Methylation Dynamics of RASSF1A and Its Impact on Cancer
Abstract
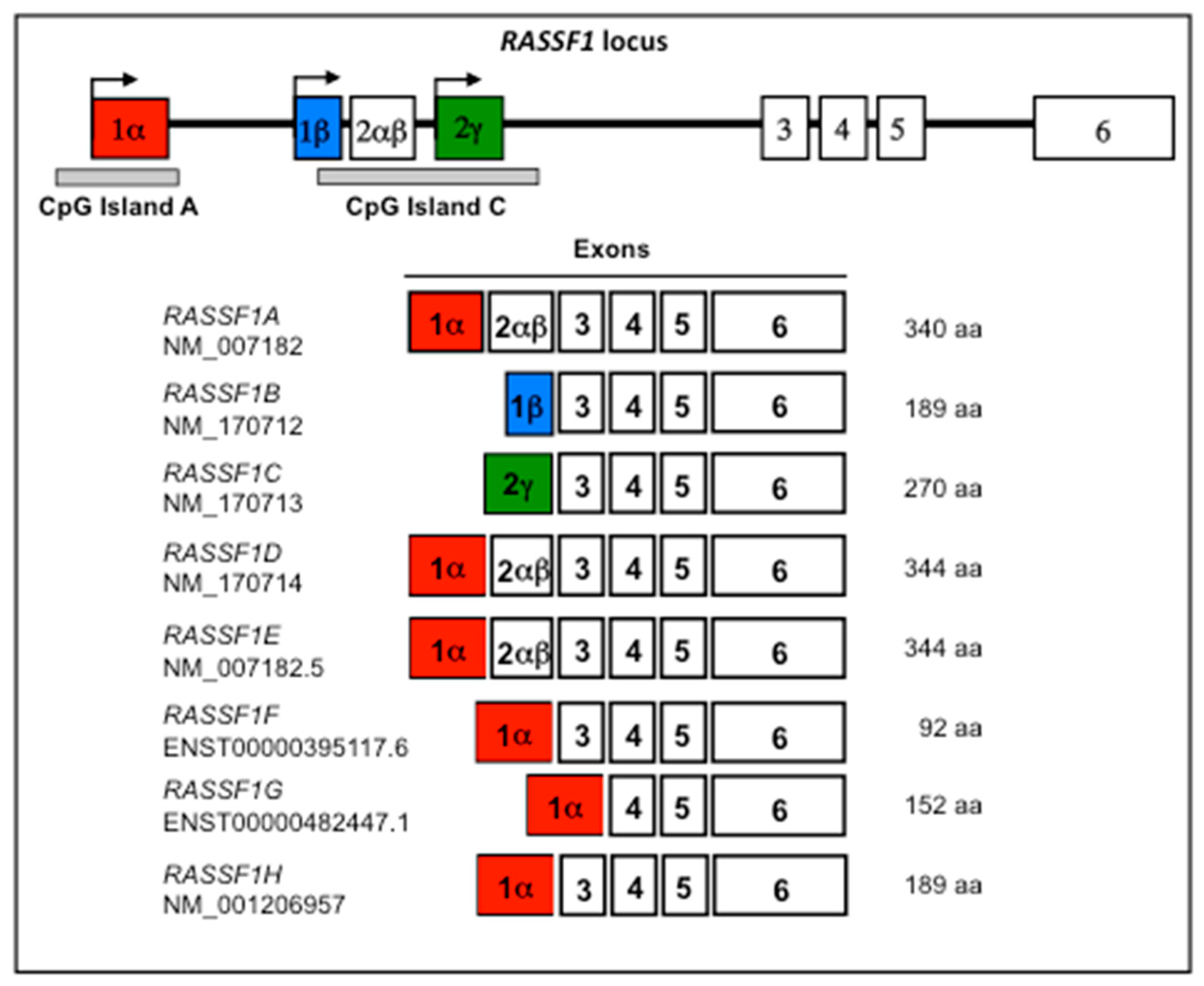
1. The Tumor Suppressor RASSF1A
2. Biological Role of RASSF1C
3. DNA Methylation Changes
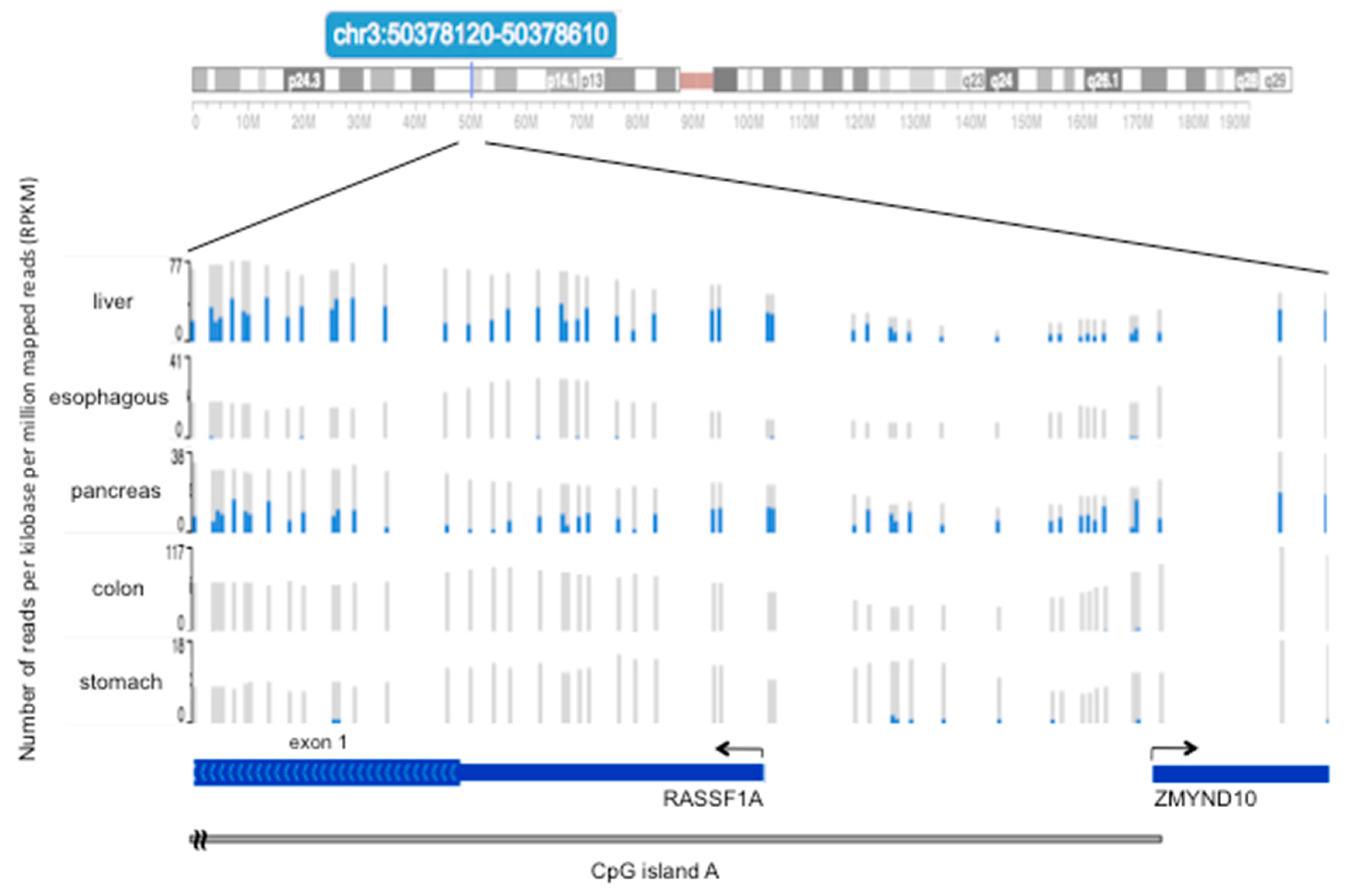
4. Methylation of RASSF1A in Normal Tissues of the Gastroenteric System
5. Mechanisms of RASSF1A Methylation in Cancer and Aging
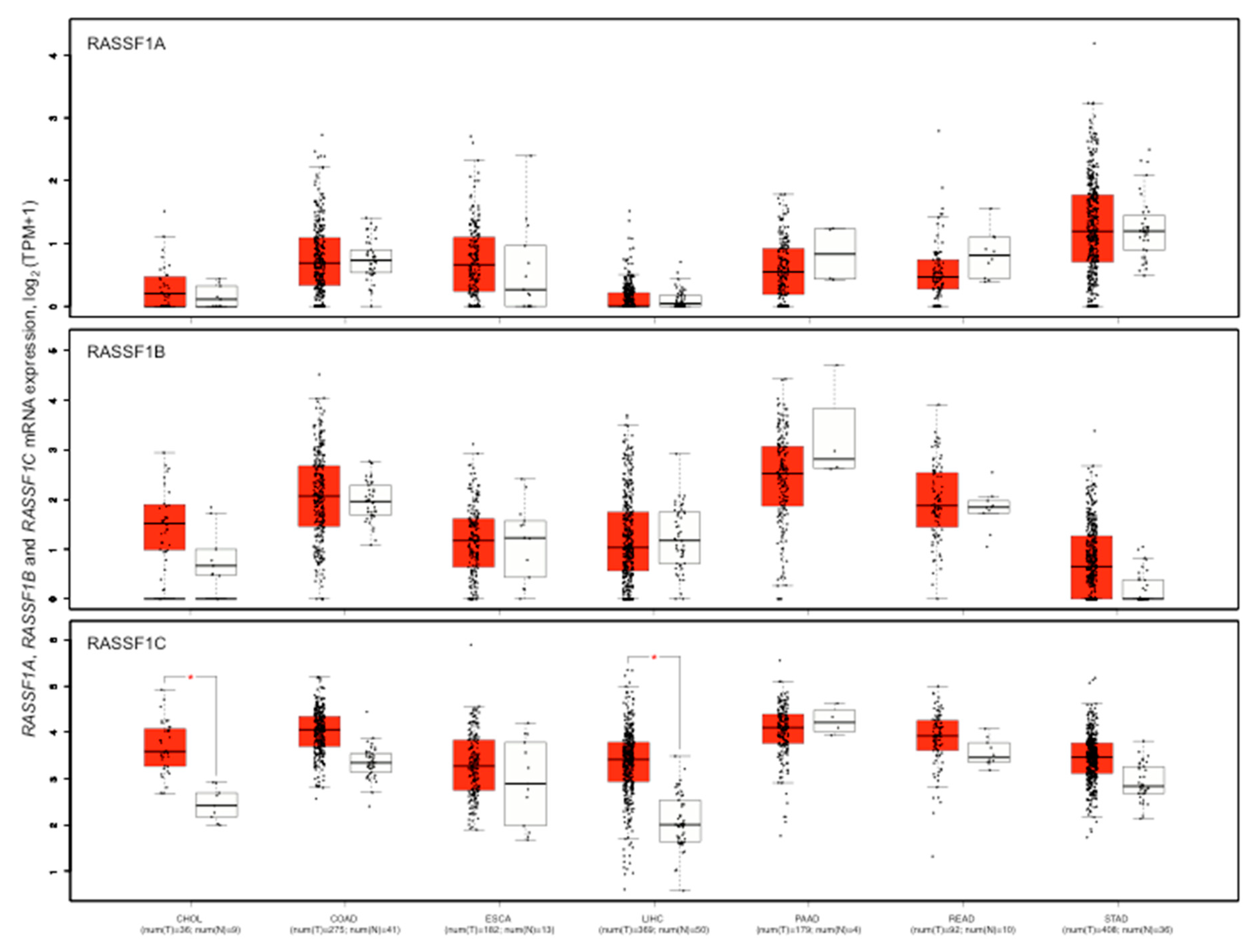
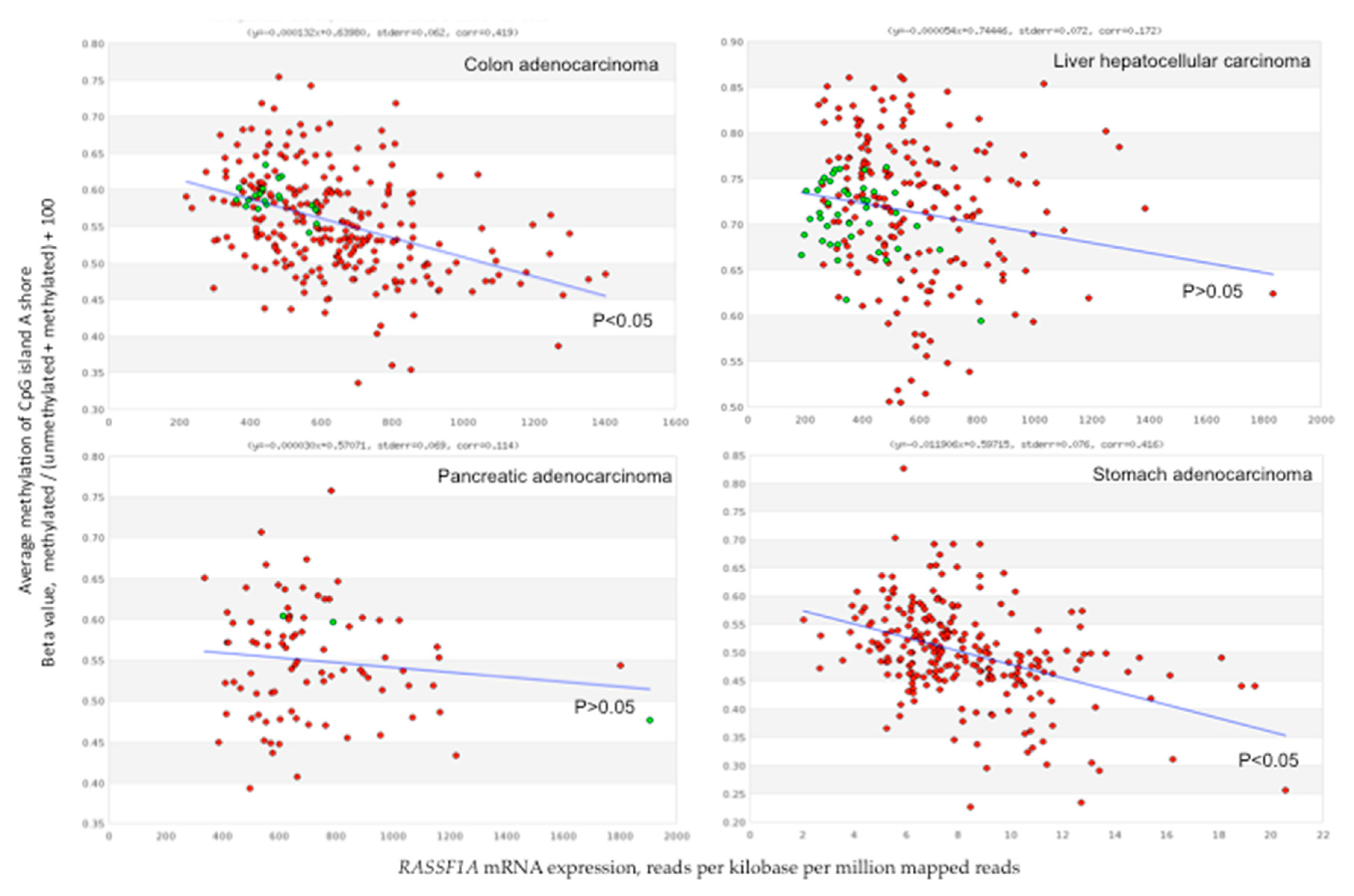
6. Relationship between RASSF1A Methylation and Expression
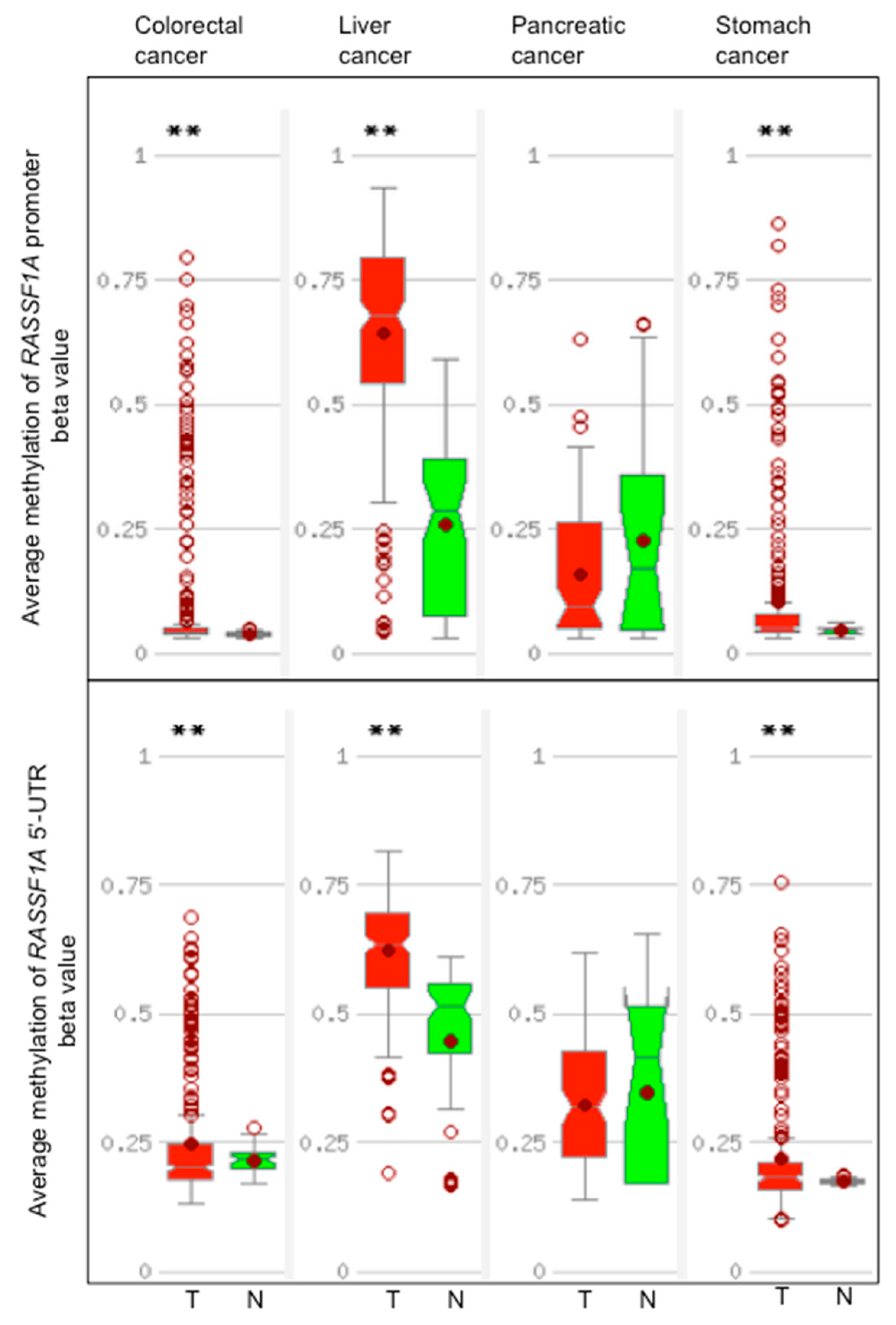
7. RASSF1A Methylation and Expression in Gastrointestinal Cancers
8. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Donninger, H.; Vos, M.D.; Clark, G.J. The RASSF1A tumor suppressor. J. Cell Sci. 2007, 120, 3163–3172. [Google Scholar] [CrossRef] [PubMed]
- Van der Weyden, L.; Adams, D.J. The Ras-association domain family(RASSF) members and their role in human tumourigenesis. Biochim. Biophys. Acta 2007, 1776, 58–85. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Hsu, H.S.; Ni, H.J.; Chuang, C.T.; Hsiung, C.H.; Huang, T.H.; Wang, Y.C. Distinct epigenetic domains separated by a CTCF bound insulator between the tandem genes, BLU and RASSF1A. PLoS ONE 2010, 5, e12847. [Google Scholar] [CrossRef] [PubMed]
- Hesson, L.; Bieche, I.; Krex, D.; Criniere, E.; Hoang-Xuan, K.; Maher, E.R.; Latif, F. Frequent epigenetic inactivation of RASSF1A and BLU genes located within the critical 3p21.3 region in gliomas. Oncogene 2004, 23, 2408–2419. [Google Scholar] [CrossRef] [PubMed]
- Senchenko, V.N.; Liu, J.; Loginov, W.; Bazov, I.; Angeloni, D.; Seryogin, Y.; Ermilova, V.; Kazubskaya, T.; Garkavtseva, R.; Zabarovska, V.I.; et al. Discovery of frequent homozygous deletions in chromosome 3p21.3 LUCA and AP20 regions in renal, lung and breast carcinomas. Oncogene 2004, 23, 5719–5728. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Ito, G.; Kondo, M.; Uchiyama, M.; Fukui, T.; Mori, S.; Yoshioka, H.; Ueda, Y.; Shimokata, K.; Sekido, Y. Frequent inactivation of RASSF1A, BLU, and SEMA3B on 3p21.3 by promoter hypermethylation and allele loss in non-small cell lung cancer. Cancer Lett. 2005, 225, 131–139. [Google Scholar] [CrossRef]
- Amato, E.; Barbi, S.; Malpeli, G.; Bersani, S.; Pelosi, G.; Capelli, P.; Scarpa, A. Chromosome 3p alterations in pancreatic endocrine neoplasia. Virchows Arch. 2011, 458, 39–45. [Google Scholar] [CrossRef]
- Hesson, L.B.; Cooper, W.N.; Latif, F. The role of RASSF1A methylation in cancer. Dis. Mark. 2007, 23, 73–87. [Google Scholar] [CrossRef]
- Vos, M.D.; Ellis, C.A.; Bell, A.; Birrer, M.J.; Clark, G.J. Ras uses the novel tumor suppressor RASSF1 as an effector to mediate apoptosis. J. Biol. Chem. 2000, 275, 35669–35672. [Google Scholar] [CrossRef]
- Dammann, R.; Li, C.; Yoon, J.H.; Chin, P.L.; Bates, S.; Pfeifer, G.P. Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3. Nat. Genet. 2000, 25, 315–319. [Google Scholar] [CrossRef]
- Baksh, S.; Tommasi, S.; Fenton, S.; Yu, V.C.; Martins, L.M.; Pfeifer, G.P.; Latif, F.; Downward, J.; Neel, B.G. The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to Bax conformational change and cell death. Mol. Cell 2005, 18, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Pefani, D.E.; O’Neill, E. Safeguarding genome stability: RASSF1A tumor suppressor regulates BRCA2 at stalled forks. Cell Cycle 2015, 14, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Song, S.J.; Ayad, N.G.; Chang, J.S.; Lee, J.H.; Hong, H.K.; Lee, H.; Choi, N.; Kim, J.; Kim, H.; et al. The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC-Cdc20 complex. Nat. Cell Biol. 2004, 6, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Dittfeld, C.; Richter, A.M.; Steinmann, K.; Klagge-Ulonska, A.; Dammann, R.H. The SARAH Domain of RASSF1A and Its Tumor Suppressor Function. Mol. Biol. Int. 2012, 2012, 196715. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tommasi, S.; Lee, D.H.; Dammann, R.; Pfeifer, G.P. Control of microtubule stability by the RASSF1A tumor suppressor. Oncogene 2003, 22, 8125–8136. [Google Scholar] [CrossRef] [PubMed]
