Regulation of Cell Signaling Pathways by Berberine in Different Cancers: Searching for Missing Pieces of an Incomplete Jig-Saw Puzzle for an Effective Cancer Therapy
Abstract
:1. Introduction
2. Berberine Mediated Restoration of TRAIL-Mediated Apoptosis
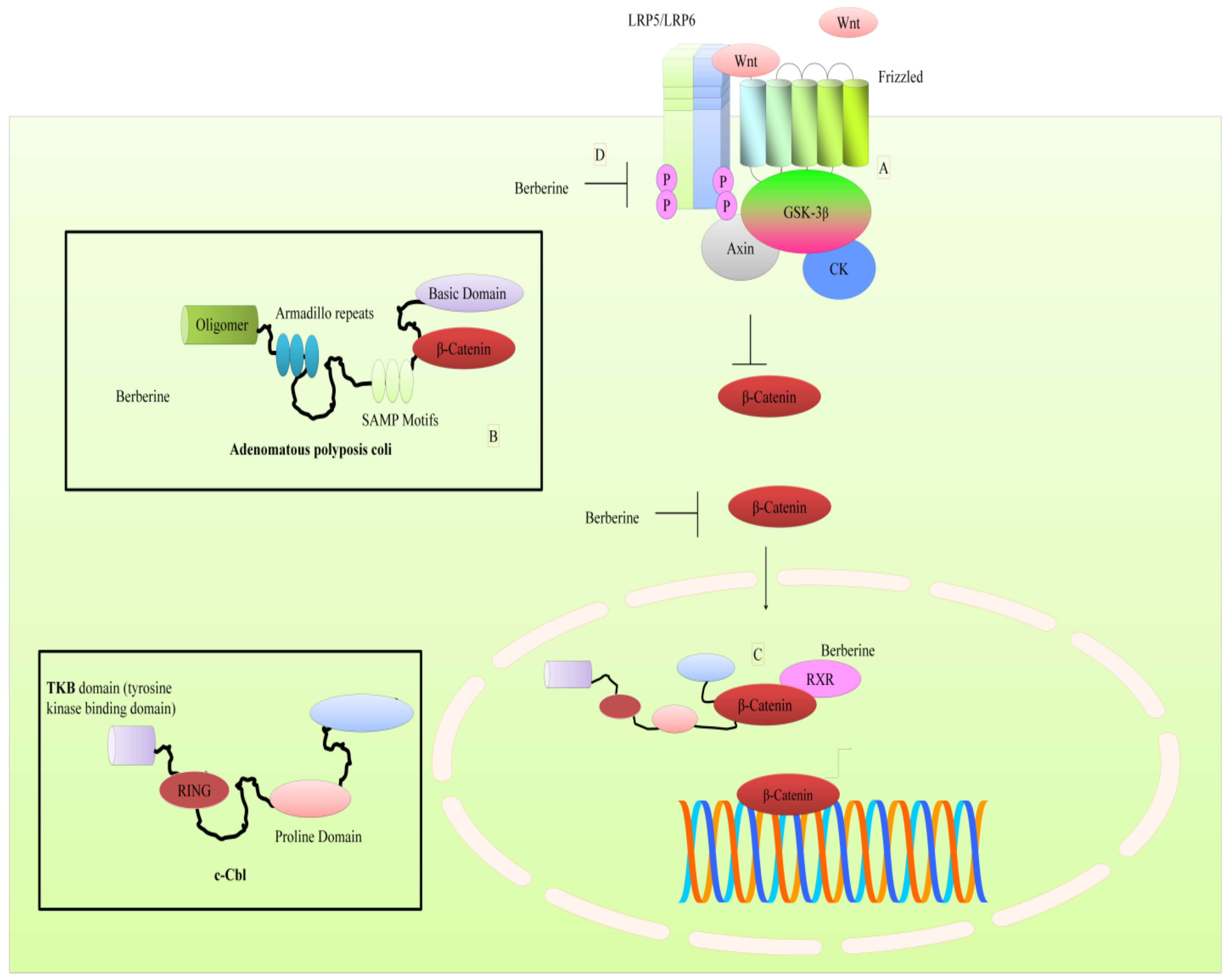
3. Regulation of WNT Pathway by Berberine
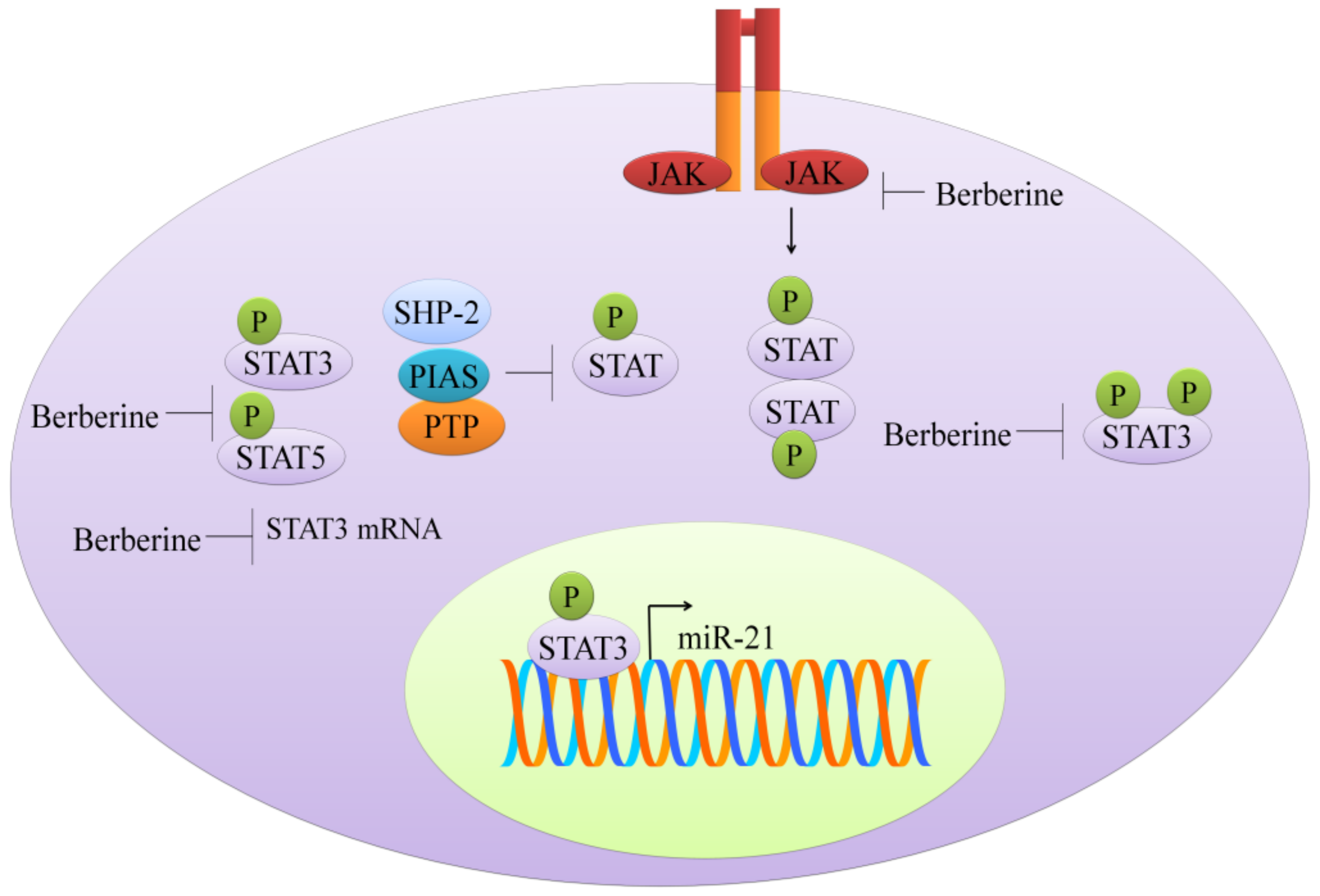
4. Targeting of JAK-STAT Pathway
5. Targeting of the mTOR Pathway: Could Berberine Modify Extracellular Vesicle-Composition?
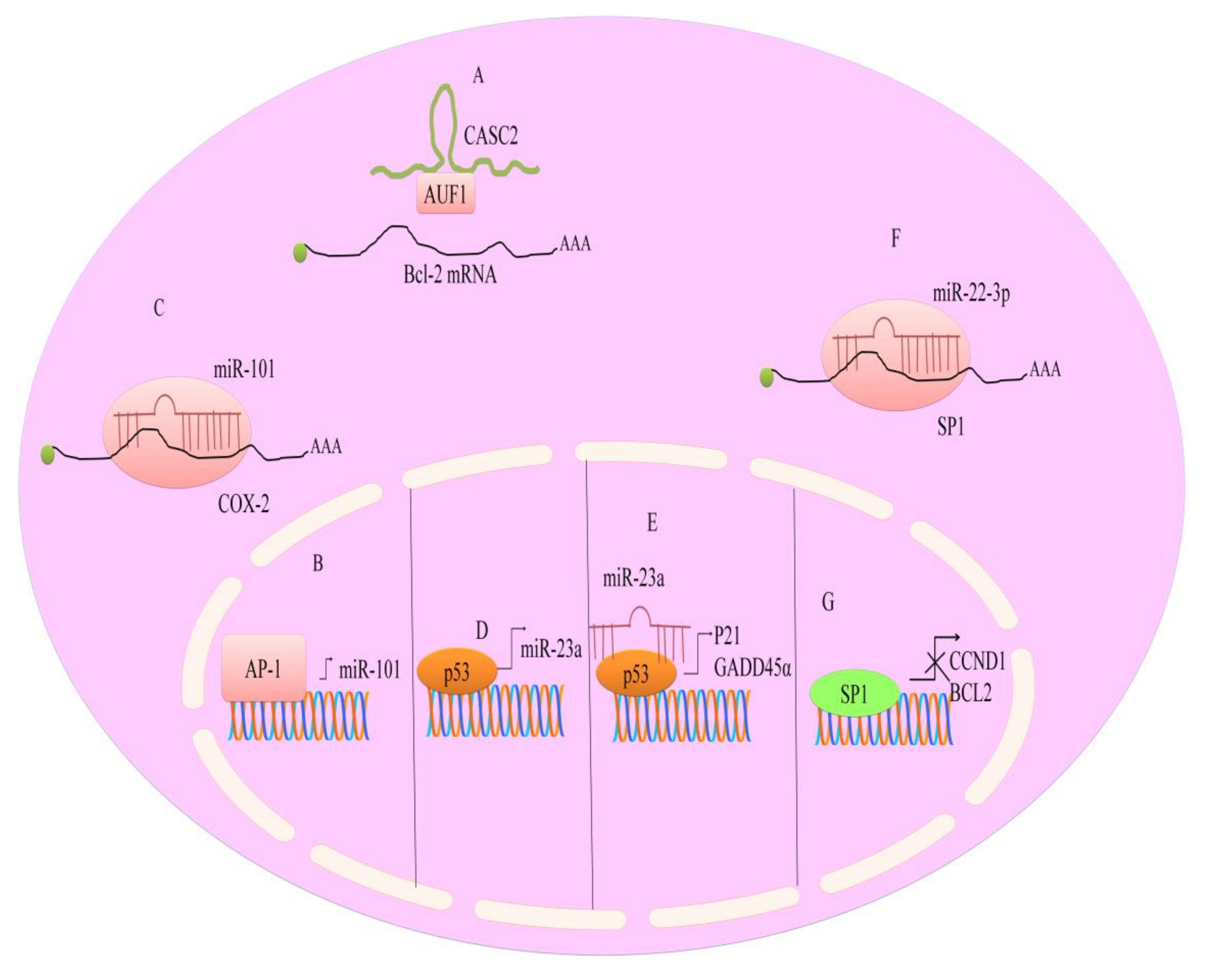
6. Regulation of Epigenetic Modulators by Berberine
7. Regulation of microRNAs by Berberine
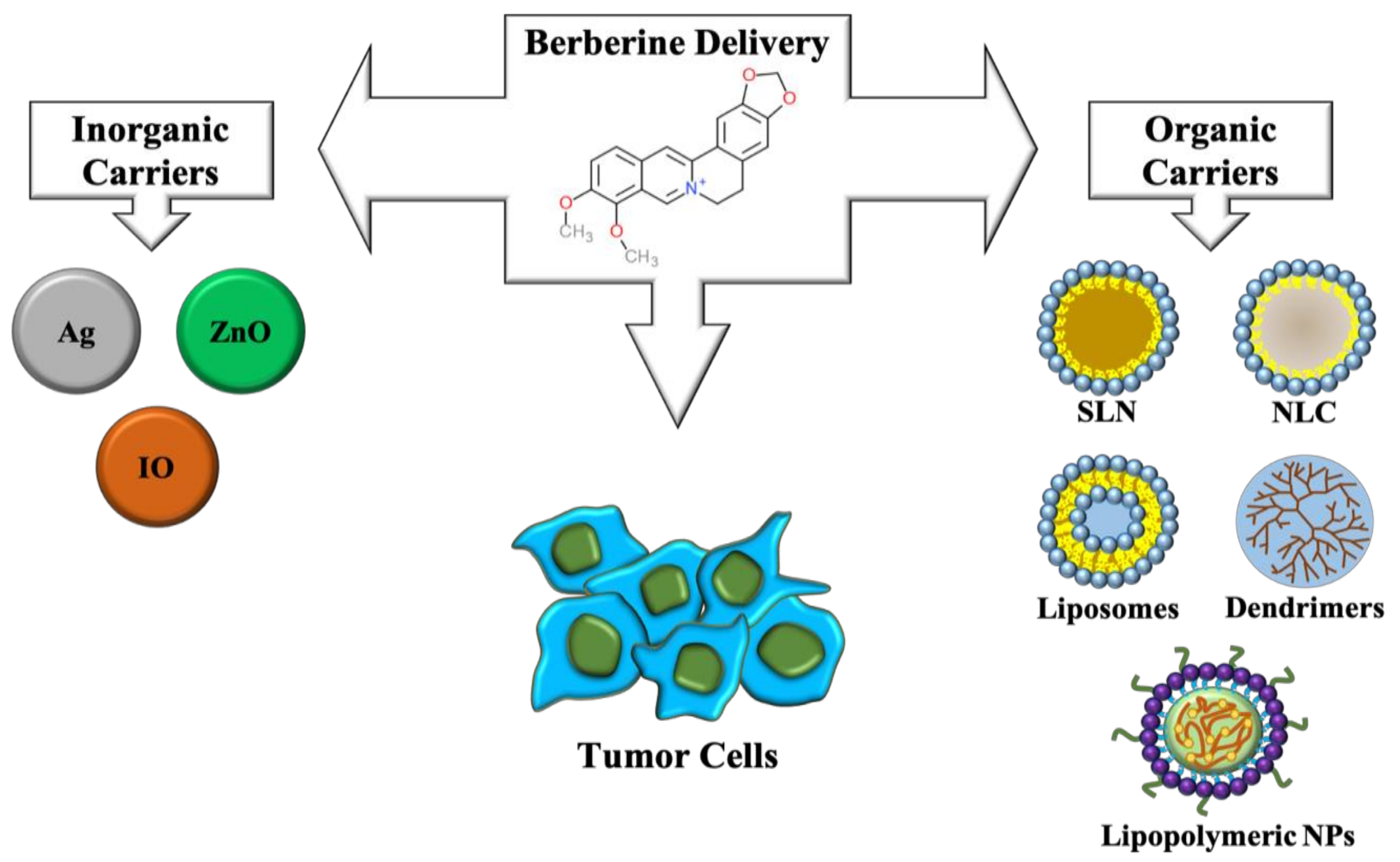
8. Nanotechnological Strategies to Improve the Delivery of Berberine
9. Conclusions
Funding
Conflicts of Interest
Abbreviations
| Breast cancer cells | MDA-MB-231, MCF-7, MDA-MB-468 |
| hepatocellular carcinoma cells | SNU-182, HepG2, Hep3B, H22 SMMC-7721 |
| gastric cancer cells | SGC7901, AGS |
| lung carcinoma | A549 |
| myeloma | U266 cell line |
| ovarian cancer cell line | SKOV3 |
| Akt | protein kinase B |
| AMPK | AMP-activated protein kinase |
| AQP-1 | aquaporin 1 |
| APC | adenomatous polyposis coli |
| Bcl-2 | B-cell lymphoma 2 |
| CCND1 | cyclin D1 |
| c-FLIP | cellular FADD like interdeukin-1-p-converting enzyme inhibitory protein |
| CK1 | casein kinase 1 |
| COX2 | cyclooxygenase-2 |
| CREB | cAMP response element-binding protein |
| DISC | death inducing signaling complex |
| DNMT1 | DNA (cytosine-5)-methyltransferase 1 |
| DR | death receptor |
| EGFR | epidermal growth factor receptor |
| ERK | extracellular signal-regulated kinases |
| EVs | extracellular vesicles |
| GADD45α | growth arrest and DNA damage 45α |
| GLUT1 | glucose transporter 1 |
| HIF-1α | hypoxia-inducible factor 1α |
| HK2 | hexokinase 2 |
| HUVECs | human umbilical vein endothelial cells |
| IO | iron oxide |
| JAK-STAT | Janus kinases-signal transducer and activator of transcription proteins |
| LDHA | lactate dehydrogenase A |
| lncRNAs | long noncoding RNA |
| LRP | low-density lipoprotein receptor-related protein |
| MAPK | mitogen-activated protein kinases |
| MARCKS | myristoylated alanine-rich C-kinase substrate |
| MMP | matrix metalloproteinases |
| mTOR | mammalian target of rapamycin |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLC | nanostructured lipid carriers |
| NP | nanoparticles |
| NPC | nasopharyngeal carcinoma |
| PI3K | phosphoinositide 3-kinases |
| RXRα | retinoid X receptor α |
| SIRT1 | sirtuin 1 |
| SLN | solid lipid nanoparticles |
| T2D | type 2 diabetes |
| TAFs | tumor-associated fibroblasts |
| TARID | TCF21 antisense RNA inducing demethylation |
| TNF | tumor necrosis factor |
| TRAIL | TNF-related apoptosis-inducing ligand |
| ULK1 | Unc-51 like autophagy activating kinase |
| VEGF | vascular endothelial growth factor |
| ZnO | zinc oxide. |
References
- De Miguel, D.; Lemke, J.; Anel, A.; Walczak, H.; Martinez-Lostao, L. Onto better TRAILs for cancer treatment. Cell Death Differ. 2016, 23, 733–747. [Google Scholar] [CrossRef]
- Lemke, J.; von Karstedt, S.; Zinngrebe, J.; Walczak, H. Getting TRAIL back on track for cancer therapy. Cell Death Differ. 2014, 21, 1350–1364. [Google Scholar] [CrossRef]
- Von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef]
