The Role of AMP-Activated Protein Kinase as a Potential Target of Treatment of Hepatocellular Carcinoma
Abstract
1. Introduction
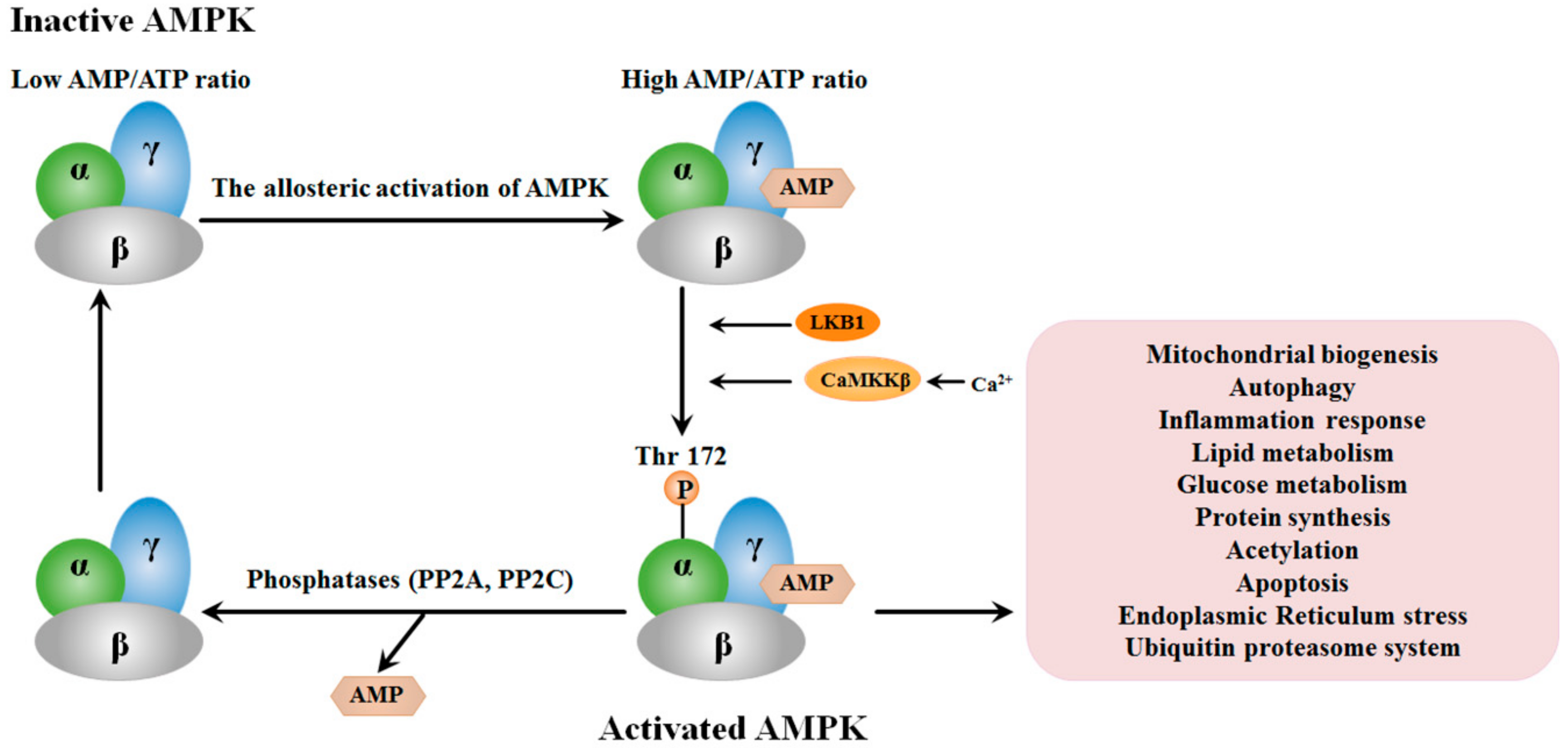
2. Structure and the Activation of AMPK
3. Dysregulation of AMPK in HCC
4. The Regulation of AMPK on HCC
4.1. Regulation on Cell Proliferation
4.2. Regulation on Cell Death
4.3. Regulation on Cell Invasion and Metastasis
4.4. Regulation of Cancer Metabolism
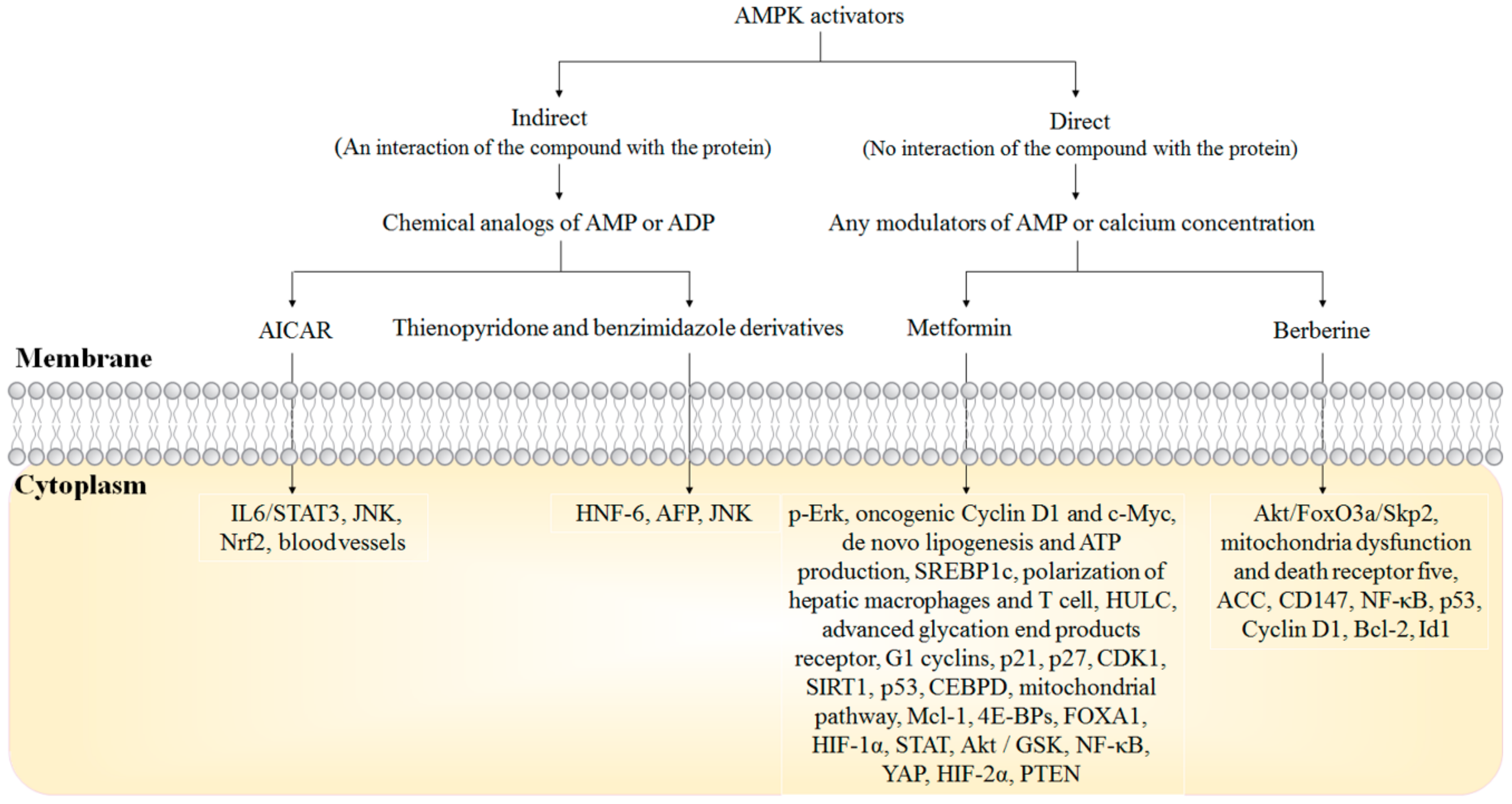
5. AMPK Activators in HCC Treatment
5.1. Metformin
5.2. Berberine
5.3. AICAR
5.4. Thienopyridone and Benzimidazole Derivatives
6. Clinical Perspective of AMPK Activation in HCC Treatment
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| ACC | Acetyl-CoA carboxylase carboxylase |
| AFP | α-fetoprotein |
| AICAR | 5-aminoimidazole-4-carboxamide riboside |
| bFGF | Basic fibroblast growth factor |
| CaMKK | Ca2+/calmodulin-dependent protein kinase |
| CDKs | Cyclin-dependent kinases |
| CDKIs | CDK inhibitors |
| CEBPD | CCAAT/enhancer-binding protein delta |
| GSPs | Growth suppressor proteins |
| HCC | Hepatocellular carcinoma |
| MAT | Methionine adenosyltransferase |
| MBIC | Methyl 2-(5-fluoro-2-hydroxyphenyl)-1H-benzo[d]imidazole-5-carboxylate |
| NAFLD | Nonalcoholic fatty liver disease |
| NF-κB | Nuclear factor kappa-B |
| PP5 | Protein phosphatase 5 |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| PGC-1α | Proliferator-activated receptor gamma coactivator 1-alpha |
| STAT3 | Signal transducer and activator of transcription 3 |
| TACE | Transcatheter arterial chemoembolization |
| TNM | Tumor–node–metastasis |
References
- Xie, W.; Qiao, X.; Shang, L.; Dou, J.; Yang, X.; Qiao, S.; Wu, Y. Knockdown of znf233 suppresses hepatocellular carcinoma cell proliferation and tumorigenesis. Gene 2018, 679, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the american association for the study of liver diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global epidemiology of hepatocellular carcinoma: An emphasis on demographic and regional variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef]
- Li, P.; Huang, W.; Wang, F.; Ke, Y.F.; Gao, L.; Shi, K.Q.; Zhou, M.T.; Chen, B.C. Nomograms based on inflammatory biomarkers for predicting tumor grade and micro-vascular invasion in stage i/ii hepatocellular carcinoma. Biosci. Rep. 2018, 38, 38. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA: A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Gao, H.; Xie, J.; Yuan, Y.P.; Yuan, Q.; Gao, M.Q.; Liu, K.L.; Chen, X.H.; Han, Y.T.; Han, Z.W. Hispidulin induces er stress-mediated apoptosis in human hepatocellular carcinoma cells in vitro and in vivo by activating ampk signaling pathway. Acta Pharmacol. Sin. 2018, 40, 666–676. [Google Scholar] [CrossRef]
- Cheng, Z.; Lei, Z.; Yang, P.; Si, A.; Xiang, D.; Zhou, J.; Huser, N. Long non-coding rna thor promotes cell proliferation and metastasis in hepatocellular carcinoma. Gene 2018, 678, 129–136. [Google Scholar] [CrossRef]
- Nies, A.T.; Konig, J.; Pfannschmidt, M.; Klar, E.; Hofmann, W.J.; Keppler, D. Expression of the multidrug resistance proteins mrp2 and mrp3 in human hepatocellular carcinoma. Int. J. Cancer 2001, 94, 492–499. [Google Scholar] [CrossRef]
- Murray, G.I.; Paterson, P.J.; Weaver, R.J.; Ewen, S.W.; Melvin, W.T.; Burke, M.D. The expression of cytochrome p-450, epoxide hydrolase, and glutathione s-transferase in hepatocellular carcinoma. Cancer 1993, 71, 36–43. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the asia-pacific region with advanced hepatocellular carcinoma: A phase iii randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The ampk signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. Amp-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef]
- Zheng, L.; Yang, W.; Wu, F.; Wang, C.; Yu, L.; Tang, L.; Qiu, B.; Li, Y.; Guo, L.; Wu, M.; et al. Prognostic significance of ampk activation and therapeutic effects of metformin in hepatocellular carcinoma. Clin. Cancer Res. 2013, 19, 5372–5380. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. Ampk: A key regulator of energy balance in the single cell and the whole organism. Int. J. Obes. 2008, 32 (Suppl. 4), S7–S12. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. Ampk, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Williams, M.R.; Ryffel, B. Amp-activated protein kinase regulation of the nlrp3 inflammasome during aging. Trends Endocrinol. Metab. 2018, 29, 8–17. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Amp-activated protein kinase as a reprogramming strategy for hypertension and kidney disease of developmental origin. Int. J. Mol. Sci. 2018, 19, 1744. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Lu, Q.; Ren, D.; Sun, X.; Rousselle, T.; Tan, Y.; Li, J. Ampk: A therapeutic target of heart failure-not only metabolism regulation. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of ampk. Am. J. Physiol.-Endocrinol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, S.; Higurashi, T.; Nakajima, A. Ampk: Therapeutic target for diabetes and cancer prevention. Curr. Pharm. Des. 2017, 23, 3629–3644. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Li, J.; Hao, Q.; Vadgama, J.V.; Wu, Y. Amp-activated protein kinase: A potential therapeutic target for triple-negative breast cancer. Breast Cancer Res. 2019, 21, 29. [Google Scholar] [CrossRef]
- Kim, A.S.; Miller, E.J.; Young, L.H. Amp-activated protein kinase: A core signalling pathway in the heart. Acta Physiol. (Oxf.) 2009, 196, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Andreelli, F.; Jorgensen, S.B.; Perrin, C.; Flamez, D.; Mu, J.; Wojtaszewski, J.F.P.; Schuit, F.C.; Birnbaum, M.; Richter, E.; et al. Physiological role of amp-activated protein kinase (ampk): Insights from knockout mouse models. Biochem. Soc. Trans. 2003, 31, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Bairwa, S.C.; Parajuli, N.; Dyck, J.R. The role of ampk in cardiomyocyte health and survival. Biochimica et Biophysica Acta 2016, 1862, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Zaha, V.G.; Young, L.H. Amp-activated protein kinase regulation and biological actions in the heart. Circ. Res. 2012, 111, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Carling, D.; Gamblin, S.J. Amp-activated protein kinase: Also regulated by adp? Trends Biochem. Sci. 2011, 36, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Sanz, P.; Rubio, T.; Garcia-Gimeno, M.A. Ampkbeta subunits: More than just a scaffold in the formation of ampk complex. FEBS J. 2013, 280, 3723–3733. [Google Scholar] [CrossRef]
- Fogarty, S.; Hardie, D.G. Development of protein kinase activators: Ampk as a target in metabolic disorders and cancer. Biochimica et Biophysica Acta 2010, 1804, 581–591. [Google Scholar] [CrossRef]
- Davies, S.P.; Helps, N.R.; Cohen, P.T.; Hardie, D.G. 5’-amp inhibits dephosphorylation, as well as promoting phosphorylation, of the amp-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2c alpha and native bovine protein phosphatase-2ac. FEBS Lett. 1995, 377, 421–425. [Google Scholar]
- Marin, T.L.; Gongol, B.; Zhang, F.; Martin, M.; Johnson, D.A.; Xiao, H.; Wang, Y.; Subramaniam, S.; Chien, S.; Shyy, J.Y.J. Ampk promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors dnmt1, rbbp7, and hat1. Sci. Signal. 2017, 10, eaaf7478. [Google Scholar] [CrossRef]
- Lee, S.R.; Kwon, S.W.; Lee, Y.H.; Kaya, P.; Kim, J.M.; Ahn, C.; Jung, E.M.; Lee, G.S.; An, B.S.; Jeung, E.B.; et al. Dietary intake of genistein suppresses hepatocellular carcinoma through ampk-mediated apoptosis and anti-inflammation. BMC Cancer 2019, 19, 12. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Zhen, Z.; Ma, X.; Yu, W.; Zeng, H.; Li, L. Cxcl17 promotes cell metastasis and inhibits autophagy via the lkb1-ampk pathway in hepatocellular carcinoma. Gene 2019, 690, 129–136. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Li, M.; Wu, H.; Wang, Y.; You, Y.; Li, P.; Ding, X.; Liu, C.; Gong, J. Predictive and preventive significance of ampk activation on hepatocarcinogenesis in patients with liver cirrhosis. Cell Death Dis. 2018, 9, 264. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Wu, L.; Lu, J.; Zou, D.J.; Huang, Q. Expression of phosphorylated amp-activated protein kinase predicts response to transarterial chemoembolization in postoperative cases of hepatocellular carcinoma. Medicine 2016, 95, e2908. [Google Scholar] [CrossRef]
- Cai, C.; Wang, W.; Tu, Z. Aberrantly DNA methylated-differentially expressed genes and pathways in hepatocellular carcinoma. J. Cancer 2019, 10, 355–366. [Google Scholar] [CrossRef]
- Huang; Li, T.; Wang, L.; Zhang, L.; Yan, R.; Li, K.; Xing, S.; Wu, G.; Hu, L.; Jia, W.; et al. Hepatocellular carcinoma redirects to ketolysis for progression under nutrition deprivation stress. Cell Res. 2016, 26, 1112–1130. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Hong, D.; Nam, S.H.; Kim, J.M.; Paik, Y.H.; Joh, J.W.; Kwon, C.H.; Park, J.B.; Choi, G.S.; Jang, K.Y.; et al. Sirt1 regulates oncogenesis via a mutant p53-dependent pathway in hepatocellular carcinoma. J. Hepatol. 2015, 62, 121–130. [Google Scholar] [CrossRef]