- Fausti, F.; Di Agostino, S.; Sacconi, A.; Strano, S.; Blandino, G. Hippo and rassf1a Pathways: A Growing Affair. Mol. Biol. Int. 2012, 2012, 307628. [Google Scholar] [CrossRef] [PubMed]
- Foley, C.J.; Freedman, H.; Choo, S.L.; Onyskiw, C.; Fu, N.Y.; Yu, V.C.; Tuszynski, J.; Pratt, J.C.; Baksh, S. Dynamics of RASSF1A/MOAP-1 association with death receptors. Mol. Cell. Biol. 2008, 28, 4520–4535. [Google Scholar] [CrossRef]
- Law, J.; Salla, M.; Zare, A.; Wong, Y.; Luong, L.; Volodko, N.; Svystun, O.; Flood, K.; Lim, J.; Sung, M.; et al. Modulator of apoptosis 1(MOAP-1) is a tumor suppressor protein linked to the RASSF1A protein. J. Biol. Chem. 2015, 290, 24100–24118. [Google Scholar] [CrossRef]
- Papaspyropoulos, A.; Bradley, L.; Thapa, A.; Leung, C.Y.; Toskas, K.; Koennig, D.; Pefani, D.E.; Raso, C.; Grou, C.; Hamilton, G.; et al. RASSF1A uncouples Wnt from Hippo signalling and promotes YAP mediated differentiation via p73. Nat. Commun. 2018, 9, 424. [Google Scholar] [CrossRef]
- Tommasi, S.; Dammann, R.; Zhang, Z.; Wang, Y.; Liu, L.; Tsark, W.M.; Wilczynski, S.P.; Li, J.; You, M.; Pfeifer, G.P. Tumor susceptibility of Rassf1a knockout mice. Cancer Res. 2005, 65, 92–98. [Google Scholar]
- Wang, W.G.; Chen, S.J.; He, J.S.; Li, J.S.; Zang, X.F. The tumor suppressive role of RASSF1A in osteosarcoma through the Wnt signaling pathway. Tumour Biol. 2016, 37, 8869–8877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, C.; Wu, X.; Li, A.X.; Liu, L.; Tsark, W.; Dammann, R.; Shen, H.; Vonderfecht, S.L.; Pfeifer, G.P. Analysis of liver tumor-prone mouse models of the hippo kinase scaffold proteins RASSF1A and SAV1. Cancer Res. 2016, 76, 2824–2835. [Google Scholar] [CrossRef] [PubMed]
- Teng, I.W.; Hou, P.C.; Lee, K.D.; Chu, P.Y.; Yeh, K.T.; Jin, V.X.; Tseng, M.J.; Tsai, S.J.; Chang, Y.S.; Wu, C.S.; et al. Targeted methylation of two tumor suppressor genes is sufficient to transform mesenchymal stem cells into cancer stem/initiating cells. Cancer Res. 2011, 71, 4653–4663. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.E.; Firek, M.; Chen, S.T.; Amaar, Y. The RASSF1 Gene and the Opposing Effects of the RASSF1A and RASSF1C Isoforms on Cell Proliferation and Apoptosis. Mol. Biol. Int. 2013, 2013, 145096. [Google Scholar] [CrossRef] [PubMed]
- Agathanggelou, A.; Bieche, I.; Ahmed-Choudhury, J.; Nicke, B.; Dammann, R.; Baksh, S.; Gao, B.; Minna, J.D.; Downward, J.; Maher, E.R.; et al. Identification of novel gene expression targets for the Ras association domain family 1(RASSF1A) tumor suppressor gene in non-small cell lung cancer and neuroblastoma. Cancer Res. 2003, 63, 5344–5351. [Google Scholar] [PubMed]
- Dallol, A.; Agathanggelou, A.; Tommasi, S.; Pfeifer, G.P.; Maher, E.R.; Latif, F. Involvement of the RASSF1A tumor suppressor gene in controlling cell migration. Cancer Res. 2005, 65, 7653–7659. [Google Scholar] [CrossRef] [PubMed]
- Dreijerink, K.; Braga, E.; Kuzmin, I.; Geil, L.; Duh, F.M.; Angeloni, D.; Zbar, B.; Lerman, M.I.; Stanbridge, E.J.; Minna, J.D.; et al. The candidate tumor suppressor gene, RASSF1A, from human chromosome 3p21.3 is involved in kidney tumorigenesis. Proc. Natl. Acad. Sci. USA 2001, 98, 7504–7509. [Google Scholar] [CrossRef]
- Reu, F.J.; Leaman, D.W.; Maitra, R.R.; Bae, S.I.; Cherkassky, L.; Fox, M.W.; Rempinski, D.R.; Beaulieu, N.; MacLeod, A.R.; Borden, E.C. Expression of RASSF1A, an epigenetically silenced tumor suppressor, overcomes resistance to apoptosis induction by interferons. Cancer Res. 2006, 66, 2785–2793. [Google Scholar] [CrossRef]
- Xue, W.J.; Li, C.; Zhou, X.J.; Guan, H.G.; Qin, L.; Li, P.; Wang, Z.W.; Qian, H.X. RASSF1A expression inhibits the growth of hepatocellular carcinoma from Qidong County. J. Gastroenterol. Hepatol. 2008, 23, 1448–1458. [Google Scholar] [CrossRef]
- Zhou, P.H.; Zheng, J.B.; Wei, G.B.; Wang, X.L.; Wang, W.; Chen, N.Z.; Yu, J.H.; Yao, J.F.; Wang, H.; Lu, S.Y.; et al. Lentivirus-mediated RASSF1A expression suppresses aggressive phenotypes of gastric cancer cells in vitro and in vivo. Gene Ther. 2015, 22, 793–801. [Google Scholar] [CrossRef]
- Pelosi, G.; Fumagalli, C.; Trubia, M.; Sonzogni, A.; Rekhtman, N.; Maisonneuve, P.; Galetta, D.; Spaggiari, L.; Veronesi, G.; Scarpa, A.; et al. Dual role of RASSF1 as a tumor suppressor and an oncogene in neuroendocrine tumors of the lung. Anticancer Res. 2010, 30, 4269–4281. [Google Scholar] [PubMed]