- Stuckey, D.W.; Shah, K. TRAIL on trial: Preclinical advances in cancer therapy. Trends Mol. Med. 2013, 19, 685–694. [Google Scholar] [CrossRef]
- Ke, R.; Vishnoi, K.; Viswakarma, N.; Santha, S.; Das, S.; Rana, A.; Rana, B. Involvement of AMP-activated protein kinase and death receptor 5 in TRAIL-berberine-induced apoptosis of cancer cells. Sci. Rep. 2018, 8, 5521. [Google Scholar] [CrossRef] [PubMed]
- Refaat, A.; Abdelhamed, S.; Saiki, I.; Sakurai, H. Inhibition of p38 mitogen-activated protein kinase potentiates the apoptotic effect of berberine/tumor necrosis factor-related apoptosis-inducing ligand combination therapy. Oncol. Lett. 2015, 10, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Refaat, A.; Abdelhamed, S.; Yagita, H.; Inoue, H.; Yokoyama, S.; Hayakawa, Y.; Saiki, I. Berberine enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast cancer. Oncol. Lett. 2013, 6, 840–844. [Google Scholar] [CrossRef]
- Lee, S.J.; Noh, H.; Sung, E.G.; Song, I.H.; Kim, J.Y.; Kwon, T.K.; Lee, T.J. Berberine sensitizes TRAIL-induced apoptosis through proteasome-mediated downregulation of c-FLIP and Mcl-1 proteins. Int. J. Oncol. 2011, 38, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, H.; Zhang, B.; Cao, H.; Xu, X.; Ruan, H.; Yi, T.; Tan, L.; Qu, R.; Song, G.; et al. Berberine potently attenuates intestinal polyps growth in ApcMin mice and familial adenomatous polyposis patients through inhibition of Wnt signaling. J. Cell. Mol. Med. 2013, 17, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Ma, Y.; Yang, T.; Fan, M.; Yu, R.; Su, Q.; Wang, H.; Liu, F.; Yang, C.; Zhang, Y. Synergistic effect of berberine and HMQ1611 impairs cell proliferation and migration by regulating Wnt signaling pathway in hepatocellular carcinoma. Phytother. Res. 2019, 18. [Google Scholar] [CrossRef]
- Hu, Q.; Li, L.; Zou, X.; Xu, L.; Yi, P. Berberine attenuated proliferation, invasion and migration by targeting the AMPK/HNF4α/WNT5A pathway in gastric carcinoma. Front. Pharmacol. 2018, 9, 1150. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Zhang, W.; Wu, G.; Ren, J.; Lu, H.; Li, Z.; Han, X. Synergistic anti-cancer effects of galangin and berberine through apoptosis induction and proliferation inhibition in oesophageal carcinoma cells. Biomed. Pharmacother. 2016, 84, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yang, Q.; Mu, Y.; Zhou, L.; Liu, Y.; Zhou, Q.; He, B. Berberine inhibits the proliferation of colon cancer cells by inactivating Wnt/β-catenin signaling. Int. J. Oncol. 2012, 41, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Zhan, Y.Y.; Hou, J.; Xu, B.; Chen, B.; Tian, Y.; Wu, D.; Zhao, Y.; Zhang, Y.; Chen, X.; et al. Berberine binds RXRα to suppress β-catenin signaling in colon cancer cells. Oncogene 2017, 36, 6906–6918. [Google Scholar] [CrossRef] [PubMed]
- Puthdee, N.; Seubwai, W.; Vaeteewoottacharn, K.; Boonmars, T.; Cha’on, U.; Phoomak, C.; Wongkham, S. Berberine induces cell cycle arrest in cholangiocarcinoma cell lines via inhibition of NF-κB and STAT3 pathways. Biol. Pharm. Bull. 2017, 40, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.M.; Cheung, Y.C.; Lui, V.W.; Yip, Y.L.; Zhang, G.; Lin, V.W.; Cheung, K.C.; Feng, Y.; Tsao, S.W. Berberine suppresses tumorigenicity and growth of nasopharyngeal carcinoma cells by inhibiting STAT3 activation induced by tumor associated fibroblasts. BMC Cancer 2013, 13, 619. [Google Scholar] [CrossRef]