- Chen, Y.L.; Hung, M.H.; Chu, P.Y.; Chao, T.I.; Tsai, M.H.; Chen, L.J.; Hsiao, Y.J.; Shih, C.T.; Hsieh, F.S.; Chen, K.F. Protein phosphatase 5 promotes hepatocarcinogenesis through interaction with amp-activated protein kinase. Biochem. Pharmacol. 2017, 138, 49–60. [Google Scholar] [CrossRef]
- Hsieh, F.S.; Chen, Y.L.; Hung, M.H.; Chu, P.Y.; Tsai, M.H.; Chen, L.J.; Hsiao, Y.J.; Shih, C.T.; Chang, M.J.; Chao, T.I.; et al. Palbociclib induces activation of ampk and inhibits hepatocellular carcinoma in a cdk4/6-independent manner. Mol. Oncol. 2017, 11, 1035–1049. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, T.; Li, Y.; Guo, Y.; Zhu, Y.; Wang, Q.; Tan, X.; Chen, W.; Zhang, Y.; Cheng, W.; et al. Amp-activated protein kinase suppresses the in vitro and in vivo proliferation of hepatocellular carcinoma. PLoS ONE 2014, 9, e93256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grana, X.; Reddy, E.P. Cell cycle control in mammalian cells: Role of cyclins, cyclin dependent kinases (cdks), growth suppressor genes and cyclin-dependent kinase inhibitors (ckis). Oncogene 1995, 11, 211–219. [Google Scholar] [PubMed]
- Tuo, L.; Xiang, J.; Pan, X.; Hu, J.; Tang, H.; Liang, L.; Xia, J.; Hu, Y.; Zhang, W.; Huang, A.; et al. Pck1 negatively regulates cell cycle progression and hepatoma cell proliferation via the ampk/p27(kip1) axis. J. Exp. Clin. Cancer Res. 2019, 38, 50. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Ho, H.J.; Lin, J.T.; Shieh, J.J.; Wu, C.Y. Simvastatin-induced cell cycle arrest through inhibition of stat3/skp2 axis and activation of ampk to promote p27 and p21 accumulation in hepatocellular carcinoma cells. Cell Death Dis. 2017, 8, e2626. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.S.; Zhang, J.Y.; Zhang, D.H. Fatsioside ainduced apoptotic death of hepg2 cells requires activation of ampactivated protein kinase. Mol. Med. Rep. 2015, 12, 5679–5684. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Hayakawa, M.; Koga, H.; Torimura, T. Effects of fucoidan on proliferation, amp-activated protein kinase, and downstream metabolism- and cell cycle-associated molecules in poorly differentiated human hepatoma hlf cells. Int. J. Oncol. 2015, 46, 2216–2222. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Xing, Y.; Yan, Y.; Guo, P.; Zhuang, J.; Qin, F.; Zhang, J. Physcion 8-o-beta-glucopyranoside induces apoptosis, suppresses invasion and inhibits epithelial to mesenchymal transition of hepatocellular carcinoma hepg2 cells. Biomed. Pharmacother. 2016, 83, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, H.; Tong, D.; Wang, C.; Sun, L.; Zhao, C.; Li, Y.; Zhu, L.; Wu, D. Physcion induces apoptosis in hepatocellular carcinoma by modulating mir-370. Am. J. Cancer Res. 2016, 6, 2919–2931. [Google Scholar] [PubMed]
- Yie, Y.; Zhao, S.; Tang, Q.; Zheng, F.; Wu, J.; Yang, L.; Deng, S.; Hann, S.S. Ursolic acid inhibited growth of hepatocellular carcinoma hepg2 cells through ampkalpha-mediated reduction of DNA methyltransferase 1. Mol. Cell. Biochem. 2015, 402, 63–74. [Google Scholar] [CrossRef]
- Stein, U.; Arlt, F.; Walther, W.; Smith, J.; Waldman, T.; Harris, E.D.; Mertins, S.D.; Heizmann, C.W.; Allard, D.; Birchmeier, W.; et al. The metastasis-associated gene s100a4 is a novel target of beta-catenin/t-cell factor signaling in colon cancer. Gastroenterology 2006, 131, 1486–1500. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, Y.K.; Kim, H.J.; Park, O.J.; Kim, Y.M. Ampk interacts with beta-catenin in the regulation of hepatocellular carcinoma cell proliferation and survival with selenium treatment. Oncol. Rep. 2016, 35, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.C.; Lin, S.C.; Pan, S.L.; Kuo, C.H.; Tsai, I.L.; Kuo, M.T.; Wen, W.C.; Chen, P.; Guh, J.H. Antroquinonol displays anticancer potential against human hepatocellular carcinoma cells: A crucial role of ampk and mtor pathways. Biochem. Pharmacol. 2010, 79, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; He, Z.; Zhou, X.; Xian, L.; Yuan, T.; Jia, X.; Hong, J.; He, L.; Liu, J. Low concentration of metformin induces a p53-dependent senescence in hepatoma cells via activation of the ampk pathway. Int. J. Oncol. 2013, 43, 1503–1510. [Google Scholar] [CrossRef]
- Sun, X.L.; Gao, L.; Chien, H.Y.; Li, W.C.; Zhao, J.J. The regulation and function of the nuak family. J. Mol. Endocrinol. 2013, 51, R15–R22. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.C.; Pepe-Mooney, B.; Galli, G.G.; Dill, M.T.; Huang, H.T.; Hao, M.F.; Wang, Y.M.; Liang, H.; Calogero, R.A.; Camargo, F.D. Nuak2 is a critical yap target in liver cancer. Nat. Commun. 2018, 9, 12. [Google Scholar] [CrossRef]
