Oxaliplatin-Based Chemotherapy in Patients with Metastatic Colorectal Cancer Aged at Least 75 Years: A Post-Hoc Subgroup Analysis of Three Phase II Trials
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Treatment Cycles and Dose Reductions
2.3. Efficacy
2.4. Safety
3. Discussion
4. Patients and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Gallego, R.; Sanchez, N.; Maurel, J. Chemotherapy for elderly patients with advanced colorectal carcinoma. Expert Rev. Anticancer Ther. 2006, 6, 795–800. [Google Scholar] [CrossRef]
- Taieb, J. How best to treat older patients with metastatic colorectal cancer? Lancet Gastroenterol.Hepatol. 2019, 6, 30076–30077. [Google Scholar] [CrossRef]
- Yancik, R.; Wesley, M.N.; Ries, L.A.; Havlik, R.J.; Long, S.; Edwards, B.K.; Yates, J.W. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: A population-based study. Cancer 1998, 82, 2123–2134. [Google Scholar] [CrossRef]
- Yancik, R.; Ries, L.A. Cancer in older persons. Magnitude of the problem—How do we apply what we know? Cancer 1994, 74 (Suppl. 7), 1995–2003. [Google Scholar] [CrossRef]
- Kemeny, M.M.; Peterson, B.L.; Kornblith, A.B.; Muss, H.B.; Wheeler, J.; Levine, E.; Bartlett, N.; Fleming, G.; Cohen, H.J. Barriers to clinical trial participation by older women with breast cancer. J. Clin. Oncol. 2003, 21, 2268–2275. [Google Scholar] [CrossRef]
- Papamichael, D.; Audisio, R.A.; Glimelius, B.; de Gramont, A.; Glynne-Jones, R.; Haller, D.; Köhne, C.H.; Rostoft, S.; Lemmens, V.; Mitry, E.; et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann. Oncol. 2015, 26, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Rosati, G.; Cordio, S.; Bordonaro, G.; Caputo, G.; Novello, G.; Reggiardo, G.; Manzione, L. Capecitabine in combination with oxaliplatin or irinotecan in elderly patients with advanced colorectal cancer: Results of a randomized phase II study. Ann. Oncol. 2010, 21, 781–786. [Google Scholar] [CrossRef]
- Goldberg, R.M.; Tabah-Fisch, I.; Bleiberg, H.; de Gramont, A.; Tournigand, C.; Andre, T.; Rothenberg, M.L.; Green, E.; Sargent, D.J. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J. Clin. Oncol. 2006, 24, 4085–4091. [Google Scholar] [CrossRef]
- Figer, A.; Perez-Staub, N.; Carola, E.; Tournigand, C.; Lledo, G.; Flesch, M.; Barcelo, R.; Cervantes, A.; André, T.; Colin, P.; et al. FOLFOX in patients aged between 76 and 80 years with metastatic colorectal cancer. An exploratory cohort of the OPTIMOX1 study. Cancer 2007, 110, 2666–2671. [Google Scholar] [CrossRef]
- McCleary, N.; Odejide, O.; Szymonifka, J.; Ryan, D.; Hezel, A.; Meyerhardt, J.A. Safety and effectiveness of oxaliplatin-based chemotherapy regimens in adults 75 years and older with colorectal cancer. Clin. Colorectal Cancer 2013, 12, 62–69. [Google Scholar] [CrossRef][Green Version]
