Contribution of Anoctamins to Cell Survival and Cell Death
Abstract
:1. Introduction
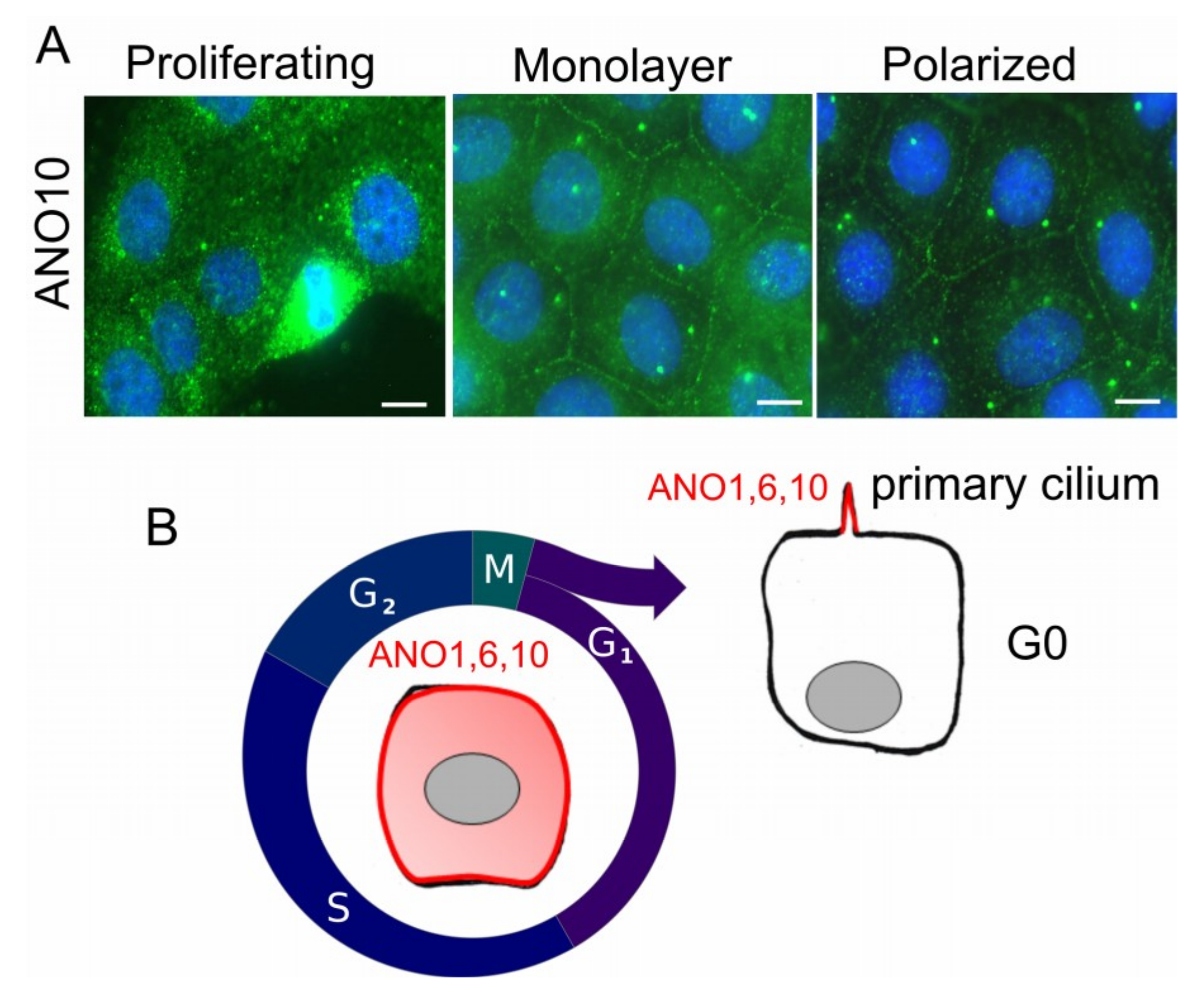
2. Anoctamins and Their Cellular Localization
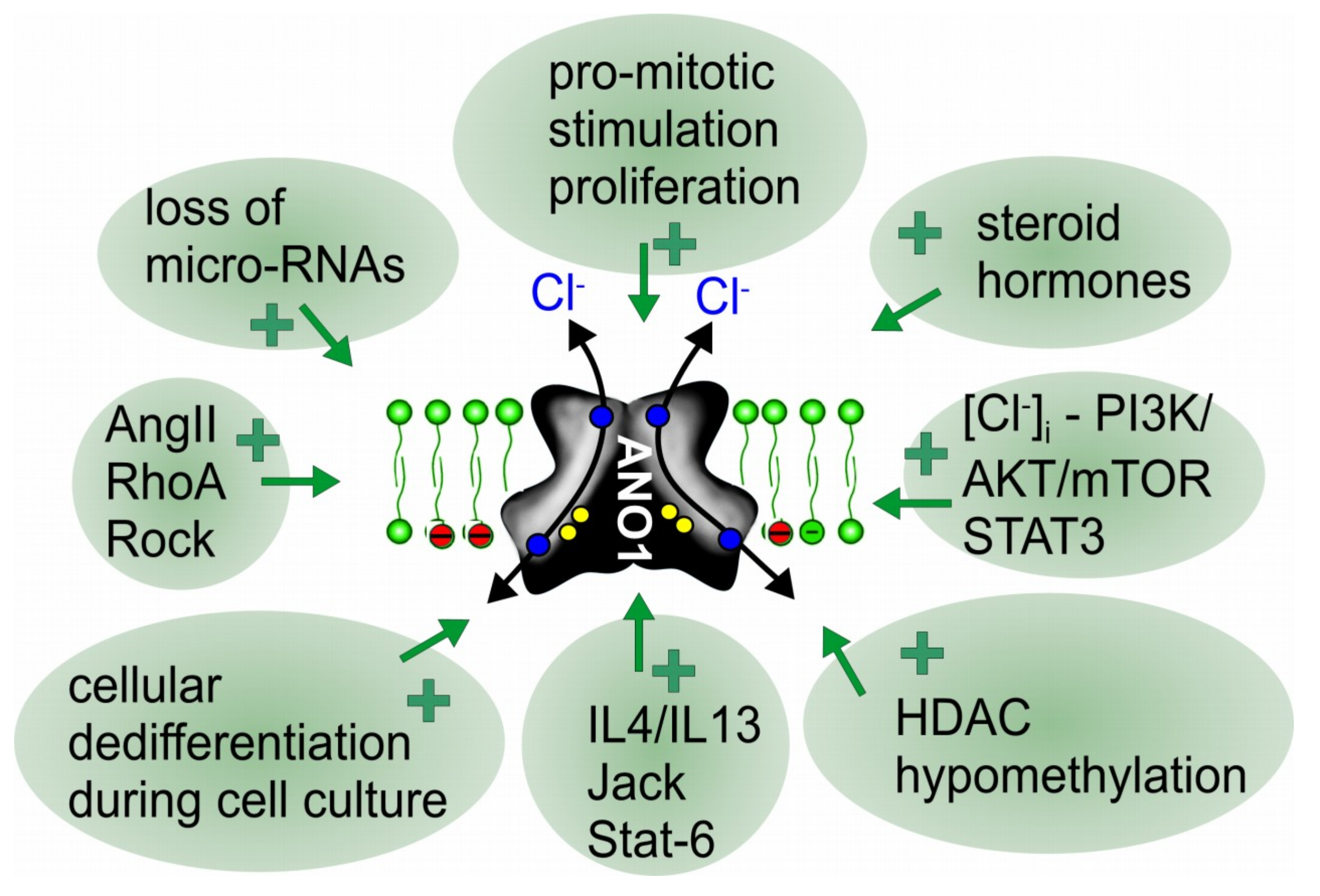
3. How Is ANO1 Upregulated during Cell Proliferation and Cancer Development?
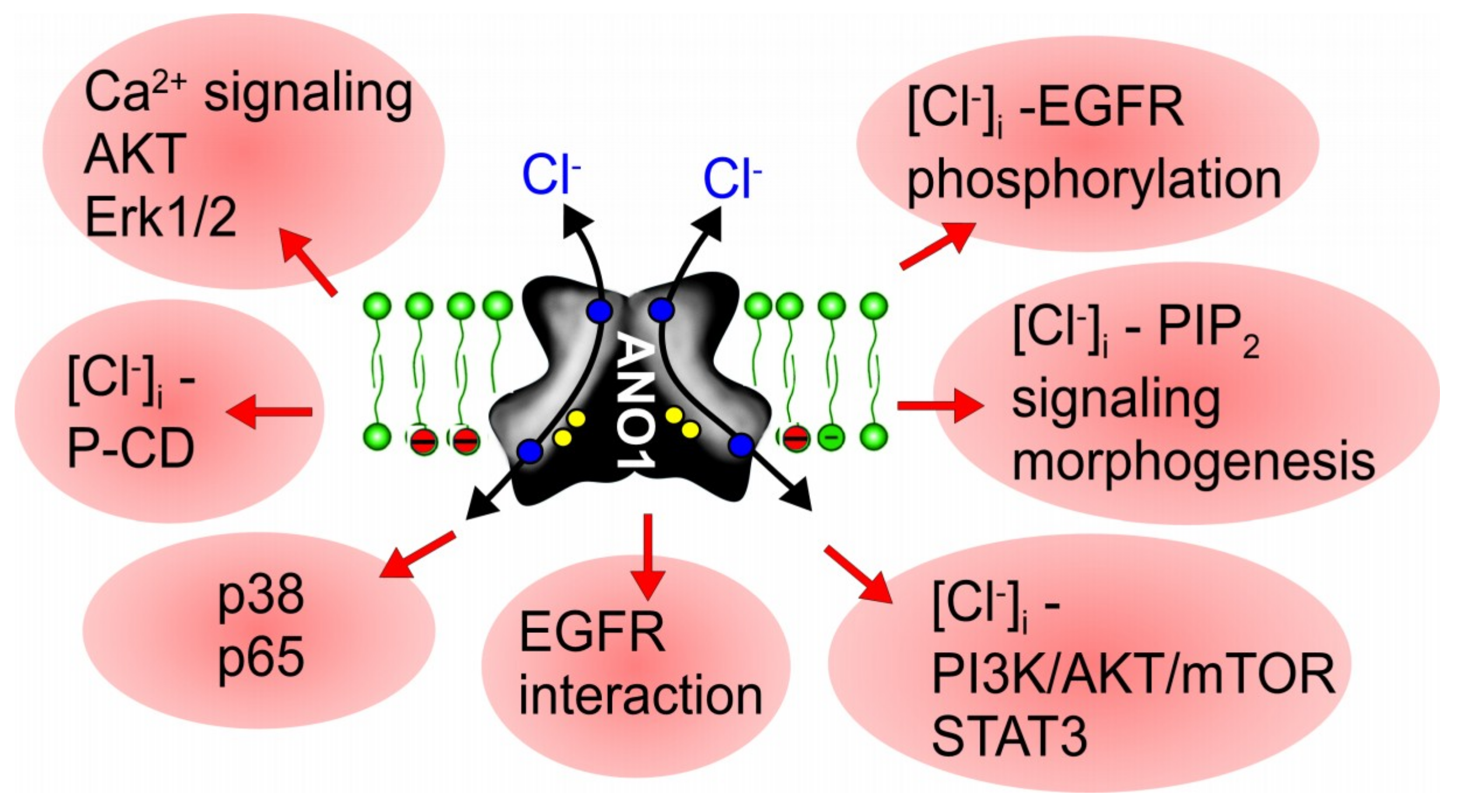
4. ANO1, Cell Proliferation and Tumor Growth: How Does It Work?
5. ANO5, 6, 7, and 9 in Cancer and Cell Proliferation
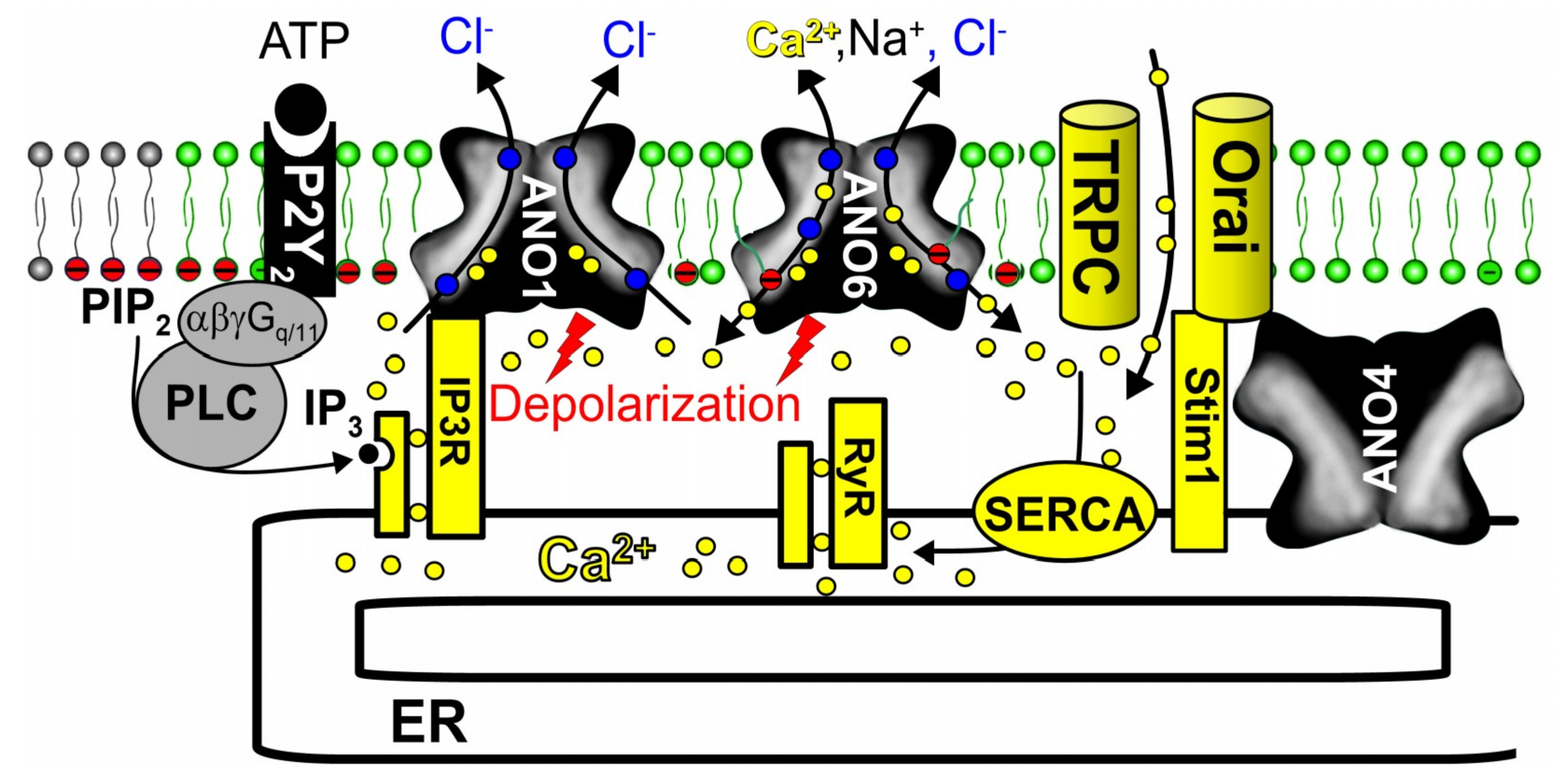
6. Anoctamins Control Intracellular Ca2+ Levels
7. The Role of Anoctamins in Controlling Intracellular Cl− Concentration, Exocytosis, Organ Growth and Microvesicular Signaling
8. ANO1 Is Upregulated during Inflammation
9. Relationship of Anoctamins to the Tumor Associated Cl− Channel VRAC
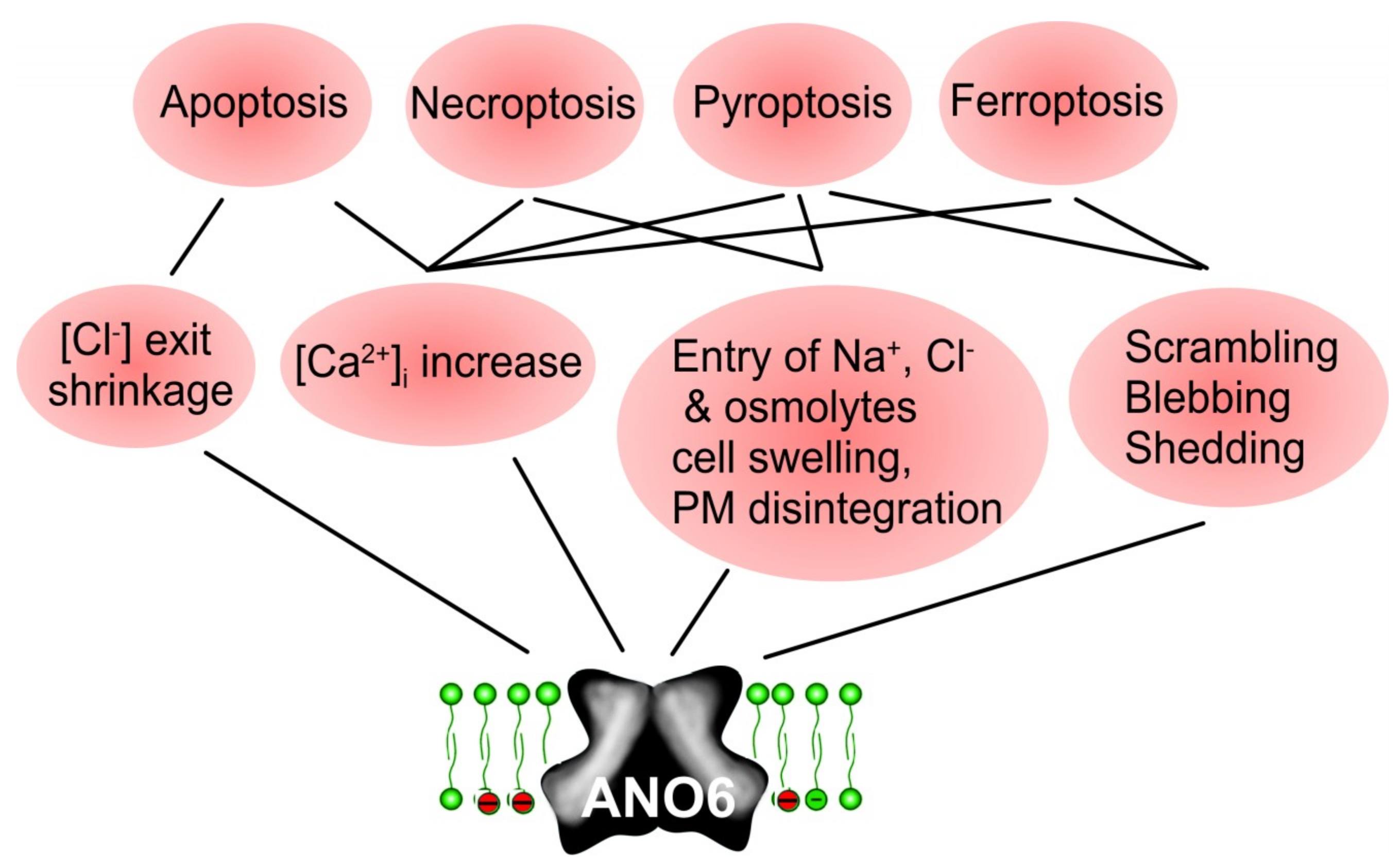
10. Role of Anoctamins in Cell Death
11. Activation of Anoctamins and Ferroptotic Cell Death in Cancer
12. Conclusions
Funding
Conflicts of Interest
References
- Hartzell, H.C.; Yu, K.; Xiao, Q.; Chien, L.T.; Qu, Z. Anoctamin/TMEM16 family members are Ca2+-activated Cl-channels. J. Physiol. 2008, 587, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J. Calcium-activated chloride channels: (un)known, (un)loved? Proc. Am. Thorac. Soc. 2004, 1, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, K.; Kongsuphol, P.; Chootip, K.; Toledo, C.; Martins, J.R.; Almaca, J.; Tian, Y.; Witzgall, R.; Ousingsawat, J.; Schreiber, R. Role of the Ca(2+)-activated Cl(-) channels bestrophin and anoctamin in epithelial cells. Biol. Chem. 2011, 392, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.D.; Cho, H.; Koo, J.Y.; Tak, M.H.; Cho, Y.; Shim, W.S.; Park, S.P.; Lee, J.; Lee, B.; Kim, B.M.; et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 2008, 455, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.C.; Cheng, T.; Jan, Y.N.; Jan, L.Y. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 2008, 134, 1019–1029. [Google Scholar] [CrossRef] [Green Version]
- Caputo, A.; Caci, E.; Ferrera, L.; Pedemonte, N.; Barsanti, C.; Sondo, E.; Pfeffer, U.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L.J. TMEM16A, A Membrane Protein Associated With Calcium-Dependent Chloride Channel Activity. Science 2008, 322, 590–594. [Google Scholar] [CrossRef]
- Sala-Rabanal, M.; Yurtsever, Z.; Nichols, C.G.; Brett, T.J. Secreted CLCA1 modulates TMEM16A to activate Ca(2+)-dependent chloride currents in human cells. Elife 2015, 4, e05875. [Google Scholar] [CrossRef]
- Loewen, M.E.; Forsyth, G.W. Structure and function of CLCA proteins. Physiol. Rev. 2005, 85, 1061–1092. [Google Scholar] [CrossRef]
