Abstract
The intestinal epithelium interacts dynamically with the immune system to maintain its barrier function to protect the host, while performing the physiological roles in absorption of nutrients, electrolytes, water and minerals. The importance of lysophosphatidic acid (LPA) and its receptors in the gut has been progressively appreciated. LPA signaling modulates cell proliferation, invasion, adhesion, angiogenesis, and survival that can promote cancer growth and metastasis. These effects are equally important for the maintenance of the epithelial barrier in the gut, which forms the first line of defense against the milieu of potentially pathogenic stimuli. This review focuses on the LPA-mediated signaling that potentially contributes to inflammation and tumor formation in the gastrointestinal tract.
1. Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in the United States and the fourth leading cause of cancer-related deaths in the world [1]. Tumorigenesis is a multistep process in which each step reflects genetic alterations that drive the progressive transformation of normal cells into highly malignant derivatives [2]. A model for the genetic basis of colorectal neoplasia that includes mutational alterations in oncogenes and tumor suppressor genes was first described by Fearon and Vogelstein in 1990 [3]. Since then, many efforts have been made to discover additional genetic and cellular changes for colorectal tumorigenesis [4,5].
The intestine, which is topically exposed to the exterior, is lined with a monolayer of epithelial cells that forms a physical and functional barrier separating the internal milieu of the body from the lumen. This epithelial barrier allows absorption of essential nutrients, electrolytes and water, while preventing absorption of toxins and the invasion of commensal and pathogenic microorganisms. Damage to the epithelial barrier results in increased intestinal permeability and allows the entry of noxious substances, which in turn leads to intestinal inflammation [6]. As first hypothesized by Virchow in 1863, chronic inflammation potentiates and promotes neoplastic risk [7]. Examples of chronic inflammation potentiating neoplastic development include Helicobacter pylori infection in gastric adenocarcinoma and hepatitis B and C in hepatocellular carcinoma. Consistently, there is an increased risk of colitis-associated cancer (CAC) in inflammatory bowel disease (IBD), particularly in patients with ulcerative pancolitis and colonic CD [8,9]. More than 20% IBD patients develop CAC within 30 years of disease onset, and > 50% of these will die from CAC [10]. Oncogenic activation, while driving proliferation of cancer cells, results in the expression of transcription factors that produce inflammatory mediators, which promote angiogenesis, inhibit adaptive immunity, and alter response to hormones and growth factors (Figure 1) [11]. Thus, not only does inflammation promote cancer, but cancer induces inflammation.
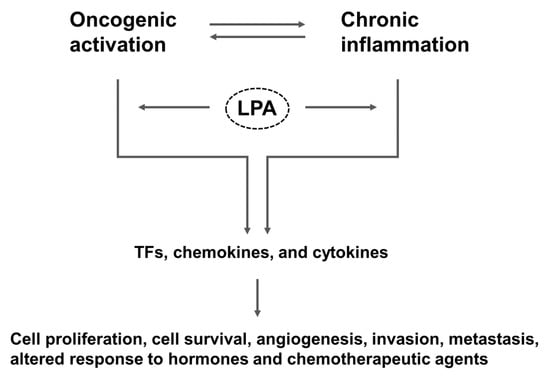
Figure 1.
Overall scheme that connects LPA to inflammation and cancer. LPA-mediated signaling activates both oncogenic and inflammatory pathways that promote tumorigenesis. TFs, transcription factors.
Cancer is a disease where cancer cells sustain proliferation, evade growth suppressors, resist cell death, enable replicative immortality, induce angiogenesis, and activate invasion and metastasis [12]. Cancer cells can achieve the perpetual growth through release of its own growth signals and mutational changes that over-express or constitutively activate cell surface receptors [13]. Studies have shown that lysophosphatidic acid (LPA) is capable of merging intrinsic oncogenic activation and extrinsic inflammatory pathways to sustain cell proliferation and promote cell survival, invasion, and metastasis (Figure 1). These inflammatory and proliferative effects of LPA are associated with the pathogenesis of cancer, fibrosis, and rheumatoid arthritis [14]. Instead of a comprehensive examination of pro-inflammatory and pro-tumorigenic roles of LPA and LPA receptors, this review focuses on the physiological and pathological effects of LPA in the gut.
2. LPA metabolism and signaling
LPA is a naturally occurring lipid mediator that consists of a glycerol backbone, a single acyl chain, and a phosphate group [15]. LPA is generated by multiple mechanisms. Extracellular LPA is derived from two pathways. First involves hydrolysis of the fatty acid moiety from the membrane derived phosphatidic acid (PA) by phospholipase A1 (PLA1) and phospholipase A2 (PLA2). The second pathway that produces majority of extracellular LPA involves the removal of choline moiety from lysophosphatidylcholine (LPC) by lysophospholipase D, also known as autotaxin (ATX) [16,17]. Intracellular LPA is produced as an intermediate product of the phospholipids and triacylglycerol synthesis [18]. LPA is turned over rapidly in the plasma by a family of lipid phosphate phosphatases (LPPs), which dephosphorylate LPA and decrease LPA available for receptor binding and activation [19,20].
LPA signals through six distinct G protein-coupled receptors, termed LPA1–LPA6 [21,22]. LPA1–LPA3 belong to the endothelial differentiation gene (EDG) subfamily, whereas LPA4–LPA6 bear resemblance to purinergic receptor [23]. The genes encoding these proteins are designated as LPAR1–6 in humans and Lpar1–6 in mice [24]. In addition, there are other orphan receptors that respond to LPA, including GPR35 [25], GPR87 [26], P2Y10 [27]. Of these, GPR35 is of interest to the gastrointestinal (GI) system. GPR35 has a number of ligands, including kynurenic acid and 2-acyl LPA, and GPR35 has been associated with a number of pathologies, including cardiovascular disease and diabetes [28]. A genome-wide association study has identified a single nucleotide polymorphism in GPR35 among UC patients [29], potentially linking the regulation of GPR35 to IBD. Recent studies have suggested GPR35 participates in mucosal repair regulation [30,31]. A role of GPR87 in cancer growth and metastasis is emerging too [32,33]. In addition to LPA, P2Y10 is activated by sphingosine-1-phosphate and lysophosphatidylserine [27,34]. These early studies are promising in defining the pathological importance of these receptors, but whether these effects are indeed mediated by LPA is not known.
LPA receptors transmit downstream signals to activate various cellular targets through heterotrimeric G proteins, including Gαi/o, Gαq/11, Gα12/13, Gαs (Table 1), and Gβγ. LPA receptor expression varies widely among different tissues and cell types, and this variation in LPA receptor expression in part accounts for the dichotomy of LPA-mediated effects observed in different tissues and cells [21]. Multiple LPA receptors are present in the GI tract with LPA1 and LPA5 being the highest in the intestinal epithelial cells (IECs) [35,36]. In addition to IECs, LPA receptors are found in the gut mucosal immune system, although a full profile of LPA receptor expression in the immune cells is not yet available. LPA2 is expressed by human CD4+ T cells and CD19+ B cells, but not in CD8+ T cells [37]. It was shown that LPA5 is highly expressed in the intraepithelial CD8+ T cells in the mouse intestine [38]. LPA5 is also abundantly expressed in human mast cells [39]. Immature and mature dendritic cells express LPA1–LPA3 [40,41].

Table 1.
LPA receptors in human and mouse.
3. Autotaxin
Bioactive LPA in serum and plasma is mainly produced by ATX, which is encoded by the Enpp2 gene that belongs to the ectonucleotide pyrophosphatase/phosphodiesterase (ENPP) family [44]. ATX is a unique ENPP member that possess lysophospholipase D (lysoPLD) activity that converts lysophosphatidylcholine (LPC) into LPA [45,46,47]. ATX is expressed as a pre-proenzyme, which is secreted upon proteolytic cleavage of the N-terminal signal peptide and further trimming by a furine-type protease [48]. Secreted ATX binds to integrin or heparan sulfate on the cell surface that enables the localization of ATX on the target cells [49,50]. Because LPA has a short half-life of ~ 3 min, this interaction of ATX with cell surface molecules provides a means for localized production of LPA close to its cognate receptors [49,50,51,52].
Studies using ATX knockout mice have revealed the essential role of ATX in vascular and neural development [17]. Homozygous knockout of the ATX gene (Enpp2−/−) results in embryonic lethality with profound vascular defects in the yolk sac and embryo, demonstrating the indispensable role of LPA in the development of blood vessels. ATX heterozygous (Enpp2+/−) mice are phenotypically normal, and the half-normal ATX activity and plasma LPA levels underscore the role of ATX as the major LPA producing enzyme in vivo [17]. A recent study has shown that LPA promotes vascular network formation in tumors with improved functional vascular permeability [53]. Remarkably, transgenic expression of ATX in mouse embryos results in vascular instability leading to decreased vessel branching and lethality [54]. The latter study suggests that the role of the ATX-LPA axis on vasculature integrity is complex and the LPA level must be regulated tightly [54].
Unlike embryonic deletion of ATX, deletion of ATX in adults does not result in lethality [55]. We have recently used Enpp2f/f;R26CreERT mice, which express Cre recombinase under the transcriptional control of the ubiquitous Rosa26 locus [56]. In contrast to the previous study [55], we found that deletion of ATX with tamoxifen (TAM) in adult Enpp2f/f;R26CreERT mice resulted in significant body weight loss. Most of TAM-treated mice gradually recovered body weights over the next several days, but about of a quarter of mice failed to recover [57]. There was evidence of increased inflammation with immune cell infiltration and elevated levels of TNF-α and INF-γ. Intestinal epithelium was damaged, correlating with weight loss. The reasons for the discrepancies between two studies are not clear but may relate to the use of different genetic backgrounds. Another potential explanation is the difference in gut microbiota known to influence the pathophysiology and histological appearance of animals [58]. However, we ruled out this possibility since we observed similar phenotypic changes in mice housed at two different locations over 3 years. Despite the discrepancy between the two studies, our study suggests that acute and near complete depletion of ATX may induce an adverse response in the intestinal tract.
ATX is among the 40 most up-regulated genes in highly metastatic cancers [59]. Elevated ATX expression has been detected in various types of cancer, including glioblastoma, breast cancer, non-small-cell lung cancer, and thyroid cancer [60,61,62,63]. Nakai et al. [64] have reported that serum ATX activities in patients with malignant pancreatic cancer were markedly elevated compared to those with benign pancreatic cyst, but serum ATX activities in gastric or colorectal cancer were not significantly different. Up-regulation of ATX in malignancies correlates with invasiveness and metastatic potential of cancer cells [65,66]. Indeed, ATX expression positively correlates with micro-vessel vascular density, macroscopic depression and tumor angiogenesis in the early stage of CRC [67].
