Connexins and Gap Junctions in Cancer of the Urinary Tract
Abstract
1. Introduction
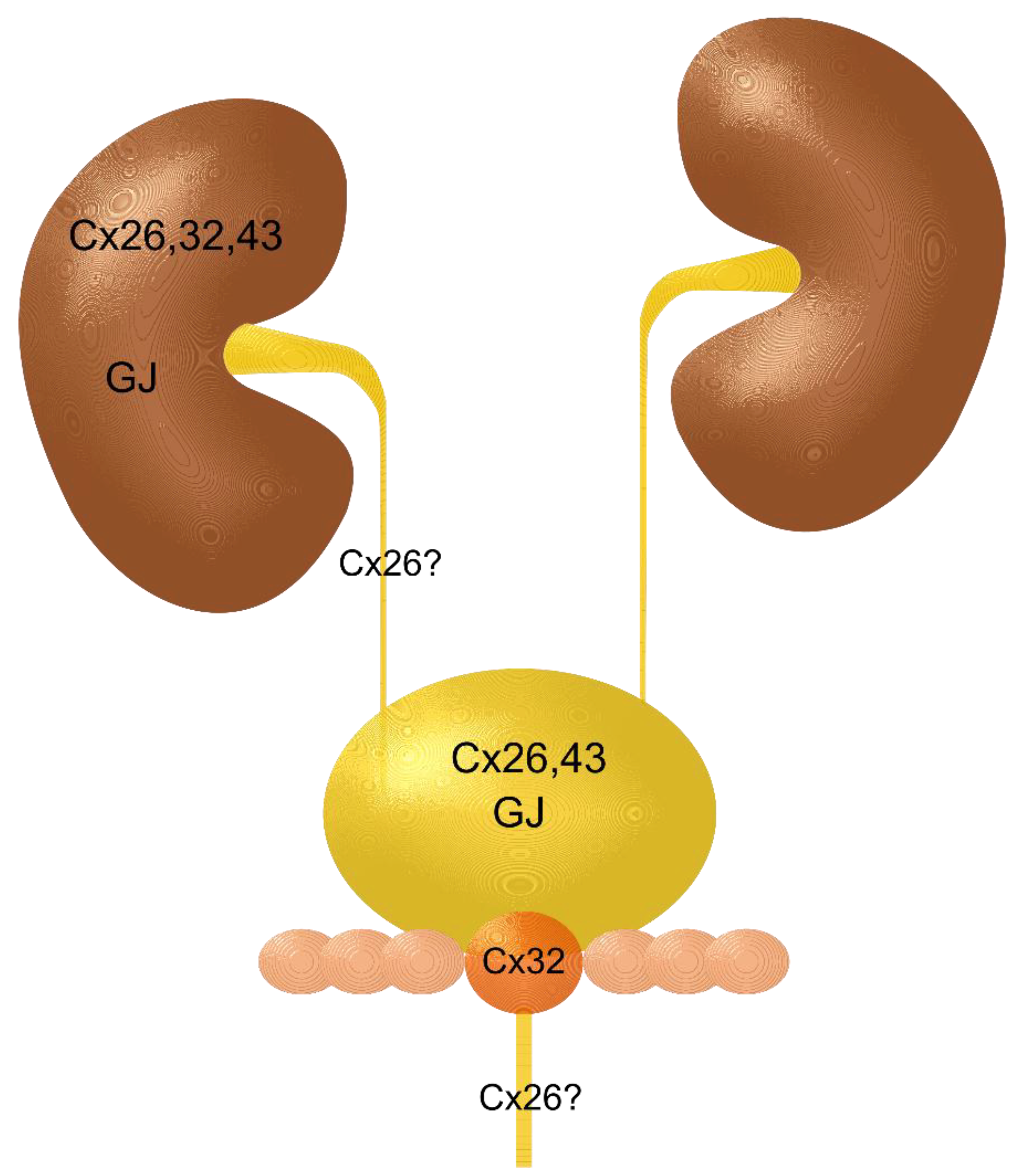
2. Kidney and Renal Cell Carcinoma
3. Renal Pelvis, Ureter, and Urethra and Their Carcinomas
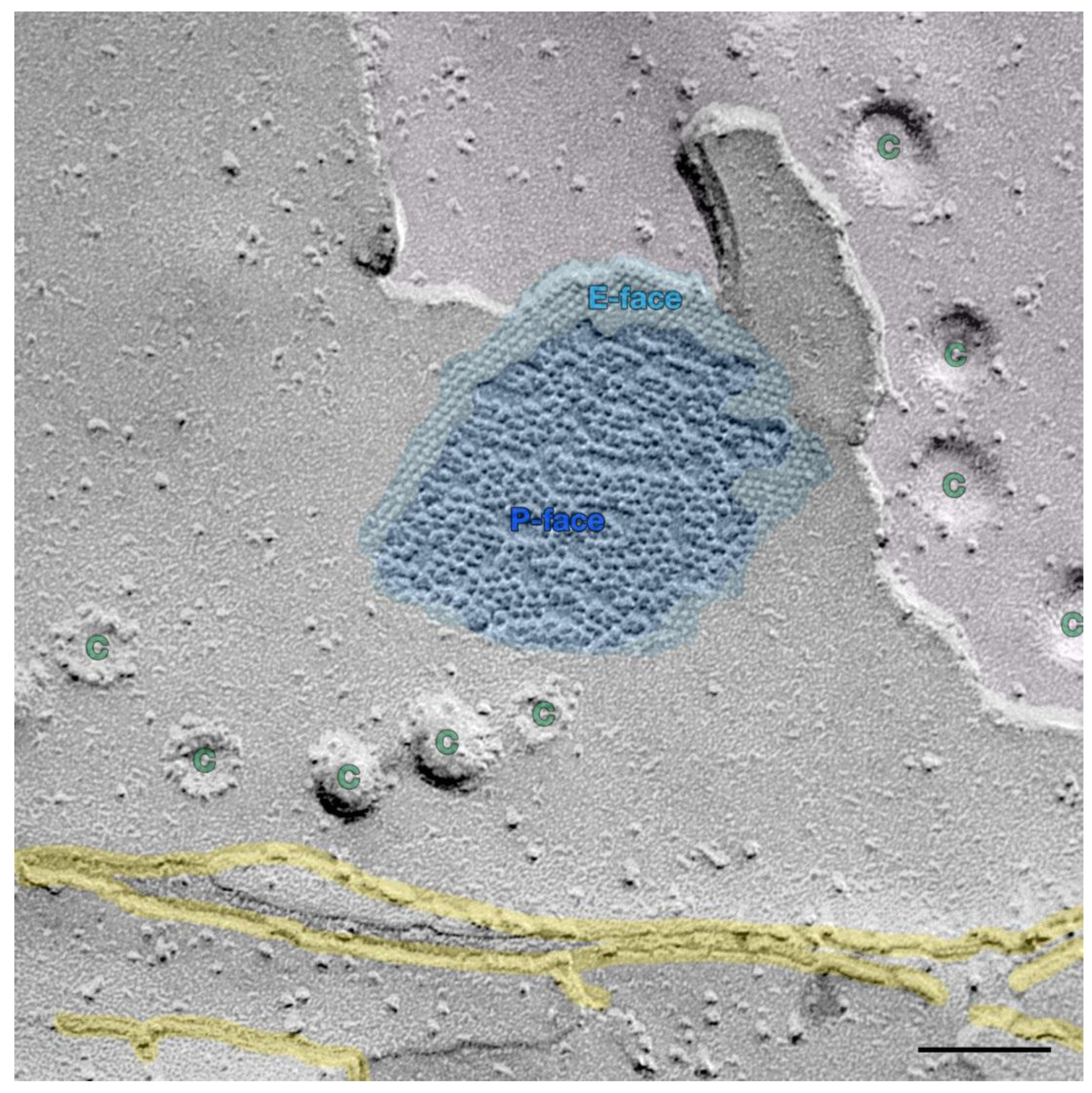
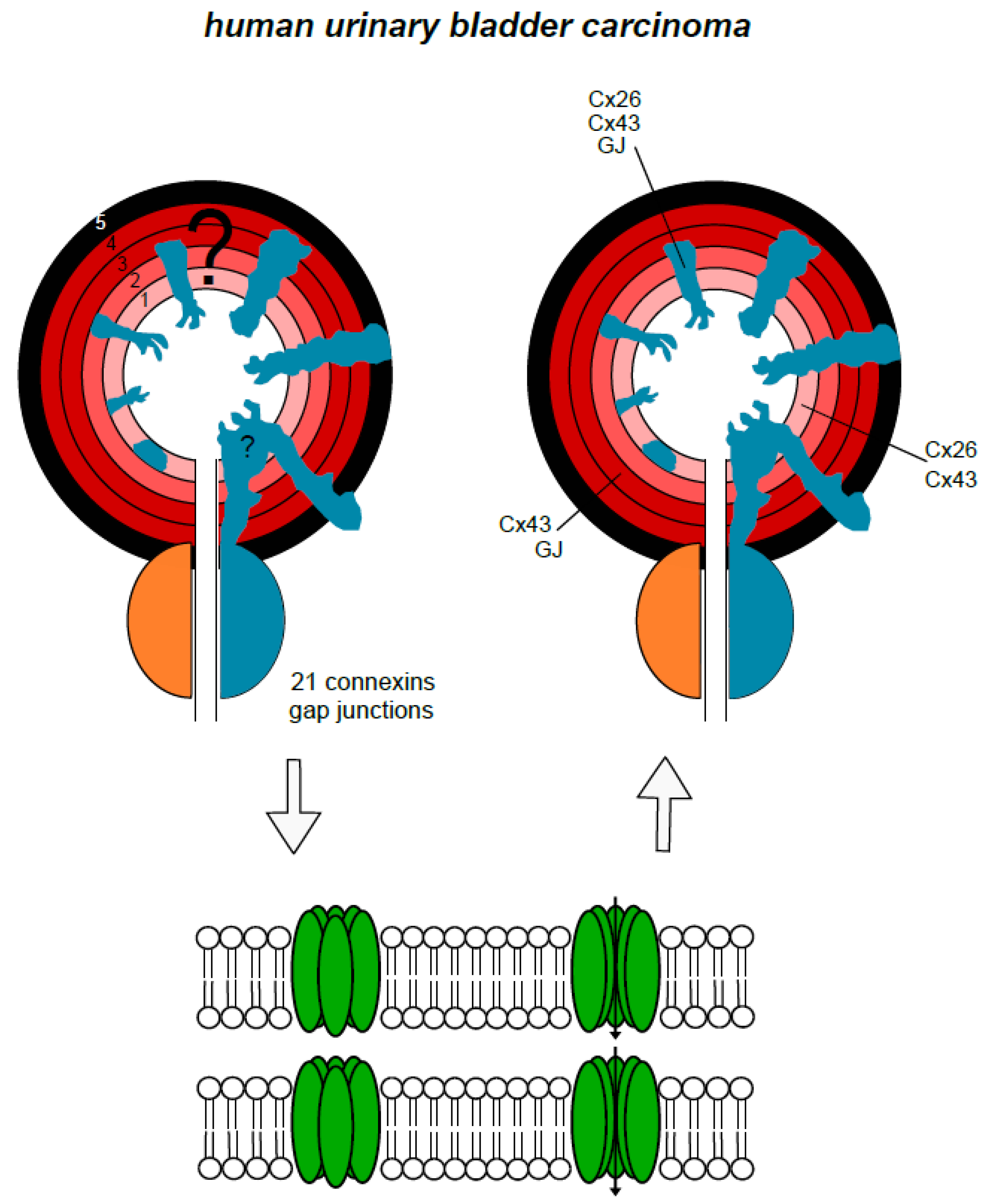
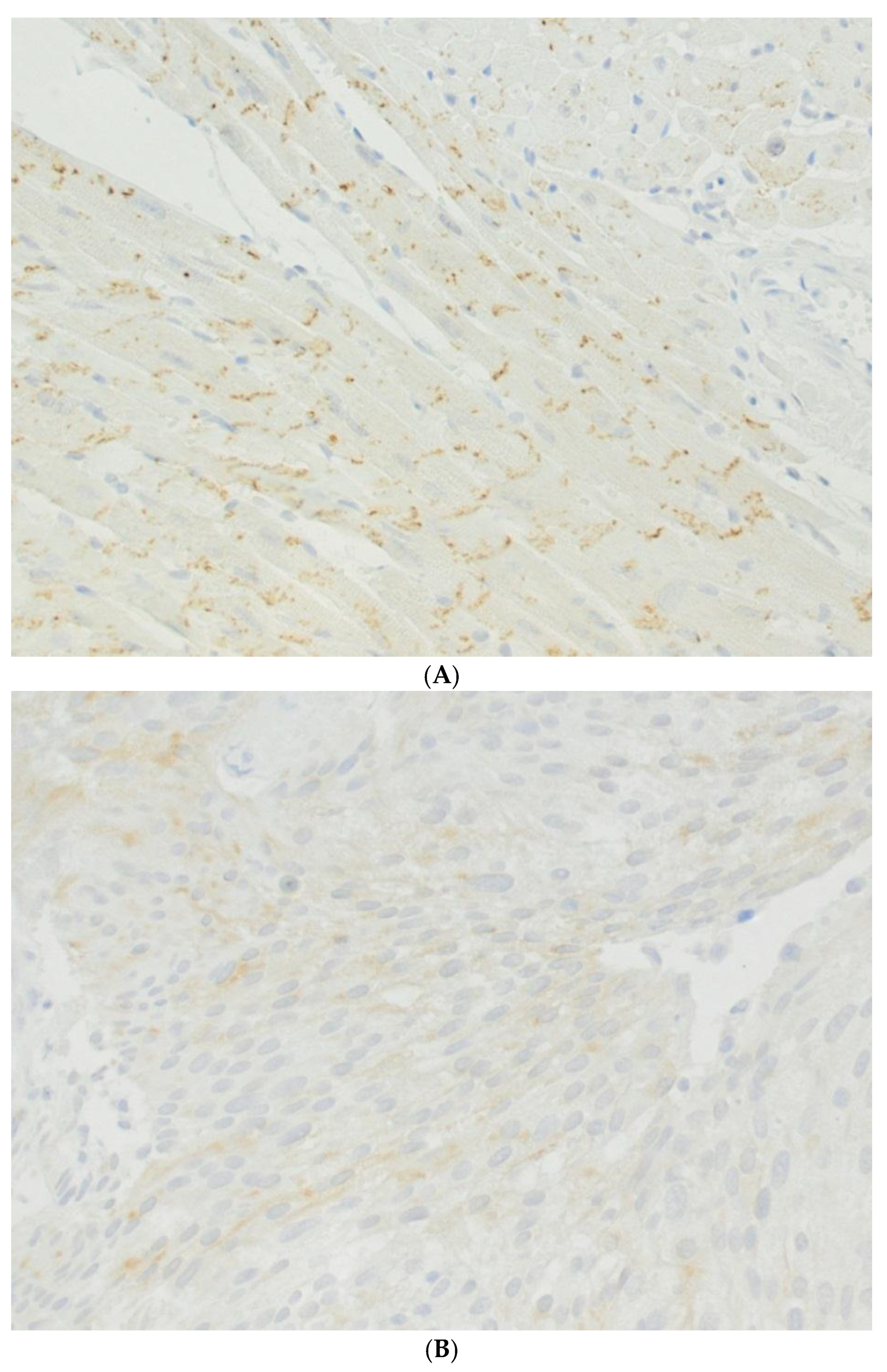
4. Urinary Bladder and Urinary Bladder Carcinoma
5. Prostate and Seminal Vesicles and Their Carcinomas
6. Urinary Tract Carcinomas, Related Toxic Agents and Connexons, Gap Junctions, and Gap Junctional Intercellular Communication
7. Putting it Together
8. Concluding Remark
Funding
Acknowledgments
Conflicts of Interest
References
- Loewenstein, W.R. Junctional intercellular communication and the control of growth. Biochim. Biophys. Acta 1979, 560, 1–65. [Google Scholar] [CrossRef]
- Trosko, J.E. Cancer prevention and therapy of two types of gap junctional intercellular communication(-)deficient “cancer stem cell”. Cancers 2019, 11, 87. [Google Scholar] [CrossRef]
- Upham, B.L.; Blaha, L.; Babica, P.; Park, J.S.; Sovadinova, I.; Pudrith, C.; Rummel, A.M.; Weis, L.M.; Sai, K.; Tithof, P.K.; et al. Tumor promoting properties of a cigarette smoke prevalent polycyclic aromatic hydrocarbon as indicated by the inhibition of gap junctional intercellular communication via phosphatidylcholine-specific phospholipase C. Cancer Sci. 2008, 99, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Aasen, T.; Johnstone, S.; Vidal-Brime, L.; Lynn, K.S.; Koval, M. Connexins: Synthesis, post-translational modifications, and trafficking in health and disease. Int. J. Mol. Sci. 2018, 19, 1296. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, R.; Willecke, K. Connexin-caused genetic diseases and corresponding mouse models. Antioxid. Redox Signal. 2009, 11, 283–295. [Google Scholar] [CrossRef]
- Retamal, M.A.; Reyes, E.P.; Garcia, I.E.; Pinto, B.; Martinez, A.D.; Gonzalez, C. Diseases associated with leaky hemichannels. Front. Cell Neurosci. 2015, 9, 267. [Google Scholar] [CrossRef]