- Goto, K.; Lin, W.Y.; Zhang, L.L.; Jilg, N.; Shao, R.X.; Schaefer, E.A.K.; Zhao, H.; Fusco, D.N.; Peng, L.F.; Kato, N.; et al. The ampk-related kinase snark regulates hepatitis c virus replication and pathogenesis through enhancement of tgf-beta signaling. J. Hepatol. 2013, 59, 942–948. [Google Scholar] [CrossRef]
- Goto, K.; Kato, N.; Chung, R.T. Anti-hepatocellular carcinoma properties of the anti-alcoholism drug disulfiram discovered to enzymatically inhibit the ampk-related kinase snark in vitro. Oncotarget 2016, 7, 74987–74999. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Garcia-Rodriguez, J.L.; Varela-Rey, M.; Gutierrez, V.; Fernandez-Ramos, D.; Beraza, N.; Aransay, A.M.; Schlangen, K.; Lozano, J.J.; Aspichueta, P.; et al. Hepatoma cells from mice deficient in glycine n-methyltransferase have increased ras signaling and activation of liver kinase b1. Gastroenterology 2012, 143, 787–798e713. [Google Scholar] [CrossRef]
- Liu, X.; Hu, X.; Kuang, Y.; Yan, P.; Li, L.; Li, C.; Tao, Q.; Cai, X. Bclb, methylated in hepatocellular carcinoma, is a starvation stress sensor that induces apoptosis and autophagy through the ampk-mtor signaling cascade. Cancer Lett. 2017, 395, 63–71. [Google Scholar] [CrossRef]
- Zhang, X.; Han, K.; Yuan, D.H.; Meng, C.Y. Overexpression of nad(p)h: Quinone oxidoreductase 1 inhibits hepatocellular carcinoma cell proliferation and induced apoptosis by activating ampk/pgc-1alpha pathway. DNA Cell Biol. 2017, 36, 256–263. [Google Scholar] [CrossRef]
- Han, M.; Gao, H.; Ju, P.; Gao, M.Q.; Yuan, Y.P.; Chen, X.H.; Liu, K.L.; Han, Y.T.; Han, Z.W. Hispidulin inhibits hepatocellular carcinoma growth and metastasis through ampk and erk signaling mediated activation of ppargamma. Biomed. Pharmacother. 2018, 103, 272–283. [Google Scholar] [CrossRef]
- Kim, Y.W.; Jang, E.J.; Kim, C.H.; Lee, J.H. Sauchinone exerts anticancer effects by targeting ampk signaling in hepatocellular carcinoma cells. Chem.-Biol. Interact. 2017, 261, 108–117. [Google Scholar] [CrossRef]
- Pan, X.P.; Wang, C.; Li, Y.; Huang, L.H. Physcion induces apoptosis through triggering endoplasmic reticulum stress in hepatocellular carcinoma. Biomed. Pharmacother. 2018, 99, 894–903. [Google Scholar] [CrossRef]
- Yang, X.; Huang, N. Berberine induces selective apoptosis through the ampkmediated mitochondrial/caspase pathway in hepatocellular carcinoma. Mol. Med. Rep. 2013, 8, 505–510. [Google Scholar] [CrossRef]
- Lee, C.W.; Wong, L.L.; Tse, E.Y.; Liu, H.F.; Leong, V.Y.; Lee, J.M.; Hardie, D.G.; Ng, I.O.; Ching, Y.P. Ampk promotes p53 acetylation via phosphorylation and inactivation of sirt1 in liver cancer cells. Cancer Res. 2012, 72, 4394–4404. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hong, H.H.; Chen, S.P.; Ma, C.Q.; Liu, H.Y.; Yao, Y.C. Activation of ampk/mnsod signaling mediates anti-apoptotic effect of hepatitis b virus in hepatoma cells. World J. Gastroenterol. 2016, 22, 4345–4353. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Lai, H.Y.; Chen, Y.C.; Li, C.F.; Huang, H.S.; Liu, H.S.; Tsai, Y.S.; Wang, J.M. Metformin promotes apoptosis in hepatocellular carcinoma through the cebpd-induced autophagy pathway. Oncotarget 2017, 8, 13832–13845. [Google Scholar] [CrossRef]
- Zhong, J.; Dong, X.; Xiu, P.; Wang, F.; Liu, J.; Wei, H.; Xu, Z.; Liu, F.; Li, T.; Li, J. Blocking autophagy enhances meloxicam lethality to hepatocellular carcinoma by promotion of endoplasmic reticulum stress. Cell Prolif. 2015, 48, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.M.; Jiang, Q.H.; Cai, C.; Qu, M.; Shen, W. Scd1 negatively regulates autophagy-induced cell death in human hepatocellular carcinoma through inactivation of the ampk signaling pathway. Cancer Lett. 2015, 358, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, Y.; Zhu, M.; Siu, F.M.; Ng, K.M.; Che, C.M. A novel mechanism of xiap degradation induced by timosaponin aiii in hepatocellular carcinoma. Biochim. Biophys. Acta 2013, 1833, 2890–2899. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Xu, H.; Liu, G.; Wu, J.; Li, C.; Wang, X.; Zhang, S.; Xu, H.; Ju, S.; Cheng, W.; et al. Mita1, a novel energy stress-inducible lncrna, promotes hepatocellular carcinoma metastasis. Hepatology 2019. [Google Scholar] [CrossRef]
- Li, M.; Jin, C.; Xu, M.; Zhou, L.; Li, D.; Yin, Y. Bifunctional enzyme atic promotes propagation of hepatocellular carcinoma by regulating ampk-mtor-s6 k1 signaling. Cell Commun. Signal. 2017, 15, 52. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Z.X.; Huang, F.; Yuan, X.W.; Deng, L.; Tang, D. Microrna-1271 functions as a potential tumor suppressor in hepatitis b virus-associated hepatocellular carcinoma through the ampk signaling pathway by binding to ccna1. J. Cell. Physiol. 2019, 234, 3555–3569. [Google Scholar] [CrossRef]
