Abstract
Before anoctamins (TMEM16 proteins) were identified as a family of Ca2+-activated chloride channels and phospholipid scramblases, the founding member anoctamin 1 (ANO1, TMEM16A) was known as DOG1, a marker protein for gastrointestinal stromal tumors (GIST). Meanwhile, ANO1 has been examined in more detail, and the role of ANO1 in cell proliferation and the development of different types of malignomas is now well established. While ANO5, ANO7, and ANO9 may also be relevant for growth of cancers, evidence has been provided for a role of ANO6 (TMEM16F) in regulated cell death. The cellular mechanisms by which anoctamins control cell proliferation and cell death, respectively, are just emerging; however, the pronounced effects of anoctamins on intracellular Ca2+ levels are likely to play a significant role. Recent results suggest that some anoctamins control membrane exocytosis by setting Ca2+i levels near the plasma membrane, and/or by controlling the intracellular Cl− concentration. Exocytosis and increased membrane trafficking induced by ANO1 and ANO6 may enhance membrane expression of other chloride channels, such as CFTR and volume activated chloride channels (VRAC). Notably, ANO6-induced phospholipid scrambling with exposure of phosphatidylserine is pivotal for the sheddase function of disintegrin and metalloproteinase (ADAM). This may support cell death and tumorigenic activity of IL-6 by inducing IL-6 trans-signaling. The reported anticancer effects of the anthelminthic drug niclosamide are probably related to the potent inhibitory effect on ANO1, apart from inducing cell cycle arrest through the Let-7d/CDC34 axis. On the contrary, pronounced activation of ANO6 due to a large increase in intracellular calcium, activation of phospholipase A2 or lipid peroxidation, can lead to ferroptotic death of cancer cells. It therefore appears reasonable to search for both inhibitors and potent activators of TMEM16 in order to interfere with cancer growth and metastasis.
Keywords:
anoctamin; ANO1; ANO6; TMEM16A; TMEM16F; cancer; proliferation; apoptosis; Ca2+ signaling; inflammation 1. Introduction
Cl− currents activated by an increase in intracellular Ca2+ (CaCC) have been known for more than 40 years. The human homologue of Drosophila tweety and the bestrophin family of channels were shown to operate as Ca2+ activated Cl− channels (reviewed in [1,2,3]). However, they behave differently from the “classical” receptor-operated CaCC, identified 11 years ago as anoctamin 1 (ANO1; TMEM16A) [4,5,6]. ANO1 is particularly expressed in acinar cells of secretory glands and is regulated by CLCA1 [7,8]. Apart from glands, CaCCs have long been known to be present primarily in proliferating cells in culture and various types of cancer cells [9,10,11]. After identification of ANO1 as Ca2+ activated Cl− channel, it became clear that the protein is identical to DOG1, a significant and reliable tumor marker in gastrointestinal stromal tumors (GIST) and head and neck cancers [12,13,14] (Table 1). Meanwhile, ANO1 has been found in a number of different malignant tumors. Apart from ANO1, other members of the anoctamin family were also correlated with cell proliferation and cancer development, like ANO5 (TMEM16E), ANO7 (TMEM16G) and ANO9 (TMEM16J) (Table 1). Anoctamins could have tumor-specific functions, or may support cell proliferation and possible development towards malignancy in any cell-type. The latter assumption is supported by the fact that ANO1 is present in many different types of proliferating cells and tumor tissues [15] (Table 1). Notably, the ANO1-knockout mouse is hypotrophic when compared to wild type littermates [16]. ANO1 and its role in proliferation and cancer development has been reported repeatedly, but we are still far from any comprehensive understanding. Compared to Ano1, much less is known for other anoctamin paralogues regarding their potential role in proliferation and tumor development (Table 1). Moreover, some anoctamins, like ANO6, may even promote cell death, rather than growth.

Table 1.
Anoctamins in Cancer and Proliferation.
2. Anoctamins and Their Cellular Localization
Anoctamins form a family of Ca2+-activated proteins, consisting of phospholipid scramblases and ion channels [90,91]. The 10 proteins (ANO1-10; TMEM16A-K) are broadly expressed in epithelial and non-epithelia tissues [15]. ANO1 appears to operate as a relatively selective anion channel [92], while ANO6 is a phospholipid scramblase, i.e., it moves phosphatidylserine from the inner to the outer plasma membrane leaflet, when activated by a large increase in intracellular Ca2+ [93,94]. However, ANO6 is also permeable for chloride ions [95,96,97]. Previous work suggests that it becomes increasingly nonselective with increasing concentrations of intracellular free Ca2+ [98]. Although it is now clear that most anoctamins operate as phospholipid scramblases [99,100,101], our earlier work may suggest that all anoctamins also conduct ions, when co-expressed with purinergic receptors and activated by stimulation with ATP [102]. A subsequent study on the role of ANO5 for muscle repair presented strong evidence that ANO5 is a scramblase and conducts ions as well [103].
It is not entirely clear to what extent anoctamins operate as channels/scramblases in the apical plasma membrane of polarized cells, and what fraction of the protein resides in intracellular membranous compartments, or in the basolateral plasma membrane. For example, ANO1 is apical in pancreas, salivary gland, and airways, but it is basolateral in mouse colonic epithelia [104,105,106,107]. Cellular location of ANO1 may therefore depend on the cell type, and maybe on the cell function and differentiation. For example, ANO5 is mostly found intracellularly, but it can be also detected in the plasma membrane where it produces a non-selective whole cell current [15,102]. Endogenous and overexpressed ANO10 is typically intracellular, and co-localizes with acetylated tubulin [108,109,110]. However, expression and localization appears tissue dependent and may dependent on the cell cycle. For example, endogenous ANO10 in rapidly proliferating Fisher Rat Thyroid (FTR) cells is mostly intracellular and appears upregulated during mitosis [108] (Figure 1). Once FRT cells form a dense monolayer and stop proliferating in serum free media, some ANO10 moves into the cell membrane and co-localizes with the centrioles [108] (Figure 1A). Non-proliferating cells on permeable supports and in the absence of serum seem to lower expression of ANO10, which is now preferentially expressed close to the centriole and probably in the primary cilium (Figure 1). Expression in the primary cilium has also been observed for ANO1 and ANO6 in renal and retinal pigment epithelial cells [111,112,113]. We may therefore hypothesize a dynamic regulation of expression and localization of anoctamins, depending on proliferation and on the cell cycle (Figure 1B). As discussed below, upregulation of ANO1 is correlated with enhanced proliferation, e.g., in polycystic kidney disease, in many rapidly growing cell lines, as well as in different types of tumors [73,114] (Table 1).
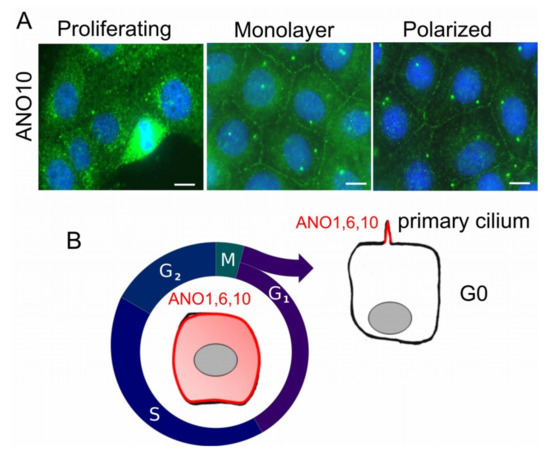
Figure 1.
Proliferation-dependent expression of ANO10 in FRT cells. (A) FRT cells were grown in FCS-containing media at 70% density (Proliferating), as confluent monolayer in FCS-free media (Monolayer), or as polarized monolayer on permeable supports and in FCS-free media (Polarized). Expression of endogenous ANO10 (green fluorescence) was intracellular in dividing cells (Proliferating), but was detected in the plasma membrane and in the primary cilium in densely grown cells (Monolayer). ANO10 was more prominent in plasma membrane and primary cilium in polarized cells (Polarized). For further details and references, see main text. (B) Hypothetical model proposing variable cellular locations of ANO10 depending on cell proliferation or cell polarization. ANO10 is found primarily intracellularly, but is also in the plasma membrane during cell cycle. Reduced expression of ANO10 and translocation into the primary cilium is observed once cells move into G0. Bar, 20 µm [108].
3. How Is ANO1 Upregulated during Cell Proliferation and Cancer Development?
Most studies on ANO1 have been performed on cultured cells, particularly in ANO1 overexpressing cells. Under these conditions, ANO1 currents are generally of large size and may show some properties that are different to currents expressed endogenously [95,115]. Although ANO1 is widely expressed and particularly abundant in epithelial cells [15,90,91], we observed that non-proliferating epithelial cells in culture or freshly isolated (non-cultured) cells from airways, kidney and intestine show very little Ca2+ activated Cl− currents [116,117,118]. However, ANO1 currents are quickly upregulated once cells have been isolated from the tissue and are maintained in serum-containing media under proliferating conditions. Upregulation of ANO1-currents can be reversed by growing the cells on permeable supports and removing the serum so that cells stop proliferating [11,106,116,117,119,120]. Thus, removing the cells from their physiological environment, cellular reorganization and pro-mitotic stimulation may all contribute to upregulation of ANO1. Moreover, transcriptional stimulation via the IL4/IL13-Jack-STAT3-STAT6 axis, steroid hormones such as testosterone, activation of histone deacetylase (HDCA), promotor hypo- methylation, as well as downregulation of inhibitory micro-RNAs have been shown to upregulate ANO1 expression (reviewed in [73,74,76,121,122] (Figure 2).
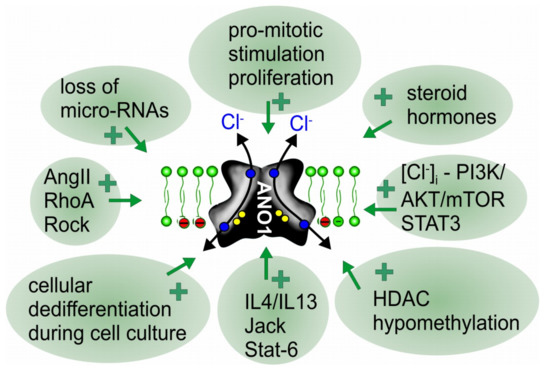
Figure 2.
Upregulation and redistribution of ANO1 during proliferation and cancer. Scheme summarizing reported factors and signaling pathways that lead to upregulation of expression of ANO1 and cellular redistribution during proliferation and cancer growth (Table 1). For further details and references, see main text.
4. ANO1, Cell Proliferation and Tumor Growth: How Does It Work?
ANO1 was found to increase proliferation in many different tissues [14,26,30,71,72,75,114,123,124,125] (Table 1). Apart from increasing proliferation, additional pro-apoptotic effects of ANO1 have also been reported, based on studies using ANO1-inhibitors. It should be noted, however, that ANO1-inhibitors might exert non-specific effects, when used at higher concentrations. In contrast, inhibition of proliferation by knockout of ANO1-expression or inhibition of ANO1 using low concentrations of ANO1 inhibitors have been shown in a number of studies [73,126,127]. This can also be demonstrated in experiments using nanomolar concentrations of the recently identified potent ANO1 inhibitor niclosamide, which however, has a number of additional anti-cancer effects (c.f. below) [128]. Nevertheless, other ANO1-inhibitors also blocked cell proliferation and cancer growth [30,72,127,129].
Niclosamide is a FDA-approved drug and was shown to inhibit Notch signaling [130], a pathway that is well known to participate in tumorigenesis [131]. In a number of reports, additional antineoplastic mechanisms of niclosamide have been described. Thus, niclosamide was shown to inhibit nuclear factor kappa B (NF-κB), Wnt/ß-catenin signaling, the IL-6-JAK1-STAT3-pathway, GSK-3 and more [132,133,134,135,136,137,138,139,140]. A recent paper suggests cell cycle arrest by niclosamide, through activation of the Let-7d/CDC34 axis [41]. Notably, blockade of notch signaling inhibits goblet cell metaplasia in asthmatic mice, which could be part of the mechanism how niclosamide inhibits mucus production [128,141,142]. Moreover, mucus production is also inhibited by other ANO1 inhibitors, such as niflumic acid (NFA) and CaCCinhAO1, or in ANO1 knockout mice [107,143,144]. Although the antiproliferative effects of niclosamide correspond well to its inhibitory effect on ANO1, this relationship is not well recognized. Niclosamide has been used in a number of preclinical studies and even in clinical trials with prostate and colorectal cancer patients [135,137,145,146,147,148,149]. Taken together, the multiple anti-cancer effects described for the ANO1-inhibitor niclosamide, may correspond to the wide range of pro-cancerous mechanisms by ANO1 (Figure 3).
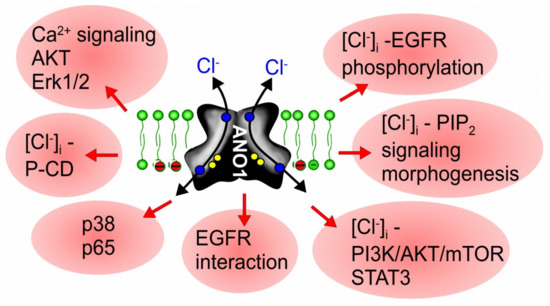
Figure 3.
Mechanisms for ANO1-induced cell proliferation and cancer development. Scheme summarizing reported mechanisms for ANO1-induced cell proliferation and development of cancer. All pathways are inhibited by niclosamide and other inhibitors of anoctamins (Table 1). For further details and references, see main text.
5. ANO5, 6, 7, and 9 in Cancer and Cell Proliferation
Other members of the anoctamin family were also associated with cell proliferation, embryogenesis and cancer growth. ANO5 (TMEM16E) is now known for its role in myoblast proliferation and muscle repair [80,103], while gain of function mutations of ANO5 cause gnathodiaphyseal dysplasia [150]. In a collaborative effort, we identified an essential role of ANO6 for embryogenesis [151]. Similar to ANO5 also ANO6 was also reported to control myoblast proliferation [80]. ANO7 (TMEM16G, NGEP) is a marker for prostate cancer [85,86]. For ANO9 (TMEM16J) an inverse correlation of expression and progression of colorectal cancer was described [89], while it may also promote pancreatic cancer [88]. Interestingly, enhanced phospho-Erk1,2 activity was correlated with the cellular effects of ANO1, 6, and 9, but a possible common mechanism remains obscure [30,80,88].
