Clinical Management of Blood–Brain Barrier Disruptions after Active Raster-Scanned Carbon Ion Re-Radiotherapy in Patients with Recurrent Head-and-Neck Cancer
Abstract
1. Introduction
2. Results
2.1. Tumor Features
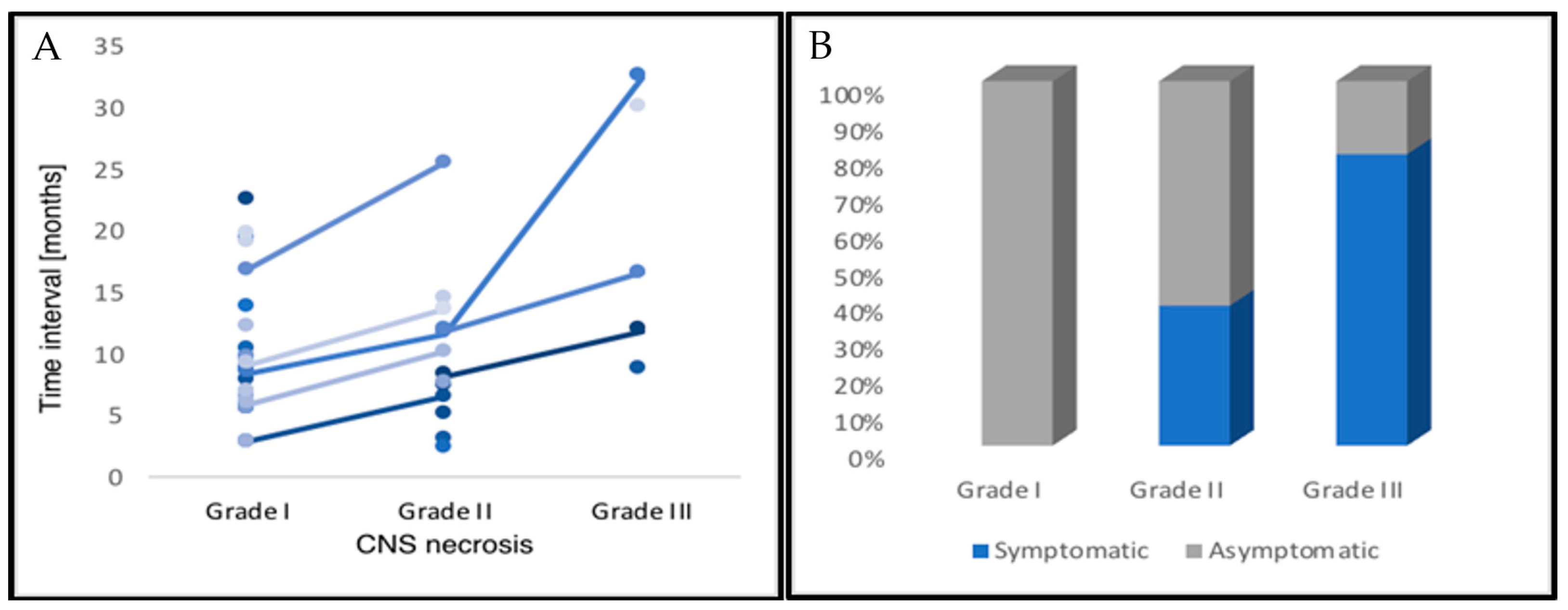
2.2. CNS Necrosis
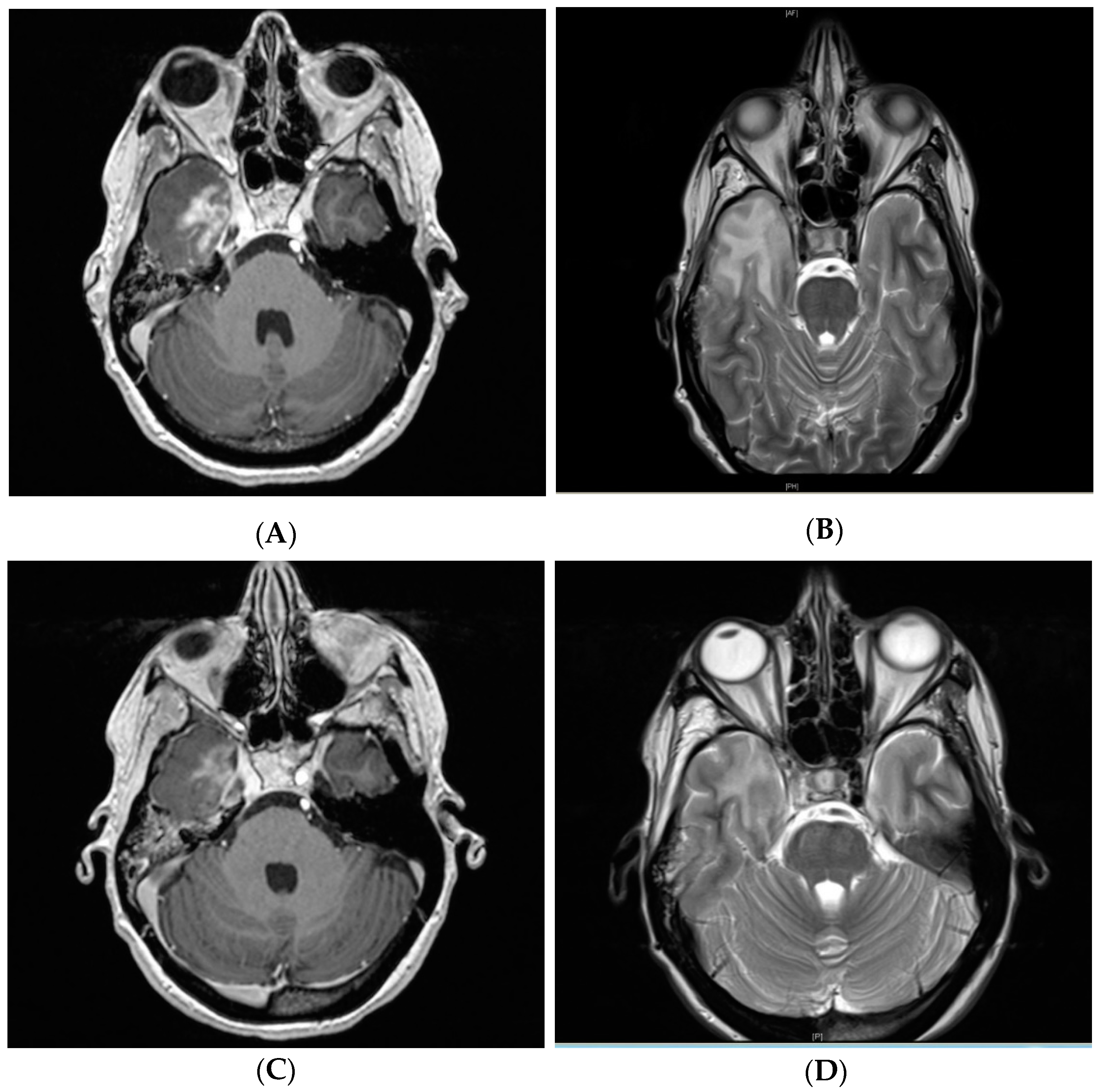
2.3. Clinical Management and Radiographic Response
3. Discussion
4. Materials and Methods
4.1. Patient Selection
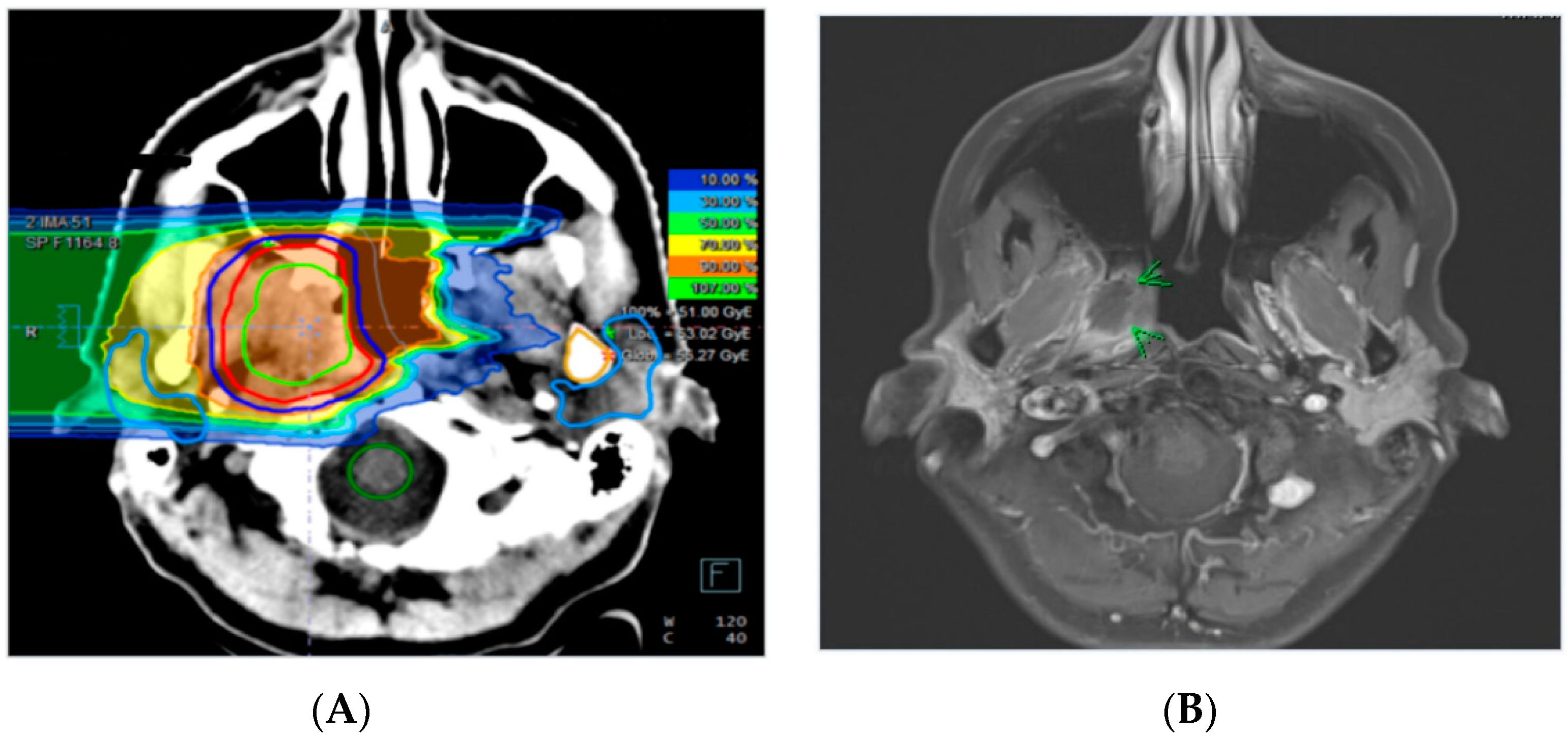
4.2. Treatment Planning and Radiotherapy
4.3. Radiographic and Clinical Evaluation
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giglio, P.; Gilbert, M.R. Cerebral radiation necrosis. Neurologist 2003, 9, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Bao, C.; Gao, J.; Guan, X.; Hu, W.; Yang, J.; Hu, C.; Kong, L.; Lu, J.J. Salvage treatment using carbon ion radiation in patients with locoregionally recurrent nasopharyngeal carcinoma: Initial results. Cancer 2018, 124, 2427–2437. [Google Scholar] [CrossRef]
- Belka, C.; Budach, W.; Kortmann, R.D.; Bamberg, M. Radiation induced cns toxicity – molecular and cellular mechanisms. Br. J. Cancer 2001, 85, 1233. [Google Scholar] [CrossRef] [PubMed]
- Yancopoulos, G.D.; Davis, S.; Gale, N.W.; Rudge, J.S.; Wiegand, S.J.; Holash, J. Vascular-specific growth factors and blood vessel formation. Nature 2000, 407, 242–248. [Google Scholar] [CrossRef]
- Cheung, M.C.; Chan, A.S.; Law, S.C.; Chan, J.H.; Tse, V.K. Impact of radionecrosis on cognitive dysfunction in patients after radiotherapy for nasopharyngeal carcinoma. Cancer 2003, 97, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.R.; McHugh, B.J.; Bi, W.L.; Minja, F.J.; Knisely, J.P.; Chiang, V.L. A comprehensive review of mr imaging changes following radiosurgery to 500 brain metastases. Ajnr. Am. J. Neuroradiol. 2011, 32, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Karger, C.P.; Peschke, P. Rbe and related modeling in carbon-ion therapy. Phys. Med. Biol. 2017, 63, 01tr02. [Google Scholar] [CrossRef] [PubMed]
