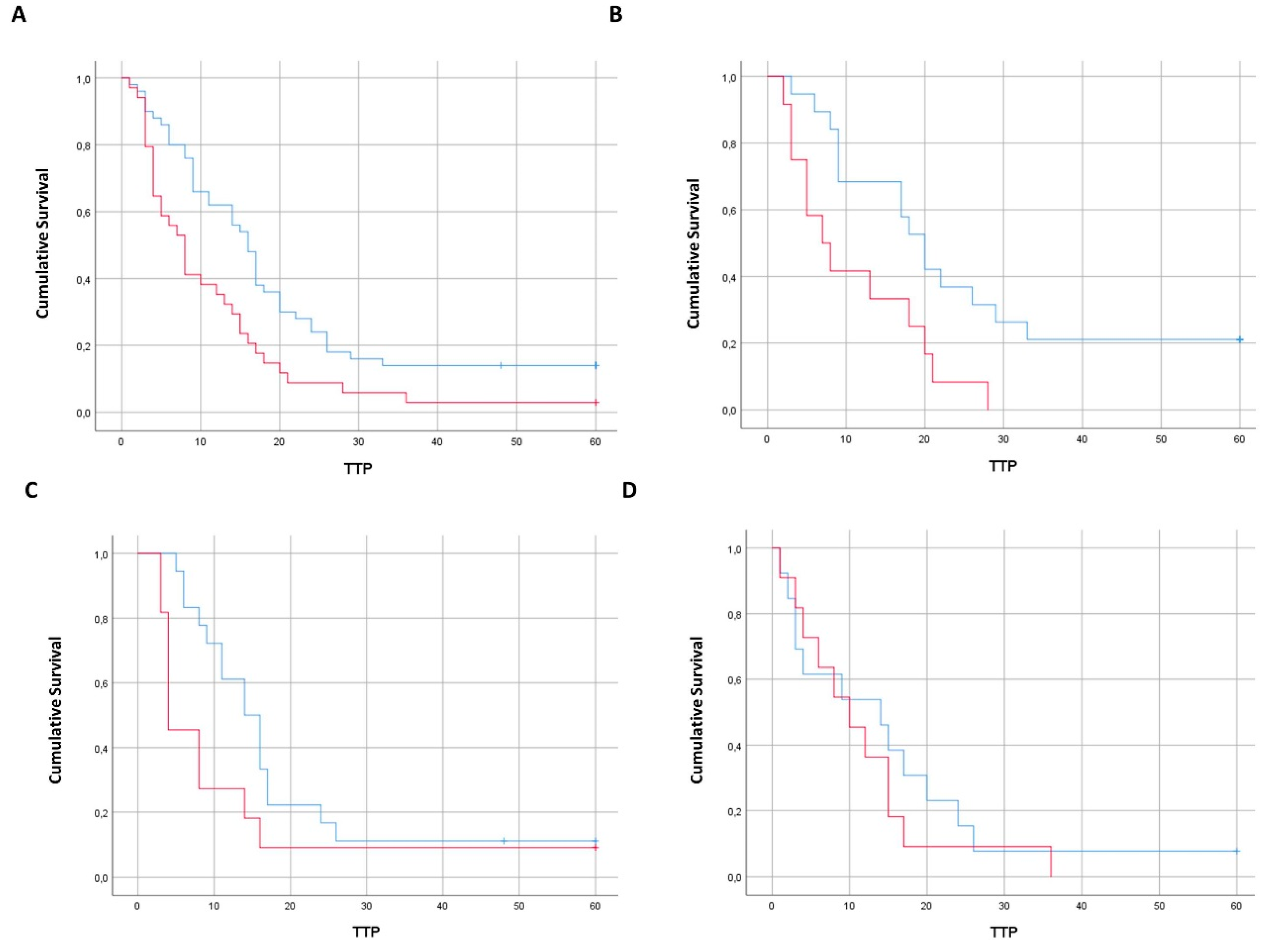
2.1. Enumeration of CTC According to Tumor Sidedness
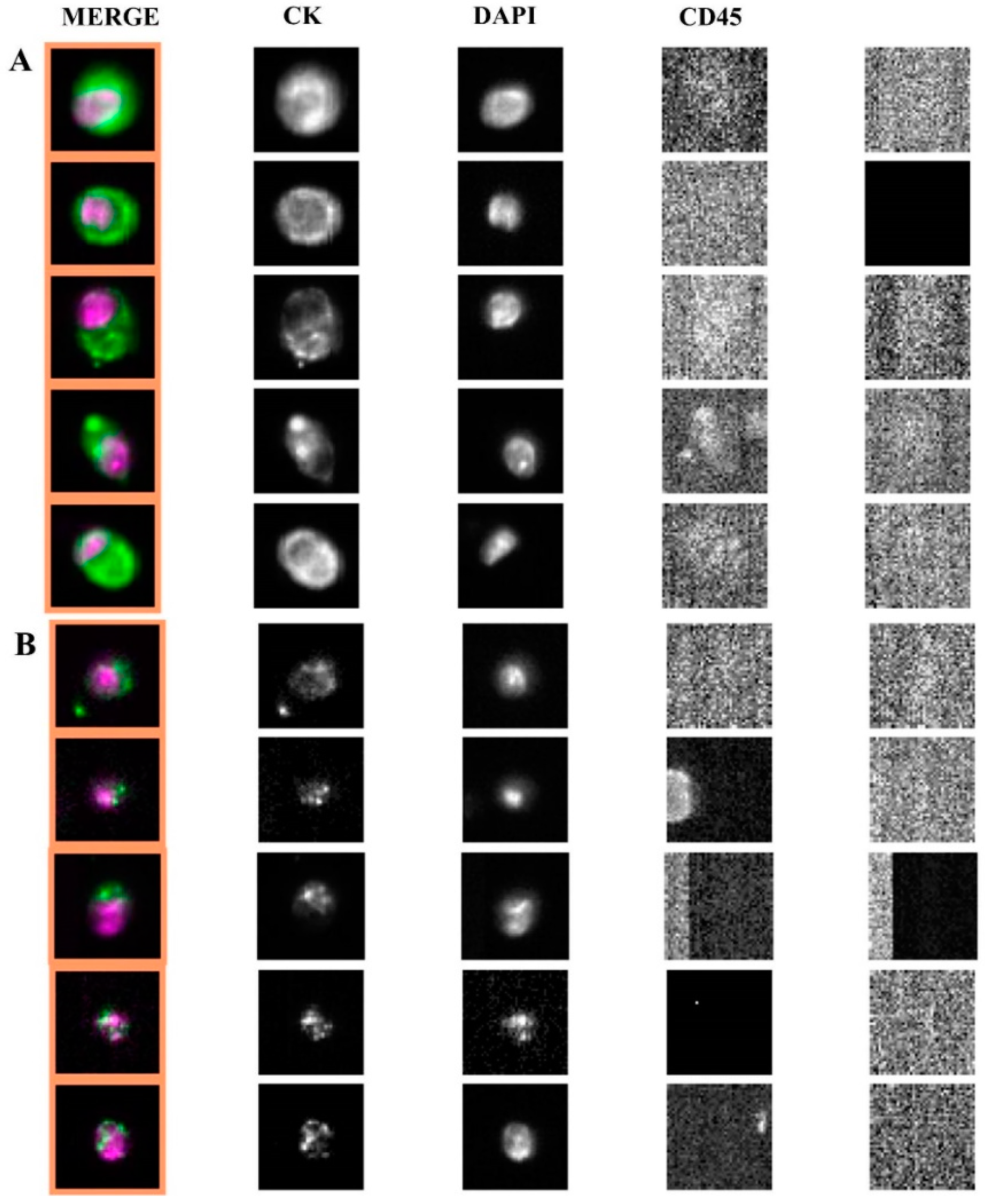
2.2. Apoptotic Morphology of CTC
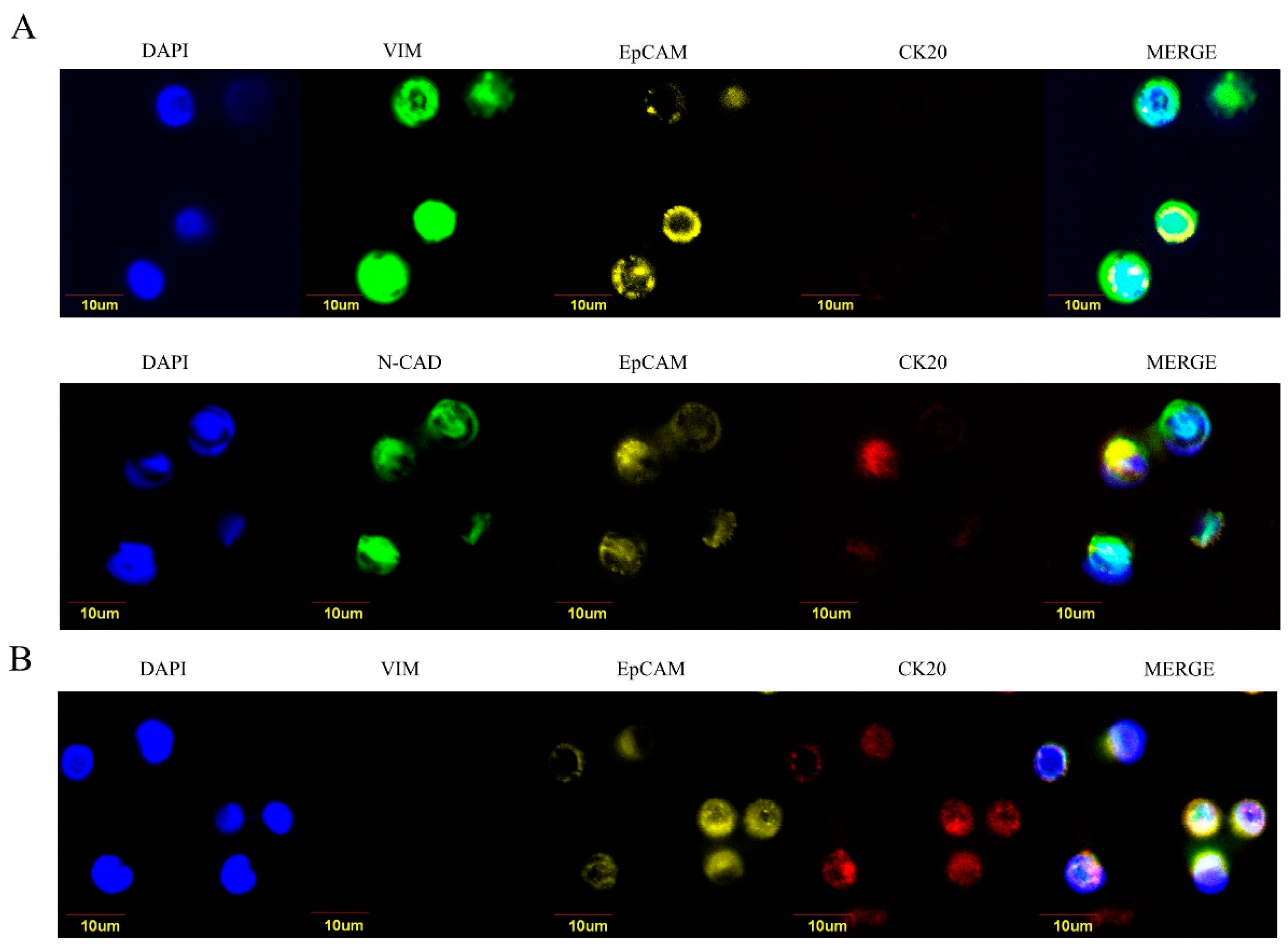
2.3. Epithelial-Like and Mesenchymal-Like Features of CTC
3. Discussion
4. Materials and Methods
4.1. CTC Enumeration
4.2. Epithelial-Like and Mesenchymal-Like CTC
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dienstmann, R.; Vermeulen, L.; Guinney, J.; Kopetz, S.; Tejpar, S.; Tabernero, J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer 2017, 17, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Mussolin, B.; Buscarino, M.; Corti, G.; Cassingena, A.; Crisafulli, G.; Ponzetti, A.; Cremolini, C.; Amatu, A.; Lauricella, C.; et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat. Med. 2015, 21, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Venesio, T.; Siravegna, G.; Bardelli, A.; Sapino, A. Liquid Biopsies for Monitoring Temporal Genomic Heterogeneity in Breast and Colon Cancers. Pathobiology 2018, 8, 5146–5154. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, S.; Tejpar, S.; Gibbs, P.; Thiebach, L.; Lenz, H.J. Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes. Eur. J. Cancer 2017, 84, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Holch, J.W.; Demmer, M.; Lamersdorf, C.; Mich, M.; Schulz, C.; von Einem, J.C.; Modest, D.P.; Heinemann, V. Pattern and Dynamics of Distant Metastases in Metastatic Colorectal Cancer. Visc. Med. 2017, 33, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Hugen, N.; Nagtegaal, I.D. Distinct metastatic patterns in colorectal cancer patients based on primary tumour location. Eur. J. Cancer 2017, 75, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, C.; Nicolazzo, C.; Gradilone, A.; Giannini, G.; De Falco, E.; Chimenti, I.; Varriale, E.; Hauch, S.; Plappert, L.; Cortesi, E.; et al. Circulating tumor cells: exploring intratumor heterogeneity of colorectal cancer. Cancer Biol. Ther. 2014, 15, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, D.S.; Haber, D.A.; Maheswaran, S. Cancer metastasis through the prism of epithelial-to-mesenchymal transition in circulating tumor cells. Mol. Oncol. 2017, 11, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Andree, K.C.; van Dalum, G.; Terstappen, L.W. Challenges in circulating tumor cell detection by the CellSearch system. Mol. Oncol. 2016, 10, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Tomasello, G.; Borgonovo, K.; Ghidini, M.; Turati, L.; Dallera, P.; Passalacqua, R.; Sgroi, G.; Barni, S. Prognostic Survival Associated With Left-Sided vs Right-Sided Colon Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 3, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Cabel, L.; Proudhon, C.; Gortais, H.; Loirat, D.; Coussy, F.; Pierga, J.Y.; Bidard, F.C. Circulating tumor cells: clinical validity and utility. Int. J. Clin. Oncol. 2017, 22, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.; Lenz, H.J.; Xiu, J.; El-Deiry, W.S.; Swensen, J.; El Ghazal, H.; Gatalica, Z.; Hwang, J.J.; Philip, P.A.; Shields, A.F.; et al. Molecular variances between rectal and left-sided colon cancers. J. Clin. Oncol. 2017, 35. abstract 522. [Google Scholar] [CrossRef]