- Li, X.; Lynn, B.D.; Olson, C.; Meier, C.; Davidson, K.G.; Yasumura, T.; Rash, J.E.; Nagy, J.I. Connexin29 expression, immunocytochemistry and freeze-fracture replica immunogold labelling (FRIL) in sciatic nerve. Eur. J. Neurosci. 2002, 16, 795–806. [Google Scholar] [CrossRef]
- Meier, C.; Dermietzel, R.; Davidson, K.G.; Yasumura, T.; Rash, J.E. Connexin32-containing gap junctions in Schwann cells at the internodal zone of partial myelin compaction and in Schmidt-Lanterman incisures. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 3186–3198. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, D.A. Gap junction dynamics and intercellular communication. Pharmacol. Rev. 1978, 30, 383–392. [Google Scholar]
- Hertzberg, E.L.; Lawrence, T.S.; Gilula, N.B. Gap junctional communication. Annu. Rev. Physiol. 1981, 43, 479–491. [Google Scholar] [CrossRef]
- Lambiase, P.D.; Tinker, A. Connexins in the heart. Cell Tissue Res. 2015, 360, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, W.R.; Rose, B. The cell-cell channel in the control of growth. Semin. Cell Biol. 1992, 3, 59–79. [Google Scholar] [CrossRef]
- Aasen, T.; Mesnil, M.; Naus, C.C.; Lampe, P.D.; Laird, D.W. Gap junctions and cancer: Communicating for 50 years. Nat. Rev. Cancer 2016, 16, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.V.; Jiang, J.X.; Mesnil, M. Connexins and pannexins: Important players in tumorigenesis, metastasis and potential therapeutics. Int. J. Mol. Sci. 2018, 19, 1645. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Y.; Li, Q.Q.; Gao, Y.F.; Zhou, H.H.; Liu, Z.Q.; Jin, W.L. Gap junction as an intercellular glue: Emerging roles in cancer EMT and metastasis. Cancer Lett. 2016, 381, 133–137. [Google Scholar] [CrossRef]
- John, H.; Wang, X.; Wehrli, E.; Hauri, D.; Maake, C. Evidence of gap junctions in the stable nonobstructed human bladder. J. Urol. 2003, 169, 745–749. [Google Scholar] [CrossRef]