- Liu, X.; Ji, Q.; Ye, N.; Sui, H.; Zhou, L.; Zhu, H.; Fan, Z.; Cai, J.; Li, Q. Berberine inhibits invasion and metastasis of colorectal cancer cells via COX-2/PGE2 mediated JAK2/STAT3 signaling pathway. PLoS ONE 2015, 8, e0123478. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, T.; Zhao, H.J.; Sun, T.T.; Chen, H.M.; Chen, H.Y.; An, H.F.; Weng, Y.R.; Yu, J.; Li, M.; et al. Berberine may rescue Fusobacterium nucleatum-induced colorectal tumorigenesis by modulating the tumor microenvironment. Oncotarget 2015, 6, 32013–32026. [Google Scholar] [CrossRef]
- Zhu, T.; Li, L.L.; Xiao, G.F.; Luo, Q.Z.; Liu, Q.Z.; Yao, K.T.; Xiao, G.H. Berberine increases doxorubicin sensitivity by suppressing STAT3 in lung cancer. Am. J. Chin. Med. 2015, 43, 1487–1502. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Vishnoi, K.; Mahata, S.; Tripathi, S.C.; Misra, S.P.; Misra, V.; Mehrotra, R.; Dwivedi, M.; Bharti, A.C. Berberine and curcumin target Survivin and STAT3 in gastric cancer cells and synergize actions of standard chemotherapeutic 5-Fluorouracil. Nutr. Cancer 2015, 67, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism, and disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Conciatori, F.; Bazzichetto, C.; Falcone, I.; Pilotto, S.; Bria, E.; Cognetti, F.; Milella, M.; Ciuffreda, L. Role of mTOR signaling in tumor microenvironment: An overview. Int. J. Mol. Sci. 2018, 19, 2453. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T.; Hengstschlager, M.; Linke, M. Regulation of innate immune cell function by mTOR. Nat. Rev. Immunol. 2015, 15, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bajraszewski, N.; Wu, E.; Wang, H.; Moseman, A.P.; Dabora, S.L.; Griffin, J.D.; Kwiatkowski, D.J. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J. Clin. Investig. 2007, 117, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Liang, H.; Tian, D.; Du, J.; Wang, Q.; Xi, P.; Wang, H.; Li, Y. mTOR remains unchanged in diet-resistant (DR) rats despite impaired LKB1/AMPK cascade in adipose tissue. Biochem. Biophys. Res. Commun. 2016, 476, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qi, Q.; Feng, Z.; Zhang, X.; Huang, B.; Chen, A.; Prestegarden, L.; Li, X.; Wang, J. Berberine induces autophagy in glioblastoma by targeting the AMPK/mTOR/ULK1-pathway. Oncotarget 2016, 7, 66944–66958. [Google Scholar] [CrossRef] [PubMed]
- Knebel, B.; Lehr, S.; Hartwig, S.; Haas, J.; Kaber, G.; Dicken, H.D.; Susanto, F.; Bohne, L.; Jacob, S.; Nitzgen, U.; et al. Phosphorylation of sterol regulatory element-binding protein (SREBP)-1c by p38 kinases, ERK and JNK influences lipid metabolism and the secretome of human liver cell line HepG2. Arch. Physiol. Biochem. 2014, 120, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Saran, U.; Foti, M.; Dufour, J.F. Cellular and molecular effects of the mTOR inhibitor everolimus. Clin. Sci. (Lond.) 2015, 129, 895–914. [Google Scholar] [CrossRef]
- Bukowski, R.M. Temsirolimus: A safety and efficacy review. Expert Opin. Drug Saf. 2012, 11, 861–879. [Google Scholar] [CrossRef] [PubMed]
- Hambright, H.G.; Batth, I.S.; Xie, J.; Ghosh, R.; Kumar, A.P. Palmatine inhibits growth and invasion in prostate cancer cell: Potential role for rpS6/NFkappaB/FLIP. Mol. Carcinog. 2015, 54, 1227–1234. [Google Scholar] [CrossRef]