- Hartzell, H.C.; Putzier, I.; Arreola, J. Calcium-Activated Chloride Channels. Annu. Rev. Physiol. 2005, 67, 719–758. [Google Scholar] [CrossRef]
- Schreiber, R. Ca2+ signaling, intracellular pH and cell volume in cell proliferation. J. Membr. Biol. 2005, 205, 129–137. [Google Scholar] [CrossRef]
- Kunzelmann, K. Ion channels and cancer. J. Membr. Biol. 2005, 205, 159–173. [Google Scholar] [CrossRef]
- Espinosa, I.; Lee, C.H.; Kim, M.K.; Rouse, B.T.; Subramanian, S.; Montgomery, K.; Varma, S.; Corless, C.L.; Heinrich, M.C.; Smith, K.S.; et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am. J. Surg. Pathol. 2008, 32, 210–218. [Google Scholar] [CrossRef]
- West, R.B.; Corless, C.L.; Chen, X.; Rubin, B.P.; Subramanian, S.; Montgomery, K.; Zhu, S.; Ball, C.A.; Nielsen, T.O.; Patel, R.; et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am. J. Pathol. 2004, 165, 107–113. [Google Scholar] [CrossRef]
- Ruiz, C.; Martins, J.R.; Rudin, F.; Schneider, S.; Dietsche, T.; Fischer, C.A.; Tornillo, L.; Terracciano, L.M.; Schreiber, R.; Bubendorf, L.; et al. Enhanced Expression of ANO1 in Head and Neck Squamous Cell Carcinoma Causes Cell Migration and Correlates with Poor Prognosis. PLoS ONE 2012, 7, e43265. [Google Scholar] [CrossRef]
- Schreiber, R.; Uliyakina, I.; Kongsuphol, P.; Warth, R.; Mirza, M.; Martins, J.R.; Kunzelmann, K. Expression and Function of Epithelial Anoctamins. J. Biol. Chem. 2010, 285, 7838–7845. [Google Scholar] [CrossRef]
- Kunzelmann, K.; Schreiber, R.; Kmit, A.; Jantarajit, W.; Martins, J.R.; Faria, D.; Kongsuphol, P.; Ousingsawat, J.; Tian, Y. Expression and function of epithelial anoctamins. Exp. Physiol. 2012, 97, 184–192. [Google Scholar] [CrossRef]
- Carles, A.; Millon, R.; Cromer, A.; Ganguli, G.; Lemaire, F.; Young, J.; Wasylyk, C.; Muller, D.; Schultz, I.; Rabouel, Y.; et al. Head and neck squamous cell carcinoma transcriptome analysis by comprehensive validated differential display. Oncogene 2006, 25, 1821–1831. [Google Scholar] [CrossRef]
- Carneiro, A.; Isinger, A.; Karlsson, A.; Johansson, J.; Jonsson, G.; Bendahl, P.O.; Falkenback, D.; Halvarsson, B.; Nilbert, M. Prognostic impact of array-based genomic profiles in esophageal squamous cell cancer. BMC Cancer 2008, 8, 98. [Google Scholar] [CrossRef]
- Liegl, B.; Hornick, J.L.; Corless, C.L.; Fletcher, C.D. Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am. J. Surg. Pathol. 2009, 33, 437–446. [Google Scholar] [CrossRef]
- Miwa, S.; Nakajima, T.; Murai, Y.; Takano, Y.; Sugiyama, T. Mutation assay of the novel gene DOG1 in gastrointestinal stromal tumors (GISTs). J. Gastroenterol. 2008, 43, 531–537. [Google Scholar] [CrossRef]