Despite the increasing evidence associating ATX to cancer, direct evidence suggesting ATX as a potential therapeutic target for GI tumor is lacking. It was described that BrP-LPA, which acts as both pan-LPA GPCR antagonist and ATX inhibitor, was effective in reducing liver metastasis of HCT116 human colon cancer cells, but a full account of this study is yet to be reported [68]. Instead, the relevant evidence linking ATX to CRC comes from the studies of colitis. Hozumi et al. [69] observed that ATX mRNA levels are considerably elevated in inflamed mucosa from CD and UC patients. Similarly, ATX mRNA expression is elevated in dextran sodium sulfate (DSS)-induced rodent model of colitis and SAMP1/Fc mice, a mouse model of CD-like ileitis [57,69,70]. Pharmacological inhibition of ATX mitigated disease activity in DSS-induced model of IBD [69]. Consistently, deletion of Enpp2 gene decreased disease severity in both acute and chronic colitis induced by DSS with significant decreases in mucosal damage and inflammation [57].
The lumen of the GI tract is populated with potentially pathogenic microorganisms. The GI tract is heavily laden with lymphocytes, macrophages, and other cells that participate in immune responses [71]. ATX is highly expressed in high endothelial venules (HEVs) in lymph node and Peyer’s patches [72]. Secreted ATX by HEVs binds to lymphocytes to promote transendothelial migration of T cells and facilitate T cell extravasation to secondary lymphoid organs [72]. ATX promotes lymphocyte-HEV interactions by generating LPA locally, while ATX inhibition abolishes T cell entry into lymph node by arresting the transendothelial migration of T cells [73,74]. In keeping with these studies, there was a significant decrease in CD45+ or CD3+ cells in DSS-treated Enpp2−/− mice [57].
CD can occur anywhere between the mouth and the anus as such CD is often associated with malnutrition due to inflammation in the small intestine [75]. Pharmacological inhibition of ATX using the ATX inhibitor PF-8380 in SAMP1/Fc mice resulted in increased body weight with a decrease in Th2 cytokine expression, including IL-4, IL5 and IL-13 [70]. The improvement in body weight by PF-8380 treatment was associated with enhanced intestinal epithelial cell differentiation and increased Na+-dependent glucose transporter 1 (SGLT1), which is the major glucose and galactose transporter in the small intestine [70,76].
ATX mRNA is widely expressed but its expression has been observed in limited cell types that include adipocytes, oligodendrocytes, choroid plexus and brochial epithelial cells [61,77,78]. The secretion of ATX by adipocytes and elevated visceral fat ATX expression in obese humans has linked adipose tissue ATX to metabolic diseases [77,79,80]. Recent studies have shown that ATX derived from mammary adipose tissue stimulates breast cancer progression [81,82]. Moreover, the obesity promoting western diet has been shown to elevate unsaturated LPA levels in mouse small intestine, and adipocyte-specific deletion of ATX prevents high fat diet-associated hepatic steatosis [83,84]. Because there is a strong relationship between obesity and CRC, and the locale of visceral fat close to the GI tract, it is likely that adipose ATX influences gastrointestinal tumorigenesis and targeting adipose ATX could a potential therapeutic strategy for CRC.
Despite the general acceptance that ATX expression is elevated in various types of cancer, ATX expression in epithelial cancer cells is relatively low or undetectable by immunoblotting or immunohistochemistry [61,66,85]. Similarly, Enpp2 gene expression in most of colon cancer cell lines is low with an exception of Colo320 cells, which has a neuroendocrine origin [57,61,86]. Boiler et al. [87] recently found that enteroendocrine cells in human intestine express ATX, but not in the intestine of rodents [87]. By cell fractionation, we have recently found that B cells are a major ATX expressing cell type in mouse gut. Human B cells also express ATX, suggesting that both B cells and enteroendocrine cells secrete ATX in human intestine. ATX secreted from splenic B cells is able to increase ERK phosphorylation in HCT116 colon cancer cells and induce migration of Jurket T cells [57]. The relative importance of ATX secreted from B cells, enteroendocrine cells, or adipose tissues in initiating and promoting inflammation or CRC remains to be determined.
4. LPA1
The intestinal tract is lined primarily with columnar epithelial cells that are regenerated every 4–5 days in rodents. The rapid turnover of the intestinal epithelium is maintained by stem cells residing at the crypt base. Turnover of the intestinal epithelium involves a series of actions that include proliferation at the crypt base, migration and differentiation along the crypt-luminal axis, and ultimately regulated shedding at the luminal surface [88]. In the mouse intestinal tract, LPA1 is the highest based on transcript expression [35]. Lpar1−/− mice show fewer numbers of proliferation cells in the intestinal crypts and decreased mobility of these cells from the crypt to the luminal surface, suggesting defects in IEC homeostasis [89].
Migration is a key aspect of many physiological and pathological processes, including wound healing and tumor cell metastasis [90], and a body of evidence demonstrates that LPA regulates migration of various types of cells [21,91]. Migrating cells undergo transition in cell shape that is orchestrated by the RhoA family of GTPase, actin cytoskeletal reorganization, and focal adhesion kinase (FAK). LPA rapidly induces reorganization of the actin cytoskeleton that forms lamellipodial protrusions in the leading edge [92]. FAK plays a crucial role in LPA-induced assembly of focal adhesions and migration of IECs [92,93]. LPA induces proliferation and migration of non-transformed intestinal cell lines, including rat IEC-6, Young adult mouse colonic epithelium (YAMC), and mouse small intestinal epithelium (MSIE) cells, which have high levels of Lpar1 and Lpar2 genes. The sensitivity to Ki16425, an antagonist for LPA1 and LPA3, suggests that the effects in these cells are mediated by LPA1 [89]. LPA1 couples with Gi/o, Gq/11, and G12/13 [94]. In YAMC cells, activation of LPA1 translocates Gαq to the nucleus where it interacts with PLC-β1 to stimulate cell cycle programing. PLC-β2, on the other hand, activates Rac1 at the plasma membrane contributing to cell migration [89].
Several studies in rodents have demonstrated that LPA regulates the wound healing process. A topical application of LPA ameliorated mucosal damage induced by trinitrobenzene [95]. Oral administration of DSS induces reversible damage to the epithelial monolayer lining the colon, which is accompanied by onset of inflammation. Once DSS is removed, inflammation in the colon subsides and the colonic epithelia begin to recover by replacing damaged epithelial cells. [96]. Ki16425 delayed the recovery from DSS-induced colitis, suggesting the role of LPA1 in wound repair [89,97]. We have shown that orally administered LPA facilitates the closure of a wound on the mucosal surface induced by a mechanical device. The wound closure by LPA in mouse mucosa is mediated via LPA1, as LPA has no effect in Lpar1−/− mice [89].
Despite the overwhelming evidence for tumorigenic effects of LPA, a number of studies have suggested the therapeutic potential of LPA for the treatment of wounds. The GI tract is the primary site of digestion, and high contents of LPA are found in several types of foodstuffs, including cabbages, soybean, tomatoes, and eggs [98,99,100]. In addition, PA, which is a precursor of LPA, is converted to LPA during mastication in the mouth by salivary PLA2 [101]. LPA derived from cabbage leaves facilitated proliferation and motility of fibroblasts and gastric epithelial cells, which was inhibited by Ki16425 [101]. In addition, a number of studies have demonstrated the wound healing or anti-inflammatory effects of LPA derived from medicinal herbs [102,103,104,105]. On the other hand, oral administration of LPA to ApcMin mice, which are predisposed to intestinal adenoma formation due to a mutant allele of the Apc gene, increased the number of adenomas [36]. Similarly, a high-fat diet together with LPA-rich soybean phospholipid had a tumor promoting effect than the high-fat diet alone in rats exposed to the tumor causing combination of azoxymethane (AOM) and DSS [106]. The beneficial (wound healing) vs harmful (tumor promoting) effects of LPA present in the intestinal lumen requires further studies, but the context dependency (normal vs cancer cells, somatic mutations, LPA receptor expression, etc) needs to be considered.
In addition to the defect in IEC proliferation and migration, LPA1-deficiency compromises the intestinal epithelial barrier function with aberrant apical junctional complex. Increased intestinal epithelial permeability renders Lpar1−/− mice more permissible to the intestinal penetration of gut microorganisms and bacterial products such as liposaccharide [107]. Increased intestinal permeability is associated with chronic inflammatory diseases of the gut, including IBD, celiac disease, and infectious diarrheal disease [108,109,110]. Indeed, Lpar1−/− mice are more susceptible to colitis induced by DSS with increased inflammation and mortality [107]. Notably, LPA protects IECs from TNF-α-induced epithelial barrier dysfunction in vitro and in vivo via a LPA1-dependent mechanism [107].
Elevated levels of LPAR1 expression have been observed in various types of tumors and cancer cell lines, including ovarian, breast, prostate, and bladder cancer [111,112,113,114]. Frequent mutations in LPAR1 has been observed in rat liver tumors and the role of ATX-LPA1 axis in lung carcinogenesis has recently been implicated [115,116]. Although LPAR1 mRNA expression levels were reported to be low in human CRC tissues and colon cancer cell lines [117,118,119], a recent study of GWAS meta-analysis has identified LPAR1 as one of the 40 new CRC risk loci [5].
LPA1 seems to have a pervasive effect on cancer cell motility. LPA enhanced migration and adhesion of DLD1 human carcinoma cell line via LPA1 [118]. LPA1 activation stimulated migration of NUGC-3 and MKN1 human gastric cancer cells [120]. LPA stimulated scattering of LPA1-expressing DLD1 cells and AGS human gastric carcinoma cell line, but no apparent effect on LPA2-expressing MKN74 cells was observed [121]. Moreover, attenuation of invasion or metastasis of breast cancer cells, pancreatic cancer cells, breast cancer cells, and melanoma cells by LPA1 antagonism has been reported [122,123,124,125]. LPA1 may or may not stimulate colon cancer cell proliferation. Yang et al. [126] reported that knockdown of LPA1 did not affect proliferation of HCT116 and LS174T colon cancer cells, but LPA stimulated proliferation of DLD1 cells via LPA1 [118].
There is evidence that LPA1 activation participates in drug resistance. It has been reported that LPA1 activation increases nuclear factor erythroid 2-related factor 2 (Nrf2) stability, with subsequent increases in multidrug-resistant transporters and antioxidant genes that protect breast cancer cells from doxorubicin-induced death [127]. A recent study reported that LPAR1 gene expression was elevated in fluorouracil (5-FU) resistant DLD1 cells and LPAR1 gene knockdown ablated the formation of colonies on soft agar [128]. Similarly, long-term treatment of PANC-1 pancreatic cells with cisplatin elevated LPAR1 and LPAR3 expression [129]. Drug resistance is a major obstacle in conventional cancer therapies and LPA1 is emerging as a potential target to improve chemotherapy.