- Giovannini, L.; Bianchi, S. Role of nutraceutical SIRT1 modulators in AMPK and mTOR pathway: Evidence of a synergistic effect. Nutrition 2017, 34, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Jeong, H.; Lee, M.N.; Koh, A.; Kwon, O.; Yang, Y.R.; Noh, J.; Suh, P.-G.; Park, H.; Ryu, S.H. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci. Rep. 2016, 6, 21772. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hua, B.; Saud, S.M.; Lin, H.; Hou, W.; Matter, M.S.; Jia, L.; Colburn, N.H.; Young, M.R. Berberine regulates AMP-activated protein kinase signaling pathways and inhibits colon tumorigenesis in mice. Mol. Carcinog. 2015, 54, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Chen, Q.; Gong, K.; Xu, X.; Xie, Y.; Zhang, W.; Cao, H.; Hu, T.; Hong, X.; Zhan, Y.-Y. Berberine decelerates glucose metabolism via suppression of mTORdependent HIF1alpha protein synthesis in colon cancer cells. Oncol. Rep. 2018, 39, 2436–2442. [Google Scholar] [PubMed]
- Wang, N.; Feng, Y.; Zhu, M.; Tsang, C.M.; Man, K.; Tong, Y.; Tsao, S.-W. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: The cellular mechanism. J. Cell. Biochem. 2010, 111, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Paquette, M.; El-Houjeiri, L.; Pause, A. mTOR pathways in cancer and autophagy. Cancers 2018, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Kosaka, N.; Tominaga, N.; Yoshioka, Y.; Takeshita, F.; Takahashi, R.; Yoshida, M.; Tsuda, H.; Tamura, K.; Ochiya, T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal. 2014, 7, ra63. [Google Scholar] [CrossRef]
- Guo, N.; Yan, A.; Gao, X.; Chen, Y.; He, X.; Hu, Z.; Mi, M.; Tang, X.; Gou, X. Berberine sensitizes rapamycinmediated human hepatoma cell death in vitro. Mol. Med. Rep. 2014, 10, 3132–3138. [Google Scholar] [CrossRef]
- Meng, M.; Geng, S.; Du, Z.; Yao, J.; Zheng, Y.; Li, Z.; Zhang, Z.; Li, J.; Duan, Y.; Du, G. Berberine and cinnamaldehyde together prevent lung carcinogenesis. Oncotarget 2017, 8, 76385–76397. [Google Scholar] [CrossRef]
- Pucci, M.; Reclusa Asiain, P.; Durendez Saez, E.; Jantus-Lewintre, E.; Malarani, M.; Khan, S.; Fontana, S.; Naing, A.; Passiglia, F.; Raez, LE.; et al. Extracellular vesicles as miRNA nano-shuttles: Dual role in tumor progression. Target. Oncol. 2018, 13, 175–187. [Google Scholar] [CrossRef]
- Reclusa, P.; Taverna, S.; Pucci, M.; Durendez, E.; Calabuig, S.; Manca, P.; Serrano, M.J.; Sober, L.; Pauwels, P.; Russo, A.; et al. Exosomes as diagnostic and predictive biomarkers in lung cancer. J. Thorac. Dis. 2017, 9, S1373–S1382. [Google Scholar] [CrossRef] [PubMed]
- Ruivo, C.F.; Adem, B.; Silva, M.; Melo, S.A. The biology of cancer exosomes: Insights and new perspectives. Cancer Res. 2017, 77, 6480–6488. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Thery, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Endzelins, E.; Abols, A.; Buss, A.; Zandberga, E.; Palviainen, M.; Siljander, P.; Linē, A. Extracellular vesicles derived from hypoxic colorectal cancer cells confer metastatic phenotype to non-metastatic cancer cells. Anticancer Res. 2018, 38, 5139–5147. [Google Scholar] [CrossRef]