- Sui, G.P.; Rothery, S.; Dupont, E.; Fry, C.H.; Severs, N.J. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int. 2002, 90, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Corteggio, A.; Florio, J.; Roperto, F.; Borzacchiello, G. Expression of gap junction protein connexin 43 in bovine urinary bladder tumours. J. Comp. Pathol. 2011, 144, 86–90. [Google Scholar] [CrossRef]
- Briere, N.; Cabana, C.; Magny, P. Freeze-fracture observations on human fetal kidney in serum-free organ culture. Anat. Rec. 1991, 230, 249–260. [Google Scholar] [CrossRef]
- Wilgenbus, K.K.; Kirkpatrick, C.J.; Knuechel, R.; Willecke, K.; Traub, O. Expression of Cx26, Cx32 and Cx43 gap junction proteins in normal and neoplastic human tissues. Int. J. Cancer 1992, 51, 522–529. [Google Scholar] [CrossRef]
- Blackburn, J.G.; Hazen-Martin, D.J.; Detrisac, C.J.; Sens, D.A. Electrophysiology and ultrastructure of cultured human proximal tubule cells. Kidney Int. 1988, 33, 508–516. [Google Scholar] [CrossRef]
- Noguchi, M.; Nomata, K.; Watanabe, J.I.; Sato, H.; Kanetake, H.; Saito, Y. Disruption of gap junctional intercellular communication in human renal cancer cell lines. Urology 1999, 53, 218–222. [Google Scholar] [CrossRef]
- Watanabe, J.; Nomata, K.; Noguchi, M.; Satoh, H.; Kanda, S.; Kanetake, H.; Saito, Y. All-trans retinoic acid enhances gap junctional intercellular communication among renal epithelial cells in vitro treated with renal carcinogens. Eur. J. Cancer 1999, 35, 1003–1008. [Google Scholar] [CrossRef]
- Evans, W.H.; Martin, P.E. Gap junctions: Structure and function (Review). Mol. Membr. Biol. 2002, 19, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Schenone, A.; Mancardi, G.L. Molecular basis of inherited neuropathies. Curr. Opin. Neurol. 1999, 12, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Willecke, K.; Temme, A.; Teubner, B.; Ott, T. Characterization of targeted connexin32-deficient mice: A model for the human Charcot-Marie-Tooth (X-type) inherited disease. Ann. N. Y. Acad. Sci. 1999, 883, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Wanke, I.; Wulbrand, U.; Moennikes, O.; Buchmann, A. Role of connexin32 and beta-catenin in tumor promotion in mouse liver. Toxicol. Pathol. 2003, 31, 99–102. [Google Scholar] [CrossRef]