- Rong, R.; Jin, W.; Zhang, J.; Sheikh, M.S.; Huang, Y. Tumor suppressor RASSF1A is a microtubule-binding protein that stabilizes microtubules and induces G2/M arrest. Oncogene 2004, 23, 8216–8230. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.D.; Martinez, A.; Elam, C.; Dallol, A.; Taylor, B.J.; Latif, F.; Clark, G.J. A role for the RASSF1A tumor suppressor in the regulation of tubulin polymerization and genomic stability. Cancer Res. 2004, 64, 4244–4250. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, D.; Kajiho, H.; Negishi, T.; Ura, S.; Watanabe, T.; Wada, T.; Ichijo, H.; Katada, T.; Nishina, H. Release of RASSF1C from the nucleus by Daxx degradation links DNA damage and SAPK/JNK activation. EMBO J. 2006, 25, 3286–3297. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, F.; Protopopov, A.; Malyukova, A.; Kashuba, V.; Minna, J.D.; Lerman, M.I.; Klein, G.; Zabarovsky, E. Inactivation of RASSF1C during in vivo tumor growth identifies it as a tumor suppressor gene. Oncogene 2004, 23, 5941–5949. [Google Scholar] [CrossRef]
- Estrabaud, E.; Lassot, I.; Blot, G.; Le Rouzic, E.; Tanchou, V.; Quemeneur, E.; Daviet, L.; Margottin-Goguet, F.; Benarous, R. RASSF1C, an isoform of the tumor suppressor RASSF1A, promotes the accumulation of beta-catenin by interacting with betaTrCP. Cancer Res. 2007, 67, 1054–1061. [Google Scholar] [CrossRef]
- Amaar, Y.G.; Minera, M.G.; Hatran, L.K.; Strong, D.D.; Mohan, S.; Reeves, M.E. Ras association domain family 1C protein stimulates human lung cancer cell proliferation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L1185–L1190. [Google Scholar] [CrossRef]
- Reeves, M.E.; Firek, M.; Chen, S.T.; Amaar, Y.G. Evidence that RASSF1C stimulation of lung cancer cell proliferation depends on IGFBP-5 and PIWIL1 expression levels. PLoS ONE 2014, 9, e101679. [Google Scholar] [CrossRef]
- Bestor, T.H. The DNA methyltransferases of mammals. Hum. Mol. Genet. 2000, 9, 2395–2402. [Google Scholar] [CrossRef]
- Esteve, P.O.; Chang, Y.; Samaranayake, M.; Upadhyay, A.K.; Horton, J.R.; Feehery, G.R.; Cheng, X.; Pradhan, S. A methylation and phosphorylation switch between an adjacent lysine and serine determines human DNMT1 stability. Nat. Struct. Mol. Biol. 2011, 18, 42–48. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, H.; Xu, Z.; Liu, Q.; Liang, Y.; Wang, L.; Cai, X.; Zhang, L.; Hu, L.; Wang, G.; et al. Phosphatidylinositol 3-kinase/protein kinase B pathway stabilizes DNA methyltransferase I protein and maintains DNA methylation. Cell. Signal. 2007, 19, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Lyko, F. The DNA methyltransferase family: A versatile toolkit for epigenetic regulation. Nat. Rev. Genet. 2018, 19, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Koivunen, P.; Laukka, T. The TET enzymes. Cell. Mol. Life Sci. 2018, 75, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Pradhan, K.; Campbell, N.; Mazdo, J.; Vasantkumar, A.; Maqbool, S.; Bhagat, T.D.; Gupta, S.; Suzuki, M.; Yu, Y.; et al. Altered hydroxymethylation is seen at regulatory regions in pancreatic cancer and regulates oncogenic pathways. Genome Res. 2017, 27, 1830–1842. [Google Scholar] [CrossRef]
- Du, C.; Kurabe, N.; Matsushima, Y.; Suzuki, M.; Kahyo, T.; Ohnishi, I.; Tanioka, F.; Tajima, S.; Goto, M.; Yamada, H.; et al. Robust quantitative assessments of cytosine modifications and changes in the expressions of related enzymes in gastric cancer. Gastric Cancer 2015, 18, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Qi, T.; Wang, S.; Hao, M.; Sakhawat, A.; Liang, T.; Zhang, L.; Cong, X.; Huang, Y. Tet methylcytosine dioxygenase 1 promotes hypoxic gene induction and cell migration in colon cancer. J. Cell. Physiol. 2019, 234, 6286–6297. [Google Scholar] [CrossRef]
- Murata, A.; Baba, Y.; Ishimoto, T.; Miyake, K.; Kosumi, K.; Harada, K.; Kurashige, J.; Iwagami, S.; Sakamoto, Y.; Miyamoto, Y.; et al. TET family proteins and 5-hydroxymethylcytosine in esophageal squamous cell carcinoma. Oncotarget 2015, 6, 23372–23382. [Google Scholar] [CrossRef]