- Deep, G.; Panigrahi, G.K. Hypoxia-induced signaling promotes prostate cancer progression: Exosomes role as messenger of hypoxic response in tumor microenvironment. Crit. Rev. Oncog. 2015, 20, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, K.; Babashah, S.; Sadeghizadeh, M.; Mowla, S.J.; Mossahebi-Mohammadi, M.; Ataei, F.; Dana, N.; Javan, M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1alpha/VEGF signaling axis in breast cancer cells. Cell. Oncol. (Dordr.) 2017, 40, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Taverna, S.; Fontana, S.; Monteleone, F.; Pucci, M.; Saieva, L.; De Caro, V.; Cardinale, V.G.; Giallombardo, M.; Vicario, E.; Rolfo, C.; et al. Curcumin modulates chronic myelogenous leukemia exosomes composition and affects angiogenic phenotype via exosomal miR-21. Oncotarget 2016, 7, 30420–30439. [Google Scholar] [CrossRef] [PubMed]
- Taverna, S.; Giallombardo, M.; Pucci, M.; Flugy, A.; Manno, M.; Raccosta, S.; Rolfo, C.; De Leo, G.; Alessandro1, R. Curcumin inhibits in vitro and in vivo chronic myelogenous leukemia cells growth: A possible role for exosomal disposal of miR-21. Oncotarget 2015, 6, 21918–21933. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Mancuso, A.; Bui, T.V.; Tong, X.; Gruber, J.J.; Swider, C.R.; Sanchez, P.V.; Lum, J.J.; Sayed, N.; Melo, J.V.; et al. Imatinib resistance associated with BCR-ABL upregulation is dependent on HIF-1alpha-induced metabolic reprograming. Oncogene 2010, 29, 2962–2972. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, F.; Taverna, S.; Alessandro, R.; Fontana, S. SWATH-MS based quantitative proteomics analysis reveals that curcumin alters the metabolic enzyme profile of CML cells by affecting the activity of miR-22/IPO7/HIF-1alpha axis. J. Exp. Clin. Cancer Res. 2018, 37, 170. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Peng, L.; Pan, N.; Hu, X.; Zhang, Y. The anti-atherogenic effects of berberine on foam cell formation are mediated through the upregulation of sirtuin 1. Int. J. Mol. Med. 2014, 34, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Y.; Li, K.P.; Wang, X.Y.; Liu, Y.; Lu, Z.G.; Dong, R.H.; Guo, H.B.; Zhang, M.X. Set9, NF-κB, and microRNA-21 mediate berberine-induced apoptosis of human multiple myeloma cells. Acta Pharmacol. Sin. 2013, 34, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xiao, Y.; Yin, J.; Hou, W.; Yu, X.; Shen, L.; Liu, F.; Wei, L.; Jia, W. Berberine promotes glucose consumption independently of AMP-activated protein kinase activation. PLoS ONE 2014, 9, e103702. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, J.S.; Duarte, F.V.; Gomes, A.P.; Varela, A.T.; Peixoto, F.M.; Rolo, A.P.; Palmeira, C.M. Berberine reverts hepatic mitochondrial dysfunction in high-fat fed rats: A possible role for SirT3 activation. Mitochondrion 2013, 13, 637–646. [Google Scholar] [CrossRef]
- Arab, K.; Park, Y.J.; Lindroth, A.M.; Schäfer, A.; Oakes, C.; Weichenhan, D.; Lukanova, A.; Lundin, E.; Risch, A.; Meister, M.; et al. Long noncoding RNA TARID directs demethylation and activation of the tumor suppressor TCF21 via GADD45A. Mol. Cell 2014, 55, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gu, T.P.; Weber, A.R.; Shen, J.Z.; Li, B.Z.; Xie, Z.G.; Yin, R.; Guo, F.; Liu, X.; Tang, F.; et al. Gadd45a promotes DNA demethylation through TDG. Nucleic Acids Res. 2015, 43, 3986–3997. [Google Scholar] [CrossRef] [PubMed]
- Kienhöfer, S.; Musheev, M.U.; Stapf, U.; Helm, M.; Schomacher, L.; Niehrs, C.; Schäfer, A. GADD45a physically and functionally interacts with TET1. Differentiation 2015, 90, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Long, Q.; Wang, T.; Zhao, D.; Zhou, Y.; Qi, J.; Wu, Y.; Li, S.; Chen, C.; Zeng, X.; et al. Gadd45a is a heterochromatin relaxer that enhances iPS cell generation. EMBO Rep. 2016, 17, 1641–1656. [Google Scholar] [CrossRef] [PubMed]