LPA1, LPA2 and LPA5 have the Class I PSD-95, DlgA, and ZO-1 (PDZ) binding motif sequence X-(S/T)-X-(V/I/L)-COOH (where X is any amino acid) at the carboxyl terminus. The interaction of PDZ-domain containing proteins modulates the nature of LPA-mediated signaling and effects. LPA1 in particular interacts with leukemia-associated Rho guanine nucleotide exchange factor (RhoGEF), and PDZ-RhoGEF via the carboxyl-terminal SVV sequence to activate RhoA [130]. In addition, the PDZ protein GIPC regulates endocytic trafficking of LPA1. Depletion of GIPC perturbs trafficking of LPA1 to early endosomes and prolongs LPA1 signaling [131]. Indeed, LPA1 lacking the carboxyl PDZ-binding domain stimulates B103 neuroblastoma cell proliferation with persistent activation of the PI3K-Akt pathway [132].
5. LPA2
The relevance of LPA2 to carcinogenesis has been suggested early on by the findings that LPAR2 gene expression is elevated in ovarian cancer and thyroid cancer [133,134,135]. Since then, increased LPAR2 gene or LPA2 expression has been observed in carcinoma tissues of breast, liver and uterus, and multiple cancer cell lines [136,137,138]. Shida et al. [117] have found elevated expression of LPAR2 gene in CRC patients. The altered expression of LPAR2 is a common occurrence in several colon cancer cell lines [117,119]. In ApcMin/+ mice, a mouse model of familial polyposis, LPAR2 mRNA level increases with increased size of adenomas, and oral administration of LPA increases adenomas growth [35]. The importance of LPA2 in carcinogenesis in the intestinal tract has been experimentally demonstrated where the absence of LPA2 reduces tumor burden in experimental models of intestinal cancer [35,36]. On the other hand, transgenic expression of LPA2 driven by the Villin promoter resulted in intestinal dysplasia [139].
Activation of LPA2 and LPA3 promotes proliferation and migration of human colon cancer cells, including HCT116, LS174T, SW480, and LoVo [126,140]. LPA also stimulates migration of SGC-7901 gastric cancer cells via LPA2 [141]. The most commonly mutated gene in CRC is the Apc gene, which results in accumulation of β-catenin and subsequent transcriptional activation of proto-oncogenes [142]. Several studies have shown that LPA2-mediated colon cancer cell growth involves activation of β-catenin. The initial implication that LPA may regulate β-catenin came from a study demonstrating phosphorylation of glycogen synthase kinase 3β (GSK-3β) at Ser21 by LPA [143]. Although this study was not aimed at β-catenin regulation by LPA, inhibition of GSK-3β is linked to activation of the Wnt/β-catenin pathway by stabilization of β-catenin and subsequent target gene transcription by complexing with T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) [144]. It was shown later that LPA2 and LPA3, but not LPA1, enhanced nuclear translocation of β-catenin and target gene transcription in HCT116 and LS174T colon cancer cells [126]. In addition to phosphorylation of GSK at Ser9, LPA2 can activate β-catenin by phosphorylation at Ser552 and Ser675, which induces nuclear translocation of β-catenin and interaction with TCF4 [126,145].
Another major transcription factor that play a significant role in LPA2-mediated oncogenic effect is Kruppel-like factor 5 (KLF5). KLF5 is highly expressed in proliferating intestinal crypt cells, and studies in knockout mice have linked KLF5 to intestinal crypt epithelial cell proliferation and transformation [146]. A recent study has identified KLF5 gene as a CRC risk gene [5]. LPA2 induces KLF5 expression, and knockdown of KLF5 reduces proliferation of SW480 and HCT116 colon cancer cells by impeding cell cycle [140]. KLF5 enhances the interaction between β-catenin and TCF4 complex increasing β-catenin activity, without affecting β-catenin nuclear translocation [145].
Hypoxia-inducible factor 1 (HIF-1) is a pivotal regulator of multiple aspects of tumorigenesis, including cancer cell proliferation, angiogenesis, metastasis and chemotherapy resistance [147]. HIF-1α is a transcription factor that mediates adaptive responses to changes in tissue oxygenation, but several growth factors, activated oncogenes, and LPA can induce HIF-1α expression under nonhypoxic conditions [148,149]. LPA induces HIF-1α expression in several types of cancer cells, including colon cancer cells [150,151]. In colon cancer cells, LPA2 induces HIF-1α gene expression via dynamic interaction of Hif-1α promoter with KLF5 and p53 [151]. Once synthesized, HIF-1α protein is stabilized via its interaction with macrophage migration inhibitory factor (MIF), hence retarding oxygen-dependent hydroxylation and proteasomal degradation [148,151,152]. MIF, which is transcriptionally regulated by LPA, is important for LPA-mediated colon cancer cell invasion, metastasis, and growth [153,154].
As described earlier, LPA2 has PDZ binding sequences at the carboxyl terminus. LPA2 interacts with several PDZ domain-containing proteins, including Na+/H+ exchanger regulatory factor 2 (NHERF2), membrane-associated guanylate kinase with inverted orientation-3 (MAGI-3), RhoGEF, and PDZ-RhoGEF [130,155,156]. In addition, the proximal region of LPA2 carboxyl-terminus associates with several zinc figure proteins, including the LIM-domain containing TRIP6 and the pro-apoptotic Siva-1 protein [157,158]. These interactions modulate the activation of several key proteins, including Akt, Erk, PLCβ, Cox-2, RhoA and NF-κB [119,130,155,159,160]. In 1998, An et al. identified a LPAR2 cDNA clone from an ovarian tumor library, which was shown later to have a frameshift mutation near the 3′ end of the coding region [161,162]. Because of this form of LPA2 lacks the C-terminal PDZ-binding motif, it remains an intriguing speculation that this mutation could cause aberrant LPA2 signaling, similar to the C-terminal truncated LPA1 [132].
LPA prevents apoptosis induced by chemotherapeutic drugs and radiation injury through inhibiting the mitochondrial apoptosis pathway [163,164]. LPA enhances cell survival post-irradiation, and the ability of LPA2 to interact with TRIP6, Siva-1 or NHERF2 is critical in LPA-mediated protection of cells [158,163,165,166]. Lpar2−/− mice have defects in intestinal epithelial recovery from radiation-induced injury, and LPA2 agonists show a therapeutic potential against radiation-induced injury [167,168].
6. LPA3
The expression level of Lpar3 mRNA in the mouse intestinal epithelia and adenomatous lesions of ApcMin/+ mice is low compared to Lpar1 mRNA levels [35]. Similarly, LPAR3 mRNA expression in human colonic tissues is relatively low and its expression is not significantly altered in human CRC biopsies [117]. Probably related to the low LPA3 expression, studies on LPA3 in colon cancer are limited. LPA3 activation increases proliferation of HCT116 and LS174T colon cancer cells via stimulation of the β-catenin pathway [126]. Surprisingly, Fukui et al. [169] reported that LPA3 has a negative effect on HCT116 cells as LPA3-deficient HCT116 cells have increased cell motility associated with elevated expression of VEGF gene. However, experimental evidence from studies on other tissues and organs points to cancer-promoting effects of LPA3. The motility and invasive properties of pancreatic cancer cells are inhibited by LPA3 knockdown [170]. Long-term culturing of PANC-1 cells in the presence of cisplatin resulted in increased expression of LPAR3, suggesting the role of LPA3 in drug resistance in pancreatic cancer [129]. There appears a strong correlation between LPAR3 expression and the triple receptor-negative breast cancers, and LPAR3 knockdown attenuate motility and invasion of breast and pancreatic cancer cells [170,171]. In addition, the embryo implantation defect in Lpar3−/− mice is attributed to downregulation of COX-2, which is associated with carcinogenesis [172,173]. These warrant further studies on the significance of LPA3 in CRC.
7. LPA4
LPA4 is the first non-EDG LPA receptor cloned [174]. LPA4 expression in the intestinal tract is low in both human and mouse [35,174]. Contrary to cell motility promoting LPA1-LPA3, a number of studies have shown a negative effect of LPA4 on cell motility. Lpar4-deficient embryonic fibroblasts are hypersensitive to LPA-mediated cell migration [175]. Knockdown of LPA4 in DLD1 and HCT116 cells was shown to increase the cell motility, whereas overexpression of LPA4 in DLD1 cells is sufficient to mitigate LPA-driven migration and invasion of DLD1 cells [175,176]. Similarly, LPA4 depletion increases invasive activities of PANC pancreatic cancer cells with elevated MMP-9 activity. On the other hand, LPA4 is necessary for the formation of invadopodia that stimulates invasion and metastasis of HT1080 human fibrosarcoma cells, suggesting cell-type dependent roles of LPA4 [177].
The abnormality in blood vessel formation in ATX deficiency that results in embryonic lethality has demonstrated the pivotal role of the ATX-LPA axis in vasculature [17]. Similarly, LPA4 deficient embryos display abnormalities in the blood vascular system, which cause lethality of embryos and neonates [178]. A recent study by Takara et al. [53] reported that LPA4 activation promotes vasculature network formation in tumors by enhancing maturation of blood vessels. This study points to the possibility of using LPA4 agonism to enhance drug deliver in tumors [53]. Keeping with this notion, a recent study showed that LPA4 activation promoted the formation of fine vascular structures in brain tumors by inducing tightening of endothelial cell-to-cell adhesion and increased lymphocyte infiltration into the tumor [179].
8. LPA5
Lpar5 mRNA expression is abundant in the GI tract [180], and our previous studies have demonstrated the role of LPA5 in activation of intestinal Na+ and fluid absorption by Na+/H+ exchanger 3 (NHE3) [181,182]. Imbalance in electrolyte and water absorption and secretion not only leads to diarrhea, but also contributes to chronic inflammation. For example, NHE3 expression is often down-regulated in IBD and the absence of NHE3 alters the gut microbiota and renders mice more sensitive to DSS-induced colitis [183]. In this context, the activation of NHE3 via LPA5 can potentially regulates inflammatory responses via augmentation of the epithelial barrier.
Unlike primary intestinal tissues and cells, Lpar5 mRNA expression in human colon cancer cells is relatively low [181]. Notably, the Lpar5 mRNA expression levels in several cancer cells correlate with the methylation status of the Lpar5 5′ region [184]. We found that exogenous expression of LPA5 in MSIE cells attenuated cell proliferation and Lpar5−/− colonoids grew more rapidly than control colonoids in Matrigel. Recently, studies using melanoma and pancreatic cancer cell lines have shown anti-migratory and anti-metastatic effects of LPA5 [42,125]. These preliminary studies suggest that LPA5 has a negative effect on cell proliferation and migration.
LPA5 expression in the intestine is not limited to epithelial cells, but also present in CD8+ T cells, B cells and mast cells [38,39,43]. LPA5 activation in CD8+ T cells suppresses T-cell activation and proliferation, and Lpar5−/− CD8+ T cell transfer suppresses tumor growth in a mouse model of melanoma [185]. LPA5 also negatively regulates B cell antigen receptor signaling via Gα12/13 pathways [43]. On the other hand, LPA5 is the main LPA receptor in mast cells responsible for macrophage inflammatory protein (MIP)-1β release [39].