- Sajadian, S.O.; Ehnert, S.; Vakilian, H.; Koutsouraki, E.; Damm, G.; Seehofer, D.; Thasler, W.; Dooley, S.; Baharvand, H.; Sipos, B.; et al. Induction of active demethylation and 5hmC formation by 5-azacytidine is TET2 dependent and suggests new treatment strategies against hepatocellular carcinoma. Clin. Epigenetics 2015, 7, 98. [Google Scholar] [CrossRef]
- Tian, Y.; Pan, F.; Sun, X.; Gan, M.; Lin, A.; Zhang, D.; Zhu, Y.; Lai, M. Association of TET1 expression with colorectal cancer progression. Scand. J. Gastroenterol. 2017, 52, 312–320. [Google Scholar] [CrossRef]
- Wang, K.C.; Kang, C.H.; Tsai, C.Y.; Chou, N.H.; Tu, Y.T.; Li, G.C.; Lam, H.C.; Liu, S.I.; Chang, P.M.; Lin, Y.H.; et al. Ten-eleven translocation 1 dysfunction reduces 5-hydroxymethylcytosine expression levels in gastric cancer cells. Oncol. Lett. 2018, 15, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, C.; Manzo, M.; Baubec, T. Dynamics and Context-Dependent Roles of DNA Methylation. J. Mol. Biol. 2017, 429, 1459–1475. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, X.; Hu, K.; Liu, B.; Wang, H.; Li, A.; Lin, F.; Zhang, L.; Sun, X.; Du, Z.; et al. Silencing DNA methyltransferase 1(DNMT1) inhibits proliferation, metastasis and invasion in ESCC by suppressing methylation of RASSF1A and DAPK. Oncotarget 2016, 7, 44129–44141. [Google Scholar] [CrossRef] [PubMed]
- Gjoneska, E.; Pfenning, A.R.; Mathys, H.; Quon, G.; Kundaje, A.; Tsai, L.H.; Kellis, M. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer’s disease. Nature 2015, 518, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bhutani, M.; Pathak, A.K.; Lang, W.; Ren, H.; Jelinek, J.; He, R.; Shen, L.; Issa, J.P.; Mao, L. Delta DNMT3B variants regulate DNA methylation in a promoter-specific manner. Cancer Res. 2007, 67, 10647–10652. [Google Scholar] [CrossRef]
- Wang, X.Z.; Cheng, Y.; Wang, K.L.; Liu, R.; Yang, X.L.; Wen, H.M.; Chai, C.; Liang, J.Y.; Wu, H. Peperomin E reactivates silenced tumor suppressor genes in lung cancer cells by inhibition of DNA methyltransferase. Cancer Sci. 2016, 107, 1506–1519. [Google Scholar] [CrossRef]
- Palakurthy, R.K.; Wajapeyee, N.; Santra, M.K.; Gazin, C.; Lin, L.; Gobeil, S.; Green, M.R. Epigenetic silencing of the RASSF1A tumor suppressor gene through HOXB3-mediated induction of DNMT3B expression. Mol. Cell 2009, 36, 219–230. [Google Scholar] [CrossRef]
- Li, H.; Rauch, T.; Chen, Z.X.; Szabo, P.E.; Riggs, A.D.; Pfeifer, G.P. The histone methyltransferase SETDB1 and the DNA methyltransferase DNMT3A interact directly and localize to promoters silenced in cancer cells. J. Biol. Chem. 2006, 281, 19489–19500. [Google Scholar] [CrossRef]
- Strunnikova, M.; Schagdarsurengin, U.; Kehlen, A.; Garbe, J.C.; Stampfer, M.R.; Dammann, R. Chromatin inactivation precedes de novo DNA methylation during the progressive epigenetic silencing of the RASSF1A promoter. Mol. Cell. Biol. 2005, 25, 3923–3933. [Google Scholar] [CrossRef]
- Boyes, J.; Bird, A. DNA methylation inhibits transcription indirectly via a methyl-CpG binding protein. Cell 1991, 64, 1123–1134. [Google Scholar] [CrossRef]
- Stirzaker, C.; Song, J.Z.; Ng, W.; Du, Q.; Armstrong, N.J.; Locke, W.J.; Statham, A.L.; French, H.; Pidsley, R.; Valdes-Mora, F.; et al. Methyl-CpG-binding protein MBD2 plays a key role in maintenance and spread of DNA methylation at CpG islands and shores in cancer. Oncogene 2017, 36, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, Y.A.; Khamis, A.M.; Kulakovskiy, I.V.; Ba-Alawi, W.; Bhuyan, M.S.; Kawaji, H.; Lassmann, T.; Harbers, M.; Forrest, A.R.; Bajic, V.B.; et al. Effects of cytosine methylation on transcription factor binding sites. BMC Genom. 2014, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Shin, J.Y.; Tonge, P.D.; Puri, M.C.; Lee, S.; Park, H.; Lee, W.C.; Hussein, S.M.; Bleazard, T.; Yun, J.Y.; et al. An epigenomic roadmap to induced pluripotency reveals DNA methylation as a reprogramming modulator. Nat. Commun. 2014, 5, 5619. [Google Scholar] [CrossRef] [PubMed]