- Ayati, S.H.; Fazeli, B.; Momtazi-Borojeni, A.A.; Cicero, A.F.G.; Pirro, M.; Sahebkar, A. Regulatory effects of berberine on microRNome in cancer and other conditions. Crit. Rev. Oncol. Hematol. 2017, 116, 147–158. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Xu, J.; Li, J.; Li, S.; Zhang, M.; Yang, D. Systematic identification of non-coding pharmacogenomic landscape in cancer. Nat. Commun. 2018, 9, 3192. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, J.; Tang, X.; Li, Y.; Xia, P.; Gao, X. Berberine ameliorates nonalcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J. Transl. Med. 2015, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Biersack, B. Non-coding RNA/microRNA-modulatory dietary factors and natural products for improved cancer therapy and prevention: Alkaloids, organosulfur compounds, aliphatic carboxylic acids and water-soluble vitamins. Non-Coding RNA Res. 2016, 1, 51–63. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Abrams, S.L.; Lertpiriyapong, K.; Cocco, L.; Ratti, S.; Martelli, A.M.; Candido, S.; Libra, M.; Murata, R.M.; Rosalen, P.L.; et al. Effects of berberine, curcumin, resveratrol alone and in combination with chemotherapeutic drugs and signal transduction inhibitors on cancer cells—Power of nutraceuticals. Adv. Biol. Regul. 2018, 67, 190–211. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Lertpiriyapong, K.; Steelman, L.S.; Abrams, S.L.; Yang, L.V.; Murata, R.M.; Rosalen, P.L.; Scalisi, A.; Neri, L.M.; Cocco, L.; et al. Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging (Albany, NY) 2017, 9, 1477–1536. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Noncoding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Luo, X.; Gu, C.; Li, Y.; Zhu, X.; Fei, J. Systematic analysis of berberine-induced signaling pathway between miRNA clusters and mRNAs and identification of mir-99a-125b cluster function by seed-targeting inhibitors in multiple myeloma cells. RNA Biol. 2015, 12, 82–91. [Google Scholar] [CrossRef]
- Chen, J.; Wu, F.X.; Luo, H.L.; Liu, J.J.; Luo, T.; Bai, T.; Li, L.Q.; Fan, X.H. Berberine upregulates miR-22-3p to suppress hepatocellular carcinoma cell proliferation by targeting Sp1. Am. J. Transl. Res. 2016, 8, 4932–4941. [Google Scholar]
- Luo, X.; Gu, J.; Zhu, R.; Feng, M.; Zhu, X.; Li, Y.; Fei, J. Integrative analysis of differential miRNA and functional study of miR-21 by seed-targeting inhibition in multiple myeloma cells in response to berberine. BMC Syst. Biol. 2014, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Wang, N.; Tan, H.Y.; Tsao, S.W.; Feng, Y. MicroRNAs and Chinese medicinal herbs: New possibilities in cancer therapy. Cancers 2015, 7, 1643–1657. [Google Scholar] [CrossRef] [PubMed]
- Tillhon, M.; Guamán Ortiz, L.M.; Lombardi, P.; Scovassi, A.I. Berberine: New perspectives for old remedies. Biochem. Pharmacol. 2012, 84, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.F.; Tsai, W.C.; Chen, S.T. MicroRNA-21-3p, a berberine-induced miRNA, directly down-regulates human methionine adenosyltransferases 2A and 2B and inhibits hepatoma cell growth. PLoS ONE 2013, 8, e75628. [Google Scholar] [CrossRef]