- Sato, H.; Hagiwara, H.; Ohde, Y.; Senba, H.; Virgona, N.; Yano, T. Regulation of renal cell carcinoma cell proliferation, invasion and metastasis by connexin 32 gene. J. Membr. Biol. 2007, 216, 17–21. [Google Scholar] [CrossRef]
- Yano, T.; Fujimoto, E.; Hagiwara, H.; Sato, H.; Yamasaki, H.; Negishi, E.; Ueno, K. Connexin 32 as an anti-invasive and anti-metastatic gene in renal cell carcinoma. Biol. Pharm. Bull. 2006, 29, 1991–1994. [Google Scholar] [CrossRef][Green Version]
- Sato, A.; Sekine, M.; Kobayashi, M.; Virgona, N.; Ota, M.; Yano, T.; Sato, H.; Hagiwara, H.; Ohde, Y.; Senba, H.; et al. Induction of the connexin 32 gene by epigallocatechin-3-gallate potentiates vinblastine-induced cytotoxicity in human renal carcinoma cells. Chemotherapy 2013, 59, 192–199. [Google Scholar] [CrossRef]
- Gee, J.; Tanaka, M.; Grossman, H.B. Connexin 26 is abnormally expressed in bladder cancer. J. Urol. 2003, 169, 1135–1137. [Google Scholar] [CrossRef]
- Sancho, M.; Triguero, D.; Garcia-Pascual, A. Direct coupling through gap junctions is not involved in urethral neurotransmission. Am. J. Physiol. Ren. Physiol. 2011, 300, F864–F872. [Google Scholar] [CrossRef]
- Farci, F.; Manassero, F.; Baldesi, R.; Bartolucci, A.; Boldrini, L.; Selli, C.; Faviana, P. Primary small cell carcinoma of the ureter: Case report and review of the literature. Medicine 2018, 97, e11113. [Google Scholar] [CrossRef] [PubMed]
- Hensley, P.J.; Bhalodi, A.A.; Gupta, S. Primary upper urinary tract small cell carcinoma: A case series and literature review. J. Endourol. Case Rep. 2017, 3, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Church, D.N.; Bahl, A. Clinical review—Small cell carcinoma of the bladder. Cancer Treat. Rev. 2006, 32, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Letasiova, S.; Medve’ova, A.; Sovcikova, A.; Dusinska, M.; Volkovova, K.; Mosoiu, C.; Bartonova, A. Bladder cancer, a review of the environmental risk factors. Environ. Health A Glob. Access Sci. Source 2012, 11 (Suppl. 