- Fatima, N.; Cohen, C.; Siddiqui, M.T. DOG1 utility in diagnosing gastrointestinal stromal tumors on fine-needle aspiration. Cancer Cytopathol. 2011, 119, 202–208. [Google Scholar] [CrossRef]
- Kang, G.H.; Srivastava, A.; Kim, Y.E.; Park, H.J.; Park, C.K.; Sohn, T.S.; Kim, S.; Kang, D.Y.; Kim, K.M. DOG1 and PKC-theta are useful in the diagnosis of KIT-negative gastrointestinal stromal tumors. Modern Pathol. 2011, 24, 866–875. [Google Scholar] [CrossRef]
- Hwang, D.G.; Qian, X.; Hornick, J.L. DOG1 antibody is a highly sensitive and specific marker for gastrointestinal stromal tumors in cytology cell blocks. Am. J. Clin. Pathol. 2011, 135, 448–453. [Google Scholar] [CrossRef]
- Novelli, M.; Rossi, S.; Rodriguez-Justo, M.; Taniere, P.; Seddon, B.; Toffolatti, L.; Sartor, C.; Hogendoorn, P.C.; Sciot, R.; Van Glabbeke, M.; et al. DOG1 and CD117 are the antibodies of choice in the diagnosis of gastrointestinal stromal tumours. Histopathology 2010, 57, 259–270. [Google Scholar] [CrossRef]
- Wong, N.A.; Shelley-Fraser, G. Specificity of DOG1 (K9 clone) and protein kinase C theta (clone 27) as immunohistochemical markers of gastrointestinal stromal tumour. Histopathology 2010, 57, 250–258. [Google Scholar] [CrossRef]
- Ayoub, C.; Wasylyk, C.; Li, Y.; Thomas, E.; Marisa, L.; Robe, A.; Roux, M.; Abecassis, J.; de Reynies, A.; Wasylyk, B. ANO1 amplification and expression in HNSCC with a high propensity for future distant metastasis and its functions in HNSCC cell lines. Br. J. Cancer 2010, 103, 715–726. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Liang, C.W.; Espinosa, I. The utility of discovered on gastrointestinal stromal tumor 1 (DOG1) antibody in surgical pathology-the GIST of it. Adv. Anat. Pathol. 2010, 17, 222–232. [Google Scholar] [CrossRef]
- Kleist, B.; Lasota, J.; Miettinen, M. Gastrointestinal stromal tumor and gastric adenocarcinoma collision tumors. Hum. Pathol. 2010, 41, 1034–1039. [Google Scholar] [CrossRef]
- Miettinen, M.; Wang, Z.F.; Lasota, J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: A study of 1840 cases. Am. J. Surg. Pathol. 2009, 33, 1401–1408. [Google Scholar] [CrossRef]
- Duvvuri, U.; Shiwarski, D.J.; Xiao, D.; Bertrand, C.; Huang, X.; Edinger, R.S.; Rock, J.R.; Harfe, B.D.; Henson, B.J.; Kunzelmann, K.; et al. TMEM16A, induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res. 2012, 72, 3270–3281. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kojima, A.; Nagata, S.; Tomita, Y.; Takahashi, S.; Oda, Y. KIT-negative gastrointestinal stromal tumor of the abdominal soft tissue: A clinicopathologic and genetic study of 10 cases. Am. J. Surg. Pathol. 2011, 35, 1287–1295. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Hong, S. ANO1 as a marker of oral squamous cell carcinoma and silencing ANO1 suppresses migration of human SCC-25 cells. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e313–e319. [Google Scholar] [CrossRef]