- Ruben, J.D.; Dally, M.; Bailey, M.; Smith, R.; McLean, C.A.; Fedele, P. Cerebral radiation necrosis: Incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 499–508. [Google Scholar] [CrossRef]
- Rahmathulla, G.; Marko, N.F.; Weil, R.J. Cerebral radiation necrosis: A review of the pathobiology, diagnosis and management considerations. J. Clin. Neurosci. 2013, 20, 485–502. [Google Scholar] [CrossRef]
- Lam, T.-C.; Wong, F.C.S.; Leung, T.-W.; Ng, S.H.; Tung, S.Y. Clinical outcomes of 174 nasopharyngeal carcinoma patients with radiation-induced temporal lobe necrosis. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e57–e65. [Google Scholar] [CrossRef]
- Gonzalez, J.; Kumar, A.J.; Conrad, C.A.; Levin, V.A. Effect of bevacizumab on radiation necrosis of the brain. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Levin, V.A.; Bidaut, L.; Hou, P.; Kumar, A.J.; Wefel, J.S.; Bekele, B.N.; Prabhu, S.; Loghin, M.; Gilbert, M.R.; Jackson, E.F. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1487–1495. [Google Scholar] [CrossRef]
- Xu, Y.; Rong, X.; Hu, W.; Huang, X.; Li, Y.; Zheng, D.; Cai, Z.; Zuo, Z.; Tang, Y. Bevacizumab monotherapy reduces radiation-induced brain necrosis in nasopharyngeal carcinoma patients: A randomized controlled trial. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1087–1095. [Google Scholar] [CrossRef]