6. Anoctamins Control Intracellular Ca2+ Levels
Growth hormone receptors signal via Ras/Raf/Erk1,2, PI3K/Akt, and DAG/IP3 [152], while intracellular Ca2+ signals are essential regulators of cell proliferation [153]. We showed that anoctamins control compartmentalized Ca2+ signals ([Ca2+]i), and therefore proposed this as a major mechanism by which ANO1, and possibly other anoctamins, affect cell proliferation and a number of other cellular properties [108,110,154,155]. It is important to note that ANO1 is homologous to yeast Ist2, known to tether the peripheral cortical endoplasmic reticulum (ER) to the plasma membrane [156]. Gamper and collaborators convincingly showed that the relatively low Ca2+ sensitivity of ANO1 at (physiological) negative membrane voltages, requires this mechanism in order to concentrate Ca2+ near the plasma membrane and in close proximity of ANO1 [157,158]. ER-localized inositol trisphosphate receptors interact with ANO1 and tether the ER to ANO1 containing plasma membranes [157]. Moreover, IP3-induced Ca2+ store release is augmented by ANO1 [110]. Anoctamin-controlled Ca2+ compartments could be relevant for expression and activation of Erk1,2 [30,159]. Transient rise in intracellular Ca2+ followed by sustained activation of the Ras/Raf/Erk pathway is a central aspect of cell proliferation in many systems [154,160,161,162].
Although detailed mechanisms are currently not fully understood, it is clear that also other anoctamins affect [Ca2+]i, i.e., basal [Ca2+]i as well as receptor mediated Ca2+ signals depend on expression of anoctamins [110]. Apart from compartmentalization, protein interaction [163] and membrane depolarization, anoctamins may also contribute to cell proliferation and cell growth by operating as counter-ion channels. Counter ion movement of K+ or Cl− over the ER membrane is necessary for charge compensation to allow for efficient Ca2+ transport out of the ER via release channels, and for re-uptake of Ca2+ into the ER by the sarcoplasmic endoplasmic reticulum Ca2+-ATPase (SERCA) [164,165]. Given the Ca2+ permeability of some anoctamins, they may also serve as plasma membrane localized Ca2+ channels [90,166,167,168] or ER Ca2+ leakage channels [103,110,154,169,170,171]. Disturbed intracellular Ca2+ signals with changes of cellular properties are detectable in naïve tissues and primary cells from mice with knockout of anoctamins [107,108,110,155,172,173,174] (Figure 4).
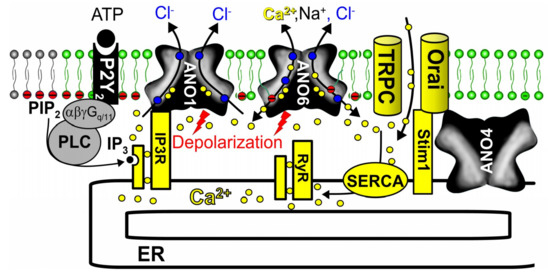
Figure 4.
Compartmentalized Ca2+ signaling by anoctamins. Scheme illustrating the effects of anoctamins on Ca2+ signaling. ANO1 tethers ER Ca2+ stores close to the plasma membrane, which leads to improved ATP-induced apical Ca2+ signaling. Activation of both ANO1 and ANO6 induce plasma membrane depolarization, supporting release of Ca2+ from ER stores via inositol trisphosphate receptors (IP3R) and ryanodine receptors (RyR). In addition, Ca2+ store content was found to be enhanced by ANO1. ANO6 is permeable for Ca2+ and therefore supports Ca2+ entry. ANO4 localized in the ER interacts with Orai1 [110]. For further details and references, see main text.
7. The Role of Anoctamins in Controlling Intracellular Cl− Concentration, Exocytosis, Organ Growth and Microvesicular Signaling
Recent reports suggest additional mechanisms whereby anoctamins may augment proliferation and cell growth. He and coworkers proposed an interesting concept in which ANO1 controls cytoplasmic Cl− levels that affect phosphoinositide levels in the inner plasma membrane leaflet, such as PtdIns(4,5)P2 in membrane microdomains [175]. Although the proposed concept requires further validation, it could contribute to attenuated purinergic Ca2+ signals found in tissues isolated from conditional ANO1 knockout animals or in ANO1 knockout cells (c.f. above). Similar to He et al., we also detected shortened motile cilia in the respiratory epithelium of ANO1-knockout mice, as well as a reduced length of non-motile primary cilia in renal collecting ducts of ANO1 knockout mice. While motile cilia from wt animals measured 6.1 ± 0.4 µm, those from animals with a ANO1-knockout in ciliated epithelial cells had a length of only 3.6 ± 0.4 µm (n = 7). Finally, the data by Ruppersburg and Hartzell convincingly demonstrate the importance of ANO1 for primary ciliogenesis [111]. siRNA-suppression of ANO6 expression and expression of other anoctamins suggested a contribution to basal Cl− conductance [102]. In contrast, overexpression of ANO1 and ANO6 enhanced basal Cl− conductance when analyzed at 37 °C [95]. In contrast to He et al., we found that ongoing activation of ANO1 (or ANO6) by either ionomycin or purinergic stimulation increased intracellular Cl− concentrations in HEK293 and HeLa cells [102]. Nevertheless the proposed concept that intracellular Cl− levels determine vesicular endocytosis/exocytosis, control apical membrane delivery and morphogenesis [175], is interesting and corresponds well to the role of ANO1 in exocytosis and normal renal development detected in recent studies [107,175,176,177] (Figure 5). It is also noteworthy that intracellular Cl− regulation by ANO1 has been shown to participate in transcription of human epidermal growth factor receptor 2, which mediates PI3K/AKT/mTOR and JAK/STAT3 signaling pathways [52].
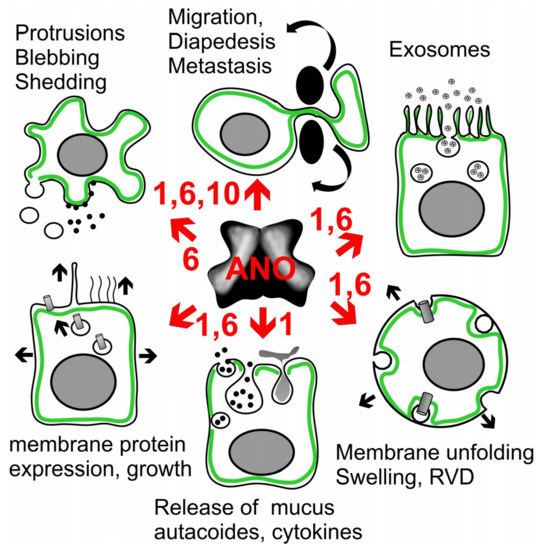
Figure 5.
Potential action of anoctamins on exocytosis, growth and microvesicular signaling. ANO1 and ANO6 determine the extent of membrane protrusions and membrane blebbing in macrophages and other cell types, and support cell migration, diapedesis and cancer metastasis. Exosome release and paracrine signaling by epithelial cells is probably anoctamin-dependent. Support of membrane unfolding, cell swelling and subsequent activation of VRAC could be a general property of anoctamins. Mucus secretion and release of inflammatory mediators such as autacoids and cytokines was shown to be ANO1-dependent. Exocytosis leads to enhanced expression of membrane proteins, cell growth, and extensions such as motile cilia and the primary cilium, as proposed for ANO1. For further details and references, see main text.
We observed that animals lacking expression of ANO1 in epithelial cells of airways and intestine accumulate mucus in club (Clara) and goblet cells [107]. We found that ANO1 is essential for secretion of mucus, probably by controlling mucus release from club/goblet cells, and by controlling release of prosecretory cytokines from ciliated cells [107]. IL-13-induced production and secretion of Muc5AC was inhibited by the ANO1 blocker and antiproliferative/anticancer drug, niclosamide. Along the same line, release of IL-8 induced by lipopolysaccharide (LPS) was significantly reduced by knockdown of ANO1. A recent paper by Hilgemann and colleagues demonstrates massive membrane expansion with activation of ANO6, with subsequent membrane shedding [178]. These results are reminiscent to our earlier observations of ANO6-depending blebbing and membrane shedding in macrophages [98]. Taken together, there is now evidence that anoctamins, particularly ANO1 and ANO6, control endolysosomal trafficking [98,107,108,175,179,180], membrane exocytosis, increase in membrane surface area and insertion of proteins into the plasma membrane [155,176,181] (Figure 5). As outlined above, ANO1 and ANO6 control [Ca2+]i, which is an essential regulator of exocytosis. Thus compartmentalized [Ca2+]i close to the plasma membrane is required for docking of exocytic vesicles and granules, respectively. This process requires the so-called Munc13 proteins and the soluble N-ethylmaleimide-sensitive factor-attachment protein receptor machinery [182,183].
Anoctamins were shown to have additional impact on cancer related events that involve plasma membrane function. Both ANO1 and ANO6 support cell migration and metastasis [14,26,30,55,61,73,184]. Endogenous ANO6 expressed in macrophages, or ANO6 overexpressed in HEK293 cells, induced massive membrane blebbing when activated by the P2X7-agonist ATP [98]. ANO6 also supported apoptosis, movement, and formation of protrusions, as well as phagocytic activity and bacterial killing by macrophages [98,108]. Importantly, phosphatidylserine exposure by ANO6 is required for the function of ADAM17 and ADAM10, both members of the family of cell bound disintegrin and metalloproteases. These enzymes regulate a plethora of biological functions, including proliferation and cell death [185,186,187]. The role of ANO1 for organ development, cell growth and extension of motile cilia and primary cilia has been discussed above. This is in line with its contribution to exocytosis and release of mucus or cytokines [107,155,176,188,189]. Moreover, other papers report a function of ANO6 [178] and ANO1 [190] for the release of microvesicles and exosomes, which could represent a paracrine control of neighbor cells in airways and intestine [107,191,192,193,194]. Notably, ANO1 is excreted in human urinary exosomes [195]. Interestingly, for both tissue repair [196] and necroptotic cell death [197] a role of the endosomal-sorting complex required for transport (ESCRT) has been described. Correspondingly, repair of muscle membrane requires ANO5 [103,198], while ANO6 has a role in necroptotic cell death [197,199]. Finally, our previous work suggests a role of ANO1, ANO6 and ANO10 in both membrane swelling and volume regulation by regulatory volume decrease, which is related to membrane unfolding and phospholipid metabolism [109,176,200] (Figure 5). This will be described in more detail below.
8. ANO1 Is Upregulated during Inflammation
The current data suggest that upregulated ANO1 in rapidly growing cells and tumors, supports proliferation, while expression in differentiated non-proliferating cells is generally much lower and may enable cells to perform specific tasks such as signaling, contraction, or secretion of electrolytes and mucus. Proliferation and inflammation/hypoxia are intimately connected through multiple signaling pathways including JACK/STAT [201,202,203]. Thus, it is not surprising that ANO1 is strongly upregulated during inflammation, which enabled its molecular identification as CaCC [6,204]. ANO1 is strongly upregulated in inflammatory airway diseases such as CF, COPD and asthma, which parallels goblet cell metaplasia and mucus hypersecretion [143,181,205]. It is also upregulated during bacterial inflammation [206]. Upregulation of TMEM16A is predominant in mucus producing cells and to a lesser degree in ciliated airway epithelial cells [107,155,205,207]. ANO1 may participate in the transition from inflammation to proliferation, which explains its strong impact in wound healing and tissue repair [14,103,208].
9. Relationship of Anoctamins to the Tumor Associated Cl− Channel VRAC
All living cells are able to maintain a constant cell volume. According to a general concept, regulatory volume decrease (RVD) prevents cell swelling and necrotic cell death, while regulatory volume increase (RVI) prevents cell shrinkage and apoptotic cell death [209,210]. The volume regulated or swelling activated anion channel (VRAC) is activated during RVD. Excessive activation of VRAC may support apoptotic cell death, while its upregulation leads to cellular resistance towards anti-cancer drugs [209,211,212,213,214,215,216,217]. Recent experiments suggest that ANO1 and ANO6 also contribute to volume activated whole cell currents, which may indicate a possible functional link between anoctamins and VRAC [123,176,200,218]. Although broadly expressed, there has been a long controversy concerning the molecular identity of VRAC, which was finally solved in 2014 [219,220,221]. Structural analysis by cryo-EM demonstrated a hexameric assembly of LRRC8 subunits, which form a typical ion channel with a central pore, structurally related to the connexin family of channels [222,223,224]. Conserved charged amino acid residues at the extracellular domain determine the permeability towards anions and other osmolytes. Two structurally different populations of VRAC have been shown by Kasuya et al., corresponding to a compact and a relaxed conformation. These conformations may correlate to closed and open states of the channel [224].
Although rather abundant, LRRC8/VRAC may not be essential for RVD and thus cells may be able to control their cell volume in the absence of VRAC [225,226,227]. Lack of functional VRAC leads to increased prenatal and postnatal mortality, growth retardation, and multiple tissue abnormalities, including abnormal function of B- and T-cells [228,229,230,231]. Additional, LRRC8-independent and cell-specific mechanisms may exist that enable RVD. These mechanisms comprise other Cl− channels such CFTR, bestrophin, and anoctamins, as well as electroneutral KCl co-transporter [218,225,226,227]. An inverse relationship exists between the magnitudes of VRAC and of Ca2+ activated ANO1 Cl− currents: With increased activation of CaCC, VRAC decreases and vice versa [232,233]. We could not activate ANO1 after maximal activation of VRAC [123], while Zholos et al showed a reduced probability for activation of VRAC after activation of CaCC [233]. A loss of expression of LRRC8A not only inhibited VRAC, but also attenuated Ca2+ activated Cl− currents. Vice versa, overexpression of LRRC8A enhanced Ca2+ activated Cl− currents. Because LRRC8A and ANO1 could be co-immunoprecipitated, a co-localization of both anion channels is proposed, with membrane insertion of LRRC8A being supported by ANO1 [176]. Apart from ANO1 and ANO6, also VRAC is blocked by niclosamide [95]. Because VRAC induces resistance towards cisplatin and other anticancer drugs and leads to metastasis and bad patient outcome, inhibition of VRAC may be another mechanism how niclosamide inhibits growth of cancer [215,234,235].
Taken together, a functional relationship exists between VRAC and ANO1, possibly because activation of both channels involves release of Ca2+ from the ER-store [200,236]. As VRAC controls survival of cells, the functional crosstalk with ANO1 is highly relevant for tumor biology [215,234,236].