- Simon, S.; Grabellus, F.; Ferrera, L.; Galietta, L.; Schwindenhammer, B.; Muhlenberg, T.; Taeger, G.; Eilers, G.; Treckmann, J.; Breitenbuecher, F.; et al. DOG1 regulates growth and IGFBP5 in gastrointestinal stromal tumors. Cancer Res. 2013, 73, 3661–3670. [Google Scholar] [CrossRef]
- Berglund, E.; Akcakaya, P.; Berglund, D.; Karlsson, F.; Vukojevic, V.; Lee, L.; Bogdanovic, D.; Lui, W.O.; Larsson, C.; Zedenius, J.; et al. Functional role of the Ca-activated Cl channel DOG1/TMEM16A in gastrointestinal stromal tumor cells. Exp. Cell Res. 2014, 326, 315–325. [Google Scholar] [CrossRef]
- Slavik, T.; du Plessis, J.; Sparaco, A.; van der Merwe, S.W. Duodenal gastrointestinal stromal tumor with epithelioid and neural features mimicking a primary pancreas head neuroendocrine tumor. Pancreas 2014, 43, 482–483. [Google Scholar] [CrossRef]
- Rodrigo, J.P.; Menendez, S.T.; Hermida-Prado, F.; Alvarez-Teijeiro, S.; Villaronga, M.A.; Alonso-Duran, L.; Vallina, A.; Martinez-Camblor, P.; Astudillo, A.; Suarez, C.; et al. Clinical significance of Anoctamin-1 gene at 11q13 in the development and progression of head and neck squamous cell carcinomas. Sci. Rep. 2015, 5, 15698. [Google Scholar] [CrossRef] [Green Version]
- Bill, A.; Gutierrez, A.; Kulkarni, S.; Kemp, C.; Bonenfant, D.; Voshol, H.; Duvvuri, U.; Gaither, L.A. ANO1/TMEM16A interacts with EGFR and correlates with sensitivity to EGFR-targeting therapy in head and neck cancer. Oncotarget 2015, 6, 9173–9188. [Google Scholar] [CrossRef] [Green Version]
- Reddy, R.B.; Bhat, A.R.; James, B.L.; Govindan, S.V.; Mathew, R.; Ravindra, D.R.; Hedne, N.; Illiayaraja, J.; Kekatpure, V.; Khora, S.S.; et al. Meta-Analyses of Microarray Datasets Identifies ANO1 and FADD as Prognostic Markers of Head and Neck Cancer. PLoS ONE 2016, 11, e0147409. [Google Scholar] [CrossRef]
- Dixit, R.; Kemp, C.; Kulich, S.; Seethala, R.; Chiosea, S.; Ling, S.; Ha, P.K.; Duvvuri, U. TMEM16A/ANO1 is differentially expressed in HPV-negative versus HPV-positive head and neck squamous cell carcinoma through promoter methylation. Sci. Rep. 2015, 5, 16657. [Google Scholar] [CrossRef] [Green Version]
- Godse, N.R.; Khan, N.I.; Yochum, Z.A.; Gomez-Casal, R.; Kemp, C.; Shiwarski, D.J.; Seethala, R.; Kulich, S.; Seshadri, M.; Burns, T.F.; et al. TMEM16A/ANO1 inhibits apoptosis via down-regulation of Bim expression. Clin. Cancer Res. 2017. [Google Scholar] [CrossRef]
- Han, Z.; Li, Q.; Wang, Y.; Wang, L.; Li, X.; Ge, N.; Wang, Y.; Guo, C. Niclosamide Induces Cell Cycle Arrest in G1 Phase in Head and Neck Squamous Cell Carcinoma Through Let-7d/CDC34 Axis. Front. Pharmacol. 2018, 9, 1544. [Google Scholar] [CrossRef]