9. LPA6
The status of LPA6 expression in CRC is not yet known, but higher LPA6 mRNA level in hepatocellular carcinoma is associated with microvascular invasion [137]. It was reported that in human umbilical vein endothelial cells (HUVECs) LPA6 activation induces actin stress fiber formation that enhances vasculature permeability [186]. On the other hand, knockdown of LPA6 stimulates mobility of DLD1 and HCT116 cells [176]. Although LPAR6 gene expression was elevated in cisplatin-resistant DLD1 cells, knockdown of LPA6 resulted in larger colonies, further suggesting an anticancer role of LPA6 [128].
10. Conclusions
A balance between the external stimuli and immune response of either tolerance or defense finely regulates intestinal homeostasis. In this context, the emerging idea that LPA is involved on the regulation of more activities than merely the proliferation and migration of cell in the gut has provided new insights into the significance of the ATX-LPA axis in the gut. Studies in the past two decades have decoded many roles that each LPA receptor plays. The current and past studies of LPA in the gut have largely focused on how LPA modulate epithelial cell fate, but much remains to be learned on receptor expression and effects on non-epithelial cells, including lymphoid cells, myeloid cells and enteric neurons within the gut. In addition, many questions about the dynamic nature of LPA signaling in the gut remain, including how LPA modulates the interaction between different population of cells, such as the epithelia and immune system, and whether the influence varies over time and at different regions within the gut. Recent studies have shown a promising outcome of LPA receptor inhibition on the treatment of idiopathic pulmonary fibrosis [187]. Although in vitro and animal studies have suggested potential benefits of blocking ATX-LPA, clinical data that support the relevance of ATX and LPA to the development and progression of IBD or CRC are still limited. Further investigation into these aspects will provide better strategies for the highly anticipated therapeutic targeting of ATX-LPA signaling.
Funding
This work was supported by NIH grants R01DK071597, R01DK116799 and the VA Merit award I01BX002540.
Conflicts of Interest
The author declares no conflict of interest.
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [CrossRef] [PubMed]
- Huyghe, J.R.; Bien, S.A.; Harrison, T.A.; Kang, H.M.; Chen, S.; Schmit, S.L.; Conti, D.V.; Qu, C.; Jeon, J.; Edlund, C.K.; et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat. Genet. 2019, 51, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Jess, T.; Rungoe, C.; Peyrin-Biroulet, L. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of population-based cohort studies. Clin. Gastroenterol. Hepatol. 2012, 10, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Kappelman, M.D.; Farkas, D.K.; Long, M.D.; Erichsen, R.; Sandler, R.S.; Sorensen, H.T.; Baron, J.A. Risk of cancer in patients with inflammatory bowel diseases: A nationwide population-based cohort study with 30 years of follow-up evaluation. Clin. Gastroenterol. Hepatol. 2014, 12, 265–273.e1. [Google Scholar] [CrossRef]
- Lakatos, P.L.; Lakatos, L. Risk for colorectal cancer in ulcerative colitis: Changes, causes and management strategies. World J. Gastroenterol. 2008, 14, 3937–3947. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Ramaswami, R.; Harding, V.; Newsom-Davis, T. Novel cancer therapies: Treatments driven by tumour biology. Postgrad. Med. J. 2013, 89, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. LPA receptor signaling: Pharmacology, physiology, and pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.E.; Herr, D.R.; Chun, J. Lysophosphatidic acid (LPA) receptors: Signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 2010, 91, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J. Mechanisms of lysophosphatidic acid production. Semin. Cell Dev. Biol. 2004, 15, 477–489. [Google Scholar] [CrossRef]
- van Meeteren, L.A.; Ruurs, P.; Stortelers, C.; Bouwman, P.; van Rooijen, M.A.; Pradere, J.P.; Pettit, T.R.; Wakelam, M.J.; Saulnier-Blache, J.S.; Mummery, C.L.; et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell. Biol. 2006, 26, 5015–5022. [Google Scholar] [CrossRef]
- Takeuchi, K.; Reue, K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1195–E1209. [Google Scholar] [CrossRef]
- Brindley, D.N.; Pilquil, C. Lipid phosphate phosphatases and signaling. J. Lipid Res. 2009, 50, S225–S230. [Google Scholar] [CrossRef]
- Benesch, M.G.K.; Tang, X.; Venkatraman, G.; Bekele, R.T.; Brindley, D.N. Recent advances in targeting the autotaxin-lysophosphatidate-lipid phosphate phosphatase axis in vivo. J. Biomed. Res. 2016, 30, 272–284. [Google Scholar]
- Choi, J.W.; Herr, D.R.; Noguchi, K.; Yung, Y.C.; Lee, C.W.; Mutoh, T.; Lin, M.E.; Teo, S.T.; Park, K.E.; Mosley, A.N.; et al. LPA receptors: Subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Houben, A.J.; Moolenaar, W.H. Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev. 2011, 30, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, K.; Kurikawa, Y.; Shimizu, T.; Ishii, S. Current progress in non-Edg family LPA receptor research. Biochim. Biophys. Acta 2013, 1831, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Hla, T.; Lynch, K.R.; Spiegel, S.; Moolenaar, W.H. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid Receptor Nomenclature. Pharmacol. Rev. 2010, 62, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Ota, R.; Shima, M.; Yamashita, A.; Sugiura, T. GPR35 is a novel lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun. 2010, 395, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Tabata, K.I.; Baba, K.; Shiraishi, A.; Ito, M.; Fujita, N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun. 2007, 363, 861–866. [Google Scholar] [CrossRef]
- Murakami, M.; Shiraishi, A.; Tabata, K.; Fujita, N. Identification of the orphan GPCR, P2Y(10) receptor as the sphingosine-1-phosphate and lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun. 2008, 371, 707–712. [Google Scholar] [CrossRef]
- MacKenzie, A.; Lappin, J.; Taylor, D.; Nicklin, S.; Milligan, G. GPR35 as a Novel Therapeutic Target. Front. Endocrinol. 2011, 2, 68. [Google Scholar] [CrossRef]
- Imielinski, M.; Baldassano, R.N.; Griffiths, A.; Russell, R.K.; Annese, V.; Dubinsky, M.; Kugathasan, S.; Bradfield, J.P.; Walters, T.D.; Sleiman, P.; et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat. Genet. 2009, 41, 1335–1340. [Google Scholar] [CrossRef]
- Farooq, S.M.; Hou, Y.; Li, H.; O’Meara, M.; Wang, Y.; Li, C.; Wang, J.-M. Disruption of GPR35 Exacerbates Dextran Sulfate Sodium-Induced Colitis in Mice. Dig. Dis. Sci. 2018, 63, 2910–2922. [Google Scholar] [CrossRef]
- Tsukahara, T.; Hamouda, N.; Utsumi, D.; Matsumoto, K.; Amagase, K.; Kato, S. G protein-coupled receptor 35 contributes to mucosal repair in mice via migration of colonic epithelial cells. Pharmacol. Res. 2017, 123, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Li, H.; Zhu, M.; Zhao, F.; Zhang, L.; Chen, T.; Jiang, G.; Xie, H.; Cui, Y.; Yao, M.; et al. G Protein-Coupled Receptor 87 (GPR87) Promotes the Growth and Metastasis of CD133+ Cancer Stem-Like Cells in Hepatocellular Carcinoma. PLoS ONE 2013, 8, e61056. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, W.; Zhong, Y.; Huo, Y.; Fan, P.; Zhan, S.; Xiao, J.; Jin, X.; Gou, S.; Yin, T.; et al. Overexpression of G protein-coupled receptor GPR87 promotes pancreatic cancer aggressiveness and activates NF-κB signaling pathway. Mol. Cancer 2017, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.L.; Jiang, J.K.; Yang, S.H.; Huang, T.S.; Lan, H.Y.; Teng, H.W.; Yang, C.Y.; Tsai, Y.P.; Lin, C.H.; Wang, H.W.; et al. MicroRNA-146a directs the symmetric division of Snail-dominant colorectal cancer stem cells. Nat. Cell Biol. 2014, 16, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lee, S.J.; Shim, H.; Chun, J.; Yun, C.C. The Absence of LPA receptor 2 Reduces the Tumorigenesis by ApcMin Mutation in the Intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1128–G1138. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, D.; Iyer, S.; Ghaleb, A.M.; Shim, H.; Yang, V.W.; Chun, J.; Yun, C.C. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology 2009, 136, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Kong, Y.; Voice, J.K. Cutting edge: Differential constitutive expression of functional receptors for lysophosphatidic acid by human blood lymphocytes. J. Immunol. 2000, 164, 4996–4999. [Google Scholar] [CrossRef] [PubMed]
- Kotarsky, K.; Boketoft, A.; Bristulf, J.; Nilsson, N.E.; Norberg, A.; Hansson, S.; Owman, C.; Sillard, R.; Leeb-Lundberg, L.M.; Olde, B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J. Pharmacol. Exp. Ther. 2006, 318, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Lundequist, A.; Boyce, J.A. LPA5 Is Abundantly Expressed by Human Mast Cells and Important for Lysophosphatidic Acid Induced MIP-1β Release. PLoS ONE 2011, 6, e18192. [Google Scholar] [CrossRef] [PubMed]