- Kumar, R.; Jain, K.; Beeke, C.; Price, T.J.; Townsend, A.R.; Padbury, R.; Roder, D.; Young, G.P.; Richards, A.; Karapetis, C.S. A population-based study of metastatic colorectal cancer in individuals aged > 80 years. Cancer 2013, 119, 722–728. [Google Scholar] [CrossRef]
- Fukuchi, M.; Ishibashi, K.; Tajima, Y.; Okada, N.; Yokoyama, M.; Chika, N.; Hatano, S.; Matsuzawa, T.; Kunamoto, K.; Kumagai, Y.; et al. Oxaliplatin-based chemotherapy in patients aged 75 years or older with metastatic colorectal cancer. Anticancer Res. 2013, 33, 4627–4630. [Google Scholar]
- Munemoto, Y.; Kanda, M.; Ishibashi, K.; Hata, T.; Kobayashi, M.; Hasegawa, J.; Fukunaga, M.; Takagane, A.; Otsuji, T.; Miyake, Y.; et al. Capecitabine and oxaliplatin combined with bevacizumab are feasible for treating selected Japanese patients at least 75 years of age with metastatic colorectal cancer. BMC Cancer 2015, 15, 786. [Google Scholar] [CrossRef] [PubMed]
- Rosati, G.; Cordio, S.; Tucci, A.; Blanco, G.; Bordonaro, R.; Reggiardo, G.; Manzione, L. Phase II trial of oxaliplatin and tegafur/uracil and oral folinic acid for advanced or metastatic colorectal cancer in elderly patients. Oncology 2005, 69, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Rosati, G.; Avallone, A.; Aprile, G.; Butera, A.; Reggiardo, G.; Bilancia, D. XELOX and bevacizumab followed by single-agent bevacizumab as maintenance therapy as first-line treatment in elderly patients with advanced colorectal cancer: The boxe study. Cancer Chemother.Pharmacol. 2013, 71, 257–264. [Google Scholar] [CrossRef]
- Benavides, M.; Pericay, C.; Valladares-Ayerbes, M.; Gil-Calle, S.; Massutí, B.; Aparicio, J.; Dueñas, R.; González-Flores, E.; Carrato, A.; Marcuello, E.; et al. Oxaliplatin in combination with infusional 5-fluorouracil as first-line chemotherapy for elderly patients with metastatic colorectal cancer: A phase II study of the Spanish Cooperative Group for the Treatment of Digestive Tumors. Clin. Colorectal Cancer 2012, 11, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Berretta, M.; Aprile, G.; Nasti, G.; Urbani, M.; Bearz, A.; Lutrino, S.; Foltran, L.; Ferrari, L.; Talamini, R.; Fiorica, F.; et al. Oxaliplapin and capecitabine (XELOX) based chemotherapy in the treatment of metastatic colorectal cancer: The right choice in elderly patients. AntiCancer Agents Med. Chem. 2013, 13, 1344–1353. [Google Scholar] [CrossRef]
- Alam, M.; Gabriel, G.; Barton, M.; Eek, R. Discriminating factors in treatment decisions for chemotherapy in elderly patients with colorectal cancer. Cancer Forum 2008, 32. Available online: http://cancerforum.org.au/forum/2008/march/ discriminating-factors-in-treatment-decisions-for-chemotherapy-in-elderly-patients-with-colorectal-cancer (accessed on 30 March 2019).