- Lister, R.; Pelizzola, M.; Dowen, R.H.; Hawkins, R.D.; Hon, G.; Tonti-Filippini, J.; Nery, J.R.; Lee, L.; Ye, Z.; Ngo, Q.M.; et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 2009, 462, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Khavari, D.A.; Sen, G.L.; Rinn, J.L. DNA methylation and epigenetic control of cellular differentiation. Cell Cycle 2010, 9, 3880–3883. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Luu, P.L.; Stirzaker, C.; Clark, S.J. Methyl-CpG-binding domain proteins: Readers of the epigenome. Epigenomics 2015, 7, 1051–1073. [Google Scholar] [CrossRef] [PubMed]
- Malpeli, G.; Amato, E.; Dandrea, M.; Fumagalli, C.; Debattisti, V.; Boninsegna, L.; Pelosi, G.; Falconi, M.; Scarpa, A. Methylation-associated down-regulation of RASSF1A and up-regulation of RASSF1C in pancreatic endocrine tumors. BMC Cancer 2011, 11, 351. [Google Scholar] [CrossRef] [PubMed]
- Byun, D.S.; Lee, M.G.; Chae, K.S.; Ryu, B.G.; Chi, S.G. Frequent epigenetic inactivation of RASSF1A by aberrant promoter hypermethylation in human gastric adenocarcinoma. Cancer Res. 2001, 61, 7034–7038. [Google Scholar] [PubMed]
- Roadmap Epigenomics, C.; Kundaje, A.; Meuleman, W.; Ernst, J.; Bilenky, M.; Yen, A.; Heravi-Moussavi, A.; Kheradpour, P.; Zhang, Z.; Wang, J.; et al. Integrative analysis of 111 reference human epigenomes. Nature 2015, 518, 317–330. [Google Scholar]
- Rauch, T.; Wang, Z.; Zhang, X.; Zhong, X.; Wu, X.; Lau, S.K.; Kernstine, K.H.; Riggs, A.D.; Pfeifer, G.P. Homeobox gene methylation in lung cancer studied by genome-wide analysis with a microarray-based methylated CpG island recovery assay. Proc. Natl. Acad. Sci. USA 2007, 104, 5527–5532. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, Y.; Straussman, R.; Keshet, I.; Farkash, S.; Hecht, M.; Zimmerman, J.; Eden, E.; Yakhini, Z.; Ben-Shushan, E.; Reubinoff, B.E.; et al. Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat. Genet. 2007, 39, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Fraga, M.F.; Esteller, M. Epigenetics and aging: The targets and the marks. Trends Genet. 2007, 23, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Nejman, D.; Straussman, R.; Steinfeld, I.; Ruvolo, M.; Roberts, D.; Yakhini, Z.; Cedar, H. Molecular rules governing de novo methylation in cancer. Cancer Res. 2014, 74, 1475–1483. [Google Scholar] [CrossRef]
- Kuzmina, N.S.; Lapteva, N.; Rubanovich, A.V. Hypermethylation of gene promoters in peripheral blood leukocytes in humans long term after radiation exposure. Environ. Res. 2016, 146, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Waki, T.; Tamura, G.; Sato, M.; Motoyama, T. Age-related methylation of tumor suppressor and tumor-related genes: An analysis of autopsy samples. Oncogene 2003, 22, 4128–4133. [Google Scholar] [CrossRef] [PubMed]
- Baylin, S.B.; Jones, P.A. A decade of exploring the cancer epigenome—Biological and translational implications. Nat. Rev. Cancer 2011, 11, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Klutstein, M.; Nejman, D.; Greenfield, R.; Cedar, H. DNA Methylation in Cancer and Aging. Cancer Res. 2016, 76, 3446–3450. [Google Scholar] [CrossRef] [PubMed]
- Wong, I.H.; Chan, J.; Wong, J.; Tam, P.K. Ubiquitous aberrant RASSF1A promoter methylation in childhood neoplasia. Clin. Cancer Res. 2004, 10, 994–1002. [Google Scholar] [CrossRef]
- Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef]
- Weber, M.; Hellmann, I.; Stadler, M.B.; Ramos, L.; Paabo, S.; Rebhan, M.; Schubeler, D. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat. Genet. 2007, 39, 457–466. [Google Scholar] [CrossRef]
- Christensen, B.C.; Houseman, E.A.; Marsit, C.J.; Zheng, S.; Wrensch, M.R.; Wiemels, J.L.; Nelson, H.H.; Karagas, M.R.; Padbury, J.F.; Bueno, R.; et al. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet. 2009, 5, e1000602. [Google Scholar] [CrossRef] [PubMed]
- Issa, J.P.; Ahuja, N.; Toyota, M.; Bronner, M.P.; Brentnall, T.A. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res. 2001, 61, 3573–3577. [Google Scholar] [PubMed]
- Agathanggelou, A.; Cooper, W.N.; Latif, F. Role of the Ras-association domain family 1 tumor suppressor gene in human cancers. Cancer Res. 2005, 65, 3497–3508. [Google Scholar] [CrossRef] [PubMed]