- Panther, E.; Idzko, M.; Corinti, S.; Ferrari, D.; Herouy, Y.; Mockenhaupt, M.; Dichmann, S.; Gebicke-Haerter, P.; Di Virgilio, F.; Girolomoni, G.; et al. The influence of lysophosphatidic acid on the functions of human dendritic cells. J. Immunol. 2002, 169, 4129–4135. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.C.; Peters, W.; Xu, Y.; Chun, J.; Farese, R.V., Jr.; Cases, S. LPA3 receptor mediates chemotaxis of immature murine dendritic cells to unsaturated lysophosphatidic acid (LPA). J. Leukoc. Biol. 2007, 82, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Hirane, M.; Fukushima, K.; Tomimatsu, A.; Fukushima, N.; Tsujiuchi, T. Diverse effects of LPA4, LPA5 and LPA6 on the activation of tumor progression in pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2015, 461, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Oda, S.K.; Shotts, K.; Donovan, E.E.; Strauch, P.; Pujanauski, L.M.; Victorino, F.; Al-Shami, A.; Fujiwara, Y.; Tigyi, G.; et al. Lysophosphatidic Acid receptor 5 inhibits B cell antigen receptor signaling and antibody response. J. Immunol. 2014, 193, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Goding, J.W.; Grobben, B.; Slegers, H. Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochim. Biophys. Acta 2003, 1638, 1–19. [Google Scholar] [CrossRef]
- Aoki, J.; Inoue, A.; Okudaira, S. Two pathways for lysophosphatidic acid production. Biochim. Biophys. Acta 2008, 1781, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Tokumura, A.; Majima, E.; Kariya, Y.; Tominaga, K.; Kogure, K.; Yasuda, K.; Fukuzawa, K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J. Biol. Chem. 2002, 277, 39436. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, J.; Perrakis, A.; Moolenaar, W.H. Structure-function relationships of autotaxin, a secreted lysophospholipase D. Adv. Biol. Regul. 2013, 53, 112–117. [Google Scholar] [CrossRef]
- Jansen, S.; Stefan, C.; Creemers, J.W.; Waelkens, E.; Van Eynde, A.; Stalmans, W.; Bollen, M. Proteolytic maturation and activation of autotaxin (NPP2), a secreted metastasis-enhancing lysophospholipase D. J. Cell Sci. 2005, 118, 3081–3089. [Google Scholar] [CrossRef]
- Fulkerson, Z.; Wu, T.; Sunkara, M.; Kooi, C.V.; Morris, A.J.; Smyth, S.S. Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells. J. Biol. Chem. 2011, 286, 34654–34663. [Google Scholar] [CrossRef]
- Houben, A.J.; van Wijk, X.M.; van Meeteren, L.A.; van Zeijl, L.; van de Westerlo, E.M.; Hausmann, J.; Fish, A.; Perrakis, A.; van Kuppevelt, T.H.; Moolenaar, W.H. The polybasic insertion in autotaxin alpha confers specific binding to heparin and cell surface heparan sulfate proteoglycans. J. Biol. Chem. 2013, 288, 510–519. [Google Scholar] [CrossRef]
- Tomsig, J.L.; Snyder, A.H.; Berdyshev, E.V.; Skobeleva, A.; Mataya, C.; Natarajan, V.; Brindley, D.N.; Lynch, K.R. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochem. J. 2009, 419, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Albers, H.M.; Dong, A.; van Meeteren, L.A.; Egan, D.A.; Sunkara, M.; van Tilburg, E.W.; Schuurman, K.; van Tellingen, O.; Morris, A.J.; Smyth, S.S.; et al. Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation. Proc. Natl. Acad. Sci. USA 2010, 107, 7257–7262. [Google Scholar] [CrossRef] [PubMed]
- Takara, K.; Eino, D.; Ando, K.; Yasuda, D.; Naito, H.; Tsukada, Y.; Iba, T.; Wakabayashi, T.; Muramatsu, F.; Kidoya, H.; et al. Lysophosphatidic Acid Receptor 4 Activation Augments Drug Delivery in Tumors by Tightening Endothelial Cell-Cell Contact. Cell Rep. 2017, 20, 2072–2086. [Google Scholar] [CrossRef] [PubMed]
- Yukiura, H.; Kano, K.; Kise, R.; Inoue, A.; Aoki, J. Autotaxin Overexpression Causes Embryonic Lethality and Vascular Defects. PLoS ONE 2015, 10, e0126734. [Google Scholar] [CrossRef] [PubMed]
- Katsifa, A.; Kaffe, E.; Nikolaidou-Katsaridou, N.; Economides, A.N.; Newbigging, S.; McKerlie, C.; Aidinis, V. The Bulk of Autotaxin Activity Is Dispensable for Adult Mouse Life. PLoS ONE 2015, 10, e0143083. [Google Scholar] [CrossRef] [PubMed]
- Seibler, J.; Zevnik, B.; Kuter-Luks, B.; Andreas, S.; Kern, H.; Hennek, T.; Rode, A.; Heimann, C.; Faust, N.; Kauselmann, G.; et al. Rapid generation of inducible mouse mutants. Nucleic Acids Res. 2003, 31, e12. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Haque, A.; Raeman, R.; Guo, L.; He, P.; Denning, T.L.; El-Rayes, B.; Moolenaar, W.H.; Yun, C.C. Autotaxin determines colitis severity in mice and is secreted by B cells in the colon. FASEB J. 2019, 33, 3623–3635. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Euer, N.; Schwirzke, M.; Evtimova, V.; Burtscher, H.; Jarsch, M.; Tarin, D.; Weidle, U.H. Identification of genes associated with metastasis of mammary carcinoma in metastatic versus non-metastatic cell lines. Anticancer Res. 2002, 22, 733–740. [Google Scholar] [PubMed]
- Benesch, M.G.; Ko, Y.M.; Tang, X.; Dewald, J.; Lopez-Campistrous, A.; Zhao, Y.Y.; Lai, R.; Curtis, J.M.; Brindley, D.N.; McMullen, T.P. Autotaxin is an inflammatory mediator and therapeutic target in thyroid cancer. Endocr. Relat. Cancer 2015, 22, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Okudaira, S.; Tanaka, M.; Hama, K.; Shida, D.; Kitayama, J.; Yamori, T.; Aoki, J.; Fujimaki, T.; Arai, H. Autotaxin is overexpressed in glioblastoma multiforme and contributes to cell motility of glioblastoma by converting lysophosphatidylcholine to lysophosphatidic acid. J. Biol. Chem. 2006, 281, 17492–17500. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Lee, J.; Park, C.G.; Kim, S.; Hong, S.Y.; Chung, H.C.; Min, S.K.; Han, J.W.; Lee, H.W.; Lee, H.Y. Expression of autotaxin (NPP-2) is closely linked to invasiveness of breast cancer cells. Clin. Exp. Metastasis 2002, 19, 603–608. [Google Scholar] [CrossRef]
- Yang, Y.; Mou, L.-j.; Liu, N.; Tsao, M.-S. Autotaxin Expression in Non-Small-Cell Lung Cancer. Am. J. Respir. Cell Mol. Biol. 1999, 21, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Ikeda, H.; Nakamura, K.; Kume, Y.; Fujishiro, M.; Sasahira, N.; Hirano, K.; Isayama, H.; Tada, M.; Kawabe, T.; et al. Specific increase in serum autotaxin activity in patients with pancreatic cancer. Clin. Biochem. 2011, 44, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, R.; Lee, S.C.; David, M.; Bordet, J.C.; Norman, D.D.; Patil, R.; Miller, D.; Sahay, D.; Ribeiro, J.; Clezardin, P.; et al. Interaction of platelet-derived autotaxin with tumor integrin alphaVbeta3 controls metastasis of breast cancer cells to bone. Blood 2014. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Wannecq, E.; Descotes, F.; Jansen, S.; Deux, B.; Ribeiro, J.; Serre, C.-M.; Grès, S.; Bendriss-Vermare, N.; Bollen, M.; et al. Cancer Cell Expression of Autotaxin Controls Bone Metastasis Formation in Mouse through Lysophosphatidic Acid-Dependent Activation of Osteoclasts. PLoS ONE 2010, 5, e9741. [Google Scholar] [CrossRef] [PubMed]
- Kazama, S.; Kitayama, J.; Aoki, J.; Mori, K.; Nagawa, H. Immunohistochemical detection of autotaxin (ATX)/lysophospholipase D (lysoPLD) in submucosal invasive colorectal cancer. J. Gastrointest. Cancer 2011, 42, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, G.D.; Gajewiak, J.; Zhang, H.; Xu, X.; Yang, G.; Serban, M. Phosphatase-resistant analogues of lysophosphatidic acid: Agonists promote healing, antagonists and autotaxin inhibitors treat cancer. Biochim. Biophys. Acta 2008, 1781, 588–594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hozumi, H.; Hokari, R.; Kurihara, C.; Narimatsu, K.; Sato, H.; Sato, S.; Ueda, T.; Higashiyama, M.; Okada, Y.; Watanabe, C.; et al. Involvement of autotaxin/lysophospholipase D expression in intestinal vessels in aggravation of intestinal damage through lymphocyte migration. Lab. Invest. 2013, 93, 508–519. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, P.; Haque, A.; Lin, S.; Cominelli, F.; Yun, C.C. Inhibition of autotaxin alleviates inflammation and increases the expression of sodium-dependent glucose cotransporter 1 and Na(+)/H(+) exchanger 3 in SAMP1/Fc mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G762–G771. [Google Scholar] [CrossRef] [PubMed]
- Nagler-Anderson, C. Man the barrier! Strategic defences in the intestinal mucosa. Nat. Rev. Immunol. 2001, 1, 59–67. [Google Scholar] [PubMed]
- Kanda, H.; Newton, R.; Klein, R.; Morita, Y.; Gunn, M.D.; Rosen, S.D. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat. Immunol. 2008, 9, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Nakasaki, T.; Tanaka, T.; Okudaira, S.; Hirosawa, M.; Umemoto, E.; Otani, K.; Jin, S.; Bai, Z.; Hayasaka, H.; Fukui, Y.; et al. Involvement of the Lysophosphatidic Acid-Generating Enzyme Autotaxin in Lymphocyte-Endothelial Cell Interactions. Am. J. Pathol. 2008, 173, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.C.; Krummel, M.F.; Rosen, S.D. Autotaxin through lysophosphatidic acid stimulates polarization, motility, and transendothelial migration of naive T cells. J. Immunol. 2012, 189, 3914–3924. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Rossi, R.E.; Cavalcoli, F.A.; Della Valle, S.; Fraquelli, M.; Conte, D. Nutritional deficiencies in inflammatory bowel disease: Therapeutic approaches. Clin. Nutr. 2013. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M. The intestinal Na+/glucose cotransporter. Annu. Rev. Physiol. 1993, 55, 575–589. [Google Scholar] [CrossRef]
- Dusaulcy, R.; Rancoule, C.; Grès, S.; Wanecq, E.; Colom, A.; Guigné, C.; van Meeteren, L.A.; Moolenaar, W.H.; Valet, P.; Saulnier-Blache, J.S. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J. Lipid Res. 2011, 52, 1247–1255. [Google Scholar] [CrossRef]