- Foster, J.A.; Salinas, G.D.; Mansell, D.; Williamson, J.C.; Casebeer, L.L. How does older age influence oncologists’ cancer management? Oncologist 2010, 15, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Shayne, M.; Culakova, E.; Poniewierski, M.S.; Wolff, D.; Dale, D.C.; Crawford, J.; Lyman, G.H. Dose intensity and hematologic toxicity in older cancer patients receiving systemic chemotherapy. Cancer 2007, 110, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, M.; Pietrantonio, F.; Biondani, P.; de Braud, F. Adjuvant treatment of colorectal cancer in the elderly: Where do we come from and where are we going? J. Solid Tumors 2012, 2, 38–46. [Google Scholar] [CrossRef][Green Version]
- Reddy, N.; Yu, J.; Fakih, M.G. Toxicities and survival among octogenarians and nonagenarians with colorectal cancer treated with chemotherapy or concurrent chemoradiation therapy. Clin. Colorectal Cancer 2007, 6, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Seymour, M.T.; Thompson, L.C.; Wasan, H.S.; Middleton, G.; Brewster, A.E.; Shepherd, S.F.; O’Mahony, M.S.; Maughan, T.S.; Parmar, M.; Langley, R.E. FOCUS2 Investigators; National Cancer Research Institute Colorectal Cancer Clinical Studies Group. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): An open-label, randomised factorial trial. Lancet 2011, 377, 1749–1759. [Google Scholar] [CrossRef]
- Extermann, M.; Aapro, M.; Bernabei, R.; Cohen, H.J.; Droz, J.P.; Lichtman, S.; Mor, V.; Monfardini, S.; Repetto, L.; Sørbye, L.; et al. Task Force on CGA of the International Society of Geriatric Oncology. Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit. Rev. Oncol.Hematol. 2005, 55, 241–252. [Google Scholar] [CrossRef] [PubMed]