1) (Suppl. 1), S11. [Google Scholar] [CrossRef]
- Kandouz, M.; Batist, G. Gap junctions and connexins as therapeutic targets in cancer. Expert Opin. Targets 2010, 14, 681–692. [Google Scholar] [CrossRef]
- Mesnil, M.; Crespin, S.; Avanzo, J.L.; Zaidan-Dagli, M.L. Defective gap junctional intercellular communication in the carcinogenic process. Biochim. Biophys. Acta 2005, 1719, 125–145. [Google Scholar] [CrossRef]
- Asamoto, M.; Takahashi, S.; Imaida, K.; Shirai, T.; Fukushima, S. Increased gap junctional intercellular communication capacity and connexin 43 and 26 expression in rat bladder carcinogenesis. Carcinogenesis 1994, 15, 2163–2166. [Google Scholar] [CrossRef] [PubMed]
- Comberg, D.; Gauer, A.; Tschernig, T. First findings of gap junction proteins in human urothelial carcinoma. World J. Urol. 2016, 34, 145–147. [Google Scholar] [CrossRef]
- Poyet, C.; Buser, L.; Roudnicky, F.; Detmar, M.; Hermanns, T.; Mannhard, D.; Hohn, A.; Ruschoff, J.; Zhong, Q.; Sulser, T.; et al. Connexin 43 expression predicts poor progression-free survival in patients with non-muscle invasive urothelial bladder cancer. J. Clin. Pathol. 2015, 68, 819–824. [Google Scholar] [CrossRef]
- Tanaka, M.; Grossman, H.B. Connexin 26 gene therapy of human bladder cancer: Induction of growth suppression, apoptosis, and synergy with Cisplatin. Hum. Gene 2001, 12, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Cronier, L.; Crespin, S.; Strale, P.O.; Defamie, N.; Mesnil, M. Gap junctions and cancer: New functions for an old story. Antioxid. Redox Signal. 2009, 11, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Monvoisin, A.; Vix, J.; Mesnil, M.; Thuringer, D.; Debiais, F.; Cronier, L. Connexins, important players in the dissemination of prostate cancer cells. Biochim. Biophys. Acta Biomembr. 