- Black, K.E.; Berdyshev, E.; Bain, G.; Castelino, F.V.; Shea, B.S.; Probst, C.K.; Fontaine, B.A.; Bronova, I.; Goulet, L.; Lagares, D.; et al. Autotaxin activity increases locally following lung injury, but is not required for pulmonary lysophosphatidic acid production or fibrosis. FASEB J. 2016, 30, 2435–2450. [Google Scholar] [CrossRef]
- Gesta, S.; Simon, M.-F.; Rey, A.; Sibrac, D.; Girard, A.; Lafontan, M.; Valet, P.; Saulnier-Blache, J.S. Secretion of a lysophospholipase D activity by adipocytes: Involvement in lysophosphatidic acid synthesis. J. Lipid Res. 2002, 43, 904–910. [Google Scholar]
- Rancoule, C.; Dusaulcy, R.; Treguer, K.; Gres, S.; Guigne, C.; Quilliot, D.; Valet, P.; Saulnier-Blache, J.S. Depot-specific regulation of autotaxin with obesity in human adipose tissue. J. Physiol. Biochem. 2012, 68, 635–644. [Google Scholar] [CrossRef]
- Benesch, M.G.K.; Tang, X.; Dewald, J.; Dong, W.-F.; Mackey, J.R.; Hemmings, D.G.; McMullen, T.P.W.; Brindley, D.N. Tumor-induced inflammation in mammary adipose tissue stimulates a vicious cycle of autotaxin expression and breast cancer progression. FASEB J. 2015, 29, 3990–4000. [Google Scholar] [CrossRef] [PubMed]
- Volden, P.A.; Skor, M.N.; Johnson, M.B.; Singh, P.; Patel, F.N.; McClintock, M.K.; Brady, M.J.; Conzen, S.D. Mammary Adipose Tissue-Derived Lysophospholipids Promote Estrogen Receptor–Negative Mammary Epithelial Cell Proliferation. Cancer Prev. Res. 2016, 9, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Hough, G.; Buga, G.M.; Su, F.; Wagner, A.C.; Meriwether, D.; Chattopadhyay, A.; Gao, F.; Grijalva, V.; Danciger, J.S.; et al. Transgenic 6F tomatoes act on the small intestine to prevent systemic inflammation and dyslipidemia caused by Western diet and intestinally derived lysophosphatidic acid. J. Lipid Res. 2013, 54, 3403–3418. [Google Scholar] [CrossRef] [PubMed]
- Brandon, J.A.; Kraemer, M.; Vandra, J.; Halder, S.; Ubele, M.; Morris, A.J.; Smyth, S.S. Adipose-derived autotaxin regulates inflammation and steatosis associated with diet-induced obesity. PLoS ONE 2019, 14, e0208099. [Google Scholar] [CrossRef] [PubMed]
- Vidot, S.; Witham, J.; Agarwal, R.; Greenhough, S.; Bamrah, H.S.; Tigyi, G.J.; Kaye, S.B.; Richardson, A. Autotaxin delays apoptosis induced by carboplatin in ovarian cancer cells. Cell. Signal. 2010, 22, 926–935. [Google Scholar] [CrossRef]
- Quinn, L.A.; Moore, G.E.; Morgan, R.T.; Woods, L.K. Cell lines from human colon carcinoma with unusual cell products, double minutes, and homogeneously staining regions. Cancer Res. 1979, 39, 4914–4924. [Google Scholar]
- Bolier, R.; Tolenaars, D.; Kremer, A.E.; Saris, J.; Pares, A.; Verheij, J.; Bosma, P.J.; Beuers, U.; Oude Elferink, R.P.J. Enteroendocrine cells are a potential source of serum autotaxin in men. Biochim. Biophys. Acta 2016, 1862, 696–704. [Google Scholar] [CrossRef]
- Crosnier, C.; Stamataki, D.; Lewis, J. Organizing cell renewal in the intestine: Stem cells, signals and combinatorial control. Nat. Rev. Genet. 2006, 7, 349–359. [Google Scholar] [CrossRef]
- Lee, S.J.; Leoni, G.; Neumann, P.A.; Chun, J.; Nusrat, A.; Yun, C.C. Distinct phospholipase C-beta isozymes mediate lysophosphatidic acid receptor 1 effects on intestinal epithelial homeostasis and wound closure. Mol. Cell. Biol. 2013, 33, 2016–2028. [Google Scholar] [CrossRef]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef]
- Mills, G.B.; Moolenaar, W.H. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer 2003, 3, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Hines, O.J.; Ryder, N.; Chu, J.; McFadden, D. Lysophosphatidic Acid Stimulates Intestinal Restitution via Cytoskeletal Activation and Remodeling. J. Surg. Res. 2000, 92, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Cetin, S.; Ford, H.R.; Sysko, L.R.; Agarwal, C.; Wang, J.; Neal, M.D.; Baty, C.; Apodaca, G.; Hackam, D.J. Endotoxin inhibits intestinal epithelial restitution through activation of Rho-GTPase and increased focal adhesions. J. Biol. Chem. 2004, 279, 24592–24600. [Google Scholar] [CrossRef] [PubMed]
- Ishii, I.; Fukushima, N.; Ye, X.; Chun, J. Lysophospholipid receptors: Signaling and biology. Annu. Rev. Biochem. 2004, 73, 321–354. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Sudermann, T.; Schulte, K.M.; Goebell, H.; Dignass, A.U. Modulation of intestinal epithelial wound healing in vitro and in vivo by lysophosphatidic acid. Gastroenterology 1999, 117, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- Ohta, H.; Sato, K.; Murata, N.; Damirin, A.; Malchinkhuu, E.; Kon, J.; Kimura, T.; Tobo, M.; Yamazaki, Y.; Watanabe, T.; et al. Ki16425, a Subtype-Selective Antagonist for EDG-Family Lysophosphatidic Acid Receptors. Mol. Pharmacol. 2003, 64, 994–1005. [Google Scholar] [CrossRef]
- Tanaka, T.; Kassai, A.; Ohmoto, M.; Morito, K.; Kashiwada, Y.; Takaishi, Y.; Urikura, M.; Morishige, J.; Satouchi, K.; Tokumura, A. Quantification of phosphatidic acid in foodstuffs using a thin-layer-chromatography-imaging technique. J. Agric. Food Chem. 2012, 60, 4156–4161. [Google Scholar] [CrossRef]
- Lee, B.H.; Choi, S.H.; Kim, H.J.; Jung, S.W.; Kim, H.K.; Nah, S.Y. Plant Lysophosphatidic Acids: A Rich Source for Bioactive Lysophosphatidic Acids and Their Pharmacological Applications. Biol. Pharm. Bull. 2016, 39, 156–162. [Google Scholar] [CrossRef]
- Nakane, S.; Tokumura, A.; Waku, K.; Sugiura, T. Hen egg yolk and white contain high amounts of lysophosphatidic acids, growth factor-like lipids: Distinct molecular species compositions. Lipids 2001, 36, 413–419. [Google Scholar] [CrossRef]
- Tanaka, T.; Horiuchi, G.; Matsuoka, M.; Hirano, K.; Tokumura, A.; Koike, T.; Satouchi, K. Formation of lysophosphatidic acid, a wound-healing lipid, during digestion of cabbage leaves. Biosci. Biotechnol. Biochem. 2009, 73, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Horiuchi, G.; Ikematsu, N.; Tanaka, T.; Terao, J.; Satouchi, K.; Tokumura, A. Intragastrically administered lysophosphatidic acids protect against gastric ulcer in rats under water-immersion restraint stress. Dig. Dis. Sci. 2011, 56, 2252–2261. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Morito, K.; Kinoshita, M.; Ohmoto, M.; Urikura, M.; Satouchi, K.; Tokumura, A. Orally administered phosphatidic acids and lysophosphatidic acids ameliorate aspirin-induced stomach mucosal injury in mice. Dig. Dis. Sci. 2013, 58, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Afroz, S.; Yagi, A.; Fujikawa, K.; Rahman, M.M.; Morito, K.; Fukuta, T.; Watanabe, S.; Kiyokage, E.; Toida, K.; Shimizu, T.; et al. Lysophosphatidic acid in medicinal herbs enhances prostaglandin E2 and protects against indomethacin-induced gastric cell damage in vivo and in vitro. Prostaglandins Other Lipid Mediat. 2018, 135, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kim, J.Y.; Lee, B.-H.; Choi, S.-H.; Rhim, H.; Kim, H.-C.; Ahn, S.-Y.; Jeong, S.-W.; Jang, M.; Cho, I.-H.; et al. Gintonin, an exogenous ginseng-derived LPA receptor ligand, promotes corneal wound healing. J. Vet. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, T.; Inoue, M.; Okamoto, Y.; Ishihara, A.; Tokumura, A. Daily Intake of High-Fat Diet with Lysophosphatidic Acid-Rich Soybean Phospholipids Augments Colon Tumorigenesis in Kyoto Apc Delta Rats. Dig. Dis. Sci. 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Han, Y.; Jenkin, K.; Lee, S.J.; Sasaki, M.; Klapproth, J.M.; He, P.; Yun, C.C. Lysophosphatidic Acid Receptor 1 Is Important for Intestinal Epithelial Barrier Function and Susceptibility to Colitis. Am. J. Pathol. 2018, 188, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Hollander, D.; Vadheim, C.M.; Brettholz, E.; Petersen, G.M.; Delahunty, T.; Rotter, J.I. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann. Intern. Med. 1986, 105, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Yu, S.; Murph, M.M.; Lu, Y.; Liu, S.; Hall, H.S.; Liu, J.; Stephens, C.; Fang, X.; Mills, G.B. Lysophosphatidic Acid Receptors Determine Tumorigenicity and Aggressiveness of Ovarian Cancer Cells. J. Natl. Cancer Inst. 2008, 100, 1630–1642. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Towers, L.N.; O’Connor, K.L. LPA2 (EDG4) mediates Rho-dependent chemotaxis with lower efficacy than LPA1 (EDG2) in breast carcinoma cells. Am. J. Physiol. Cell Physiol. 2007, 292, C1927–C1933. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Kasbohm, E.A.; Arora, P.; Sample, C.J.; Baban, B.; Sud, N.; Sivashanmugam, P.; Moniri, N.H.; Daaka, Y. Expression and function of lysophosphatidic acid LPA1 receptor in prostate cancer cells. Endocrinology 2006, 147, 4883–4892. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.; Ishibashi, K.; Kumagai, S.; Yanagida, T.; Aikawa, K.; Chiba, H.; Kojima, Y. Expression and Function of LPA1 in Bladder Cancer. J. Urol. 2015, 194, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Obo, Y.; Yamada, T.; Furukawa, M.; Hotta, M.; Honoki, K.; Fukushima, N.; Tsujiuchi, T. Frequent mutations of lysophosphatidic acid receptor-1 gene in rat liver tumors. Mutat. Res. 2009, 660, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Magkrioti, C.; Oikonomou, N.; Kaffe, E.; Mouratis, M.A.; Xylourgidis, N.; Barbayianni, I.; Megadoukas, P.; Harokopos, V.; Valavanis, C.; Chun, J.; et al. The Autotaxin-Lysophosphatidic Acid Axis Promotes Lung Carcinogenesis. Cancer Res. 2018, 78, 3634–3644. [Google Scholar] [CrossRef]