- Salem, M.E.; Yin, J.; Renfro, L.A.; Weinberg, B.A.; Maughan, T.; Richard Adams, R.; Van Cutsem, E.; Falcone, A.; Tebbutt, N.C.; Seymour, M.T.; et al. Rectal versus left-sided colon cancers: Clinicopathological differences observed in a pooled analysis of 4182 patients enrolled to 8 clinical trials from the ARCAD database. J. Clin. Oncol. 2017, 35. abstract 675. [Google Scholar]
- Li, F.; Lai, M. Colorectal cancer, one entity or three. J. Zhejiang Univ. Sci. B 2009, 10, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Alexander, S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, M.; Jain, R.K.; Munn, L.L. Active versus passive mechanisms in metastasis: do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 2007, 8, 444–448. [Google Scholar] [CrossRef]
- Ulivi, P.; Scarpi, E.; Chiadini, E.; Marisi, G.; Valgiusti, M.; Capelli, L.; Casadei Gardini, A.; Monti, M.; Ruscelli, S.; Frassineti, G.L.; et al. Right- vs. Left-Sided Metastatic Colorectal Cancer: Differences in Tumor Biology and Bevacizumab Efficacy. Int. J. Mol. Sci. 2017, 18, 1240. [Google Scholar] [CrossRef]
- Deutsch, T.M.; Riethdorf, S.; Nees, J.; Hartkopf, A.D.; Schönfisch, B.; Domschke, C.; Sprick, M.R.; Schütz, F.; Brucker, S.Y.; Stefanovic, S.; et al. Impact of apoptotic circulating tumor cells (aCTC) in metastatic breast cancer. Breast Cancer Res. Treat. 2016, 160, 277–290. [Google Scholar] [CrossRef]