2018, 1860, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, P.; Aaltonen, V.; Koskela, S.; Lehenkari, P.; Korkiamaki, T.; Peltonen, J. Impaired gap junction formation and intercellular calcium signaling in urinary bladder cancer cells can be improved by Go6976. Cell Commun. Adhes. 2007, 14, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Alroy, J.; Roganovic, D.; Banner, B.F.; Jacobs, J.B.; Merk, F.B.; Ucci, A.A.; Kwan, P.W.; Coon, J.S.T.; Miller, A.W., 3rd. Primary adenocarcinomas of the human urinary bladder: Histochemical, immunological and ultrastructural studies. Virchows Arch. Pathol. Anat. Histol. 1981, 393, 165–181. [Google Scholar] [CrossRef]
- Mehta, P.P.; Lokeshwar, B.L.; Schiller, P.C.; Bendix, M.V.; Ostenson, R.C.; Howard, G.A.; Roos, B.A. Gap-junctional communication in normal and neoplastic prostate epithelial cells and its regulation by cAMP. Mol. Carcinog. 1996, 15, 18–32. [Google Scholar] [CrossRef]
- Xu, N.; Chen, H.J.; Chen, S.H.; Xue, X.Y.; Chen, H.; Zheng, Q.S.; Wei, Y.; Li, X.D.; Huang, J.B.; Cai, H.; et al. Reduced Connexin 43 expression is associated with tumor malignant behaviors and biochemical recurrence-free survival of prostate cancer. Oncotarget 2016, 7, 67476–67484. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.S.; Williams, C.J.; Williams, C.B.; Bonilla, I.V.; Klauber-DeMore, N.; Phillips, S.L. Dysregulated connexin 43 in HER2-positive drug resistant breast cancer cells enhances proliferation and migration. Oncotarget 2017, 8, 109358–109369. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Conzelmann, C.; Riaz, M.A.; Noll, T.; Gunduz, D. Inhibition of Cx43 attenuates ERK1/2 activation, enhances the expression of Cav1 and suppresses cell proliferation. Int. J. Mol. Med. 2018, 42, 2811–2818. [Google Scholar] [CrossRef]