- Ardeleanu, C.; Arsene, D.; Hinescu, M.; Andrei, F.; Gutu, D.; Luca, L.; Popescu, L.M. Pancreatic Expression of DOG1: A Novel Gastrointestinal Stromal Tumor (GIST) Biomarker. Appl. Immunohistochem. Mol. Morphol. 2009, 17, 413–418. [Google Scholar] [CrossRef]
- Bergmann, F.; Andrulis, M.; Hartwig, W.; Penzel, R.; Gaida, M.M.; Herpel, E.; Schirmacher, P.; Mechtersheimer, G. Discovered on gastrointestinal stromal tumor 1 (DOG1) is expressed in pancreatic centroacinar cells and in solid-pseudopapillary neoplasms-novel evidence for a histogenetic relationship. Hum. Pathol. 2011, in press. [Google Scholar] [CrossRef]
- Sauter, D.R.; Novak, I.; Pedersen, S.F.; Larsen, E.H.; Hoffmann, E.K. ANO1 (TMEM16A) in pancreatic ductal adenocarcinoma (PDAC). Pflug. Arch. 2014, 467, 1495–1508. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Lu, M.; Liu, B.; Huang, Y.; Wang, K. Inhibition of Ca(2+)-activated Cl(-) channel ANO1/TMEM16A expression suppresses tumor growth and invasiveness in human prostate carcinoma. Cancer Lett. 2012, 326, 41–51. [Google Scholar] [CrossRef]
- Seo, Y.; Ryu, K.; Park, J.; Jeon, D.K.; Jo, S.; Lee, H.K.; Namkung, W. Inhibition of ANO1 by luteolin and its cytotoxicity in human prostate cancer PC-3 cells. PLoS ONE 2017, 12, e0174935. [Google Scholar] [CrossRef]
- Song, Y.; Gao, J.; Guan, L.; Chen, X.; Gao, J.; Wang, K. Inhibition of ANO1/TMEM16A induces apoptosis in human prostate carcinoma cells by activating TNF-alpha signaling. Cell Death Dis. 2018, 9, 703. [Google Scholar] [CrossRef]
- Ubby, I.; Bussani, E.; Colonna, A.; Stacul, G.; Locatelli, M.; Scudieri, P.; Galietta, L.J.; Pagani, F. TMEM16A alternative splicing coordination in breast cancer. Mol. Cancer 2013, 12, 75. [Google Scholar] [CrossRef]
- Britschgi, A.; Bill, A.; Brinkhaus, H.; Rothwell, C.; Clay, I.; Duss, S.; Rebhan, M.; Raman, P.; Guy, C.T.; Wetzel, K.; et al. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc. Natl. Acad. Sci. USA 2013, 110, E1026–E1034. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Guan, S.; Sun, M.; Yu, Z.; Zhao, L.; He, M.; Zhao, H.; Yao, W.; Wang, E.; Jin, F.; et al. Ano1/TMEM16A Overexpression Is Associated with Good Prognosis in PR-Positive or HER2-Negative Breast Cancer Patients following Tamoxifen Treatment. PLoS ONE 2015, 10, e0126128. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, S.; Qu, Z.; Zhou, S.; Ruan, Q. Novel Use for DOG1 in Discriminating Breast Invasive Carcinoma from Noninvasive Breast Lesions. Dis. Mark. 2016, 2016, 5628176. [Google Scholar] [CrossRef]