10. Role of Anoctamins in Cell Death
Sustained large increase in intracellular Ca2+ can lead to senescence or cell death [237,238]. We showed earlier that P2X7-mediated increase of intracellular Ca2+ leads to cell death of macrophages and lymphocytes expressing endogenous ANO6, and of HEK293 cells overexpressing ANO6 [98,239]. ANO6 is a component of the so-called outwardly rectifying Cl− channel ORCC or ICOR, and has a role in cell shrinkage and programmed cell death [218,239,240,241]. Expression of ANO6 is dominant in the surface epithelium of large intestine, were aged enterocytes die and dead cells are exfoliated. ANO6 is not found in intestinal crypts, where enterocytes are produced from stem cells [239]. TUNEL assays performed in mouse intestinal epithelium lacking ANO6 expression, unmask reduced cell death, when compared to wt mice. In addition, ANO10 is important for spontaneous and TNFα-induced cell death in mouse intestinal epithelium, peritoneal macrophages, and THP1 macrophages [108]. Moreover, knockdown of ANO6 impaired apoptosis and formation of cyst lumen in 3D cultures of MDCK renal cysts. ANO6 is normally expressed in apoptotic cells within the center of growing cysts formed by MDCK cells and human polycystic kidneys [113].
Although many studies demonstrated the pro-proliferative role of ANO1, a pro-apoptotic function of ANO1 has also been reported [125,242,243]. Almaca et all showed that activation of ANO1 can lead to apoptotic cell shrinkage [123]. Interestingly, the genes for ANO1 and for apoptosis associated Fas associated via death domain (FADD), are located on a common amplicon located on chromosome 11q13. Surprisingly, both proteins were associated with better survival of HNSCC patients [38]. In contrast to ANO1, which is unable to scramble membrane phospholipids, ANO6-induced cell death is probably related to its ability to scramble membrane phospholipid [99]. Interestingly, we found that expression of ANO1 enhanced ionomycin-induced scrambling performed by endogenous ANO6 in HEK293 cells. This may point to a synergism between both anoctamins, which has also been detected earlier for Cl− currents produced by both proteins [244].
Meanwhile, a number of independent regulated cell death pathways have been identified [245]. Initially, ANO6 has been reported in the context of apoptosis, but is now shown to be activated also during necroptosis, pyroptosis and ferroptosis [95,199,246,247,248]. Thus, activation of anoctamins, particularly of ANO6, might be a possibility to induce cell death in cancer cells.
11. Activation of Anoctamins and Ferroptotic Cell Death in Cancer
Pro-apoptotic Cl− currents have been activated in cells overexpressing ANO1, ANO6, ANO9 and ANO10 [95,123,227]. A potent activator of anoctamins is the bee venom melittin, which stimulates phospholipase A2 (PLA2) [95,200]. Noteworthy, melittin has been widely used as anti-cancer therapy, and PLA2-dependent activation of metalloproteinase is essential for this effect [249,250,251].
Anoctamins are also activated through reactive oxygen species and by lipid peroxidation. This may lead to inflammation and proliferation, ion secretion and ferroptosis, depending on the cell type, the anoctamin paralogue being activated, and the strength of peroxidation [95,118,247,252,253]. Ferroptosis is induced by accumulation of intracellular iron, and is distinct from apoptosis, necrosis, and other forms of regulated cell death [254]. Ferroptosis is triggered by an increase in reactive oxygen species (ROS) and an overwhelming lipid peroxidation that ultimately leads to cell death by disintegration of the plasma membrane. Experimentally lipid peroxidation is also induced by erastin-inhibition of cysteine import through the transporter system Xc−. This leads to depletion of glutathione (GSH) and inactivation of the phospholipid peroxidase glutathione peroxidase 4 (GPX4). In addition, GPX4 can be directly inhibited by RSL3 [255].
Cell death can be induced in cancer cells by activation of ANO6 through melittin-induced PLA2 or through lipid peroxidation [95]. This may suggest a new potential therapeutic approach to inhibit growth of cancer [95,247]. Lipid peroxidation and ferroptosis-induced cell death was proposed earlier as a mechanism to destroy cancer cells [256]. However, the ROS buffer capacity is typically quite high in cancer cells, which will antagonize lipid peroxidation [257]. ROS levels could be enhanced to exceed the antioxidant defense of cancer cells [258]. A number of preclinical studies were performed using small molecules to inhibit cellular glutathione antioxidant activity [259,260,261,262]. Tumor cell lines that were killed by the ANO6-activator melittin were also driven into ferroptosis by erastin and RSL3. Thus ANO1 and ANO6 were shown to be activated during ferroptotic cell death [118,247].
12. Conclusions
Proteins of the anoctamin/TMEM16 family scramble membrane phospholipids and operate as Cl− and cation-permeable channels. They demonstrate impressive effects on basic cell properties, and support both cell proliferation and regulated cell death. Clearly more work is required to be able to define the cellular functions of anoctamins, and their role in proliferation and cancer development. Despite the plethora of underlying cell specific signaling pathways, it will be interesting to learn whether common mechanisms exist for the cellular effects induced by anoctamins, such as enhanced intracellular Ca2+ signaling. Blocking ANO1 appears feasible to interfere with cancer growth.
In contrast to the pro-proliferative effect of ANO1, ANO6 seem to contribute to different types of regulated cell death (Figure 6). Activation of ANO6 may cause swelling or shrinkage of cells, and does increase in intracellular Ca2+, phospholipid scrambling, membrane blebbing and membrane shedding. It may all contribute to ANO6-induced cell death. Thus, direct activation of ANO6 may be a promising new strategy to induce cell death in cancer cells.
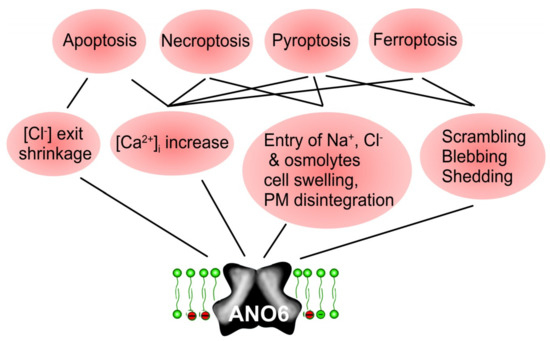
Figure 6.
ANO6-induced cell death. Scheme summarizing the contribution of ANO6 to different regulated cell death pathways such as apoptosis, necroptosis, pyroptosis, and ferroptosis. Anoctamins may contribute to regulated cell death by cell shrinkage (apoptosis), increase in compartmentalized intracellular Ca2+ (all cell death pathways), or cell swelling, scrambling, blebbing, and membrane disintegration (ferroptosis, pyroptosis). For further details and references, see main text.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft DFG KU756/14-1, DFG—Projektnummer 387509280—SFB 1350, Cystic Fibrosis Trust SRC 013, Sander Stiftung and Mukoviszidose Institute.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Hartzell, H.C.; Yu, K.; Xiao, Q.; Chien, L.T.; Qu, Z. Anoctamin/TMEM16 family members are Ca2+-activated Cl-channels. J. Physiol. 2008, 587, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J. Calcium-activated chloride channels: (un)known, (un)loved? Proc. Am. Thorac. Soc. 2004, 1, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, K.; Kongsuphol, P.; Chootip, K.; Toledo, C.; Martins, J.R.; Almaca, J.; Tian, Y.; Witzgall, R.; Ousingsawat, J.; Schreiber, R. Role of the Ca(2+)-activated Cl(-) channels bestrophin and anoctamin in epithelial cells. Biol. Chem. 2011, 392, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.D.; Cho, H.; Koo, J.Y.; Tak, M.H.; Cho, Y.; Shim, W.S.; Park, S.P.; Lee, J.; Lee, B.; Kim, B.M.; et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 2008, 455, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.C.; Cheng, T.; Jan, Y.N.; Jan, L.Y. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 2008, 134, 1019–1029. [Google Scholar] [CrossRef]
- Caputo, A.; Caci, E.; Ferrera, L.; Pedemonte, N.; Barsanti, C.; Sondo, E.; Pfeffer, U.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L.J. TMEM16A, A Membrane Protein Associated With Calcium-Dependent Chloride Channel Activity. Science 2008, 322, 590–594. [Google Scholar] [CrossRef]
- Sala-Rabanal, M.; Yurtsever, Z.; Nichols, C.G.; Brett, T.J. Secreted CLCA1 modulates TMEM16A to activate Ca(2+)-dependent chloride currents in human cells. Elife 2015, 4, e05875. [Google Scholar] [CrossRef]
- Loewen, M.E.; Forsyth, G.W. Structure and function of CLCA proteins. Physiol. Rev. 2005, 85, 1061–1092. [Google Scholar] [CrossRef]
- Hartzell, H.C.; Putzier, I.; Arreola, J. Calcium-Activated Chloride Channels. Annu. Rev. Physiol. 2005, 67, 719–758. [Google Scholar] [CrossRef]
- Schreiber, R. Ca2+ signaling, intracellular pH and cell volume in cell proliferation. J. Membr. Biol. 2005, 205, 129–137. [Google Scholar] [CrossRef]
- Kunzelmann, K. Ion channels and cancer. J. Membr. Biol. 2005, 205, 159–173. [Google Scholar] [CrossRef]
- Espinosa, I.; Lee, C.H.; Kim, M.K.; Rouse, B.T.; Subramanian, S.; Montgomery, K.; Varma, S.; Corless, C.L.; Heinrich, M.C.; Smith, K.S.; et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am. J. Surg. Pathol. 2008, 32, 210–218. [Google Scholar] [CrossRef]
- West, R.B.; Corless, C.L.; Chen, X.; Rubin, B.P.; Subramanian, S.; Montgomery, K.; Zhu, S.; Ball, C.A.; Nielsen, T.O.; Patel, R.; et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am. J. Pathol. 2004, 165, 107–113. [Google Scholar] [CrossRef]
- Ruiz, C.; Martins, J.R.; Rudin, F.; Schneider, S.; Dietsche, T.; Fischer, C.A.; Tornillo, L.; Terracciano, L.M.; Schreiber, R.; Bubendorf, L.; et al. Enhanced Expression of ANO1 in Head and Neck Squamous Cell Carcinoma Causes Cell Migration and Correlates with Poor Prognosis. PLoS ONE 2012, 7, e43265. [Google Scholar] [CrossRef]
- Schreiber, R.; Uliyakina, I.; Kongsuphol, P.; Warth, R.; Mirza, M.; Martins, J.R.; Kunzelmann, K. Expression and Function of Epithelial Anoctamins. J. Biol. Chem. 2010, 285, 7838–7845. [Google Scholar] [CrossRef]
- Kunzelmann, K.; Schreiber, R.; Kmit, A.; Jantarajit, W.; Martins, J.R.; Faria, D.; Kongsuphol, P.; Ousingsawat, J.; Tian, Y. Expression and function of epithelial anoctamins. Exp. Physiol. 2012, 97, 184–192. [Google Scholar] [CrossRef]
- Carles, A.; Millon, R.; Cromer, A.; Ganguli, G.; Lemaire, F.; Young, J.; Wasylyk, C.; Muller, D.; Schultz, I.; Rabouel, Y.; et al. Head and neck squamous cell carcinoma transcriptome analysis by comprehensive validated differential display. Oncogene 2006, 25, 1821–1831. [Google Scholar] [CrossRef]
- Carneiro, A.; Isinger, A.; Karlsson, A.; Johansson, J.; Jonsson, G.; Bendahl, P.O.; Falkenback, D.; Halvarsson, B.; Nilbert, M. Prognostic impact of array-based genomic profiles in esophageal squamous cell cancer. BMC Cancer 2008, 8, 98. [Google Scholar] [CrossRef]
- Liegl, B.; Hornick, J.L.; Corless, C.L.; Fletcher, C.D. Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am. J. Surg. Pathol. 2009, 33, 437–446. [Google Scholar] [CrossRef]
- Miwa, S.; Nakajima, T.; Murai, Y.; Takano, Y.; Sugiyama, T. Mutation assay of the novel gene DOG1 in gastrointestinal stromal tumors (GISTs). J. Gastroenterol. 2008, 43, 531–537. [Google Scholar] [CrossRef]
- Fatima, N.; Cohen, C.; Siddiqui, M.T. DOG1 utility in diagnosing gastrointestinal stromal tumors on fine-needle aspiration. Cancer Cytopathol. 2011, 119, 202–208. [Google Scholar] [CrossRef]
- Kang, G.H.; Srivastava, A.; Kim, Y.E.; Park, H.J.; Park, C.K.; Sohn, T.S.; Kim, S.; Kang, D.Y.; Kim, K.M. DOG1 and PKC-theta are useful in the diagnosis of KIT-negative gastrointestinal stromal tumors. Modern Pathol. 2011, 24, 866–875. [Google Scholar] [CrossRef]