- Yang, C.C.; Chang, S.F.; Chao, J.K.; Lai, Y.L.; Chang, W.E.; Hsu, W.H.; Kuo, W.H. Activation of amp-activated protein kinase attenuates hepatocellular carcinoma cell adhesion stimulated by adipokine resistin. BMC Cancer 2014, 14, 112. [Google Scholar] [CrossRef]
- Shen, C.; Ka, S.O.; Kim, S.J.; Kim, J.H.; Park, B.H.; Park, J.H. Metformin and aicar regulate nanog expression via the jnk pathway in hepg2 cells independently of ampk. Tumour Biol. 2016, 37, 11199–11208. [Google Scholar] [CrossRef]
- Pascale, R.M.; Feo, C.F.; Calvisi, D.F.; Feo, F. Deregulation of methionine metabolism as determinant of progression and prognosis of hepatocellular carcinoma. Transl. Gastroenterol. Hepatol. 2018, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.Y.; Kwon, J.H.; Cho, J.H.; Zhang, L.; Mansfield, B.C.; Chou, J.Y. Downregulation of pathways implicated in liver inflammation and tumorigenesis of glycogen storage disease type ia mice receiving gene therapy. Hum. Mol. Genet. 2017, 26, 1890–1899. [Google Scholar] [CrossRef]
- Lally, J.S.V.; Ghoshal, S.; DePeralta, D.K.; Moaven, O.; Wei, L.; Masia, R.; Erstad, D.J.; Fujiwara, N.; Leong, V.; Houde, V.P.; et al. Inhibition of acetyl-coa carboxylase by phosphorylation or the inhibitor nd-654 suppresses lipogenesis and hepatocellular carcinoma. Cell Metab. 2019, 29, 174–182e175. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, Y.; Tan, H.Y.; Cheung, F.; Hong, M.; Lao, L.; Nagamatsu, T. Inhibition of eukaryotic elongation factor-2 confers to tumor suppression by a herbal formulation huanglian-jiedu decoction in human hepatocellular carcinoma. J. Ethnopharmacol. 2015, 164, 309–318. [Google Scholar] [CrossRef]
- Bagga, S.; Rawat, S.; Ajenjo, M.; Bouchard, M.J. Hepatitis b virus (hbv) x protein-mediated regulation of hepatocyte metabolic pathways affects viral replication. Virology 2016, 498, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. Ampk activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, J.J.; Sheng, L.; Yuan, M.; Wu, Y.; Chen, L.; Zheng, G.H.; Qiu, Z.P. Metformin delays akt/c-met-driven hepatocarcinogenesis by regulating signaling pathways for de novo lipogenesis and atp generation. Toxicol. Appl. Pharmacol. 2019, 365, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, K.; Hwang, B.J.; Dewi, R.E.; Twaddel, W.; Goloubeva, O.G.; Wong, K.K.; Saxena, N.K.; Biswal, S.; Girnun, G.D. Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prev. Res. 2012, 5, 544–552. [Google Scholar] [CrossRef]
- Cauchy, F.; Mebarki, M.; Leporq, B.; Laouirem, S.; Albuquerque, M.; Lambert, S.; Bourgoin, P.; Soubrane, O.; Van Beers, B.E.; Faivre, S.; et al. Strong antineoplastic effects of metformin in preclinical models of liver carcinogenesis. Clin. Sci. 2017, 131, 27–36. [Google Scholar] [CrossRef]
- Jo, W.; Yu, E.S.; Chang, M.; Park, H.K.; Choi, H.J.; Ryu, J.E.; Jang, S.; Lee, H.J.; Jang, J.J.; Son, W.C. Metformin inhibits early stage diethylnitrosamineinduced hepatocarcinogenesis in rats. Mol. Med. Rep. 2016, 13, 146–152. [Google Scholar] [CrossRef]
- De Oliveira, S.; Houseright, R.A.; Graves, A.L.; Golenberg, N.; Korte, B.G.; Miskolci, V.; Huttenlocher, A. Metformin modulates innate immune-mediated inflammation and early progression of nafld-associated hepatocellular carcinoma in zebrafish. J. Hepatol. 2019, 70, 710–721. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, H. Metformin inhibits tumorigenesis in hbv-induced hepatocellular carcinoma by suppressing hulc overexpression caused by hbx. J. Cell. Biochem. 2018, 119, 4482–4495. [Google Scholar] [CrossRef] [PubMed]
- DePeralta, D.K.; Wei, L.; Ghoshal, S.; Schmidt, B.; Lauwers, G.Y.; Lanuti, M.; Chung, R.T.; Tanabe, K.K.; Fuchs, B.C. Metformin prevents hepatocellular carcinoma development by suppressing hepatic progenitor cell activation in a rat model of cirrhosis. Cancer 2016, 122, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Kato, K.; Iwama, H.; Maeda, E.; Sakamoto, T.; Fujita, K.; Toyota, Y.; Tani, J.; Nomura, T.; Mimura, S.; et al. Effect of the anti-diabetic drug metformin in hepatocellular carcinoma in vitro and in vivo. Int. J. Oncol. 2014, 45, 322–332. [Google Scholar] [CrossRef]