- CrosaraTeixeria, M.; Marques, D.F.; Ferrari, A.C.; Alves, M.F.; Alex, A.K.; Sabbaga, J.; Hoff, P.M.; Riechelmann, R.P. The effects of palliative chemotherapy in metastatic colorectal cancer patients with an ECOG performance status of 3 and 4. Clin. Colorectal Cancer 2015, 14, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Asmis, T.; Berry, S.; Cosby, R.; Chan, K.; Coburn, N.; Rother, M. Cancer Care Ontario’s Gastrointestinal Disease Site Group. Strategies of sequential therapies in unresectable metastatic colorectal cancer: A meta-analysis. Curr. Oncol. 2014, 21, 318–328. [Google Scholar] [CrossRef]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined with Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Landre, T.; Uzzan, B.; Nicolas, P.; Aparicio, T.; Zelek, L.; Mary, F.; Taleb, C.; Des Guetz, G. Doublet chemotherapy vs. single-agent therapy with 5FU in elderly patients with metastatic colorectal cancer: Ameta analysis. Int. J. Colorectal Dis. 2015, 30, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Lang, I.; Marcuello, E.; Lorusso, V.; Ocvirk, J.; Shin, D.B.; Jonker, D.; Osborne, S.; Andre, N.; Waterkamp, D.; et al. AVEX study investigators. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): An open-label, randomised phase 3 trial. Lancet Oncol. 2013, 14, 1077–1085. [Google Scholar] [CrossRef]
- Figueras, J.; Ramos, E.; López-Ben, S.; Torras, J.; Albiol, M.; Llado, L.; González, H.D.; Rafecas, A. Surgical treatment of liver metastases from colorectal carcinoma in elderly patients. When is it worthwhile? Clin. Transl. Oncol. 2007, 9, 392–400. [Google Scholar] [CrossRef] [PubMed]
- de Liguori Carino, N.; van Leeuwen, B.L.; Ghaneh, P.; Wu, A.; Audisio, R.A.; Poston, G.J. Liver resection for colorectal liver metastases in older patients. Crit. Rev. Oncol.Hematol. 