- Hwang, D.G.; Qian, X.; Hornick, J.L. DOG1 antibody is a highly sensitive and specific marker for gastrointestinal stromal tumors in cytology cell blocks. Am. J. Clin. Pathol. 2011, 135, 448–453. [Google Scholar] [CrossRef]
- Novelli, M.; Rossi, S.; Rodriguez-Justo, M.; Taniere, P.; Seddon, B.; Toffolatti, L.; Sartor, C.; Hogendoorn, P.C.; Sciot, R.; Van Glabbeke, M.; et al. DOG1 and CD117 are the antibodies of choice in the diagnosis of gastrointestinal stromal tumours. Histopathology 2010, 57, 259–270. [Google Scholar] [CrossRef]
- Wong, N.A.; Shelley-Fraser, G. Specificity of DOG1 (K9 clone) and protein kinase C theta (clone 27) as immunohistochemical markers of gastrointestinal stromal tumour. Histopathology 2010, 57, 250–258. [Google Scholar] [CrossRef]
- Ayoub, C.; Wasylyk, C.; Li, Y.; Thomas, E.; Marisa, L.; Robe, A.; Roux, M.; Abecassis, J.; de Reynies, A.; Wasylyk, B. ANO1 amplification and expression in HNSCC with a high propensity for future distant metastasis and its functions in HNSCC cell lines. Br. J. Cancer 2010, 103, 715–726. [Google Scholar] [CrossRef]
- Lee, C.H.; Liang, C.W.; Espinosa, I. The utility of discovered on gastrointestinal stromal tumor 1 (DOG1) antibody in surgical pathology-the GIST of it. Adv. Anat. Pathol. 2010, 17, 222–232. [Google Scholar] [CrossRef]
- Kleist, B.; Lasota, J.; Miettinen, M. Gastrointestinal stromal tumor and gastric adenocarcinoma collision tumors. Hum. Pathol. 2010, 41, 1034–1039. [Google Scholar] [CrossRef]
- Miettinen, M.; Wang, Z.F.; Lasota, J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: A study of 1840 cases. Am. J. Surg. Pathol. 2009, 33, 1401–1408. [Google Scholar] [CrossRef]
- Duvvuri, U.; Shiwarski, D.J.; Xiao, D.; Bertrand, C.; Huang, X.; Edinger, R.S.; Rock, J.R.; Harfe, B.D.; Henson, B.J.; Kunzelmann, K.; et al. TMEM16A, induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res. 2012, 72, 3270–3281. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kojima, A.; Nagata, S.; Tomita, Y.; Takahashi, S.; Oda, Y. KIT-negative gastrointestinal stromal tumor of the abdominal soft tissue: A clinicopathologic and genetic study of 10 cases. Am. J. Surg. Pathol. 2011, 35, 1287–1295. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Hong, S. ANO1 as a marker of oral squamous cell carcinoma and silencing ANO1 suppresses migration of human SCC-25 cells. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e313–e319. [Google Scholar] [CrossRef]
- Simon, S.; Grabellus, F.; Ferrera, L.; Galietta, L.; Schwindenhammer, B.; Muhlenberg, T.; Taeger, G.; Eilers, G.; Treckmann, J.; Breitenbuecher, F.; et al. DOG1 regulates growth and IGFBP5 in gastrointestinal stromal tumors. Cancer Res. 2013, 73, 3661–3670. [Google Scholar] [CrossRef]
- Berglund, E.; Akcakaya, P.; Berglund, D.; Karlsson, F.; Vukojevic, V.; Lee, L.; Bogdanovic, D.; Lui, W.O.; Larsson, C.; Zedenius, J.; et al. Functional role of the Ca-activated Cl channel DOG1/TMEM16A in gastrointestinal stromal tumor cells. Exp. Cell Res. 2014, 326, 315–325. [Google Scholar] [CrossRef]
- Slavik, T.; du Plessis, J.; Sparaco, A.; van der Merwe, S.W. Duodenal gastrointestinal stromal tumor with epithelioid and neural features mimicking a primary pancreas head neuroendocrine tumor. Pancreas 2014, 43, 482–483. [Google Scholar] [CrossRef]
- Rodrigo, J.P.; Menendez, S.T.; Hermida-Prado, F.; Alvarez-Teijeiro, S.; Villaronga, M.A.; Alonso-Duran, L.; Vallina, A.; Martinez-Camblor, P.; Astudillo, A.; Suarez, C.; et al. Clinical significance of Anoctamin-1 gene at 11q13 in the development and progression of head and neck squamous cell carcinomas. Sci. Rep. 2015, 5, 15698. [Google Scholar] [CrossRef]
- Bill, A.; Gutierrez, A.; Kulkarni, S.; Kemp, C.; Bonenfant, D.; Voshol, H.; Duvvuri, U.; Gaither, L.A. ANO1/TMEM16A interacts with EGFR and correlates with sensitivity to EGFR-targeting therapy in head and neck cancer. Oncotarget 2015, 6, 9173–9188. [Google Scholar] [CrossRef]
- Reddy, R.B.; Bhat, A.R.; James, B.L.; Govindan, S.V.; Mathew, R.; Ravindra, D.R.; Hedne, N.; Illiayaraja, J.; Kekatpure, V.; Khora, S.S.; et al. Meta-Analyses of Microarray Datasets Identifies ANO1 and FADD as Prognostic Markers of Head and Neck Cancer. PLoS ONE 2016, 11, e0147409. [Google Scholar] [CrossRef]
- Dixit, R.; Kemp, C.; Kulich, S.; Seethala, R.; Chiosea, S.; Ling, S.; Ha, P.K.; Duvvuri, U. TMEM16A/ANO1 is differentially expressed in HPV-negative versus HPV-positive head and neck squamous cell carcinoma through promoter methylation. Sci. Rep. 2015, 5, 16657. [Google Scholar] [CrossRef]
- Godse, N.R.; Khan, N.I.; Yochum, Z.A.; Gomez-Casal, R.; Kemp, C.; Shiwarski, D.J.; Seethala, R.; Kulich, S.; Seshadri, M.; Burns, T.F.; et al. TMEM16A/ANO1 inhibits apoptosis via down-regulation of Bim expression. Clin. Cancer Res. 2017. [Google Scholar] [CrossRef]
- Han, Z.; Li, Q.; Wang, Y.; Wang, L.; Li, X.; Ge, N.; Wang, Y.; Guo, C. Niclosamide Induces Cell Cycle Arrest in G1 Phase in Head and Neck Squamous Cell Carcinoma Through Let-7d/CDC34 Axis. Front. Pharmacol. 2018, 9, 1544. [Google Scholar] [CrossRef]
- Ardeleanu, C.; Arsene, D.; Hinescu, M.; Andrei, F.; Gutu, D.; Luca, L.; Popescu, L.M. Pancreatic Expression of DOG1: A Novel Gastrointestinal Stromal Tumor (GIST) Biomarker. Appl. Immunohistochem. Mol. Morphol. 2009, 17, 413–418. [Google Scholar] [CrossRef]
- Bergmann, F.; Andrulis, M.; Hartwig, W.; Penzel, R.; Gaida, M.M.; Herpel, E.; Schirmacher, P.; Mechtersheimer, G. Discovered on gastrointestinal stromal tumor 1 (DOG1) is expressed in pancreatic centroacinar cells and in solid-pseudopapillary neoplasms-novel evidence for a histogenetic relationship. Hum. Pathol. 2011, in press. [Google Scholar] [CrossRef]
- Sauter, D.R.; Novak, I.; Pedersen, S.F.; Larsen, E.H.; Hoffmann, E.K. ANO1 (TMEM16A) in pancreatic ductal adenocarcinoma (PDAC). Pflug. Arch. 2014, 467, 1495–1508. [Google Scholar] [CrossRef]
- Liu, W.; Lu, M.; Liu, B.; Huang, Y.; Wang, K. Inhibition of Ca(2+)-activated Cl(-) channel ANO1/TMEM16A expression suppresses tumor growth and invasiveness in human prostate carcinoma. Cancer Lett. 2012, 326, 41–51. [Google Scholar] [CrossRef]
- Seo, Y.; Ryu, K.; Park, J.; Jeon, D.K.; Jo, S.; Lee, H.K.; Namkung, W. Inhibition of ANO1 by luteolin and its cytotoxicity in human prostate cancer PC-3 cells. PLoS ONE 2017, 12, e0174935. [Google Scholar] [CrossRef]
- Song, Y.; Gao, J.; Guan, L.; Chen, X.; Gao, J.; Wang, K. Inhibition of ANO1/TMEM16A induces apoptosis in human prostate carcinoma cells by activating TNF-alpha signaling. Cell Death Dis. 2018, 9, 703. [Google Scholar] [CrossRef]
- Ubby, I.; Bussani, E.; Colonna, A.; Stacul, G.; Locatelli, M.; Scudieri, P.; Galietta, L.J.; Pagani, F. TMEM16A alternative splicing coordination in breast cancer. Mol. Cancer 2013, 12, 75. [Google Scholar] [CrossRef]
- Britschgi, A.; Bill, A.; Brinkhaus, H.; Rothwell, C.; Clay, I.; Duss, S.; Rebhan, M.; Raman, P.; Guy, C.T.; Wetzel, K.; et al. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc. Natl. Acad. Sci. USA 2013, 110, E1026–E1034. [Google Scholar] [CrossRef]
- Wu, H.; Guan, S.; Sun, M.; Yu, Z.; Zhao, L.; He, M.; Zhao, H.; Yao, W.; Wang, E.; Jin, F.; et al. Ano1/TMEM16A Overexpression Is Associated with Good Prognosis in PR-Positive or HER2-Negative Breast Cancer Patients following Tamoxifen Treatment. PLoS ONE 2015, 10, e0126128. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, S.; Qu, Z.; Zhou, S.; Ruan, Q. Novel Use for DOG1 in Discriminating Breast Invasive Carcinoma from Noninvasive Breast Lesions. Dis. Mark. 2016, 2016, 5628176. [Google Scholar] [CrossRef]
- Fujimoto, M.; Inoue, T.; Kito, H.; Niwa, S.; Suzuki, T.; Muraki, K.; Ohya, S. Transcriptional repression of HER2 by ANO1 Cl(-) channel inhibition in human breast cancer cells with resistance to trastuzumab. Biochem. Biophys. Res. Commun. 2017, 482, 188–194. [Google Scholar] [CrossRef]
- Wu, H.; Wang, H.; Guan, S.; Zhang, J.; Chen, Q.; Wang, X.; Ma, K.; Zhao, P.; Zhao, H.; Yao, W.; et al. Cell-specific regulation of proliferation by Ano1/TMEM16A in breast cancer with different ER, PR, and HER2 status. Oncotarget 2017. [Google Scholar] [CrossRef]
- Foda, A.A.; Mohamed, M.A. Aberrant expressions of c-KIT and DOG-1 in mucinous and nonmucinous colorectal carcinomas and relation to clinicopathologic features and prognosis. Ann. Diagn. Pathol. 2015, 19, 335–340. [Google Scholar] [CrossRef]
- Sui, Y.; Sun, M.; Wu, F.; Yang, L.; Di, W.; Zhang, G.; Zhong, L.; Ma, Z.; Zheng, J.; Fang, X.; et al. Inhibition of TMEM16A Expression Suppresses Growth and Invasion in Human Colorectal Cancer Cells. PLoS ONE 2014, 9, e115443. [Google Scholar] [CrossRef]
- Liu, F.; Cao, Q.H.; Lu, J.; Luo, B.; Lu, X.F.; Luo, R.C.; Wang, X.G. TMEM16A overexpression contributes to tumor invasion and poor prognosis of human gastric cancer through TGF-beta signaling. Oncotarget 2015, 6, 11585–11599. [Google Scholar]
- Cao, Q.; Liu, F.; Ji, K.; Liu, N.; He, Y.; Zhang, W.; Wang, L. MicroRNA-381 inhibits the metastasis of gastric cancer by targeting TMEM16A expression. J. Exp. Clin. Cancer Res. 2017, 36, 29. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Ren, Y.; Kang, L.; Zhang, L. Transmembrane protein with unknown function 16A overexpression promotes glioma formation through the nuclear factor-kappaB signaling pathway. Mol. Med. Rep. 2014, 9, 1068–1074. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, J.K.; Bae, Y.; Lee, B.S.; Kim, E.; Cho, C.H.; Ryoo, K.; Yoo, J.; Kim, C.H.; Yi, G.S.; et al. Suppression of 14-3-3gamma-mediated surface expression of ANO1 inhibits cancer progression of glioblastoma cells. Sci. Rep. 2016, 6, 26413. [Google Scholar] [CrossRef]
- Shang, L.; Hao, J.J.; Zhao, X.K.; He, J.Z.; Shi, Z.Z.; Liu, H.J.; Wu, L.F.; Jiang, Y.Y.; Shi, F.; Yang, H.; et al. ANO1 protein as a potential biomarker for esophageal cancer prognosis and precancerous lesion development prediction. Oncotarget 2016, 10, 24374. [Google Scholar] [CrossRef]
- Jia, L.; Liu, W.; Guan, L.; Lu, M.; Wang, K. Inhibition of Calcium-Activated Chloride Channel ANO1/TMEM16A Suppresses Tumor Growth and Invasion in Human Lung Cancer. PLoS ONE 2015, 10, e0136584. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Chen, Y.; Li, P.; Gao, L.; Zheng, Y.; Sun, Y.; Chen, J.; Qian, X. Expression of anoctamin 1 is associated with advanced tumor stage in patients with non-small cell lung cancer and predicts recurrence after surgery. Clin. Transl. Oncol. 2017. [Google Scholar] [CrossRef]
- Guo, S.; Chen, Y.; Pang, C.; Wang, X.; Shi, S.; Zhang, H.; An, H.; Zhan, Y. Matrine is a novel inhibitor of the TMEM16A chloride channel with antilung adenocarcinoma effects. J. Cell. Physiol. 2018. [Google Scholar] [CrossRef]
- Deng, L.; Yang, J.; Chen, H.; Ma, B.; Pan, K.; Su, C.; Xu, F.; Zhang, J. Knockdown of TMEM16A suppressed MAPK and inhibited cell proliferation and migration in hepatocellular carcinoma. Oncol. Targets Ther. 2016, 9, 325–333. [Google Scholar]
- Jung, I.; Gurzu, S.; Turdean, S.; Ciortea, D.; Sahlean, D.I.; Golea, M.; Bara, T. Relationship of endothelial area with VEGF-A, COX-2, maspin, c-KIT, and DOG-1 immunoreactivity in liposarcomas versus non-lipomatous soft tissue tumors. Int. J. Clin. Exp. Pathol. 2015, 8, 1776–1782. [Google Scholar]
- Sah, S.P.; McCluggage, W.G. DOG1 immunoreactivity in uterine leiomyosarcomas. J. Clin. Pathol. 2013, 66, 40–43. [Google Scholar] [CrossRef]
- Abd Raboh, N.M.; Hakim, S.A. Diagnostic role of DOG1 and p63 immunohistochemistry in salivary gland carcinomas. Int. J. Clin. Exp. Pathol. 2015, 8, 9214–9222. [Google Scholar]