- Posenato, I.; Calio, A.; Segala, D.; Sgroi, S.; Polara, A.; Brunelli, M.; Martignoni, G. Primary seminal vesicle carcinoma. The usefulness of PAX8 immunohistochemical expression for the differential diagnosis. Hum. Pathol. 2017, 69, 123–128. [Google Scholar] [CrossRef]
- Meda, P.; Pepper, M.S.; Traub, O.; Willecke, K.; Gros, D.; Beyer, E.; Nicholson, B.; Paul, D.; Orci, L. Differential expression of gap junction connexins in endocrine and exocrine glands. Endocrinology 1993, 133, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- El-Fouly, M.H.; Trosko, J.E.; Chang, C.C. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp. Cell Res. 1987, 168, 422–430. [Google Scholar] [CrossRef]
- Tai, M.H.; Upham, B.L.; Olson, L.K.; Tsao, M.S.; Reed, D.N., Jr.; Trosko, J.E. Cigarette smoke components inhibited intercellular communication and differentiation in human pancreatic ductal epithelial cells. Int. J. Cancer 2007, 120, 1855–1862. [Google Scholar] [CrossRef]
- McKarns, S.C.; Bombick, D.W.; Morton, M.J.; Doolittle, D.J. Gap junction intercellular communication and cytotoxicity in normal human cells after exposure to smoke condensates from cigarettes that burn or primarily heat tobacco. Toxicol. Vitr. Int. J. Publ. Assoc. Bibra 2000, 14, 41–51. [Google Scholar] [CrossRef]
- Ziambaras, K.; Lecanda, F.; Steinberg, T.H.; Civitelli, R. Cyclic stretch enhances gap junctional communication between osteoblastic cells. J. Bone Min. Res. 1998, 13, 218–228. [Google Scholar] [CrossRef]
- Roemer, E.; Lammerich, H.P.; Conroy, L.L.; Weisensee, D. Characterization of a gap-junctional intercellular communication (GJIC) assay using cigarette smoke. Toxicol. Lett. 2013, 219, 248–253. [Google Scholar] [CrossRef][Green Version]
- Hussain, A.; Das Sarma, S.; Babu, S.; Pal, D.; Das Sarma, J. Interaction of arsenic with gap junction protein connexin 43 alters gap junctional intercellular communication. Biochim. Biophys. Acta. Mol. Cell Res. 2018, 1865, 1423–1436. [Google Scholar] [CrossRef]