- Shida, D.; Watanabe, T.; Aoki, J.; Hama, K.; Kitayama, J.; Sonoda, H.; Kishi, Y.; Yamaguchi, H.; Sasaki, S.; Sako, A.; et al. Aberrant expression of lysophosphatidic acid (LPA) receptors in human colorectal cancer. Lab. Invest. 2004, 84, 1352–1362. [Google Scholar] [CrossRef]
- Shida, D.; Kitayama, J.; Yamaguchi, H.; Okaji, Y.; Tsuno, N.H.; Watanabe, T.; Takuwa, Y.; Nagawa, H. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res. 2003, 63, 1706–1711. [Google Scholar]
- Yun, C.C.; Sun, H.; Wang, D.; Rusovici, R.; Castleberry, A.; Hall, R.A.; Shim, H. LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am. J. Physiol. 2005, 289, C2–C11. [Google Scholar] [CrossRef]
- Shida, D.; Kitayama, J.; Yamaguchi, H.; Hama, K.; Aoki, J.; Arai, H.; Yamashita, H.; Mori, K.; Sako, A.; Konishi, T.; et al. Dual mode regulation of migration by lysophosphatidic acid in human gastric cancer cells. Exp. Cell Res. 2004, 301, 168–178. [Google Scholar] [CrossRef]
- Shin, K.J.; Kim, Y.L.; Lee, S.; Kim, D.K.; Ahn, C.; Chung, J.; Seong, J.Y.; Hwang, J.I. Lysophosphatidic acid signaling through LPA receptor subtype 1 induces colony scattering of gastrointestinal cancer cells. J. Cancer Res. Clin. Oncol. 2009, 135, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Sahay, D.; Leblanc, R.; Grunewald, T.G.; Ambatipudi, S.; Ribeiro, J.; Clezardin, P.; Peyruchaud, O. The LPA1/ZEB1/miR-21-activation pathway regulates metastasis in basal breast cancer. Oncotarget 2015, 6, 20604–20620. [Google Scholar] [CrossRef] [PubMed]
- Komachi, M.; Sato, K.; Tobo, M.; Mogi, C.; Yamada, T.; Ohta, H.; Tomura, H.; Kimura, T.; Im, D.S.; Yanagida, K.; et al. Orally active lysophosphatidic acid receptor antagonist attenuates pancreatic cancer invasion and metastasis in vivo. Cancer Sci. 2012, 103, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Ribeiro, J.; Descotes, F.; Serre, C.M.; Barbier, M.; Murone, M.; Clezardin, P.; Peyruchaud, O. Targeting lysophosphatidic acid receptor type 1 with Debio 0719 inhibits spontaneous metastasis dissemination of breast cancer cells independently of cell proliferation and angiogenesis. Int. J. Oncol. 2012, 40, 1133–1141. [Google Scholar] [CrossRef]
- Lee, S.C.; Fujiwara, Y.; Liu, J.; Yue, J.; Shimizu, Y.; Norman, D.D.; Wang, Y.; Tsukahara, R.; Szabo, E.; Patil, R.; et al. Autotaxin and LPA1 and LPA5 receptors exert disparate functions in tumor cells versus the host tissue microenvironment in melanoma invasion and metastasis. Mol. Cancer Res. MCR 2015, 13, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhong, W.W.; Srivastava, N.; Slavin, A.; Yang, J.; Hoey, T.; An, S. G protein-coupled lysophosphatidic acid receptors stimulate proliferation of colon cancer cells through the {beta}-catenin pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 6027–6032. [Google Scholar] [CrossRef]
- Venkatraman, G.; Benesch, M.G.; Tang, X.; Dewald, J.; McMullen, T.P.; Brindley, D.N. Lysophosphatidate signaling stabilizes Nrf2 and increases the expression of genes involved in drug resistance and oxidative stress responses: Implications for cancer treatment. FASEB J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Fukushima, K.; Otagaki, S.; Ishimoto, K.; Minami, K.; Fukushima, N.; Honoki, K.; Tsujiuchi, T. Effects of LPA1 and LPA6 on the regulation of colony formation activity in colon cancer cells treated with anticancer drugs. J. Recept. Signal. Transduct. Res. 2018, 38, 71–75. [Google Scholar] [CrossRef]
- Fukushima, K.; Takahashi, K.; Yamasaki, E.; Onishi, Y.; Fukushima, N.; Honoki, K.; Tsujiuchi, T. Lysophosphatidic acid signaling via LPA1 and LPA3 regulates cellular functions during tumor progression in pancreatic cancer cells. Exp. Cell Res. 2017. [Google Scholar] [CrossRef]
- Yamada, T.; Ohoka, Y.; Kogo, M.; Inagaki, S. Physical and Functional Interactions of the Lysophosphatidic Acid Receptors with PDZ Domain-containing Rho Guanine Nucleotide Exchange Factors (RhoGEFs). J. Biol. Chem. 2005, 280, 19358–19363. [Google Scholar] [CrossRef]
- Varsano, T.; Taupin, V.; Guo, L.; Baterina, O.Y., Jr.; Farquhar, M.G. The PDZ Protein GIPC Regulates Trafficking of the LPA1 Receptor from APPL Signaling Endosomes and Attenuates the Cell’s Response to LPA. PLoS ONE 2012, 7, e49227. [Google Scholar] [CrossRef]
- Shano, S.; Hatanaka, K.; Ninose, S.; Moriyama, R.; Tsujiuchi, T.; Fukushima, N. A lysophosphatidic acid receptor lacking the PDZ-binding domain is constitutively active and stimulates cell proliferation. Biochim. Biophys. Acta 2008, 1783, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Dolezalova, H.; Kong, Y.; Hu, Y.-L.; Jaffe, R.B.; Kalli, K.R.; Conover, C.A. Distinctive Expression and Functions of the Type 4 Endothelial Differentiation Gene-encoded G Protein-coupled Receptor for Lysophosphatidic Acid in Ovarian Cancer. Cancer Res. 1999, 59, 5370–5375. [Google Scholar] [PubMed]
- Fang, X.; Gaudette, D.; Furui, T.; Mao, M.; Estrella, V.; Eder, A.; Pustilnik, T.; Sasagawa, T.; Lapushin, R.; Yu, S.; et al. Lysophospholipid growth factors in the initiation, progression, metastases, and management of ovarian cancer. Ann. N. Y. Acad. Sci. 2000, 905, 188–208. [Google Scholar] [CrossRef] [PubMed]
- Schulte, K.M.; Beyer, A.; Kohrer, K.; Oberhauser, S.; Roher, H.D. Lysophosphatidic acid, a novel lipid growth factor for human thyroid cells: Over-expression of the high-affinity receptor edg4 in differentiated thyroid cancer. Int. J. Cancer 2001, 92, 249–256. [Google Scholar] [CrossRef]
- Kitayama, J.; Shida, D.; Sako, A.; Ishikawa, M.; Hama, K.; Aoki, J.; Arai, H.; Nagawa, H. Over-expression of lysophosphatidic acid receptor-2 in human invasive ductal carcinoma. Breast Cancer Res. BCR 2004, 6, R640–R646. [Google Scholar] [CrossRef] [PubMed]
- Enooku, K.; Uranbileg, B.; Ikeda, H.; Kurano, M.; Sato, M.; Kudo, H.; Maki, H.; Koike, K.; Hasegawa, K.; Kokudo, N.; et al. Higher LPA2 and LPA6 mRNA Levels in Hepatocellular Carcinoma Are Associated with Poorer Differentiation, Microvascular Invasion and Earlier Recurrence with Higher Serum Autotaxin Levels. PLoS ONE 2016, 11, e0161825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cheng, Y.; Zhang, Q.; Li, X.; Zhou, J.; Wang, J.; Wei, L. ATXLPA axis facilitates estrogeninduced endometrial cancer cell proliferation via MAPK/ERK signaling pathway. Mol. Med. Rep. 2018, 17, 4245–4252. [Google Scholar]
- Yoshida, M.; He, P.; Yun, C.C. Transgenic Expression of Human Lysophosphatidic Acid Receptor LPA2 in Mouse Intestinal Epithelial Cells Induces Intestinal Dysplasia. PLoS ONE 2016, 11, e0154527. [Google Scholar] [CrossRef]
- Zhang, H.; Bialkowska, A.; Rusovici, R.; Chanchevalap, S.; Shim, H.; Katz, J.P.; Yang, V.W.; Yun, C.C. Lysophosphatidic acid facilitates proliferation of colon cancer cells via induction of Kruppel-like factor 5. J. Biol. Chem. 2007, 282, 15541–15549. [Google Scholar] [CrossRef]
- Yang, D.; Yang, W.; Zhang, Q.; Hu, Y.; Bao, L.; Damirin, A. Migration of gastric cancer cells in response to lysophosphatidic acid is mediated by LPA receptor 2. Oncol. Lett. 2013, 5, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Morin, P.J.; Kinzler, K.W.; Sparks, A.B. beta-Catenin Mutations: Insights into the APC Pathway and the Power of Genetics. Cancer Res. 2016, 76, 5587–5589. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fang, X.; Yu, S.; Tanyi, J.L.; Lu, Y.; Woodgett, J.R.; Mills, G.B. Convergence of multiple signaling cascades at glycogen synthase kinase 3: Edg receptor-mediated phosphorylation and inactivation by lysophosphatidic acid through a protein kinase C-dependent intracellular pathway. Mol. Cell. Biol. 2002, 22, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Bienz, M.; Clevers, H. Linking colorectal cancer to Wnt signaling. Cell 2000, 103, 311–320. [Google Scholar] [CrossRef]
- Guo, L.; He, P.; No, Y.R.; Yun, C.C. Kruppel-like factor 5 incorporates into the beta-catenin/TCF complex in response to LPA in colon cancer cells. Cell. Signal. 2015, 27, 961–968. [Google Scholar] [CrossRef]
- McConnell, B.B.; Yang, V.W. Mammalian Kruppel-like factors in health and diseases. Physiol. Rev. 2010, 90, 1337–1381. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Schofield, C.J.; Ratcliffe, P.J. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 2004, 5, 343–354. [Google Scholar] [CrossRef]
- Patten, D.A.; Lafleur, V.N.; Robitaille, G.A.; Chan, D.A.; Giaccia, A.J.; Richard, D.E. Hypoxia-inducible factor-1 activation in nonhypoxic conditions: The essential role of mitochondrial-derived reactive oxygen species. Mol. Biol. Cell 2010, 21, 3247–3257. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.Y.; Lee, E.K.; Park, C.G.; Chung, H.C.; Rha, S.Y.; Kim, Y.K.; Bae, G.U.; Kim, B.K.; Han, J.W.; et al. Activation of hypoxia-inducible factor-1alpha is necessary for lysophosphatidic acid-induced vascular endothelial growth factor expression. Clin. Cancer Res. 2006, 12, 6351–6358. [Google Scholar] [CrossRef]
- Lee, S.J.; No, Y.R.; Dang, D.T.; Dang, L.H.; Yang, V.W.; Shim, H.; Yun, C.C. Regulation of hypoxia-inducible factor 1alpha (HIF-1alpha) by lysophosphatidic acid is dependent on interplay between p53 and Kruppel-like factor 5. J. Biol. Chem. 2013, 288, 25244–25253. [Google Scholar] [CrossRef] [PubMed]