- Akpalo, H.; Lange, C.; Zustin, J. Discovered on gastrointestinal stromal tumour 1 (DOG1): A useful immunohistochemical marker for diagnosing chondroblastoma. Histopathology 2012, 60, 1099–1106. [Google Scholar] [CrossRef]
- Hemminger, J.; Iwenofu, O.H. Discovered on gastrointestinal stromal tumours 1 (DOG1) expression in non-gastrointestinal stromal tumour (GIST) neoplasms. Histopathology 2012, 61, 170–177. [Google Scholar] [CrossRef]
- Chenevert, J.; Duvvuri, U.; Chiosea, S.; Dacic, S.; Cieply, K.; Kim, J.; Shiwarski, D.; Seethala, R.R. DOG1: A novel marker of salivary acinar and intercalated duct differentiation. Mod. Pathol. 2012, 25, 919–929. [Google Scholar] [CrossRef]
- Stanich, J.E.; Gibbons, S.J.; Eisenman, S.T.; Bardsley, M.R.; Rock, J.R.; Harfe, B.D.; Ordog, T.; Farrugia, G. ANO1 AS A REGULATOR OF PROLIFERATION. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G1044–G1051. [Google Scholar] [CrossRef]
- Mazzone, A.; Eisenman, S.T.; Strege, P.R.; Yao, Z.; Ordog, T.; Gibbons, S.J.; Farrugia, G. Inhibition of Cell Proliferation by a Selective Inhibitor of the Ca(2+)-activated Cl(-) Channel, Ano1. Biochem. Biophys. Res. Commun. 2012, 427, 248–253. [Google Scholar] [CrossRef]
- Wanitchakool, P.; Wolf, L.; Koehl, G.; Sirianant, L.; Gaumann, A.; Schreiber, R.; Duvvuri, U.; Kunzelmann, K. Role of Anoctamins in Cancer and Apoptosis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130096. [Google Scholar] [CrossRef]
- Bill, A.; Alex Gaither, L. The Mechanistic Role of the Calcium-Activated Chloride Channel ANO1 in Tumor Growth and Signaling. Adv. Exp. Med. Biol. 2017. [Google Scholar] [CrossRef]
- Guan, L.; Song, Y.; Gao, J.; Gao, J.; Wang, K. Inhibition of calcium-activated chloride channel ANO1 suppresses proliferation and induces apoptosis of epithelium originated cancer cells. Oncotarget 2016, 7, 78619–78630. [Google Scholar] [CrossRef]
- Wang, H.; Zou, L.; Ma, K.; Yu, J.; Wu, H.; Wei, M.; Xiao, Q. Cell-specific mechanisms of TMEM16A Ca2+-activated chloride channel in cancer. Mol. Cancer 2017, 16, 152. [Google Scholar] [CrossRef]
- Ishaque, N.; Abba, M.L.; Hauser, C.; Patil, N.; Paramasivam, N.; Huebschmann, D.; Leupold, J.H.; Balasubramanian, G.P.; Kleinheinz, K.; Toprak, U.H.; et al. Whole genome sequencing puts forward hypotheses on metastasis evolution and therapy in colorectal cancer. Nat. Commun. 2018, 9, 4782. [Google Scholar] [CrossRef]
- Hesson, L.B.; Ng, B.; Zarzour, P.; Srivastava, S.; Kwok, C.T.; Packham, D.; Nunez, A.C.; Beck, D.; Ryan, R.; Dower, A.; et al. Integrated Genetic, Epigenetic, and Transcriptional Profiling Identifies Molecular Pathways in the Development of Laterally Spreading Tumors. Mol. Cancer Res. 2016, 14, 1217–1228. [Google Scholar] [CrossRef]
- Chang, Z.; Cai, C.; Han, D.; Gao, Y.; Li, Q.; Feng, L.; Zhang, W.; Zheng, J.; Jin, J.; Zhang, H.; et al. Anoctamin5 regulates cell migration and invasion in thyroid cancer. Int. J. Oncol. 2017, 51, 1311–1319. [Google Scholar] [CrossRef]
- Zhao, P.; Torcaso, A.; Mariano, A.; Xu, L.; Mohsin, S.; Zhao, L.; Han, R. Anoctamin 6 Regulates C2C12 Myoblast Proliferation. PLoS ONE 2014, 9, e92749. [Google Scholar] [CrossRef]
- Kaikkonen, E.; Rantapero, T.; Zhang, Q.; Taimen, P.; Laitinen, V.; Kallajoki, M.; Jambulingam, D.; Ettala, O.; Knaapila, J.; Bostrom, P.J.; et al. ANO7 is associated with aggressive prostate cancer. Int. J. Cancer 2018, 143, 2479–2487. [Google Scholar] [CrossRef]
- Mohsenzadegan, M.; Shekarabi, M.; Madjd, Z.; Asgari, M.; Abolhasani, M.; Tajik, N.; Farajollahi, M.M. Study of NGEP expression pattern in cancerous tissues provides novel insights into prognostic marker in prostate cancer. Biomark. Med. 2015, 9, 391–401. [Google Scholar] [CrossRef]
- Cereda, V.; Poole, D.J.; Palena, C.; Das, S.; Bera, T.K.; Remondo, C.; Gulley, J.L.; Arlen, P.M.; Yokokawa, J.; Pastan, I.; et al. New gene expressed in prostate: A potential target for T cell-mediated prostate cancer immunotherapy. Cancer Immunol. Immunother. 2010, 59, 63–71. [Google Scholar] [CrossRef]
- Das, S.; Hahn, Y.; Walker, D.A.; Nagata, S.; Willingham, M.C.; Peehl, D.M.; Bera, T.K.; Lee, B.; Pastan, I. Topology of NGEP, a prostate-specific cell:cell junction protein widely expressed in many cancers of different grade level. Cancer Res. 2008, 68, 6306–6312. [Google Scholar] [CrossRef]
- Das, S.; Hahn, Y.; Nagata, S.; Willingham, M.C.; Bera, T.K.; Lee, B.; Pastan, I. NGEP, a prostate-specific plasma membrane protein that promotes the association of LNCaP cells. Cancer Res. 2007, 67, 1594–1601. [Google Scholar] [CrossRef]
- Bera, T.K.; Das, S.; Maeda, H.; Beers, R.; Wolfgang, C.D.; Kumar, V.; Hahn, Y.; Lee, B.; Pastan, I. NGEP, a gene encoding a membrane protein detected only in prostate cancer and normal prostate. Proc. Natl. Acad. Sci. USA 2004, 101, 3059–3064. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Vural, S.; Mishra, N.K.; Cowan, K.H.; Guda, C. Exome analysis reveals differentially mutated gene signatures of stage, grade and subtype in breast cancers. PLoS ONE 2015, 10, e0119383. [Google Scholar] [CrossRef]
- Jun, I.; Park, H.S.; Piao, H.; Han, J.W.; An, M.J.; Yun, B.G.; Zhang, X.; Cha, Y.H.; Shin, Y.K.; Yook, J.I.; et al. ANO9/TMEM16J promotes tumourigenesis via EGFR and is a novel therapeutic target for pancreatic cancer. Br. J. Cancer 2017. [Google Scholar] [CrossRef]
- Li, C.; Cai, S.; Wang, X.; Jiang, Z. Identification and characterization of ANO9 in stage II and III colorectal carcinoma. Oncotarget 2015, 6, 29324–29334. [Google Scholar] [CrossRef]
- Kunzelmann, K.; Tian, Y.; Martins, J.R.; Faria, D.; Kongsuphol, P.; Ousingsawat, J.; Thevenod, F.; Roussa, E.; Rock, J.R.; Schreiber, R. Anoctamins. Pflug. Arch. 2011, 462, 195–208. [Google Scholar] [CrossRef]
- Pedemonte, N.; Galietta, L.J. Structure and Function of TMEM16 Proteins (Anoctamins). Physiol. Rev. 2014, 94, 419–459. [Google Scholar] [CrossRef]
- Paulino, C.; Neldner, Y.; Lam, A.K.; Kalienkova, V.; Brunner, J.D.; Schenck, S.; Dutzler, R. Structural basis for anion conduction in the calcium-activated chloride channel TMEM16A. Elife 2017, 6, e26232. [Google Scholar] [CrossRef]
- Brunner, J.D.; Lim, N.K.; Schenck, S.; Duerst, A.; Dutzler, R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature 2014, 516, 207–212. [Google Scholar] [CrossRef]
- Lee, B.C.; Khelashvili, G.; Falzone, M.; Menon, A.K.; Weinstein, H.; Accardi, A. Gating mechanism of the extracellular entry to the lipid pathway in a TMEM16 scramblase. Nat. Commun. 2018, 9, 3251. [Google Scholar] [CrossRef]
- Schreiber, R.; Ousingsawat, J.; Wanitchakool, P.; Sirianant, L.; Benedetto, R.; Reiss, K.; Kunzelmann, K. Regulation of TMEM16A/ANO1 and TMEM16F/ANO6 ion currents and phospholipid scrambling by Ca2+ and plasma membrane lipid. J. Physiol. 2018, 596, 217–229. [Google Scholar] [CrossRef]
- Shimizu, T.; Iehara, T.; Sato, K.; Fujii, T.; Sakai, H.; Okada, Y. TMEM16F is a component of a Ca2+-activated Cl-channel but not a volume-sensitive outwardly rectifying Cl-channel. Am. J. Physiol. Cell Physiol.. 2013, 304, C748–C759. [Google Scholar] [CrossRef]
- Grubb, S.; Poulsen, K.A.; Juul, C.A.; Kyed, T.; Klausen, T.K.; Larsen, E.H.; Hoffmann, E.K. TMEM16F (Anoctamin 6), an anion channel of delayed Ca2+ activation. J. Gen. Physiol.. 2013, 141, 585–600. [Google Scholar] [CrossRef]
- Ousingsawat, J.; Wanitchakool, P.; Kmit, A.; Romao, A.M.; Jantarajit, W.; Schreiber, S.; Kunzelmann, K. Anoctamin 6 mediates effects essential for innate immunity downstream of P2X7-receptors in macrophages. Nat. Commun. 2015, 6, 6245. [Google Scholar] [CrossRef]
- Gyobu, S.; Ishihara, K.; Suzuki, J.; Segawa, K.; Nagata, S. Characterization of the scrambling domain of the TMEM16 family. Proc. Natl. Acad. Sci. USA 2017. [Google Scholar] [CrossRef]
- Watanabe, R.; Sakuragi, T.; Noji, H.; Nagata, S. Single-molecule analysis of phospholipid scrambling by TMEM16F. Proc. Natl. Acad. Sci. USA 2018. [Google Scholar] [CrossRef]
- Paulino, C.; Kalienkova, V.; Lam, A.K.M.; Neldner, Y.; Dutzler, R. Activation mechanism of the calcium-activated chloride channel TMEM16A revealed by cryo-EM. Nature 2017, 552, 421–425. [Google Scholar] [CrossRef]
- Tian, Y.; Schreiber, R.; Kunzelmann, K. Anoctamins are a family of Ca2+ activated Cl-channels. J. Cell Sci. 2012, 125, 4991–4998. [Google Scholar] [CrossRef]
- Whitlock, J.M.; Yu, K.; Cui, Y.Y.; Hartzell, H.C. Anoctamin 5/TMEM16E facilitates muscle precursor cell fusion. J. Gen. Physiol.. 2018. [Google Scholar] [CrossRef]
- He, Q.; Halm, S.T.; Zhang, J.; Halm, D.R. Activation of the basolateral membrane Cl conductance essential for electrogenic K secretion suppresses electrogenic Cl secretion. Exp. Physiol. 2011, 96, 305–316. [Google Scholar] [CrossRef]
- Yokoyama, T.; Takemoto, M.; Hirakawa, M.; Saino, T. Different immunohistochemical localization for TMEM16A and CFTR in acinar and ductal cells of rat major salivary glands and exocrine pancreas. Acta Histochem. 2018. [Google Scholar] [CrossRef]
- Schreiber, R.; Faria, D.; Skryabin, B.V.; Rock, J.R.; Kunzelmann, K. Anoctamins support calcium-dependent chloride secretion by facilitating calcium signaling in adult mouse intestine. Pflüg. Arch. 2014, 467, 1203–1213. [Google Scholar] [CrossRef]
- Benedetto, R.; Cabrita, I.; Schreiber, R.; Kunzelmann, K. TMEM16A is indispensable for basal mucus secretion in airways and intestine. FASEB J. 2019. [Google Scholar] [CrossRef]
- Wanitchakool, P.; Ousingsawat, J.; Sirianant, L.; Cabrita, I.; Faria, D.; Schreiber, R.; Kunzelmann, K. Cellular defects by deletion of ANO10 are due to deregulated local calcium signaling. Cell Signal. 2017, 30, 41–49. [Google Scholar] [CrossRef]
- Hammer, C.; Wanitchakool, P.; Sirianant, L.; Papiol, S.; Monnheimer, M.; Faria, D.; Ousingsawat, J.; Schramek, N.; Schmitt, C.; Margos, G.; et al. A coding variant of ANO10, affecting volume regulation of macrophages, is associated with Borrelia seropositivity. Mol. Med. 2015, 21, 26–37. [Google Scholar] [CrossRef]
- Cabrita, I.; Benedetto, R.; Fonseca, A.; Wanitchakool, P.; Sirianant, L.; Skryabin, B.V.; Schenk, L.K.; Pavenstadt, H.; Schreiber, R.; Kunzelmann, K. Differential effects of anoctamins on intracellular calcium signals. Faseb J. 2017, 31, 2123–2134. [Google Scholar] [CrossRef]
- Ruppersburg, C.C.; Hartzell, H.C. The Ca2+-activated Cl- channel ANO1/TMEM16A regulates primary ciliogenesis. Mol. Biol. Cell 2014, 25, 1793–1807. [Google Scholar] [CrossRef]
- Schreiber, R.; Kunzelmann, K. Expression of anoctamins in retinal pigment epithelium (RPE). Pflug. Arch. 2016, 468, 1921–1929. [Google Scholar] [CrossRef]
- Forschbach, V.; Goppelt-Struebe, M.; Kunzelmann, K.; Schreiber, R.; Piedagnel, R.; Kraus, A.; Eckardt, K.U.; Buchholz, B. Anoctamin 6 is localized in the primary cilium of renal tubular cells and is involved in apoptosis-dependent cyst lumen formation. Cell Death Dis. 2015, 6, e1899. [Google Scholar] [CrossRef]
- Buchholz, B.; Faria, D.; Schley, G.; Schreiber, R.; Eckardt, K.U.; Kunzelmann, K. Anoctamin 1 induces calcium-activated chloride secretion and tissue proliferation in polycystic kidney disease. Kidney Int. 2014, 85, 1058–1067. [Google Scholar] [CrossRef]
- Tian, Y.; Schreiber, R.; Wanitchakool, P.; Kongsuphol, P.; Sousa, M.; Uliyakina, I.; Palma, M.; Faria, D.; Traynor-Kaplen, A.E.; Fragata, J.I.; et al. Control of TMEM16A by INO-4995 and other inositolphosphates. Br. J. Pharmacol. 2012, 168, 253–265. [Google Scholar] [CrossRef]
- AlDehni, F.; Spitzner, M.; Martins, J.R.; Barro Soria, R.; Schreiber, R.; Kunzelmann, K. Role of bestrophin for proliferation and in epithelial to mesenchymal transition. J. Am. Soc. Nephrol. 2009, 20, 1556–1564. [Google Scholar] [CrossRef]