- Fujimoto, M.; Inoue, T.; Kito, H.; Niwa, S.; Suzuki, T.; Muraki, K.; Ohya, S. Transcriptional repression of HER2 by ANO1 Cl(-) channel inhibition in human breast cancer cells with resistance to trastuzumab. Biochem. Biophys. Res. Commun. 2017, 482, 188–194. [Google Scholar] [CrossRef]
- Wu, H.; Wang, H.; Guan, S.; Zhang, J.; Chen, Q.; Wang, X.; Ma, K.; Zhao, P.; Zhao, H.; Yao, W.; et al. Cell-specific regulation of proliferation by Ano1/TMEM16A in breast cancer with different ER, PR, and HER2 status. Oncotarget 2017. [Google Scholar] [CrossRef]
- Foda, A.A.; Mohamed, M.A. Aberrant expressions of c-KIT and DOG-1 in mucinous and nonmucinous colorectal carcinomas and relation to clinicopathologic features and prognosis. Ann. Diagn. Pathol. 2015, 19, 335–340. [Google Scholar] [CrossRef]
- Sui, Y.; Sun, M.; Wu, F.; Yang, L.; Di, W.; Zhang, G.; Zhong, L.; Ma, Z.; Zheng, J.; Fang, X.; et al. Inhibition of TMEM16A Expression Suppresses Growth and Invasion in Human Colorectal Cancer Cells. PLoS ONE 2014, 9, e115443. [Google Scholar] [CrossRef]
- Liu, F.; Cao, Q.H.; Lu, J.; Luo, B.; Lu, X.F.; Luo, R.C.; Wang, X.G. TMEM16A overexpression contributes to tumor invasion and poor prognosis of human gastric cancer through TGF-beta signaling. Oncotarget 2015, 6, 11585–11599. [Google Scholar]
- Cao, Q.; Liu, F.; Ji, K.; Liu, N.; He, Y.; Zhang, W.; Wang, L. MicroRNA-381 inhibits the metastasis of gastric cancer by targeting TMEM16A expression. J. Exp. Clin. Cancer Res. 2017, 36, 29. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Ren, Y.; Kang, L.; Zhang, L. Transmembrane protein with unknown function 16A overexpression promotes glioma formation through the nuclear factor-kappaB signaling pathway. Mol. Med. Rep. 2014, 9, 1068–1074. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, J.K.; Bae, Y.; Lee, B.S.; Kim, E.; Cho, C.H.; Ryoo, K.; Yoo, J.; Kim, C.H.; Yi, G.S.; et al. Suppression of 14-3-3gamma-mediated surface expression of ANO1 inhibits cancer progression of glioblastoma cells. Sci. Rep. 2016, 6, 26413. [Google Scholar] [CrossRef]
- Shang, L.; Hao, J.J.; Zhao, X.K.; He, J.Z.; Shi, Z.Z.; Liu, H.J.; Wu, L.F.; Jiang, Y.Y.; Shi, F.; Yang, H.; et al. ANO1 protein as a potential biomarker for esophageal cancer prognosis and precancerous lesion development prediction. Oncotarget 2016, 10, 24374. [Google Scholar] [CrossRef]
- Jia, L.; Liu, W.; Guan, L.; Lu, M.; Wang, K. Inhibition of Calcium-Activated Chloride Channel ANO1/TMEM16A Suppresses Tumor Growth and Invasion in Human Lung Cancer. PLoS ONE 2015, 10, e0136584. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Chen, Y.; Li, P.; Gao, L.; Zheng, Y.; Sun, Y.; Chen, J.; Qian, X. Expression of anoctamin 1 is associated with advanced tumor stage in patients with non-small cell lung cancer and predicts recurrence after surgery. Clin. Transl. Oncol. 2017. [Google Scholar] [CrossRef]