- Furuse, M.; Kawabata, S.; Kuroiwa, T.; Miyatake, S.-I. Repeated treatments with bevacizumab for recurrent radiation necrosis in patients with malignant brain tumors: A report of 2 cases. J. Neuro-Oncol. 2011, 102, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.T.; Huberman, M.; Lu, X.-Q.; Mahadevan, A. Bevacizumab reverses cerebral radiation necrosis. J. Clin. Oncol. 2008, 26, 5649–5650. [Google Scholar] [CrossRef]
- Yonezawa, S.; Miwa, K.; Shinoda, J.; Nomura, Y.; Asano, Y.; Nakayama, N.; Ohe, N.; Yano, H.; Iwama, T. Bevacizumab treatment leads to observable morphological and metabolic changes in brain radiation necrosis. J. Neuro-Oncol. 2014, 119, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.B.; Yorke, E.D.; Jackson, A.; Ten Haken, R.K.; Constine, L.S.; Eisbruch, A.; Bentzen, S.M.; Nam, J.; Deasy, J.O. Use of normal tissue complication probability models in the clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S10–S19. [Google Scholar] [CrossRef]
- Eyster, E.F.; Surl, L.N.; Glenn, E.S.; Charles, B.W. Cerebral radiation necrosis simulating a brain tumor. J. Neurosurg. 1974, 40, 267–271. [Google Scholar] [CrossRef]
- Kumar, A.J.; Leeds, N.E.; Fuller, G.N.; Van Tassel, P.; Maor, M.H.; Sawaya, R.E.; Levin, V.A. Malignant gliomas: Mr imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 2000, 217, 377–384. [Google Scholar] [CrossRef]
- Ricci, P.E.; Karis, J.P.; Heiserman, J.E.; Fram, E.K.; Bice, A.N.; Drayer, B.P. Differentiating recurrent tumor from radiation necrosis: Time for re-evaluation of positron emission tomography? Ajnr. Am. J. Neuroradiol. 1998, 19, 407–413. [Google Scholar]
- Prager, A.; Boothe, D.; Young, R.; Chan, T.; Yamada, Y.; Beal, K. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro-Oncology 2013, 15, 1257–1263. [Google Scholar]