- Amato, E.; Barbi, S.; Fassan, M.; Luchini, C.; Vicentini, C.; Brunelli, M.; Malleo, G.; Scarpa, A.; Malpeli, G. RASSF1 tumor suppressor gene in pancreatic ductal adenocarcinoma: Correlation of expression, chromosomal status and epigenetic changes. BMC Cancer 2016, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Malpeli, G. Potential etiologic of the epigenetic field defect in the diseases and in cancer. Edorium J. Pathol. 2015, 2, 10–13. [Google Scholar]
- Peters, I.; Rehmet, K.; Wilke, N.; Kuczyk, M.A.; Hennenlotter, J.; Eilers, T.; Machtens, S.; Jonas, U.; Serth, J. RASSF1A promoter methylation and expression analysis in normal and neoplastic kidney indicates a role in early tumorigenesis. Mol. Cancer 2007, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.S.; Shi, H.; Rahmatpanah, F.; Hsiau, T.H.; Hsiau, A.H.; Leu, Y.W.; Liu, J.C.; Huang, T.H. Differential distribution of DNA methylation within the RASSF1A CpG island in breast cancer. Cancer Res. 2003, 63, 6178–6186. [Google Scholar] [PubMed]
- Lo, K.W.; Kwong, J.; Hui, A.B.; Chan, S.Y.; To, K.F.; Chan, A.S.; Chow, L.S.; Teo, P.M.; Johnson, P.J.; Huang, D.P. High frequency of promoter hypermethylation of RASSF1A in nasopharyngeal carcinoma. Cancer Res. 2001, 61, 3877–3881. [Google Scholar] [PubMed]
- Lee, M.G.; Kim, H.Y.; Byun, D.S.; Lee, S.J.; Lee, C.H.; Kim, J.I.; Chang, S.G.; Chi, S.G. Frequent epigenetic inactivation of RASSF1A in human bladder carcinoma. Cancer Res. 2001, 61, 6688–6692. [Google Scholar]
- Jain, S.; Xie, L.; Boldbaatar, B.; Lin, S.Y.; Hamilton, J.P.; Meltzer, S.J.; Chen, S.H.; Hu, C.T.; Block, T.M.; Song, W.; et al. Differential methylation of the promoter and first exon of the RASSF1A gene in hepatocarcinogenesis. Hepatol. Res. 2015, 45, 1110–1123. [Google Scholar] [CrossRef]
- Gordon, M.; El-Kalla, M.; Zhao, Y.; Fiteih, Y.; Law, J.; Volodko, N.; Anwar-Mohamed, A.; El-Kadi, A.O.; Liu, L.; Odenbach, J.; et al. The tumor suppressor gene, RASSF1A, is essential for protection against inflammation -induced injury. PLoS ONE 2013, 8, e75483. [Google Scholar] [CrossRef] [PubMed]
- Guarasci, F.; D’Aquila, P.; Mandala, M.; Garasto, S.; Lattanzio, F.; Corsonello, A.; Passarino, G.; Bellizzi, D. Aging and nutrition induce tissue-specific changes on global DNA methylation status in rats. Mech. Ageing Dev. 2018, 174, 47–54. [Google Scholar] [CrossRef]
- Zekri, A.R.; Bahnasy, A.A.; Shoeab, F.E.; Mohamed, W.S.; El-Dahshan, D.H.; Ali, F.T.; Sabry, G.M.; Dasgupta, N.; Daoud, S.S. Methylation of multiple genes in hepatitis C virus associated hepatocellular carcinoma. J. Adv. Res. 2014, 5, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Saunderson, E.A.; Stepper, P.; Gomm, J.J.; Hoa, L.; Morgan, A.; Allen, M.D.; Jones, J.L.; Gribben, J.G.; Jurkowski, T.P.; Ficz, G. Hit-and-run epigenetic editing prevents senescence entry in primary breast cells from healthy donors. Nat. Commun. 2017, 8, 1450. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Chen, R.; Li, Z.; Liu, Y.; Cheng, D.; Zhou, Q.; Zhou, J.; Lin, Q. Hepatitis C virus core upregulates the methylation status of the RASSF1A promoter through regulation of SMYD3 in hilar cholangiocarcinoma cells. Acta Biochim. Biophys. Sin. 2011, 43, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Kader, F.; Ghai, M. DNA methylation-based variation between human populations. Mol. Genet. Genom. 2017, 292, 5–35. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Onishi, M.; Sugata, E.; Sokuza, Y.; Mori, C.; Nishikawa, T.; Honoki, K.; Tsujiuchi, T. Disturbance of DNA methylation patterns in the early phase of hepatocarcinogenesis induced by a choline-deficient L-amino acid-defined diet in rats. Cancer Sci. 2007, 98, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Starlard-Davenport, A.; Tryndyak, V.P.; James, S.R.; Karpf, A.R.; Latendresse, J.R.; Beland, F.A.; Pogribny, I.P. Mechanisms of epigenetic silencing of the Rassf1a gene during estrogen-induced breast carcinogenesis in ACI rats. Carcinogenesis 2010, 31, 376–381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rauscher, G.H.; Kresovich, J.K.; Poulin, M.; Yan, L.; Macias, V.; Mahmoud, A.M.; Al-Alem, U.; Kajdacsy-Balla, A.; Wiley, E.L.; Tonetti, D.; et al. Exploring DNA methylation changes in promoter, intragenic, and intergenic regions as early and late events in breast cancer formation. BMC Cancer 2015, 15, 816. [Google Scholar] [CrossRef]