- Barro Soria, R.; Spitzner, M.; Schreiber, R.; Kunzelmann, K. Bestrophin 1 enables Ca2+ activated Cl-conductance in epithelia. J. Biol. Chem. 2009, 284, 29405–29412. [Google Scholar] [CrossRef]
- Schreiber, R.; Buchholz, B.; Kraus, A.; Schley, G.; Scholz, J.; Ousingsawat, J.; Kunzelmann, K. Lipid peroxidation drives renal cyst growth in vitro through activation of TMEM16A. J. Am. Soc. Nephrol. JASN 2019. [Google Scholar] [CrossRef]
- Schlatter, E.; Frobe, U.; Greger, R. Ion conductances of isolated cortical collecting duct cells. Pflug. Arch. 1992, 421, 381–387. [Google Scholar] [CrossRef]
- Morris, A.P.; Frizzell, R.A. Ca(2+)-dependent Cl-channels in undifferentiated human colonic cells (HT-29). I. Single-channel properties. Am. J. Physiol. 1993, 264, C968–C976. [Google Scholar] [CrossRef]
- Qu, Z.; Yao, W.; Yao, R.; Liu, X.; Yu, K.; Hartzell, H.C. The Ca -activated Cl channel, ANO1 (TMEM16A), is a double-edged sword in cell proliferation and tumorigenesis. Cancer Med. 2014, 3, 453–461. [Google Scholar] [CrossRef]
- Fujimoto, M.; Kito, H.; Kajikuri, J.; Ohya, S. Transcriptional repression of human epidermal growth factor receptor 2 by ClC-3 Cl(-) /H(+) transporter inhibition in human breast cancer cells. Cancer Sci. 2018, 109, 2781–2791. [Google Scholar] [CrossRef]
- Almaca, J.; Tian, Y.; AlDehni, F.; Ousingsawat, J.; Kongsuphol, P.; Rock, J.R.; Harfe, B.D.; Schreiber, R.; Kunzelmann, K. TMEM16 proteins produce volume regulated chloride currents that are reduced in mice lacking TMEM16A. J. Biol. Chem. 2009, 284, 28571–28578. [Google Scholar] [CrossRef]
- Wang, M.; Yang, H.; Zheng, L.Y.; Zhang, Z.; Tang, Y.B.; Wang, G.L.; Du, Y.H.; Lv, X.F.; Liu, J.; Zhou, J.G.; et al. Downregulation of TMEM16A Calcium-Activated Chloride Channel Contributes to Cerebrovascular Remodeling during Hypertension through Promoting Basilar Smooth Muscle Cell Proliferation. Circulation 2012, 125, 697–707. [Google Scholar] [CrossRef]
- Allawzi, A.M.; Vang, A.; Clements, R.T.; Jhun, B.S.; Kue, N.R.; Mancini, T.J.; Landi, A.K.; Terentyev, D.; O-Uchi, J.; Comhair, S.A.; et al. Activation of Anoctamin-1 Limits Pulmonary Endothelial Cell Proliferation via p38-MAPK-dependent Apoptosis. Am. J. Respir. Cell Mol. Biol. 2017. [Google Scholar] [CrossRef]
- Sui, Y.; Wu, F.; Lv, J.; Li, H.; Li, X.; Du, Z.; Sun, M.; Zheng, Y.; Yang, L.; Zhong, L.; et al. Identification of the Novel TMEM16A Inhibitor Dehydroandrographolide and Its Anticancer Activity on SW620 Cells. PLoS ONE 2015, 10, e0144715. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, J.; Chang, J.; Kim, S.S.; Namkung, W.; Kim, I. Synthesis and biological evaluation of novel Ani9 derivatives as potent and selective ANO1 inhibitors. Eur. J. Med. Chem. 2018, 160, 245–255. [Google Scholar] [CrossRef]
- Miner, K.; Labitzke, K.; Liu, B.; Elliot, R.; Wang, P.; Henckels, K.; Gaida, K.; Elliot, R.; Chen, J.J.; Liu, L.; et al. The Anthelminthic Niclosamide And Related Compounds Represent Potent Tmem16a Antagonists That Fully Relax Mouse And Human Airway Rings. Froniers Pharmacol. 2019. [Google Scholar] [CrossRef]
- Seo, Y.; Park, J.; Kim, M.; Lee, H.K.; Kim, J.H.; Jeong, J.H.; Namkung, W. Inhibition of ANO1/TMEM16A Chloride Channel by Idebenone and Its Cytotoxicity to Cancer Cell Lines. PLoS ONE 2015, 10, e0133656. [Google Scholar] [CrossRef]
- Wang, A.M.; Ku, H.H.; Liang, Y.C.; Chen, Y.C.; Hwu, Y.M.; Yeh, T.S. The autonomous notch signal pathway is activated by baicalin and baicalein but is suppressed by niclosamide in K562 cells. J. Cell Biochem. 2009, 106, 682–692. [Google Scholar] [CrossRef]
- Meurette, O.; Mehlen, P. Notch Signaling in the Tumor Microenvironment. Cancer Cell 2018, 34, 536–548. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kang, J.W.; Song, X.; Kim, B.K.; Yoo, Y.D.; Kwon, Y.T.; Lee, Y.J. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell Signal. 2013, 25, 961–969. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, Z.; Ding, K.; Li, J.; Du, X.; Chen, C.; Sun, X.; Wu, Y.; Zhou, J.; Pan, J. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: Inactivation of the NF-kappaB pathway and generation of reactive oxygen species. Cancer Res. 2010, 70, 2516–2527. [Google Scholar] [CrossRef]
- Ren, X.; Duan, L.; He, Q.; Zhang, Z.; Zhou, Y.; Wu, D.; Pan, J.; Pei, D.; Ding, K. Identification of Niclosamide as a New Small-Molecule Inhibitor of the STAT3 Signaling Pathway. ACS Med. Chem. Lett. 2010, 1, 454–459. [Google Scholar] [CrossRef]
- Osada, T.; Chen, M.; Yang, X.Y.; Spasojevic, I.; Vandeusen, J.B.; Hsu, D.; Clary, B.M.; Clay, T.M.; Chen, W.; Morse, M.A.; et al. Antihelminth compound niclosamide downregulates Wnt signaling and elicits antitumor responses in tumors with activating APC mutations. Cancer Res. 2011, 71, 4172–4182. [Google Scholar] [CrossRef]
- Wang, L.H.; Xu, M.; Fu, L.Q.; Chen, X.Y.; Yang, F. The Antihelminthic Niclosamide Inhibits Cancer Stemness, Extracellular Matrix Remodeling, and Metastasis through Dysregulation of the Nuclear beta-catenin/c-Myc axis in OSCC. Sci. Rep. 2018, 8, 12776. [Google Scholar] [CrossRef]
- Arend, R.C.; Londono-Joshi, A.I.; Gangrade, A.; Katre, A.A.; Kurpad, C.; Li, Y.; Samant, R.S.; Li, P.K.; Landen, C.N.; Yang, E.S.; et al. Niclosamide and its analogs are potent inhibitors of Wnt/beta-catenin, mTOR and STAT3 signaling in ovarian cancer. Oncotarget 2016, 7, 86803–86815. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Yang, J.H.; Kim, N.H.; Lee, K.; Cha, Y.H.; Yun, J.S.; Kang, H.E.; Lee, Y.; Choi, J.; Kim, H.S.; et al. Anti-helminthic niclosamide inhibits Ras-driven oncogenic transformation via activation of GSK-3. Oncotarget 2017, 8, 31856–31863. [Google Scholar] [CrossRef]
- Chen, B.; Wei, W.; Ma, L.; Yang, B.; Gill, R.M.; Chua, M.S.; Butte, A.J.; So, S. Computational Discovery of Niclosamide Ethanolamine, a Repurposed Drug Candidate That Reduces Growth of Hepatocellular Carcinoma Cells In Vitro and in Mice by Inhibiting Cell Division Cycle 37 Signaling. Gastroenterology 2017, 152, 2022–2036. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.K.; Roberts, M.J.; Arend, R.C.; Samant, R.S.; Buchsbaum, D.J. Multi-targeted therapy of cancer by niclosamide: A new application for an old drug. Cancer Lett. 2014, 349, 8–14. [Google Scholar] [CrossRef]
- Lafkas, D.; Shelton, A.; Chiu, C.; de Leon, B.G.; Chen, Y.; Stawicki, S.S.; Siltanen, C.; Reichelt, M.; Zhou, M.; Wu, X.; et al. Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature 2015, 528, 127–131. [Google Scholar] [CrossRef]
- Danahay, H.; Pessotti, A.D.; Coote, J.; Montgomery, B.E.; Xia, D.; Wilson, A.; Yang, H.; Wang, Z.; Bevan, L.; Thomas, C.; et al. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep. 2015, 10, 239–252. [Google Scholar] [CrossRef]
- Kondo, M.; Tsuji, M.; Hara, K.; Arimura, K.; Yagi, O.; Tagaya, E.; Takeyama, K.; Tamaoki, J. Chloride ion transport and overexpression of TMEM16A in a guinea pig asthma model. Clin. Exp. Allergy 2017, 47, 795–804. [Google Scholar] [CrossRef]
- Kondo, M.; Nakata, J.; Arai, N.; Izumo, T.; Tagaya, E.; Takeyama, K.; Tamaoki, J.; Nagai, A. Niflumic acid inhibits goblet cell degranulation in a guinea pig asthma model. Allergol. Int. 2012, 61, 133–142. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Wu, Y.; Ren, Y.; Li, Z.; Yao, X.; Zhang, C.; Ye, N.; Jing, C.; Dong, J.; et al. Suppression of the Growth and Invasion of Human Head and Neck Squamous Cell Carcinomas via Regulating STAT3 Signaling and the miR-21/beta-catenin Axis with HJC0152. Mol. Cancer Ther. 2017, 16, 578–590. [Google Scholar] [CrossRef]
- Liu, C.; Lou, W.; Zhu, Y.; Nadiminty, N.; Schwartz, C.T.; Evans, C.P.; Gao, A.C. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin. Cancer Res. 2014, 20, 3198–3210. [Google Scholar] [CrossRef]
- Wieland, A.; Trageser, D.; Gogolok, S.; Reinartz, R.; Hofer, H.; Keller, M.; Leinhaas, A.; Schelle, R.; Normann, S.; Klaas, L.; et al. Anticancer effects of niclosamide in human glioblastoma. Clin. Cancer Res. 2013, 19, 4124–4136. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Haugk, K.; McKiernan, J.S.; Gulati, R.; Cheng, H.H.; Maes, J.L.; Dumpit, R.F.; Nelson, P.S.; Montgomery, B.; McCune, J.S.; et al. A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer. PLoS ONE 2018, 13, e0198389. [Google Scholar] [CrossRef]
- Burock, S.; Daum, S.; Keilholz, U.; Neumann, K.; Walther, W.; Stein, U. Phase II trial to investigate the safety and efficacy of orally applied niclosamide in patients with metachronous or sychronous metastases of a colorectal cancer progressing after therapy: The NIKOLO trial. BMC Cancer 2018, 18, 297. [Google Scholar] [CrossRef]
- Di Zanni, E.; Gradogna, A.; Scholz-Starke, J.; Boccaccio, A. Gain of function of TMEM16E/ANO5 scrambling activity caused by a mutation associated with gnathodiaphyseal dysplasia. Cell. Mol. Life Sci. 2017. [Google Scholar] [CrossRef]
- Mattheij, N.J.; Braun, A.; van Kruchten, R.; Castoldi, E.; Pircher, J.; Baaten, C.C.; Wulling, M.; Kuijpers, M.J.; Kohler, R.; Poole, A.W.; et al. Survival protein anoctamin-6 controls multiple platelet responses including phospholipid scrambling, swelling, and protein cleavage. FASEB J. 2015, 30, 727–737. [Google Scholar] [CrossRef]
- Carpenter, G. The EGF receptor: A nexus for trafficking and signaling. Bioessays 2000, 22, 697–707. [Google Scholar] [CrossRef]
- Hodeify, R.; Yu, F.; Courjaret, R.; Nader, N.; Dib, M.; Sun, L.; Adap, E.; Hubrack, S.; Machaca, K. Regulation and Role of Store-Operated Ca(2+) Entry in Cellular Proliferation. In Calcium Entry Channels in Non-Excitable Cells; Kozak, J.A., Putney, J.W., Jr., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2018; pp. 215–240. [Google Scholar]
- Kunzelmann, K.; Cabrita, I.; Wanitchakool, P.; Ousingsawat, J.; Sirianant, L.; Benedetto, R.; Schreiber, R. Modulating Ca2+signals: A common theme for TMEM16, Ist2, and TMC. Pflüg. Arch. 2016, 468, 475–490. [Google Scholar] [CrossRef]
- Benedetto, R.; Ousingsawat, J.; Wanitchakool, P.; Zhang, Y.; Holtzman, M.J.; Amaral, M.; Rock, J.R.; Schreiber, R.; Kunzelmann, K. Epithelial Chloride Transport by CFTR Requires TMEM16A. Sci. Rep. 2017, 7, 12397. [Google Scholar] [CrossRef]
- Wolf, W.; Kilic, A.; Schrul, B.; Lorenz, H.; Schwappach, B.; Seedorf, M. Yeast Ist2 recruits the endoplasmic reticulum to the plasma membrane and creates a ribosome-free membrane microcompartment. PLoS ONE 2012, 7, e39703. [Google Scholar] [CrossRef]
- Jin, X.; Shah, S.; Liu, Y.; Zhang, H.; Lees, M.; Fu, Z.; Lippiat, J.D.; Beech, D.J.; Sivaprasadarao, A.; Baldwin, S.A.; et al. Activation of the Cl- Channel ANO1 by Localized Calcium Signals in Nociceptive Sensory Neurons Requires Coupling with the IP3 Receptor. Sci. Signal 2013, 6, ra73. [Google Scholar] [CrossRef]
- Jin, X.; Shah, S.; Du, X.; Zhang, H.; Gamper, N. Activation of Ca2+-activated Cl- channel ANO1 by localized Ca2+ signals. J. Physiol. 2016, 594, 19–30. [Google Scholar] [CrossRef]
- Franklin, B.M.; Voss, S.R.; Osborn, J.L. Ion channel signaling influences cellular proliferation and phagocyte activity during axolotl tail regeneration. Mech. Dev. 2017, 146, 42–54. [Google Scholar] [CrossRef]
- Kunzelmann, K. TMEM16, LRRC8A, bestrophin: Chloride channels controlled by Ca and cell volume. Trends Biochem. Sci. 2015, 40, 535–543. [Google Scholar] [CrossRef]
- Agell, N.; Bachs, O.; Rocamora, N.; Villalonga, P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell Signal. 2002, 14, 649–654. [Google Scholar] [CrossRef]
- Darling, N.J.; Cook, S.J. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta 2014, 1843, 2150–2163. [Google Scholar] [CrossRef]