- Dawson, L.A.; Anzai, Y.; Marsh, L.; Martel, M.K.; Paulino, A.; Ship, J.A.; Eisbruch, A. Patterns of local-regional recurrence following parotid-sparing conformal and segmental intensity-modulated radiotherapy for head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 1117–1126. [Google Scholar] [CrossRef]
| CNS Necrosis | Patients | % |
|---|---|---|
| Classification | ||
| Grade IV | 1 | 2.8 |
| Grade III | 5 | 13.9 |
| Grade II | 13 | 36.1 |
| Grade I | 17 | 47.2 |
| Localization | ||
| Temporal lobe | 30 | 83.4 |
| Frontal lobe | 4 | 11.1 |
| Other | 2 | 5.5 |
| Radiographic Response * | ||
| Grade IV | n.a. | n.a. |
| Grade III | 4 | 80.0 |
| Grade II | 5 | 29.4 |
| Grade I | 4 | 16.0 |
| Median | Range | |
| Time Interval | ||
| Grade IV | n.a. | n.a. |
| Grade III | 16.6 | 8.7–32.5 |
| Grade II | 10.2 | 2.3–60.5 |
| Grade I | 9.2 | 2.8–75.0 |
| Patient Characteristics | Patients | % |
|---|---|---|
| Female/Male | 19/17 | 52.8/47.2 |
| ECOG status | ||
| 0 | 25 | 69.4 |
| 1 | 11 | 30.6 |
| Histology | ||
| ACC | 24 | 66.7 |
| Adenocarcinoma | 4 | 11.1 |
| Acinar cell carcinoma | 3 | 8.3 |
| HNSCC | 2 | 5.6 |
| Other | 3 | 8.3 |
| Tumor Site | ||
| Skull base | 17 | 47.2 |
| Orbit | 5 | 13.9 |
| Temporal bone | 4 | 11.1 |
| Cavernous sinus | 3 | 8.3 |
| Nasopharynx | 3 | 8.3 |
| Other | 4 | 11.1 |
| Treatment characteristics | Median | Range |
| Initial RT (EQD2) | 70 | 50–81 |
| Carbon ion re-irradiation (Gy (RBE)) | 51.0 | 39.0–60.0 |
| PTV re-irradiation (ccm) | 85.3 | 13.3–286.5 |
| Cumulative Dose (EQD2) | 133.9 | 112.5–152.3 |
| RT-Interval (years) | 4.1 | 0.3–46.5 |
| Prior tumor-specific treatments | 2 | 1–7 |
| Radiotherapy of Head-and-Neck or Brain Cancer | |||
|---|---|---|---|
| Target volume? | Total dose? | Dose distribution? | Systemic therapy? |
| Regular clinical and radiographic follow-up | |||
| No symptoms (Grade I) | Moderate (Grade II) | ||