- Zou, H.; Liu, X.; Han, T.; Hu, D.; Yuan, Y.; Gu, J.; Bian, J.; Liu, Z. Alpha-lipoic acid protects against cadmium-induced hepatotoxicity via calcium signalling and gap junctional intercellular communication in rat hepatocytes. J. Toxicol. Sci. 2015, 40, 469–477. [Google Scholar] [CrossRef]
- Liu, Q.; Ji, X.; Ge, Z.; Diao, H.; Chang, X.; Wang, L.; Wu, Q. Role of connexin 43 in cadmium-induced proliferation of human prostate epithelial cells. J. Appl. Toxicol. 2017, 37, 933–942. [Google Scholar] [CrossRef]
- Ferraz, E.R.; de Oliveira, G.A.; de Oliveira, D.P. The impact of aromatic amines on the environment: Risks and damages. Front. Biosci. 2012, 4, 914–923. [Google Scholar] [CrossRef]
- Takahashi, H.; Nomata, K.; Mori, K.; Matsuo, M.; Miyaguchi, T.; Noguchi, M.; Kanetake, H. The preventive effect of green tea on the gap junction intercellular communication in renal epithelial cells treated with a renal carcinogen. Anticancer Res. 2004, 24, 3757–3762. [Google Scholar]
- Liao, C.K.; Jeng, C.J.; Wang, H.S.; Wang, S.H.; Wu, J.C. Lipopolysaccharide induces degradation of connexin43 in rat astrocytes via the ubiquitin-proteasome proteolytic pathway. PLoS ONE 2013, 8, e79350. [Google Scholar] [CrossRef]
- Liao, C.K.; Wang, S.M.; Chen, Y.L.; Wang, H.S.; Wu, J.C. Lipopolysaccharide-induced inhibition of connexin43 gap junction communication in astrocytes is mediated by downregulation of caveolin-3. Int. J. Biochem. Cell Biol. 2010, 42, 762–770. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tschernig, T. Connexins and Gap Junctions in Cancer of the Urinary Tract. Cancers 2019, 11, 704. https://doi.org/10.3390/cancers11050704
Tschernig T. Connexins and Gap Junctions in Cancer of the Urinary Tract. Cancers. 2019; 11(5):704. https://doi.org/10.3390/cancers11050704
Chicago/Turabian StyleTschernig, Thomas. 2019. "Connexins and Gap Junctions in Cancer of the Urinary Tract" Cancers 11, no. 5: 704. https://doi.org/10.3390/cancers11050704
APA StyleTschernig, T. (2019). Connexins and Gap Junctions in Cancer of the Urinary Tract. Cancers, 11(5), 704. https://doi.org/10.3390/cancers11050704