- Isella, C.; Brundu, F.; Bellomo, S.E.; Galimi, F.; Zanella, E.; Porporato, R.; Petti, C.; Fiori, A.; Orzan, F.; Senetta, R.; et al. Selective analysis of cancer-cell intrinsic transcriptional traits defines novel clinically relevant subtypes of colorectal cancer. Nat. Commun. 2017, 8, 15107. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | No. of Patients (n = 84) |
|---|---|
| Sex | |
| Male | 50 |
| Female | 34 |
| Primary tumor location | |
| Right | 24 |
| Left | 31 |
| Rectum | 29 |
| Stage of disease | |
| Metastatic | 84 |
| KRAS status (tumor tissue) | |
| Wild type | 31 |
| Mutant | 29 |
| Unknown | 24 |
| Patient n. | Tumor Location | n. CTC (ScreenCell) | Epithelial-Like CTC (%) | Mesenchymal-Like CTC (%) |
|---|---|---|---|---|
| 1 | right | 20 | 16 (80) | 4 (20) |
| 2 | right | 5 | 5 (100) | 0 (0) |
| 3 | right | 16 | 10(63) | 6 (37) |
| 4 | right | 25 | 20 (80) | 5(20) |
| 5 | right | 8 | 7 (87) | 1 (13) |
| 6 | right | 30 | 25 (83) | 5 (17) |
| 7 | right | 27 | 22 (81) | 5 (19) |
| 8 | left | 4 | 0 (0) | 4 (100) |
| 9 | left | 4 | 1 (25) | 3 (75) |
| 10 | left | 10 | 1 (10) | 9 (90) |
| 11 | left | 11 | 4(36) | 7 (64) |
| 12 | left | 6 | 2(33) | 4 (67) |
| 13 | left | 16 | 5(31) | 11 (69) |
| 14 | left | 7 | 0(0) | 7 (100) |
| 15 | left | 8 | 2(25) | 6 (75) |
| 16 | left | 10 | 2(0) | 8 (100) |
| 17 | rectum | 5 | 0(0) | 5 (100) |
| 18 | rectum | 10 | 2(20) | 8 (80) |