| Observation | Corticosteroids | ||
| Radiographic or clinical progression | |||
| Severe (Grade III) | Life-threatening (Grade IV) | ||
| Bevacizumab | Surgery | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Held, T.; Akbaba, S.; Lang, K.; Harrabi, S.; Bernhardt, D.; Freudlsperger, C.; Kargus, S.; Plinkert, P.; Rieken, S.; Herfarth, K.; et al. Clinical Management of Blood–Brain Barrier Disruptions after Active Raster-Scanned Carbon Ion Re-Radiotherapy in Patients with Recurrent Head-and-Neck Cancer. Cancers 2019, 11, 383. https://doi.org/10.3390/cancers11030383
Held T, Akbaba S, Lang K, Harrabi S, Bernhardt D, Freudlsperger C, Kargus S, Plinkert P, Rieken S, Herfarth K, et al. Clinical Management of Blood–Brain Barrier Disruptions after Active Raster-Scanned Carbon Ion Re-Radiotherapy in Patients with Recurrent Head-and-Neck Cancer. Cancers. 2019; 11(3):383. https://doi.org/10.3390/cancers11030383
Chicago/Turabian StyleHeld, Thomas, Sati Akbaba, Kristin Lang, Semi Harrabi, Denise Bernhardt, Christian Freudlsperger, Steffen Kargus, Peter Plinkert, Stefan Rieken, Klaus Herfarth, and et al. 2019. "Clinical Management of Blood–Brain Barrier Disruptions after Active Raster-Scanned Carbon Ion Re-Radiotherapy in Patients with Recurrent Head-and-Neck Cancer" Cancers 11, no. 3: 383. https://doi.org/10.3390/cancers11030383
APA StyleHeld, T., Akbaba, S., Lang, K., Harrabi, S., Bernhardt, D., Freudlsperger, C., Kargus, S., Plinkert, P., Rieken, S., Herfarth, K., Debus, J., & Adeberg, S. (2019). Clinical Management of Blood–Brain Barrier Disruptions after Active Raster-Scanned Carbon Ion Re-Radiotherapy in Patients with Recurrent Head-and-Neck Cancer. Cancers, 11(3), 383. https://doi.org/10.3390/cancers11030383