- Guo, S.; Chen, Y.; Pang, C.; Wang, X.; Shi, S.; Zhang, H.; An, H.; Zhan, Y. Matrine is a novel inhibitor of the TMEM16A chloride channel with antilung adenocarcinoma effects. J. Cell. Physiol. 2018. [Google Scholar] [CrossRef]
- Deng, L.; Yang, J.; Chen, H.; Ma, B.; Pan, K.; Su, C.; Xu, F.; Zhang, J. Knockdown of TMEM16A suppressed MAPK and inhibited cell proliferation and migration in hepatocellular carcinoma. Oncol. Targets Ther. 2016, 9, 325–333. [Google Scholar] [Green Version]
- Jung, I.; Gurzu, S.; Turdean, S.; Ciortea, D.; Sahlean, D.I.; Golea, M.; Bara, T. Relationship of endothelial area with VEGF-A, COX-2, maspin, c-KIT, and DOG-1 immunoreactivity in liposarcomas versus non-lipomatous soft tissue tumors. Int. J. Clin. Exp. Pathol. 2015, 8, 1776–1782. [Google Scholar]
- Sah, S.P.; McCluggage, W.G. DOG1 immunoreactivity in uterine leiomyosarcomas. J. Clin. Pathol. 2013, 66, 40–43. [Google Scholar] [CrossRef]
- Abd Raboh, N.M.; Hakim, S.A. Diagnostic role of DOG1 and p63 immunohistochemistry in salivary gland carcinomas. Int. J. Clin. Exp. Pathol. 2015, 8, 9214–9222. [Google Scholar]
- Akpalo, H.; Lange, C.; Zustin, J. Discovered on gastrointestinal stromal tumour 1 (DOG1): A useful immunohistochemical marker for diagnosing chondroblastoma. Histopathology 2012, 60, 1099–1106. [Google Scholar] [CrossRef]
- Hemminger, J.; Iwenofu, O.H. Discovered on gastrointestinal stromal tumours 1 (DOG1) expression in non-gastrointestinal stromal tumour (GIST) neoplasms. Histopathology 2012, 61, 170–177. [Google Scholar] [CrossRef]
- Chenevert, J.; Duvvuri, U.; Chiosea, S.; Dacic, S.; Cieply, K.; Kim, J.; Shiwarski, D.; Seethala, R.R. DOG1: A novel marker of salivary acinar and intercalated duct differentiation. Mod. Pathol. 2012, 25, 919–929. [Google Scholar] [CrossRef]
- Stanich, J.E.; Gibbons, S.J.; Eisenman, S.T.; Bardsley, M.R.; Rock, J.R.; Harfe, B.D.; Ordog, T.; Farrugia, G. ANO1 AS A REGULATOR OF PROLIFERATION. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G1044–G1051. [Google Scholar] [CrossRef]
- Mazzone, A.; Eisenman, S.T.; Strege, P.R.; Yao, Z.; Ordog, T.; Gibbons, S.J.; Farrugia, G. Inhibition of Cell Proliferation by a Selective Inhibitor of the Ca(2+)-activated Cl(-) Channel, Ano1. Biochem. Biophys. Res. Commun. 2012, 427, 248–253. [Google Scholar] [CrossRef]
- Wanitchakool, P.; Wolf, L.; Koehl, G.; Sirianant, L.; Gaumann, A.; Schreiber, R.; Duvvuri, U.; Kunzelmann, K. Role of Anoctamins in Cancer and Apoptosis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130096. [Google Scholar] [CrossRef]