- Winner, M.; Koong, A.C.; Rendon, B.E.; Zundel, W.; Mitchell, R.A. Amplification of tumor hypoxic responses by macrophage migration inhibitory factor-dependent hypoxia-inducible factor stabilization. Cancer Res. 2007, 67, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Nishihira, J.; Yoshiki, T.; Kondo, M.; Sato, Y.; Sasaki, F.; Todo, S. Macrophage Migration Inhibitory Factor Promotes Tumor Invasion and Metastasis via the Rho-Dependent Pathway. Clin. Cancer Res. 2005, 11, 1050–1058. [Google Scholar] [PubMed]
- No, Y.R.; Lee, S.-J.; Kumar, A.; Yun, C.C. HIF1α-Induced by Lysophosphatidic Acid Is Stabilized via Interaction with MIF and CSN5. PLoS ONE 2015, 10, e0137513. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Ritter, S.L.; Zhang, H.; Shim, H.; Hall, R.A.; Yun, C.C. MAGI-3 competes with NHERF-2 to negatively regulate LPA2 receptor signaling in colon cancer cells. Gastroenterology 2011, 140, 924–934. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D.; Sun, H.; Hall, R.A.; Yun, C.C. MAGI-3 regulates LPA-induced activation of Erk and RhoA. Cell. Signal. 2007, 19, 261–268. [Google Scholar] [CrossRef]
- Xu, J.; Lai, Y.-J.; Lin, W.-C.; Lin, F.-T. TRIP6 Enhances Lysophosphatidic Acid-induced Cell Migration by Interacting with the Lysophosphatidic Acid 2 Receptor. J. Biol. Chem. 2004, 279, 10459–10468. [Google Scholar] [CrossRef]
- Lin, F.T.; Lai, Y.J.; Makarova, N.; Tigyi, G.; Lin, W.C. The lysophosphatidic acid 2 receptor mediates down-regulation of Siva-1 to promote cell survival. J. Biol. Chem. 2007, 282, 37759–37769. [Google Scholar] [CrossRef]
- Oh, Y.S.; Jo, N.W.; Choi, J.W.; Kim, H.S.; Seo, S.W.; Kang, K.O.; Hwang, J.I.; Heo, K.; Kim, S.H.; Kim, Y.H.; et al. NHERF2 specifically interacts with LPA2 receptor and defines the specificity and efficiency of receptor-mediated phospholipase C-beta3 activation. Mol. Cell. Biol. 2004, 24, 5069–5079. [Google Scholar] [CrossRef]
- Lai, Y.-J.; Chen, C.-S.; Lin, W.-C.; Lin, F.-T. c-Src-Mediated Phosphorylation of TRIP6 Regulates Its Function in Lysophosphatidic Acid-Induced Cell Migration. Mol. Cell. Biol. 2005, 25, 5859–5868. [Google Scholar] [CrossRef]
- An, S.; Bleu, T.; Hallmark, O.G.; Goetzl, E.J. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J. Biol. Chem. 1998, 273, 7906–7910. [Google Scholar] [CrossRef] [PubMed]
- Contos, J.J.; Chun, J. Genomic characterization of the lysophosphatidic acid receptor gene, lp(A2)/Edg4, and identification of a frameshift mutation in a previously characterized cDNA. Genomics 2000, 64, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Balazs, L.; Wang, D.A.; Van Middlesworth, L.; Tigyi, G.; Johnson, L.R. Lysophosphatidic acid protects and rescues intestinal epithelial cells from radiation- and chemotherapy-induced apoptosis. Gastroenterology 2002, 123, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Wang, D.A.; Gosmanova, E.; Johnson, L.R.; Tigyi, G. LPA protects intestinal epithelial cells from apoptosis by inhibiting the mitochondrial pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G821–G829. [Google Scholar] [CrossRef] [PubMed]
- E, S.; Lai, Y.J.; Tsukahara, R.; Chen, C.S.; Fujiwara, Y.; Yue, J.; Yu, J.H.; Guo, H.; Kihara, A.; Tigyi, G.; et al. Lysophosphatidic acid 2 receptor-mediated supramolecular complex formation regulates its antiapoptotic effect. J. Biol. Chem. 2009, 284, 14558–14571. [Google Scholar] [CrossRef] [PubMed]
- Rusovici, R.; Ghaleb, A.; Shim, H.; Yang, V.W.; Yun, C.C. Lysophosphatidic acid prevents apoptosis of Caco-2 colon cancer cells via activation of mitogen-activated protein kinase and phosphorylation of Bad. Biochim. Biophys. Acta 2007, 1770, 1194–1203. [Google Scholar] [CrossRef][Green Version]
- Deng, W.; E, S.; Tsukahara, R.; Valentine, W.J.; Durgam, G.; Gududuru, V.; Balazs, L.; Manickam, V.; Arsura, M.; Vanmiddlesworth, L.; et al. The Lysophosphatidic Acid Type 2 Receptor Is Required for Protection Against Radiation-Induced Intestinal Injury. Gastroenterology 2007, 132, 1834–1851. [Google Scholar] [CrossRef]
- Tigyi, G.J.; Johnson, L.R.; Lee, S.C.; Norman, D.D.; Szabo, E.; Balogh, A.; Thompson, K.; Boler, A.L.; McCool, S.W. Lysophosphatidic acid type 2 receptor agonists in targeted drug development offer broad therapeutic potential. J. Lipid Res. 2019. [Google Scholar] [CrossRef]
- Fukui, R.; Tanabe, E.; Kitayoshi, M.; Yoshikawa, K.; Fukushima, N.; Tsujiuchi, T. Negative regulation of cell motile and invasive activities by lysophosphatidic acid receptor-3 in colon cancer HCT116 cells. Tumour Biol. 2012, 33, 1899–1905. [Google Scholar] [CrossRef]
- Kato, K.; Yoshikawa, K.; Tanabe, E.; Kitayoshi, M.; Fukui, R.; Fukushima, N.; Tsujiuchi, T. Opposite roles of LPA1 and LPA3 on cell motile and invasive activities of pancreatic cancer cells. Tumour Biol. 2012, 33, 1739–1744. [Google Scholar] [CrossRef]
- Sun, K.; Cai, H.; Duan, X.; Yang, Y.; Li, M.; Qu, J.; Zhang, X.; Wang, J. Aberrant expression and potential therapeutic target of lysophosphatidic acid receptor 3 in triple-negative breast cancers. Clin. Exp. Med. 2015, 15, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Koehne, C.H.; Dubois, R.N. COX-2 inhibition and colorectal cancer. Semin. Oncol. 2004, 31, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Hama, K.; Contos, J.J.A.; Anliker, B.; Inoue, A.; Skinner, M.K.; Suzuki, H.; Amano, T.; Kennedy, G.; Arai, H.; et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 2005, 435, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Ishii, S.; Shimizu, T. Identification of p2y9/GPR23 as a Novel G Protein-coupled Receptor for Lysophosphatidic Acid, Structurally Distant from the Edg Family. J. Biol. Chem. 2003, 278, 25600–25606. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.; Cheng, C.T.; Zhang, H.; Subler, M.A.; Wu, J.; Mukherjee, A.; Windle, J.J.; Chen, C.K.; Fang, X. Role of LPA4/p2y9/GPR23 in negative regulation of cell motility. Mol. Biol. Cell 2008, 19, 5435–5445. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Fukushima, K.; Onishi, Y.; Inui, K.; Node, Y.; Fukushima, N.; Honoki, K.; Tsujiuchi, T. Lysophosphatidic acid (LPA) signaling via LPA4 and LPA6 negatively regulates cell motile activities of colon cancer cells. Biochem. Biophys. Res. Commun. 2017, 483, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Harper, K.; Arsenault, D.; Boulay-Jean, S.; Lauzier, A.; Lucien, F.; Dubois, C.M. Autotaxin promotes cancer invasion via the lysophosphatidic acid receptor 4: Participation of the cyclic AMP/EPAC/Rac1 signaling pathway in invadopodia formation. Cancer Res. 2010, 70, 4634–4643. [Google Scholar] [CrossRef] [PubMed]
- Sumida, H.; Noguchi, K.; Kihara, Y.; Abe, M.; Yanagida, K.; Hamano, F.; Sato, S.; Tamaki, K.; Morishita, Y.; Kano, M.R.; et al. LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood 2010, 116, 5060–5070. [Google Scholar] [CrossRef] [PubMed]
- Eino, D.; Tsukada, Y.; Naito, H.; Kanemura, Y.; Iba, T.; Wakabayashi, T.; Muramatsu, F.; Kidoya, H.; Arita, H.; Kagawa, N.; et al. LPA4-Mediated Vascular Network Formation Increases the Efficacy of Anti-PD-1 Therapy against Brain Tumors. Cancer Res. 2018, 78, 6607–6620. [Google Scholar] [CrossRef]
- Lee, C.W.; Rivera, R.; Gardell, S.; Dubin, A.E.; Chun, J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J. Biol. Chem. 2006, 281, 23589–23597. [Google Scholar] [CrossRef]
- Lin, S.; Yeruva, S.; He, P.; Singh, A.K.; Zhang, H.; Chen, M.; Lamprecht, G.; de Jonge, H.R.; Tse, M.; Donowitz, M.; et al. Lysophosphatidic acid stimulates the intestinal brush border Na+/H+ exchanger 3 and fluid absorption via LPA5 and NHERF2. Gastroenterology 2010, 138, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Jenkin, K.A.; He, P.; Yun, C.C. Expression of lysophosphatidic acid receptor 5 is necessary for the regulation of intestinal Na(+)/H(+) exchanger 3 by lysophosphatidic acid in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G433–G442. [Google Scholar] [CrossRef]
- Gurney, M.A.; Laubitz, D.; Ghishan, F.K.; Kiela, P.R. Pathophysiology of Intestinal Na(+)/H(+) exchange. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 27–40. [Google Scholar] [CrossRef]
- Okabe, K.; Hayashi, M.; Yamawaki, Y.; Teranishi, M.; Honoki, K.; Mori, T.; Fukushima, N.; Tsujiuchi, T. Possible involvement of lysophosphatidic acid receptor-5 gene in the acquisition of growth advantage of rat tumor cells. Mol. Carcinog. 2011, 50, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.K.; Strauch, P.; Fujiwara, Y.; Al-Shami, A.; Oravecz, T.; Tigyi, G.; Pelanda, R.; Torres, R.M. Lysophosphatidic acid inhibits CD8 T cell activation and control of tumor progression. Cancer Immunol. Res. 2013, 1, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Yukiura, H.; Kano, K.; Kise, R.; Inoue, A.; Aoki, J. LPP3 localizes LPA6 signalling to non-contact sites in endothelial cells. J. Cell. Sci. 2015, 128, 3871–3877. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.M.; Snyder, L.; Todd, J.L.; Soule, B.; Christian, R.; Anstrom, K.; Luo, Y.; Gagnon, R.; Rosen, G. Randomized, Double-Blind, Placebo-Controlled, Phase 2 Trial of BMS-986020, a Lysophosphatidic Acid Receptor Antagonist for the Treatment of Idiopathic Pulmonary Fibrosis. Chest 2018, 154, 1061–1069. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).