- Gao da, Y.; Zhang, B.L.; Leung, M.C.; Au, S.C.; Wong, P.Y.; Shum, W.W. Coupling of TRPV6 and TMEM16A in epithelial principal cells of the rat epididymis. J. Gen. Physiol.. 2016, 148, 161–182. [Google Scholar] [CrossRef]
- Barro Soria, R.; AlDehni, F.; Almaca, J.; Witzgall, R.; Schreiber, R.; Kunzelmann, K. ER localized bestrophin1 acts as a counter-ion channel to activate Ca2+ dependent ion channels TMEM16A and SK4. Pflüg. Arch. 2009, 459, 485–497. [Google Scholar] [CrossRef]
- Martins, J.R.; Kongsuphol, P.; Sammels, E.; Daimène, S.; AlDehni, F.; Clarke, L.; Schreiber, R.; DeSmedt, H.; Amaral, M.D.; Kunzelmann, K. F508del-CFTR increases intracellular Ca2+ signaling that causes enhanced calcium-dependent Cl-conductance in cystic fibrosis. Biochim. Biophys. Acta 2011, 1812, 1385–1392. [Google Scholar] [CrossRef]
- Yang, H.; Kim, A.; David, T.; Palmer, D.; Jin, T.; Tien, J.; Huang, F.; Cheng, T.; Coughlin, S.R.; Jan, Y.N.; et al. TMEM16F Forms a Ca(2+)-Activated Cation Channel Required for Lipid Scrambling in Platelets during Blood Coagulation. Cell 2012, 151, 111–122. [Google Scholar] [CrossRef]
- Keckeis, S.; Wernecke, L.; Salchow, D.J.; Reichhart, N.; Strauss, O. Activation of a Ca2+-dependent cation conductance with properties of TRPM2 by reactive oxygen species in lens epithelial cells. Exp. Eye Res. 2017, 161, 61–70. [Google Scholar] [CrossRef]
- Keckeis, S.; Reichhart, N.; Roubeix, C.; Strauss, O. Anoctamin2 (TMEM16B) forms the Ca(2+)-activated Cl(-) channel in the retinal pigment epithelium. Exp. Eye Res. 2017, 154, 139–150. [Google Scholar] [CrossRef]
- Sirianant, L.; Ousingsawat, J.; Tian, Y.; Schreiber, R.; Kunzelmann, K. TMC8 (EVER2) attenuates intracellular signaling by Zn2+ and Ca2+ and suppresses activation of Cl-currents. Cell Signal. 2014, 26, 2826–2833. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Lee, J.; Lee, B.; Kim, H.R.; Jung, J.; Lee, M.O.; Oh, U. Anoctamin 9/TMEM16J is a cation channel activated by cAMP/PKA signal. Cell Calcium 2018, 71, 75–85. [Google Scholar] [CrossRef]
- Gyobu, S.; Miyata, H.; Ikawa, M.; Yamazaki, D.; Takeshima, H.; Suzuki, J.; Nagata, S. A role of TMEM16E carrying a scrambling domain in sperm motility. Mol. Cell. Biol. 2015, 36, 645–659. [Google Scholar] [CrossRef]
- Kunzelmann, K.; Schreiber, R. Chloride secretion, anoctamin 1 and Ca2+ signaling. Channels (Austin) 2014, 8, 387–388. [Google Scholar] [CrossRef]
- Zak, J.D.; Grimaud, J.; Li, R.C.; Lin, C.C.; Murthy, V.N. Calcium-activated chloride channels clamp odor-evoked spike activity in olfactory receptor neurons. Sci. Rep. 2018, 8, 10600. [Google Scholar] [CrossRef]
- Malysz, J.; Gibbons, S.J.; Saravanaperumal, S.A.; Du, P.; Eisenman, S.T.; Cao, C.; Oh, U.; Saur, D.; Klein, S.; Ordog, T.; et al. Conditional genetic deletion of Ano1 in interstitial cells of Cajal impairs Ca2+ transients and slow-waves in adult mouse small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2016. [Google Scholar] [CrossRef]
- He, M.; Ye, W.; Wang, W.J.; Sison, E.S.; Jan, Y.N.; Jan, L.Y. Cytoplasmic Cl(-) couples membrane remodeling to epithelial morphogenesis. Proc. Natl. Acad. Sci. USA 2017, 114, e11161–e11169. [Google Scholar] [CrossRef]
- Benedetto, R.; Sirianant, L.; Pankonien, I.; Wanitchakool, P.; Ousingsawat, J.; Cabrita, I.; Schreiber, R.; Amaral, M.; Kunzelmann, K. Relationship between TMEM16A/anoctamin 1 and LRRC8A. Pflug. Arch. 2016, 468, 1751–1763. [Google Scholar] [CrossRef]
- Schenk, L.K.; Buchholz, B.; Henke, S.F.; Michgehl, U.; Daniel, C.; Amann, K.; Kunzelmann, K.; Pavenstadt, H.J. Nephron-specific knockout of TMEM16A leads to reduced number of glomeruli and albuminuria. Am. J. Physiol. Renal Physiol. 2018. [Google Scholar] [CrossRef]
- Bricogne, C.; Fine, M.; Pereira, P.M.; Sung, J.; Tijani, M.; Wang, Y.; Henriques, R.; Collins, M.K.; Hilgemann, D. TMEM16F activation by Ca(2+) triggers plasma membrane expansion and directs PD-1 trafficking. Sci. Rep. 2019, 9, 619. [Google Scholar] [CrossRef]
- Faria, D.; Schlatter, E.; Witzgall, R.; Grahammer, F.; Bandulik, S.; Schweda, F.; Bierer, S.; Rock, J.R.; Heitzmann, D.; Kunzelmann, K.; et al. The calcium activated chloride channel Anoctamin 1 contributes to the regulation of renal function. Kindey Int. 2014, 85, 1369–1381. [Google Scholar] [CrossRef]
- Ousingsawat, J.; Wanitchakool, P.; Schreiber, R.; Wuelling, M.; Vortkamp, A.; Kunzelmann, K. Anoctamin 6 controls bone mineralization by activating the calcium transporter NCX1. J. Biol. Chem. 2015, 290, 6270–6280. [Google Scholar] [CrossRef]
- Benedetto, R.; Ousingsawat, J.; Cabrita, I.; Pinto, M.; Lérias, J.R.; Wanitchakool, P.; Schreiber, R.; Kunzelmann, K. Plasma membrane localized TMEM16 proteins are indispensable for expression of CFTR. J. Mol. Med. 2019. [Google Scholar] [CrossRef]
- Oheim, M.; Kirchhoff, F.; Stuhmer, W. Calcium microdomains in regulated exocytosis. Cell Calcium 2006, 40, 423–439. [Google Scholar] [CrossRef]
- Chieregatti, E.; Meldolesi, J. Regulated exocytosis: New organelles for non-secretory purposes. Nat. Rev. Mol. Cell Biol. 2005, 6, 181–187. [Google Scholar] [CrossRef]
- Jacobsen, K.S.; Zeeberg, K.; Poulsen, K.A.; Hoffmann, E.K.; Schwab, A. The role of TMEM16A (ANO1) and TMEM16F (ANO6) in cell migration. Pflug. Arch. 2013, 465, 1753–1762. [Google Scholar] [CrossRef]
- Veit, M.; Koyro, K.I.; Ahrens, B.; Bleibaum, F.; Munz, M.; Rövekamp, H.; Andrä, J.; Schreiber, R.; Kunzelmann, K.; Sommer, A.; et al. Anoctamin-6 regulates ADAM sheddase function. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1598–1610. [Google Scholar] [CrossRef]
- Bleibaum, F.; Sommer, A.; Veit, M.; Rabe, B.; Andrä, J.; Kunzelmann, K.; Nehls, C.; Correa, W.; Gutsmann, T.; Grötzinger, J.; et al. ADAM10 sheddase activation is controlled by cell membrane asymmetry. J. Mol. Cell. Biol. 2018. [Google Scholar] [CrossRef]
- Sommer, A.; Kordowski, F.; Buch, J.; Maretzky, T.; Evers, A.; Andra, J.; Dusterhoft, S.; Michalek, M.; Lorenzen, I.; Somasundaram, P.; et al. Phosphatidylserine exposure is required for ADAM17 sheddase function. Nat. Commun. 2016, 7, 11523. [Google Scholar] [CrossRef]
- Crutzen, R.; Virreira, M.; Markadieu, N.; Shlyonsky, V.; Sener, A.; Malaisse, W.J.; Beauwens, R.; Boom, A.; Golstein, P.E. Anoctamin 1 (Ano1) is required for glucose-induced membrane potential oscillations and insulin secretion by murine beta-cells. Pflug. Arch. 2015, 468, 573–591. [Google Scholar] [CrossRef]
- Edlund, A.; Esguerra, J.L.; Wendt, A.; Flodstrom-Tullberg, M.; Eliasson, L. CFTR and Anoctamin 1 (ANO1) contribute to cAMP amplified exocytosis and insulin secretion in human and murine pancreatic beta-cells. BMC Med. 2014, 12, 87–12. [Google Scholar] [CrossRef]
- Tian, Y.; Kongsuphol, P.; Hug, M.J.; Ousingsawat, J.; Witzgall, R.; Schreiber, R.; Kunzelmann, K. Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel TMEM16A. FASEB J. 2011, 25, 1058–1068. [Google Scholar] [CrossRef]
- Gupta, R.; Radicioni, G.; Abdelwahab, S.; Dang, H.; Carpenter, J.; Chua, M.; Mieczkowski, P.A.; Sheridan, J.; Randell, S.H.; Kesimer, M. Intercellular Communication between Airway Epithelial Cells is Mediated by Exosome-Like Vesicles. Am. J. Respir. Cell Mol. Biol. 2018. [Google Scholar] [CrossRef]
- Whitlock, J.M.; Hartzell, H.C. Anoctamins/TMEM16 Proteins: Chloride Channels Flirting with Lipids and Extracellular Vesicles. Annu. Rev. Physiol. 2016. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Han, T.W.; Ye, W.; Bethel, N.P.; Zubia, M.; Kim, A.; Li, K.H.; Burlingame, A.L.; Grabe, M.; Jan, Y.N.; Jan, L.Y. Chemically induced vesiculation as a platform for studying TMEM16F activity. Proc. Natl. Acad. Sci. USA 2019. [Google Scholar] [CrossRef]
- Svenningsen, P.; Nielsen, M.R.; Marcussen, N.; Walter, S.; Jensen, B.L. TMEM16A is a Ca -activated Cl channel expressed in the renal collecting duct. Acta Physiol. 2014, 212, 166–174. [Google Scholar] [CrossRef]
- Jimenez, A.J.; Maiuri, P.; Lafaurie-Janvore, J.; Divoux, S.; Piel, M.; Perez, F. ESCRT machinery is required for plasma membrane repair. Science 2014, 343, 1247136. [Google Scholar] [CrossRef]
- Gong, Y.N.; Guy, C.; Olauson, H.; Becker, J.U.; Yang, M.; Fitzgerald, P.; Linkermann, A.; Green, D.R. ESCRT-III Acts Downstream of MLKL to Regulate Necroptotic Cell Death and Its Consequences. Cell 2017, 169, 286–300.e216. [Google Scholar] [CrossRef]
- Bolduc, V.; Marlow, G.; Boycott, K.M.; Saleki, K.; Inoue, H.; Kroon, J.; Itakura, M.; Robitaille, Y.; Parent, L.; Baas, F.; et al. Recessive mutations in the putative calcium-activated chloride channel Anoctamin 5 cause proximal LGMD2L and distal MMD3 muscular dystrophies. Am. J. Hum. Genet. 2010, 86, 213–221. [Google Scholar] [CrossRef]
- Ousingsawat, J.; Cabrita, I.; Wanitchakool, P.; Sirianant, L.; Krautwald, S.; Linkermann, A.; Schreiber, R.; Kunzelmann, K. Ca2+ signals, cell membrane disintegration, and activation of TMEM16F during necroptosis. Cell. Mol. Life Sci. 2016. [Google Scholar] [CrossRef]
- Sirianant, L.; Ousingsawat, J.; Wanitchakool, P.; Schreiber, R.; Kunzelmann, K. Cellular Volume regulation by Anoctamin 6:Ca2+, phospholipase A2,osmosensing. Pflüg. Arch. 2015, 468, 335–349. [Google Scholar] [CrossRef]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Pfitzner, E.; Kliem, S.; Baus, D.; Litterst, C.M. The role of STATs in inflammation and inflammatory diseases. Curr. Pharm. Des. 2004, 10, 2839–2850. [Google Scholar] [CrossRef]
- Nussinov, R.; Tsai, C.J.; Jang, H. Oncogenic Ras Isoforms Signaling Specificity at the Membrane. Cancer Res. 2018, 78, 593–602. [Google Scholar] [CrossRef]
- Galietta, L.J.; Pagesy, P.; Folli, C.; Caci, E.; Romio, L.; Costes, B.; Nicolis, E.; Cabrini, G.; Goossens, M.; Ravazzolo, R.; et al. IL-4 Is a Potent Modulator of Ion Transport in the Human Bronchial Epithelium In Vitro. J. Immunol. 2002, 168, 839–845. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, H.; Wu, M.; Yang, H.; Kudo, M.; Peters, C.J.; Woodruff, P.G.; Solberg, O.D.; Donne, M.L.; Huang, X.; et al. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc. Natl. Acad. Sci. USA 2012, 109, 16354–16359. [Google Scholar] [CrossRef]
- Caci, E.; Scudieri, P.; Di Carlo, E.; Morelli, P.; Bruno, S.; De, F.I.; Bragonzi, A.; Gianotti, A.; Sondo, E.; Ferrera, L.; et al. Upregulation of TMEM16A Protein in Bronchial Epithelial Cells by Bacterial Pyocyanin. PLoS ONE 2015, 10, e0131775. [Google Scholar] [CrossRef]
- Scudieri, P.; Caci, E.; Bruno, S.; Ferrera, L.; Schiavon, M.; Sondo, E.; Tomati, V.; Gianotti, A.; Zegarra-Moran, O.; Pedemonte, N.; et al. Association of TMEM16A chloride channel overexpression with airway goblet cells metaplasia. J. Physiol. 2012, 590, 6141–6155. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Lang, F.; Hoffmann, E.K. CrossTalk proposal: Cell volume changes are an essential step in the cell death machinery. J. Physiol. 2013, 591, 6119–6121. [Google Scholar] [CrossRef]
- Hoffmann, E.K.; Lambert, I.H.; Pedersen, S.F. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009, 89, 193–277. [Google Scholar] [CrossRef]
- Lee, E.L.; Shimizu, T.; Ise, T.; Numata, T.; Kohno, K.; Okada, Y. Impaired activity of volume-sensitive Cl- channel is involved in cisplatin resistance of cancer cells. J. Cell. Physiol. 2007, 211, 513–521. [Google Scholar] [CrossRef]
- Okada, Y.; Shimizu, T.; Maeno, E.; Tanabe, S.; Wang, X.; Takahashi, N. Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. J. Membr. Biol. 2006, 209, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Lee, E.L.; Ise, T.; Okada, Y. Volume-sensitive Cl(-) channel as a regulator of acquired cisplatin resistance. Anticancer Res. 2008, 28, 75–83. [Google Scholar] [PubMed]