- Chen, Q.; Qin, R.; Fang, Y.; Li, H. Berberine sensitizes human ovarian cancer cells to cisplatin through miR-93/PTEN/Akt signaling pathway. Cell. Physiol. Biochem. 2015, 36, 956–965. [Google Scholar] [CrossRef] [PubMed]
- You, H.Y.; Xie, X.M.; Zhang, W.J.; Zhu, H.L.; Jiang, F.Z. Berberine modulates cisplatin sensitivity of human gastric cancer cells by upregulation of miR-203. In Vitro Cell Dev. Biol. Anim. 2016, 52, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Sadeghizadeh, M.; Behmanesh, M.; Najafi, F. Dendrosomal curcumin increases expression of the long non-coding RNA gene MEG3 via up-regulation of epi-miRs in hepatocellular cancer. Phytomedicine 2015, 22, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Chang, W. Non-coding RNAs and berberine: A new mechanism of its anti-diabetic activities. Eur. J. Pharmacol. 2016, 795, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhang, M.; Yu, Y.; Lan, X.; Yao, F.; Yan, X.; Chen, L.; Hatch, G.M. Berberine attenuates development of the hepatic gluconeogenesis and lipid metabolism disorder in type 2 diabetic mice and in palmitate-incubated HepG2 cells through suppression of the HNF-4alpha miR122 pathway. PLoS ONE 2016, 11, e0152097. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, A.; Kubina, R.; Bułdak, R.J.; Skonieczna, M.; Cholewa, K. Silver nanoparticles exhibit the dose-dependent anti-proliferative effect against human squamous carcinoma cells attenuated in the presence of berberine. Molecules 2016, 21, 365. [Google Scholar] [CrossRef] [PubMed]
- Bhanumathi, R.; Vimala, K.; Shanthi, K.; Thangaraj, R.; Kannan, S. Bioformulation of silver nanoparticles as berberine carrier cum anticancer agent against breast cancer. New J. Chem. 2017, 41, 14466–14477. [Google Scholar] [CrossRef]
- Bhanumathi, R.; Manivannan, M.; Thangaraj, R.; Kannan, S. Drug-carrying capacity and anticancer effect of the folic acid- and berberine-loaded silver nanomaterial to regulate the AKT-ERK pathway in breast cancer. ACS Omega 2018, 3, 8317–8328. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.Y.; Cho, H.J. Berberine and zinc oxide-based nanoparticles for the chemo-photothermal therapy of lung adenocarcinoma. Biochem. Biophys. Res. Commun. 2018, 501, 765–770. [Google Scholar] [CrossRef]
- Sreeja, S.; Krishnan Nair, C.K. Tumor control by hypoxia-specific chemotargeting of iron-oxide nanoparticle—Berberine complexes in a mouse model. Life Sci. 2018, 195, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, H.; Wang, S.; Liu, R.; Wu, Z.; Wang, C.; Wang, Y.; Chen, M. Enhancing the antitumor activity of berberine hydrochloride by solid lipid nanoparticle encapsulation. AAPS PharmSciTech 2014, 15, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.P.; Wu, J.B.; Chen, T.S.; Zhou, Q.; Wang, Y.F. In vitro and in vivo antitumor efficacy of berberine-nanostructured lipid carriers against H22 tumor. In Biophotonics and Immune Responses X; SIPE: Bellingham, WA, USA, 2015. [Google Scholar]
- Kabary, D.M.; Helmy, M.W.; Elkhodairy, K.A.; Fang, J.Y.; Elzoghby, A.O. Hyaluronate/lactoferrin layer-by-layer-coated lipid nanocarriers for targeted co-delivery of rapamycin and berberine to lung carcinoma. Colloids Surf. B Biointerfaces 2018, 169, 183–194. [Google Scholar] [CrossRef]