- Cai, X.; Hu, X.; Cai, B.; Wang, Q.; Li, Y.; Tan, X.; Hu, H.; Chen, X.; Huang, J.; Cheng, J.; et al. Metformin suppresses hepatocellular carcinoma cell growth through induction of cell cycle g1/g0 phase arrest and p21cip and p27kip expression and downregulation of cyclin d1 in vitro and in vivo. Oncol. Rep. 2013, 30, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Han, S.; Qian, W.; Gu, Y.; Li, X.; Yang, K. Metformin induces mir-378 to downregulate the cdk1, leading to suppression of cell proliferation in hepatocellular carcinoma. Onco Targets Ther. 2018, 11, 4451–4459. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, J.; Chen, L.; Feng, X.; Liu, Z.; Hu; Zeng, Z.; Jia, X.; Liang, M.; Shi, B.; et al. Negative regulation of sirtuin 1 by amp-activated protein kinase promotes metformin-induced senescence in hepatocellular carcinoma xenografts. Cancer Lett. 2017, 411, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Lu, Q.J.; Zhao, J.; Wu, G.Y. Metformin inhibits growth of hepatocellular carcinoma cells by inducing apoptosis via mitochondrion-mediated pathway. Asian Pac. J. Cancer Prev. 2012, 13, 3275–3279. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Yanagiya, A.; Graber, T.; Razumilava, N.; Bronk, S.; Zammit, D.; Zhao, Y.; Zakaria, C.; Metrakos, P.; Pollak, M.; et al. Metformin requires 4e-bps to induce apoptosis and repress translation of mcl-1 in hepatocellular carcinoma cells. Oncotarget 2017, 8, 50542–50556. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tao, C.; Huang, X.; He, H.; Shi, H.; Zhang, Q.; Wu, H. Metformin induces apoptosis of human hepatocellular carcinoma hepg2 cells by activating an ampk/p53/mir-23a/foxa1 pathway. Onco Targets Ther. 2016, 9, 2845–2853. [Google Scholar] [PubMed]
- Qu, Z.; Zhang, Y.; Liao, M.; Chen, Y.; Zhao, J.; Pan, Y. In vitro and in vivo antitumoral action of metformin on hepatocellular carcinoma. Hepatol. Res. 2012, 42, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, J.; Yi, G.; Deng, M.; Liu, H.; Liang, M.; Shi, B.; Fu, X.; Chen, Y.; Chen, L.; et al. Metformin suppresses hypoxia-induced stabilization of hif-1alpha through reprogramming of oxygen metabolism in hepatocellular carcinoma. Oncotarget 2016, 7, 873–884. [Google Scholar] [PubMed]
- Zhao, D.; Long, X.D.; Lu, T.F.; Wang, T.; Zhang, W.W.; Liu, Y.X.; Cui, X.L.; Dai, H.J.; Xue, F.; Xia, Q. Metformin decreases il-22 secretion to suppress tumor growth in an orthotopic mouse model of hepatocellular carcinoma. Int. J. Cancer 2015, 136, 2556–2565. [Google Scholar] [CrossRef]
- Ferretti, A.C.; Hidalgo, F.; Tonucci, F.M.; Almada, E.; Pariani, A.; Larocca, M.C.; Favre, C. Metformin and glucose starvation decrease the migratory ability of hepatocellular carcinoma cells: Targeting ampk activation to control migration. Sci. Rep. 2019, 9, 2815. [Google Scholar] [CrossRef]
- Chengye, W.; Yu, T.; Ping, S.; Deguang, S.; Keyun, W.; Yan, W.; Rixin, Z.; Rui, L.; Zhenming, G.; Mingliang, Y.; et al. Metformin reverses bfgf-induced epithelial-mesenchymal transition in hcc cells. Oncotarget 2017, 8, 104247–104257. [Google Scholar] [CrossRef]
- Qu, H.; Yang, X. Metformin inhibits angiogenesis induced by interaction of hepatocellular carcinoma with hepatic stellate cells. Cell Biochem. Biophys. 2015, 71, 931–936. [Google Scholar] [CrossRef]
- Wu, W.; Yang, J.L.; Wang, Y.L.; Wang, H.; Yao, M.; Wang, L.; Gu, J.J.; Cai, Y.; Shi, Y.; Yao, D.F. Reversal of multidrug resistance of hepatocellular carcinoma cells by metformin through inhibiting nf-kappab gene transcription. World J. Hepatol. 2016, 8, 985–993. [Google Scholar] [CrossRef]
- Tian, Y.; Tang, B.; Wang, C.; Sun, D.; Zhang, R.; Luo, N.; Han, Z.; Liang, R.; Gao, Z.; Wang, L. Metformin mediates resensitivity to 5-fluorouracil in hepatocellular carcinoma via the suppression of yap. Oncotarget 2016, 7, 46230–46241. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, D.; Tian, Y.; Ling, S.; Wang, L. Metformin sensitizes hepatocellular carcinoma to arsenic trioxide-induced apoptosis by downregulating bcl2 expression. Tumour Biol. 2015, 36, 2957–2964. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Guo, X.; Yang, L. Metformin enhances the effect of regorafenib and inhibits recurrence and metastasis of hepatic carcinoma after liver resection via regulating expression of hypoxia inducible factors 2alpha (hif-2alpha) and 30 kda hiv tat-interacting protein (tip30). Med. Sci. Monit. 2018, 24, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Cao, M.; You, A.; Gao, J.; Zhou, H.; Li, H.; Cui, Y.; Fang, F.; Zhang, W.; Song, T.; et al. Metformin inhibits the prometastatic effect of sorafenib in hepatocellular carcinoma by upregulating the expression of tip30. Cancer Sci. 2016, 107, 507–513. [Google Scholar] [CrossRef]
- You, A.; Cao, M.; Guo, Z.; Zuo, B.; Gao, J.; Zhou, H.; Li, H.; Cui, Y.; Fang, F.; Zhang, W.; et al. Metformin sensitizes sorafenib to inhibit postoperative recurrence and metastasis of hepatocellular carcinoma in orthotopic mouse models. J. Hematol. Oncol. 2016, 9, 20. [Google Scholar] [CrossRef]