- Spitzwieser, M.; Holzweber, E.; Pfeiler, G.; Hacker, S.; Cichna-Markl, M. Applicability of HIN-1, MGMT and RASSF1A promoter methylation as biomarkers for detecting field cancerization in breast cancer. Breast Cancer Res. 2015, 17, 125. [Google Scholar] [CrossRef]
- Zhou, V.W.; Goren, A.; Bernstein, B.E. Charting histone modifications and the functional organization of mammalian genomes. Nat. Rev. Genet. 2011, 12, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, D.; Esteve-Codina, A.; Piferrer, F. Consistent inverse correlation between DNA methylation of the first intron and gene expression across tissues and species. Epigenetics Chromatin 2018, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Dammann, R.; Takahashi, T.; Pfeifer, G.P. The CpG island of the novel tumor suppressor gene RASSF1A is intensely methylated in primary small cell lung carcinomas. Oncogene 2001, 20, 3563–3567. [Google Scholar] [CrossRef] [PubMed]
- Dammann, R.; Yang, G.; Pfeifer, G.P. Hypermethylation of the cpG island of Ras association domain family 1A(RASSF1A), a putative tumor suppressor gene from the 3p21.3 locus, occurs in a large percentage of human breast cancers. Cancer Res. 2001, 61, 3105–3109. [Google Scholar] [PubMed]
- Freitas, M.; Ferreira, F.; Carvalho, S.; Silva, F.; Lopes, P.; Antunes, L.; Salta, S.; Diniz, F.; Santos, L.L.; Videira, J.F.; et al. A novel DNA methylation panel accurately detects colorectal cancer independently of molecular pathway. J. Transl. Med. 2018, 16, 45. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.Z.; Xu, L.W.; Jia, R.P.; Xu, Z.; Feng, Y.M.; Wu, R.; Yu, P.; Zhao, Y.; Gui, Z.L.; Tan, S.J.; et al. The association between RASSF1A promoter methylation and prostate cancer: Evidence from 19 published studies. Tumour Biol. 2014, 35, 3881–3890. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Zhang, B.; Tan, Y.; Yang, C.; Huang, C.; Wu, Q.; Zhang, Y.; Chen, X.; Zhou, M.; Shu, A. Quantitative assessment of the relationship between RASSF1A gene promoter methylation and bladder cancer(PRISMA). Medicine 2017, 96, e6097. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Shi, M.; Spivack, S.D. Site-specific methylated reporter constructs for functional analysis of DNA methylation. Epigenetics 2013, 8, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Volodko, N.; Salla, M.; Zare, A.; Abulghasem el, A.; Vincent, K.; Benesch, M.G.; McMullen, T.P.; Bathe, O.F.; Postovit, L.; Baksh, S. RASSF1A Site-Specific Methylation Hotspots in Cancer and Correlation with RASSF1C and MOAP-1. Cancers 2016, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Cui, L.; Wang, C.; Guo, Y.; Shen, S.; Kuang, G.; Dong, Z. Decreased expression of RASSF1A and up-regulation of RASSF1C is associated with esophageal squamous cell carcinoma. Clin. Exp. Metastasis 2014, 31, 521–533. [Google Scholar] [CrossRef]
- Klacz, J.; Wierzbicki, P.M.; Wronska, A.; Rybarczyk, A.; Stanislawowski, M.; Slebioda, T.; Olejniczak, A.; Matuszewski, M.; Kmiec, Z. Decreased expression of RASSF1A tumor suppressor gene is associated with worse prognosis in clear cell renal cell carcinoma. Int. J. Oncol. 2016, 48, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Van der Weyden, L.; Papaspyropoulos, A.; Poulogiannis, G.; Rust, A.G.; Rashid, M.; Adams, D.J.; Arends, M.J.; O’Neill, E. Loss of RASSF1A synergizes with deregulated RUNX2 signaling in tumorigenesis. Cancer Res. 2012, 72, 3817–3827. [Google Scholar] [CrossRef] [PubMed]