- Kabary, D.M.; Helmy, M.W.; Abdelfattah, E.Z.A.; Fang, J.Y.; Elkhodairy, K.A.; Elzoghby, A.O. Inhalable multi-compartmental phospholipid enveloped lipid core nanocomposites for localized mTOR inhibitor/herbal combined therapy of lung carcinoma. Eur. J. Pharm. Biopharm. 2018, 130, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Sharma, A.K.; Gothwal, A.; Khan, M.S.; Khinchi, M.P.; Qayum, A.; Singh, S.K.; Gupta, U. Dendrimer encapsulated and conjugated delivery of berberine: A novel approach mitigating toxicity and improving in vivo pharmacokinetics. Int. J. Pharm. 2017, 528, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Kim, J.J.; Yao, M.; Elbayoumi, T.A. Development and evaluation of vitamin E D-α-tocopheryl polyethylene glycol 1000 succinate-mixed polymeric phospholipid micelles of berberine as an anticancer nanopharmaceutical. Int. J. Nanomed. 2016, 11, 1687–1700. [Google Scholar]
- Wang, Y.; Wen, B.; Yu, H.; Ding, D.; Zhang, J.J.; Zhang, Y.; Zhao, L.; Zhang, W. Berberine hydrochloride-loaded chitosan nanoparticles effectively targets and suppresses human nasopharyngeal carcinoma. J. Biomed. Nanotechnol. 2018, 14, 1486–1495. [Google Scholar] [CrossRef]
- Agnarelli, A.; Natali, M.; Garcia-Gil, M.; Pesi, R.; Tozzi, M.G.; Ippolito, C.; Bernardini, N.; Vignali, R.; Batistoni, R.; Bianucci, A.M.; et al. Cell-specific pattern of berberine pleiotropic effects on different human cell lines. Sci. Rep. 2018, 8, 10599. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Ahmadi, Z.; Tavakol, S.; Ashrafizadeh, M. Berberine as a potential autophagy modulator. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef]
- Bianchi, S.; Giovannini, L. Inhibition of mTOR/S6K1/4E-BP1 signaling by nutraceutical SIRT1 modulators. Nutr. Cancer 2018, 70, 490–501. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooqi, A.A.; Qureshi, M.Z.; Khalid, S.; Attar, R.; Martinelli, C.; Sabitaliyevich, U.Y.; Nurmurzayevich, S.B.; Taverna, S.; Poltronieri, P.; Xu, B. Regulation of Cell Signaling Pathways by Berberine in Different Cancers: Searching for Missing Pieces of an Incomplete Jig-Saw Puzzle for an Effective Cancer Therapy. Cancers 2019, 11, 478. https://doi.org/10.3390/cancers11040478
Farooqi AA, Qureshi MZ, Khalid S, Attar R, Martinelli C, Sabitaliyevich UY, Nurmurzayevich SB, Taverna S, Poltronieri P, Xu B. Regulation of Cell Signaling Pathways by Berberine in Different Cancers: Searching for Missing Pieces of an Incomplete Jig-Saw Puzzle for an Effective Cancer Therapy. Cancers. 2019; 11(4):478. https://doi.org/10.3390/cancers11040478
Chicago/Turabian StyleFarooqi, Ammad Ahmad, Muhammad Zahid Qureshi, Sumbul Khalid, Rukset Attar, Chiara Martinelli, Uteuliyev Yerzhan Sabitaliyevich, Sadykov Bolat Nurmurzayevich, Simona Taverna, Palmiro Poltronieri, and Baojun Xu. 2019. "Regulation of Cell Signaling Pathways by Berberine in Different Cancers: Searching for Missing Pieces of an Incomplete Jig-Saw Puzzle for an Effective Cancer Therapy" Cancers 11, no. 4: 478. https://doi.org/10.3390/cancers11040478
APA StyleFarooqi, A. A., Qureshi, M. Z., Khalid, S., Attar, R., Martinelli, C., Sabitaliyevich, U. Y., Nurmurzayevich, S. B., Taverna, S., Poltronieri, P., & Xu, B. (2019). Regulation of Cell Signaling Pathways by Berberine in Different Cancers: Searching for Missing Pieces of an Incomplete Jig-Saw Puzzle for an Effective Cancer Therapy. Cancers, 11(4), 478. https://doi.org/10.3390/cancers11040478