- Hsieh, S.C.; Tsai, J.P.; Yang, S.F.; Tang, M.J.; Hsieh, Y.H. Metformin inhibits the invasion of human hepatocellular carcinoma cells and enhances the chemosensitivity to sorafenib through a downregulation of the erk/jnk-mediated nf-kappab-dependent pathway that reduces upa and mmp-9 expression. Amino Acids 2014, 46, 2809–2822. [Google Scholar] [CrossRef]
- Zhang, Q.; Kong, J.; Dong, S.; Xu, W.; Sun, W. Metformin exhibits the anti-proliferation and anti-invasion effects in hepatocellular carcinoma cells after insufficient radiofrequency ablation. Cancer Cell Int. 2017, 17, 48. [Google Scholar] [CrossRef]
- Chuang, T.Y.; Wu, H.L.; Min, J.; Diamond, M.; Azziz, R.; Chen, Y.H. Berberine regulates the protein expression of multiple tumorigenesis-related genes in hepatocellular carcinoma cell lines. Cancer Cell Int. 2017, 17, 59. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, M.F.; Wang, X.B.; Tan, H.Y.; Tsao, S.W.; Feng, Y.B. Berberine-induced tumor suppressor p53 up-regulation gets involved in the regulatory network of mir-23a in hepatocellular carcinoma. Biochim. Biophys. Acta-Gene Regul. Mech. 2014, 1839, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, M.; Zhang, Z.L.; Liu, N.; Han, X.Y.; Liu, Q.C.; Deng, W.J.; Liao, C.X. Induction of apoptosis by berberine in hepatocellular carcinoma hepg2 cells via downregulation of nf-kappa b. Oncol. Res. 2017, 25, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Dong, X.; Lin, P.; Jiang, J. Regulation of akt/foxo3a/skp2 axis is critically involved in berberine-induced cell cycle arrest in hepatocellular carcinoma cells. Int. J. Mol. Sci. 2018, 19, 327. [Google Scholar]
- Ke, R.; Vishnoi, K.; Viswakarma, N.; Santha, S.; Das, S.; Rana, A.; Rana, B. Involvement of amp-activated protein kinase and death receptor 5 in trail-berberine-induced apoptosis of cancer cells. Sci. Rep. 2018, 8, 5521. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhang, Z.Q.; Wang, B.; Jiang, H.X.; Cheng, L.; Shen, L.M. Berberine-induced apoptotic and autophagic death of hepg2 cells requires ampk activation. Cancer Cell Int. 2014, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Tang, X.; Liu, H.; Tang, J.; Yang, Y.; Jing, X.; Xiao, Q.; Wang, W.; Gou, X.; Wang, Z. Berberine induces cell death in human hepatoma cells in vitro by downregulating cd147. Cancer Sci. 2011, 102, 1287–1292. [Google Scholar] [CrossRef]
- Li, J.; Li, O.; Kan, M.; Zhang, M.; Shao, D.; Pan, Y.; Zheng, H.; Zhang, X.; Chen, L.; Liu, S. Berberine induces apoptosis by suppressing the arachidonic acid metabolic pathway in hepatocellular carcinoma. Mol. Med. Rep. 2015, 12, 4572–4577. [Google Scholar] [CrossRef]
- Lo, T.F.; Tsai, W.C.; Chen, S.T. Microrna-21-3p, a berberine-induced mirna, directly down-regulates human methionine adenosyltransferases 2a and 2b and inhibits hepatoma cell growth. PLoS ONE 2013, 8, e75628. [Google Scholar] [CrossRef]
- Chen, J.; Wu, F.X.; Luo, H.L.; Liu, J.J.; Luo, T.; Bai, T.; Li, L.Q.; Fan, X.H. Berberine upregulates mir-22-3p to suppress hepatocellular carcinoma cell proliferation by targeting sp1. Am. J. Transl. Res. 2016, 8, 4932–4941. [Google Scholar]
- Tsang, C.M.; Cheung, K.C.; Cheung, Y.C.; Man, K.; Lui, V.W.; Tsao, S.W.; Feng, Y. Berberine suppresses id-1 expression and inhibits the growth and development of lung metastases in hepatocellular carcinoma. Biochimica et Biophysica Acta 2015, 1852, 541–551. [Google Scholar] [CrossRef]
- Wang, X.; Wang, N.; Li, H.; Liu, M.; Cao, F.; Yu, X.; Zhang, J.; Tan, Y.; Xiang, L.; Feng, Y. Up-regulation of pai-1 and down-regulation of upa are involved in suppression of invasiveness and motility of hepatocellular carcinoma cells by a natural compound berberine. Int. J. Mol. Sci. 2016, 17, 577. [Google Scholar] [CrossRef] [PubMed]
- Jie, S.; Li, H.; Tian, Y.; Guo, D.; Zhu, J.; Gao, S.; Jiang, L. Berberine inhibits angiogenic potential of hep g2 cell line through vegf down-regulation in vitro. J. Gastroenterol. Hepatol. 2011, 26, 179–185. [Google Scholar] [CrossRef]
- Guo, N.; Yan, A.; Gao, X.; Chen, Y.; He, X.; Hu, Z.; Mi, M.; Tang, X.; Gou, X. Berberine sensitizes rapamycinmediated human hepatoma cell death in vitro. Mol. Med. Rep. 2014, 10, 3132–3138. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, K.; Gu, C.; Yu, G.; Zhao, D.; Mai, W.; Zhong, Y.; Liu, S.; Nie, Y.; Yang, H. Berberine, a natural plant alkaloid, synergistically sensitizes human liver cancer cells to sorafenib. Oncol. Rep. 2018, 40, 1525–1532. [Google Scholar] [CrossRef]