2008, 67, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Rosati, G.; Aprile, G.; Poletto, E.; Avallone, A. An update on the management of metastatic colorectal cancer in the elderly. Colorectal Cancer 2014, 3, 451–463. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Simon, R. Optimal two stage design for phase II clinical trials. Control. Clin. Trials 1989, 10, 1–10. [Google Scholar] [CrossRef]
| Characteristics | Oxaliplatin Plus UFT/FA (n = 21) | Oxaliplatin Plus Capecitabine (n = 27) | Oxaliplatin/Capecitabine Plus Bevacizumab (n = 19) | All (n = 67) (%) |
|---|---|---|---|---|
| Median age (range) | 76 (75–89) | 78 (75–85) | 78 (75–83) | 77 (75–89) |
| Male/female | 12/9 | 15/12 | 11/8 | 38/29 (57/43) |
| ECOG PS 0/1 | 10/11 | 16/11 | 14/5 | 40/27 (60/40) |
| Colon/rectum | 13/8 | 16/11 | 14/5 | 43/24 (64/36) |
| Liver/lung/other sites | 11/4/6 | 14/7/6 | 14/3/2 | 39/14/14 (58/21/21) |
| Involved sites ≥ 1 | 11/10 | 11/16 | 11/8 | 33/34 (49/51) |
| Adjuvant therapy | 7 | 9 | 4 | 20 (30) |
| Comorbidity | 11 | 17 | 12 | 40 (60) |
| Characteristics | <75 Years | ≥75 Years | p Value |
|---|---|---|---|
| Diarrhea | 12 (17%) | 9 (13%) | 0.57 |
| Stomatitis | 2 (3%) | 2 (3%) | 0.95 |
| Nausea/vomiting | 3 (4%) | 2 (3%) | 0.70 |
| Peripheral neuropathy | 8 (11%) | 7 (10%) | 0.88 |
| Laryngeal spasm | 3 (4%) | 2 (3%) | 0.70 |
| Fatigue/asthenia | 8 (11%) | 9 (13%) | 0.70 |
| Fever/chills | 3 (4%) | 4 (6%) | 0.64 |
| Hyperbilirubinemia | 2 (3%) | 1 (1%) | 0.59 |
| Anemia | 3 (4%) | 3 (4%) | 0.94 |
| Hand-foot syndrome | 3 (4%) | 4 (6%) | 0.64 |
| Neutropenia | 4 (6%) | 5 (7%) | 0.66 |
| Thrombocytopenia | 4 (6%) | 3 (4%) | 0.76 |
| 20% dose reduction | 27 (38%) | 21 (31%) | 0.41 |
| Early discontinuation | 9 (13%) | 7 (10%) | 0.68 |
| ≥75 Years, n = 67 | <75 Years, n = 71 | p Value | |
|---|---|---|---|
| Response rate | 30 (45%) | 36 (51%) | 0.49 |
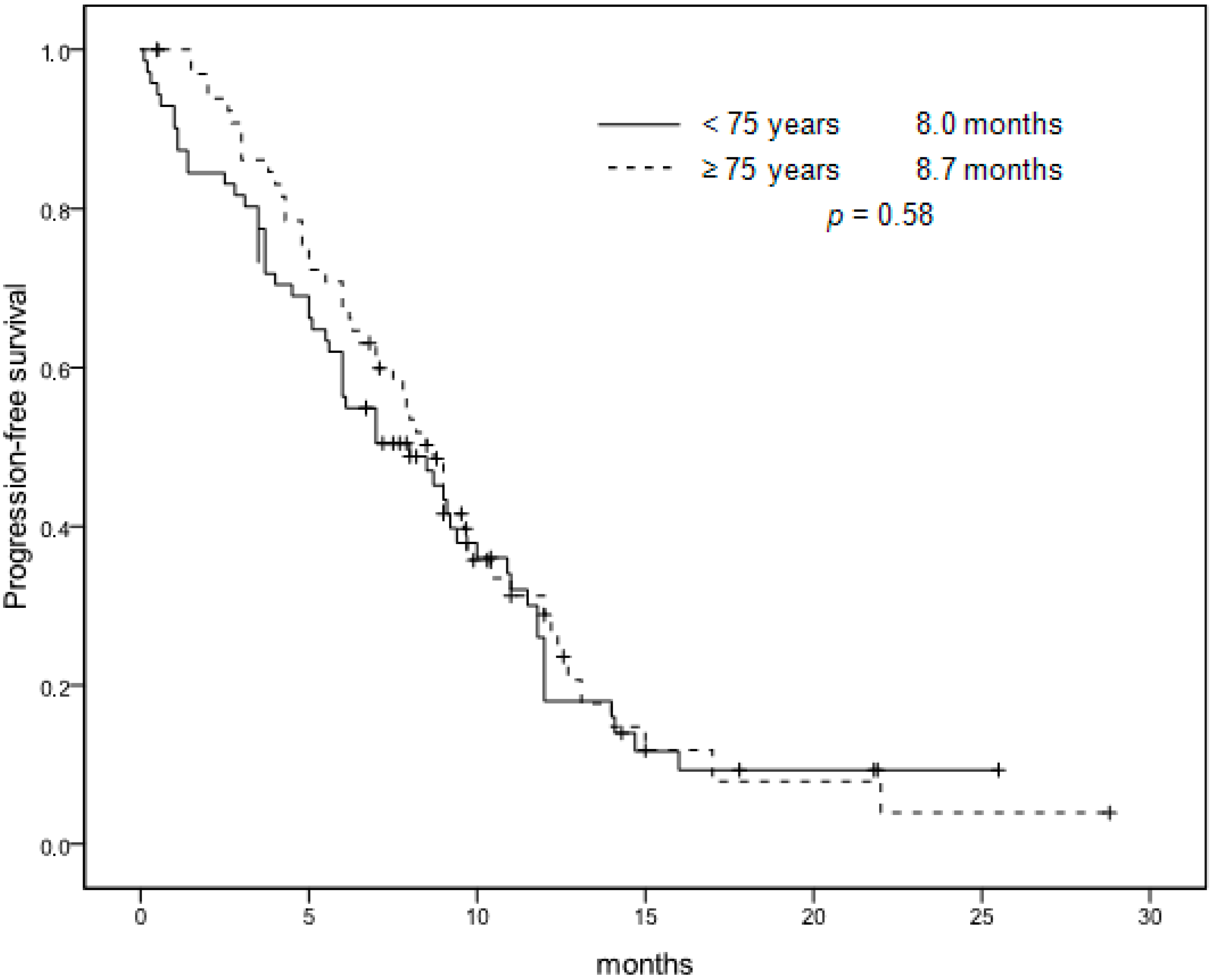