- Vlahov, N.; Scrace, S.; Soto, M.S.; Grawenda, A.M.; Bradley, L.; Pankova, D.; Papaspyropoulos, A.; Yee, K.S.; Buffa, F.; Goding, C.R.; et al. Alternate RASSF1 Transcripts Control SRC Activity, E-Cadherin Contacts, and YAP-Mediated Invasion. Curr. Biol. 2015, 25, 3019–3034. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Hsu, S.D.; Huang, H.Y.; Sun, Y.M.; Chou, C.H.; Weng, S.L.; Huang, H.D. MethHC: A database of DNA methylation and gene expression in human cancer. Nucleic Acids Res. 2015, 43, D856–D861. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Graff, J.R.; Myohanen, S.; Nelkin, B.D.; Baylin, S.B. Methylation-specific PCR: A novel PCR assay for methylation status of CpG islands. Proc. Natl. Acad. Sci. USA 1996, 93, 9821–9826. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Ahuja, N.; Ohe-Toyota, M.; Herman, J.G.; Baylin, S.B.; Issa, J.P. CpG island methylator phenotype in colorectal cancer. Proc. Natl. Acad. Sci. USA 1999, 96, 8681–8686. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.A.; Yu, M.; Baker, K.; Redman, M.; Wu, C.; Heinzerling, T.J.; Wirtz, R.M.; Charalambous, E.; Pentheroudakis, G.; Kotoula, V.; et al. The CpG island methylator phenotype is concordant between primary colorectal carcinoma and matched distant metastases. Clin. Epigenetics 2017, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Rahi, H.; Wansley, D.; Varma, S.; Shokrani, B.; Lee, E.; Daremipouran, M.; Laiyemo, A.; Goel, A.; Carethers, J.M.; et al. Toward a comprehensive and systematic methylome signature in colorectal cancers. Epigenetics 2013, 8, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Weisenberger, D.J.; Liang, G.; Lenz, H.J. DNA methylation aberrancies delineate clinically distinct subsets of colorectal cancer and provide novel targets for epigenetic therapies. Oncogene 2018, 37, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, R.A.; Ladd-Acosta, C.; Wen, B.; Wu, Z.; Montano, C.; Onyango, P.; Cui, H.; Gabo, K.; Rongione, M.; Webster, M.; et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat. Genet. 2009, 41, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Grawenda, A.M.; O’Neill, E. Clinical utility of RASSF1A methylation in human malignancies. Br. J. Cancer 2015, 113, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Joosten, S.C.; Feng, Z.; de Ruijter, T.C.; Draht, M.X.; Melotte, V.; Smits, K.M.; Veeck, J.; Herman, J.G.; Neste, L.V.; et al. Analysis of DNA methylation in cancer: Location revisited. Nat. Rev. Clin. Oncol. 2018, 15, 459. [Google Scholar] [CrossRef] [PubMed]
- Van Neste, L.; Bigley, J.; Toll, A.; Otto, G.; Clark, J.; Delree, P.; Van Criekinge, W.; Epstein, J.I. A tissue biopsy-based epigenetic multiplex PCR assay for prostate cancer detection. BMC Urol. 2012, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Aran, D.; Sabato, S.; Hellman, A. DNA methylation of distal regulatory sites characterizes dysregulation of cancer genes. Genome Biol. 2013, 14, R21. [Google Scholar] [CrossRef] [PubMed]
- Widschwendter, M.; Jones, A.; Evans, I.; Reisel, D.; Dillner, J.; Sundstrom, K.; Steyerberg, E.W.; Vergouwe, Y.; Wegwarth, O.; Rebitschek, F.G.; et al. Epigenome-based cancer risk prediction: Rationale, opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 292. [Google Scholar] [CrossRef] [PubMed]
| Cancer Type | Methylation of RASSF1A |
|---|---|
| Hepatocellular carcinoma (HCC) | 522 of 669 (78%) HCC |
| Hepatoblastoma (HB) | 46 of 133 (34.6%) HB |
| Esophageal squamous cell carcinoma (ESCC) | 442 of 884 (50%) ESCC |
| Pancreatic ductal adenocarcinoma (PDAC) | 32 of 59 (54%) PDAC |
| Pancreatic endocrine tumor (PET) | 114 of 175 (75%) PET |
| Colorectal carcinoma (CRC) | 558 of 1567 (35.6%) CRC |
| Gastric cancer (GC) | 179 of 378 (31%) GC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malpeli, G.; Innamorati, G.; Decimo, I.; Bencivenga, M.; Nwabo Kamdje, A.H.; Perris, R.; Bassi, C. Methylation Dynamics of RASSF1A and Its Impact on Cancer. Cancers 2019, 11, 959. https://doi.org/10.3390/cancers11070959
Malpeli G, Innamorati G, Decimo I, Bencivenga M, Nwabo Kamdje AH, Perris R, Bassi C. Methylation Dynamics of RASSF1A and Its Impact on Cancer. Cancers. 2019; 11(7):959. https://doi.org/10.3390/cancers11070959
Chicago/Turabian StyleMalpeli, Giorgio, Giulio Innamorati, Ilaria Decimo, Maria Bencivenga, Armel Herve Nwabo Kamdje, Roberto Perris, and Claudio Bassi. 2019. "Methylation Dynamics of RASSF1A and Its Impact on Cancer" Cancers 11, no. 7: 959. https://doi.org/10.3390/cancers11070959
APA StyleMalpeli, G., Innamorati, G., Decimo, I., Bencivenga, M., Nwabo Kamdje, A. H., Perris, R., & Bassi, C. (2019). Methylation Dynamics of RASSF1A and Its Impact on Cancer. Cancers, 11(7), 959. https://doi.org/10.3390/cancers11070959