- Sid, B.; Glorieux, C.; Valenzuela, M.; Rommelaere, G.; Najimi, M.; Dejeans, N.; Renard, P.; Verrax, J.; Calderon, P.B. Aicar induces nrf2 activation by an ampk-independent mechanism in hepatocarcinoma cells. Biochem. Pharmacol. 2014, 91, 168–180. [Google Scholar] [CrossRef]
- Gao, J.; Xiong, R.; Xiong, D.; Zhao, W.; Zhang, S.; Yin, T.; Zhang, X.; Jiang, G.; Yin, Z. The adenosine monophosphate (amp) analog, 5-aminoimidazole-4-carboxamide ribonucleotide (aicar) inhibits hepatosteatosis and liver tumorigenesis in a high-fat diet murine model treated with diethylnitrosamine (den). Med. Sci. Monit. 2018, 24, 8533–8543. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.X.; Zheng, R.L.; Zhou, T.A.; He, H.Y.; Liu, J.Y.; Zheng, Y.; Tong, A.P.; Xiang, M.L.; Song, X.R.; Yang, S.Y.; et al. Novel thienopyridine derivatives as specific anti-hepatocellular carcinoma (hcc) agents: Synthesis, preliminary structure-activity relationships, and in vitro biological evaluation. Bioorg. Med. Chem. Lett. 2010, 20, 6282–6285. [Google Scholar] [CrossRef]
- Zhou, R.; Huang, W.J.; Guo, Z.Y.; Li, L.; Zeng, X.R.; Deng, Y.Q.; Hu, F.Y.; Tong, A.P.; Yang, L.; Yang, J.L. Molecular mechanism of hepatocellular carcinoma-specific antitumor activity of the novel thienopyridine derivative tp58. Oncol. Rep. 2012, 28, 225–231. [Google Scholar]
- El-Miligy, M.M.; Rida, S.M.; Ashour, F.A.; Badr, M.H.; El-Bassiony, E.M.; El-Demellawy, M.A.; Omar, A.M. Dual inhibitors of hepatitis c virus and hepatocellular carcinoma: Design, synthesis and docking studies. Fut. Sci. OA 2018, 4, FSO252. [Google Scholar] [CrossRef]
- Dai, X.; Wang, L.; Deivasigamni, A.; Looi, C.Y.; Karthikeyan, C.; Trivedi, P.; Chinnathambi, A.; Alharbi, S.A.; Arfuso, F.; Dharmarajan, A.; et al. A novel benzimidazole derivative, mbic inhibits tumor growth and promotes apoptosis via activation of ros-dependent jnk signaling pathway in hepatocellular carcinoma. Oncotarget 2017, 8, 12831–12842. [Google Scholar] [CrossRef]
- Schulte, L.; Scheiner, B.; Voigtlander, T.; Koch, S.; Schweitzer, N.; Marhenke, S.; Ivanyi, P.; Manns, M.P.; Rodt, T.; Hinrichs, J.B.; et al. Treatment with metformin is associated with a prolonged survival in patients with hepatocellular carcinoma. Liver Int. 2019, 39, 714–726. [Google Scholar] [CrossRef]
- Seo, Y.S.; Kim, Y.J.; Kim, M.S.; Suh, K.S.; Kim, S.B.; Han, C.J.; Kim, Y.J.; Jang, W.I.; Kang, S.H.; Tchoe, H.J.; et al. Association of metformin use with cancer-specific mortality in hepatocellular carcinoma after curative resection: A nationwide population-based study. Medicine (Baltimore) 2016, 95, e3527. [Google Scholar] [CrossRef]
- Bhat, M.; Chaiteerakij, R.; Harmsen, W.S.; Schleck, C.D.; Yang, J.D.; Giama, N.H.; Therneau, T.M.; Gores, G.J.; Roberts, L.R. Metformin does not improve survival in patients with hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 15750–15755. [Google Scholar] [CrossRef]
- Chung, Y.K.; Hwang, S.; Song, G.W.; Lee, Y.J.; Kim, K.H.; Ahn, C.S.; Moon, D.B.; Ha, T.Y.; Jung, D.H.; Park, G.C.; et al. Absence of antitumor effects of metformin in sorafenib-treated patients with hepatocellular carcinoma recurrence after hepatic resection and liver transplantation. Ann. Hepatobiliary Pancreat. Surg. 2018, 22, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Casadei Gardini, A.; Faloppi, L.; De Matteis, S.; Foschi, F.G.; Silvestris, N.; Tovoli, F.; Palmieri, V.; Marisi, G.; Brunetti, O.; Vespasiani-Gentilucci, U.; et al. Metformin and insulin impact on clinical outcome in patients with advanced hepatocellular carcinoma receiving sorafenib: Validation study and biological rationale. Eur. J. Cancer 2017, 86, 106–114. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Tan, H.-Y.; Teng, S.; Chan, Y.-T.; Wang, D.; Wang, N. The Role of AMP-Activated Protein Kinase as a Potential Target of Treatment of Hepatocellular Carcinoma. Cancers 2019, 11, 647. https://doi.org/10.3390/cancers11050647
Jiang X, Tan H-Y, Teng S, Chan Y-T, Wang D, Wang N. The Role of AMP-Activated Protein Kinase as a Potential Target of Treatment of Hepatocellular Carcinoma. Cancers. 2019; 11(5):647. https://doi.org/10.3390/cancers11050647
Chicago/Turabian StyleJiang, Xue, Hor-Yue Tan, Shanshan Teng, Yau-Tuen Chan, Di Wang, and Ning Wang. 2019. "The Role of AMP-Activated Protein Kinase as a Potential Target of Treatment of Hepatocellular Carcinoma" Cancers 11, no. 5: 647. https://doi.org/10.3390/cancers11050647
APA StyleJiang, X., Tan, H.-Y., Teng, S., Chan, Y.-T., Wang, D., & Wang, N. (2019). The Role of AMP-Activated Protein Kinase as a Potential Target of Treatment of Hepatocellular Carcinoma. Cancers, 11(5), 647. https://doi.org/10.3390/cancers11050647