| Median PFS | 8.7 months | 8.0 months | 0.58 |
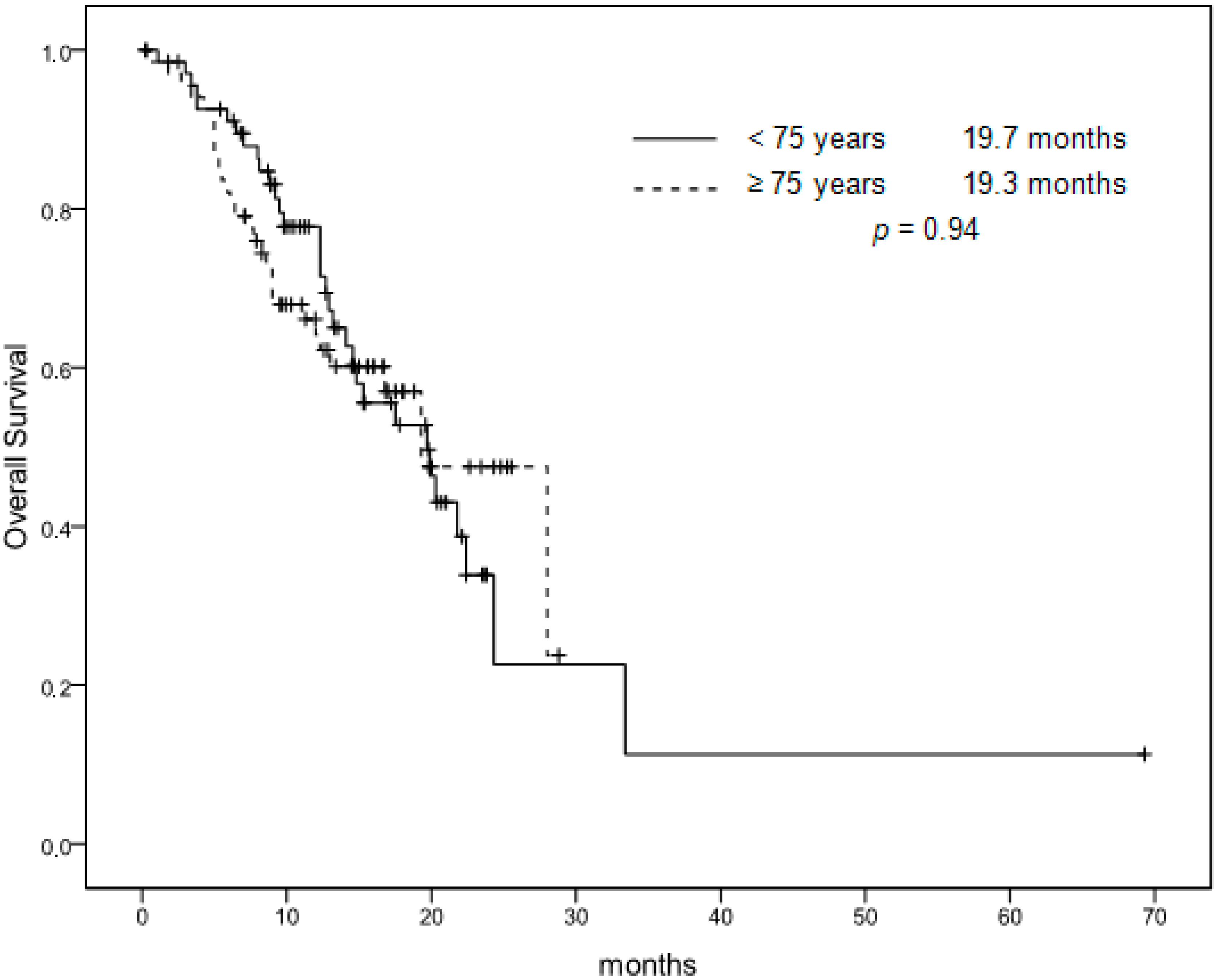
| Median OS | 19.3 months | 19.7 months | 0.94 |
| Additional therapeutic lines | 23 (34%) | 23 (32%) | 0.81 |
| Liver metastasectomy | 2 (3%) | 4 (6%) | 0.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosati, G.; Cordio, S.; Reggiardo, G.; Aprile, G.; Butera, A.; Avallone, A.; Tucci, A.; Novello, G.; Blanco, G.; Caputo, G.; et al. Oxaliplatin-Based Chemotherapy in Patients with Metastatic Colorectal Cancer Aged at Least 75 Years: A Post-Hoc Subgroup Analysis of Three Phase II Trials. Cancers 2019, 11, 578. https://doi.org/10.3390/cancers11040578
Rosati G, Cordio S, Reggiardo G, Aprile G, Butera A, Avallone A, Tucci A, Novello G, Blanco G, Caputo G, et al. Oxaliplatin-Based Chemotherapy in Patients with Metastatic Colorectal Cancer Aged at Least 75 Years: A Post-Hoc Subgroup Analysis of Three Phase II Trials. Cancers. 2019; 11(4):578. https://doi.org/10.3390/cancers11040578
Chicago/Turabian StyleRosati, Gerardo, Stefano Cordio, Giorgio Reggiardo, Giuseppe Aprile, Alfredo Butera, Antonio Avallone, Aniello Tucci, Giuseppe Novello, Giuseppina Blanco, Giuseppe Caputo, and et al. 2019. "Oxaliplatin-Based Chemotherapy in Patients with Metastatic Colorectal Cancer Aged at Least 75 Years: A Post-Hoc Subgroup Analysis of Three Phase II Trials" Cancers 11, no. 4: 578. https://doi.org/10.3390/cancers11040578
APA StyleRosati, G., Cordio, S., Reggiardo, G., Aprile, G., Butera, A., Avallone, A., Tucci, A., Novello, G., Blanco, G., Caputo, G., Bilancia, D., & Bordonaro, R. (2019). Oxaliplatin-Based Chemotherapy in Patients with Metastatic Colorectal Cancer Aged at Least 75 Years: A Post-Hoc Subgroup Analysis of Three Phase II Trials. Cancers, 11(4), 578. https://doi.org/10.3390/cancers11040578