- Lemonnier, L.; Shuba, Y.; Crepin, A.; Roudbaraki, M.; Slomianny, C.; Mauroy, B.; Nilius, B.; Prevarskaya, N.; Skryma, R. Bcl-2-dependent modulation of swelling-activated Cl- current and ClC-3 expression in human prostate cancer epithelial cells. Cancer Res. 2004, 64, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- Planells-Cases, R.; Lutter, D.; Guyader, C.; Gerhards, N.M.; Ullrich, F.; Elger, D.A.; Kucukosmanoglu, A.; Xu, G.; Voss, F.K.; Reincke, S.M.; et al. Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J. 2015, 34, 2993–3008. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.F.; Okada, Y.; Nilius, B. Biophysics and Physiology of the Volume-Regulated Anion Channel (VRAC)/Volume-Sensitive Outwardly Rectifying Anion Channel (VSOR). Pflug. Arch. 2016, 468, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Foller, M.; Lang, K.; Lang, P.; Ritter, M.; Vereninov, A.; Szabo, I.; Huber, S.M.; Gulbins, E. Cell volume regulatory ion channels in cell proliferation and cell death. Methods Enzymol. 2007, 428, 209–225. [Google Scholar] [PubMed]
- Juul, C.A.; Grubb, S.; Poulsen, K.A.; Kyed, T.; Hashem, N.; Lambert, I.H.; Larsen, E.H.; Hoffmann, E.K. Anoctamin 6 differs from VRAC and VSOAC but is involved in apoptosis and supports volume regulation in the presence of Ca. Pflug. Arch. 2014, 466, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Dubin, A.E.; Mathur, J.; Tu, B.; Reddy, K.; Miraglia, L.J.; Reinhardt, J.; Orth, A.P.; Patapoutian, A. SWELL1, a Plasma Membrane Protein, Is an Essential Component of Volume-Regulated Anion Channel. Cell 2014, 157, 447–458. [Google Scholar] [CrossRef]
- Voss, F.K.; Ullrich, F.; Munch, J.; Lazarow, K.; Lutter, D.; Mah, N.; Andrade-Navarro, M.A.; von Kries, J.P.; Stauber, T.; Jentsch, T.J. Identification of LRRC8 Heteromers as an Essential Component of the Volume-Regulated Anion Channel VRAC. Science 2014, 344, 634–638. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Klausen, T.K.; Nilius, B. The identification of VRAC (Volume Regulated Anion Channel):An amazing Odyssey. Acta Physiol. 2015, 213, 868–881. [Google Scholar] [CrossRef]
- Deneka, D.; Sawicka, M.; Lam, A.K.M.; Paulino, C.; Dutzler, R. Structure of a volume-regulated anion channel of the LRRC8 family. Nature 2018, 558, 254–259. [Google Scholar] [CrossRef]
- Kefauver, J.M.; Saotome, K.; Dubin, A.E.; Pallesen, J.; Cottrell, C.A.; Cahalan, S.M.; Qiu, Z.; Hong, G.; Crowley, C.S.; Whitwam, T.; et al. Structure of the human volume regulated anion channel. Elife 2018, 7, e38461. [Google Scholar] [CrossRef]
- Kasuya, G.; Nakane, T.; Yokoyama, T.; Jia, Y.; Inoue, M.; Watanabe, K.; Nakamura, R.; Nishizawa, T.; Kusakizako, T.; Tsutsumi, A.; et al. Cryo-EM structures of the human volume-regulated anion channel LRRC8. Nat. Struct. Mol. Biol. 2018, 25, 797–804. [Google Scholar] [CrossRef]
- Sirianant, L.; Wanitchakool, P.; Ousingsawat, J.; Benedetto, R.; Zormpa, A.; Cabrita, I.; Schreiber, R.; Kunzelmann, K. Non-essential contribution of LRRC8A to volume regulation. Pflüg. Arch. 2016, 468, 1789–1796. [Google Scholar] [CrossRef]
- Milenkovic, A.; Brandl, C.; Milenkovic, V.M.; Jendrike, T.; Sirianant, L.; Wanitchakool, P.; Zimmermann, S.; Reif, C.M.; Horling, F.; Schrewe, H.; et al. Bestrophin1 is the volume-regulated anion channel in mouse sperm and human retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2015, 112, E2630–E2639. [Google Scholar] [CrossRef]
- Wanitchakool, P.; Ousingsawat, J.; Sirianant, L.; MacAulay, N.; Schreiber, R.; Kunzelmann, K. Cl-channels in apoptosis. Eur. Biophys. J. 2016, 45, 599–610. [Google Scholar] [CrossRef]
- Kumar, L.; Chou, J.; Yee, C.S.; Borzutzky, A.; Vollmann, E.H.; von Andrian, U.H.; Park, S.Y.; Hollander, G.; Manis, J.P.; Poliani, P.L.; et al. Leucine-rich repeat containing 8A (LRRC8A) is essential for T lymphocyte development and function. J. Exp. Med. 2014, 211, 929–942. [Google Scholar] [CrossRef]
- Yamada, T.; Wondergem, R.; Morrison, R.; Yin, V.P.; Strange, K. Leucine-rich repeat containing protein LRRC8A is essential for swelling-activated Cl-currents and embryonic development in zebrafish. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef]
- Sawada, A.; Takihara, Y.; Kim, J.Y.; Matsuda-Hashii, Y.; Tokimasa, S.; Fujisaki, H.; Kubota, K.; Endo, H.; Onodera, T.; Ohta, H.; et al. A congenital mutation of the novel gene LRRC8 causes agammaglobulinemia in humans. J. Clin. Investig. 2003, 112, 1707–1713. [Google Scholar] [CrossRef]
- Bao, J.; Perez, C.J.; Kim, J.; Zhang, H.; Murphy, C.J.; Hamidi, T.; Jaubert, J.; Platt, C.D.; Chou, J.; Deng, M.; et al. Deficient LRRC8A-dependent volume-regulated anion channel activity is associated with male infertility in mice. JCI Insight 2018, 3, e99767. [Google Scholar] [CrossRef]
- Lemonnier, L.; Prevarskaya, N.; Shuba, Y.; Vanden Abeele, F.; Nilius, B.; Mazurier, J.; Skryma, R. Ca2+ modulation of volume-regulated anion channels: Evidence for colocalization with store-operated channels. FASEB J. 2002, 16, 222–224. [Google Scholar] [CrossRef]
- Zholos, A.; Beck, B.; Sydorenko, V.; Lemonnier, L.; Bordat, P.; Prevarskaya, N.; Skryma, R. Ca(2+)- and volume-sensitive chloride currents are differentially regulated by agonists and store-operated Ca2+ entry. J. Gen. Physiol. 2005, 125, 197–211. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, Z.; Zhang, D.; Li, H.; Zhang, L.; Niu, J.; Zuo, W.; Fu, R.; Fan, L.; Ye, J.H.; et al. High expression of leucinerich repeatcontaining 8A is indicative of a worse outcome of colon cancer patients by enhancing cancer cell growth and metastasis. Oncol. Rep. 2018, 40, 1275–1286. [Google Scholar]
- Sorensen, B.H.; Nielsen, D.; Thorsteinsdottir, U.A.; Hoffmann, E.K.; Lambert, I.H. Downregulation of LRRC8A protects human ovarian and alveolar carcinoma cells against Cisplatin-induced expression of p53, MDM2, p21Waf1/Cip1, and Caspase-9/-3 activation. Am. J. Physiol. Cell Physiol. 2016, 310, C857–C873. [Google Scholar] [CrossRef]
- Ghosh, A.; Khandelwal, N.; Kumar, A.; Bera, A.K. Leucine-rich repeat-containing 8B protein is associated with the endoplasmic reticulum Ca2+ leak in HEK293 cells. J. Cell Sci. 2017, 130, 3818–3828. [Google Scholar] [CrossRef]
- Borahay, M.A.; Kilic, G.S.; Yallampalli, C.; Snyder, R.R.; Hankins, G.D.; Al-Hendy, A.; Boehning, D. Simvastatin potently induces calcium-dependent apoptosis of human leiomyoma cells. J. Biol. Chem. 2014, 289, 35075–35086. [Google Scholar] [CrossRef]
- Jackisch, C.; Hahm, H.A.; Tombal, B.; McCloskey, D.; Butash, K.; Davidson, N.E.; Denmeade, S.R. Delayed micromolar elevation in intracellular calcium precedes induction of apoptosis in thapsigargin-treated breast cancer cells. Clin. Cancer Res. 2000, 6, 2844–2850. [Google Scholar]
- Martins, J.R.; Faria, D.; Kongsuphol, P.; Reisch, B.; Schreiber, R.; Kunzelmann, K. Anoctamin 6 is an essential component of the outwardly rectifying chloride channel. Proc. Natl. Acad. Sci. USA 2011, 108, 18168–18172. [Google Scholar] [CrossRef]
- Szabo, I.; Lepple-Wienhues, A.; Kaba, K.N.; Zoratti, M.; Gulbins, E.; Lang, F. Tyrosine kinase-dependent activation of a chloride channel in CD95-induced apoptosis in T lymphocytes. Proc. Natl. Acad. Sci. USA 1998, 95, 6169–6174. [Google Scholar] [CrossRef]
- Lepple-Wienhues, A.; Wieland, U.; Laun, T.; Heil, L.; Stern, M.; Lang, F. A src-like kinase activates outwardly rectifying chloride channels in CFTR-defective lymphocytes. FASEB J. 2001, 15, 927–931. [Google Scholar] [CrossRef]
- Lian, H.; Cheng, Y.; Wu, X. TMEM16A exacerbates renal injury by activating P38/JNK signaling pathway to promote podocyte apoptosis in diabetic nephropathy mice. Biochem. Biophys. Res. Commun. 2017. [Google Scholar] [CrossRef]
- Zeng, J.W.; Chen, B.Y.; Lv, X.F.; Sun, L.; Zeng, X.L.; Zheng, H.Q.; Du, Y.H.; Wang, G.L.; Ma, M.M.; Guan, Y.Y. TMEM16A Participates in Hydrogen Peroxide-Induced Apoptosis by Facilitating Mitochondria-Dependent Pathway in Vascular Smooth Muscle Cells. Br. J. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Henkel, B.; Drose, D.R.; Ackels, T.; Oberland, S.; Spehr, M.; Neuhaus, E.M. Co-expression of Anoctamins in Cilia of Olfactory Sensory Neurons. Chem. Sens. 2015, 40, 73–87. [Google Scholar] [CrossRef]
- Linkermann, A.; Stockwell, B.R.; Krautwald, S.; Anders, H.J. Regulated cell death and inflammation: An auto-amplification loop causes organ failure. Nat. Rev. Immunol. 2014, 14, 759–767. [Google Scholar] [CrossRef]
- Ousingsawat, J.; Wanitchakool, P.; Schreiber, R.; Kunzelmann, K. Contribution of TMEM16F to pyroptotic cell death. Cell Death Dis. 2018, 9, 300. [Google Scholar] [CrossRef]
- Simoes, F.; Ousingsawat, J.; Wanitchakool, P.; Fonseca, A.; Cabrita, I.; Benedetto, R.; Schreiber, R.; Kunzelmann, K. CFTR supports cell death through ROS-dependent activation of TMEM16F (anoctamin 6). Pflug. Arch. 2018, 470, 305–314. [Google Scholar] [CrossRef]
- Kunzelmann, K. Ion channels in regulated cell death. Cell. Mol. Life Sci. 2016, 73, 2387–2403. [Google Scholar] [CrossRef]
- Orsolic, N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef]
- Zheng, J.; Lee, H.L.; Ham, Y.W.; Song, H.S.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom on colon cancer cell growth by activation of death receptors and inhibition of nuclear factor kappa B. Oncotarget 2015, 6, 44437–44451. [Google Scholar] [CrossRef]
- Park, M.H.; Choi, M.S.; Kwak, D.H.; Oh, K.W.; Yoon, D.Y.; Han, S.B.; Song, H.S.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom in prostate cancer cells through activation of caspase pathway via inactivation of NF-kappaB. Prostate 2011, 71, 801–812. [Google Scholar] [CrossRef]
- Dar, H.H.; Tyurina, Y.Y.; Mikulska-Ruminska, K.; Shrivastava, I.; Ting, H.C.; Tyurin, V.A.; Krieger, J.; St Croix, C.M.; Watkins, S.; Bayir, E.; et al. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J. Clin. Investig. 2018. [Google Scholar] [CrossRef]
- Gueraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010, 44, 1098–1124. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Florean, C.; Song, S.; Dicato, M.; Diederich, M. Redox biology of regulated cell death in cancer: A focus on necroptosis and ferroptosis. Free Radic. Biol. Med. 2019. [Google Scholar] [CrossRef]
- Moreira, J.D.; Hamraz, M.; Abolhassani, M.; Bigan, E.; Peres, S.; Pauleve, L.; Nogueira, M.L.; Steyaert, J.M.; Schwartz, L. The Redox Status of Cancer Cells Supports Mechanisms behind the Warburg Effect. Metabolites 2016, 6, 33. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef]
- Pei, S.; Minhajuddin, M.; Callahan, K.P.; Balys, M.; Ashton, J.M.; Neering, S.J.; Lagadinou, E.D.; Corbett, C.; Ye, H.; Liesveld, J.L.; et al. Targeting aberrant glutathione metabolism to eradicate human acute myelogenous leukemia cells. J. Biol. Chem. 2013, 288, 33542–33558. [Google Scholar] [CrossRef]
- Trachootham, D.; Zhou, Y.; Zhang, H.; Demizu, Y.; Chen, Z.; Pelicano, H.; Chiao, P.J.; Achanta, G.; Arlinghaus, R.B.; Liu, J.; et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 2006, 10, 241–252. [Google Scholar] [CrossRef]
- Takeshita, N.; Evangelinos, M.; Zhou, L.; Serizawa, T.; Somera-Fajardo, R.A.; Lu, L.; Takaya, N.; Nienhaus, G.U.; Fischer, R. Pulses of Ca2+ coordinate actin assembly and exocytosis for stepwise cell extension. Proc. Natl. Acad. Sci. USA 2017, 114, 5701–5706. [Google Scholar] [CrossRef]
- Dolma, S.; Lessnick, S.L.; Hahn, W.C.; Stockwell, B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 2003, 3, 285–296. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).