| 19 | rectum | 14 | 2(14) | 12 (86) |
| 20 | rectum | 7 | 2(29) | 5 (71) |
| 21 | rectum | 10 | 0 (0) | 10 (100) |
| 22 | rectum | 6 | 1 (17) | 5 (83) |
| 23 | rectum | 8 | 0 (0) | 8 (100) |
| 24 | rectum | 3 | 0 (0) | 3 (100) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolazzo, C.; Raimondi, C.; Gradilone, A.; Emiliani, A.; Zeuner, A.; Francescangeli, F.; Belardinilli, F.; Seminara, P.; Loreni, F.; Magri, V.; et al. Circulating Tumor Cells in Right- and Left-Sided Colorectal Cancer. Cancers 2019, 11, 1042. https://doi.org/10.3390/cancers11081042
Nicolazzo C, Raimondi C, Gradilone A, Emiliani A, Zeuner A, Francescangeli F, Belardinilli F, Seminara P, Loreni F, Magri V, et al. Circulating Tumor Cells in Right- and Left-Sided Colorectal Cancer. Cancers. 2019; 11(8):1042. https://doi.org/10.3390/cancers11081042
Chicago/Turabian StyleNicolazzo, Chiara, Cristina Raimondi, Angela Gradilone, Alessandra Emiliani, Ann Zeuner, Federica Francescangeli, Francesca Belardinilli, Patrizia Seminara, Flavia Loreni, Valentina Magri, and et al. 2019. "Circulating Tumor Cells in Right- and Left-Sided Colorectal Cancer" Cancers 11, no. 8: 1042. https://doi.org/10.3390/cancers11081042
APA StyleNicolazzo, C., Raimondi, C., Gradilone, A., Emiliani, A., Zeuner, A., Francescangeli, F., Belardinilli, F., Seminara, P., Loreni, F., Magri, V., Tomao, S., & Gazzaniga, P. (2019). Circulating Tumor Cells in Right- and Left-Sided Colorectal Cancer. Cancers, 11(8), 1042. https://doi.org/10.3390/cancers11081042