- Bill, A.; Alex Gaither, L. The Mechanistic Role of the Calcium-Activated Chloride Channel ANO1 in Tumor Growth and Signaling. Adv. Exp. Med. Biol. 2017. [Google Scholar] [CrossRef]
- Guan, L.; Song, Y.; Gao, J.; Gao, J.; Wang, K. Inhibition of calcium-activated chloride channel ANO1 suppresses proliferation and induces apoptosis of epithelium originated cancer cells. Oncotarget 2016, 7, 78619–78630. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zou, L.; Ma, K.; Yu, J.; Wu, H.; Wei, M.; Xiao, Q. Cell-specific mechanisms of TMEM16A Ca2+-activated chloride channel in cancer. Mol. Cancer 2017, 16, 152. [Google Scholar] [CrossRef]
- Ishaque, N.; Abba, M.L.; Hauser, C.; Patil, N.; Paramasivam, N.; Huebschmann, D.; Leupold, J.H.; Balasubramanian, G.P.; Kleinheinz, K.; Toprak, U.H.; et al. Whole genome sequencing puts forward hypotheses on metastasis evolution and therapy in colorectal cancer. Nat. Commun. 2018, 9, 4782. [Google Scholar] [CrossRef]
- Hesson, L.B.; Ng, B.; Zarzour, P.; Srivastava, S.; Kwok, C.T.; Packham, D.; Nunez, A.C.; Beck, D.; Ryan, R.; Dower, A.; et al. Integrated Genetic, Epigenetic, and Transcriptional Profiling Identifies Molecular Pathways in the Development of Laterally Spreading Tumors. Mol. Cancer Res. 2016, 14, 1217–1228. [Google Scholar] [CrossRef]
- Chang, Z.; Cai, C.; Han, D.; Gao, Y.; Li, Q.; Feng, L.; Zhang, W.; Zheng, J.; Jin, J.; Zhang, H.; et al. Anoctamin5 regulates cell migration and invasion in thyroid cancer. Int. J. Oncol. 2017, 51, 1311–1319. [Google Scholar] [CrossRef]
- Zhao, P.; Torcaso, A.; Mariano, A.; Xu, L.; Mohsin, S.; Zhao, L.; Han, R. Anoctamin 6 Regulates C2C12 Myoblast Proliferation. PLoS ONE 2014, 9, e92749. [Google Scholar] [CrossRef]
- Kaikkonen, E.; Rantapero, T.; Zhang, Q.; Taimen, P.; Laitinen, V.; Kallajoki, M.; Jambulingam, D.; Ettala, O.; Knaapila, J.; Bostrom, P.J.; et al. ANO7 is associated with aggressive prostate cancer. Int. J. Cancer 2018, 143, 2479–2487. [Google Scholar] [CrossRef]
- Mohsenzadegan, M.; Shekarabi, M.; Madjd, Z.; Asgari, M.; Abolhasani, M.; Tajik, N.; Farajollahi, M.M. Study of NGEP expression pattern in cancerous tissues provides novel insights into prognostic marker in prostate cancer. Biomark. Med. 2015, 9, 391–401. [Google Scholar] [CrossRef]
- Cereda, V.; Poole, D.J.; Palena, C.; Das, S.; Bera, T.K.; Remondo, C.; Gulley, J.L.; Arlen, P.M.; Yokokawa, J.; Pastan, I.; et al. New gene expressed in prostate: A potential target for T cell-mediated prostate cancer immunotherapy. Cancer Immunol. Immunother. 2010, 59, 63–71. [Google Scholar] [CrossRef]
- Das, S.; Hahn, Y.; Walker, D.A.; Nagata, S.; Willingham, M.C.; Peehl, D.M.; Bera, T.K.; Lee, B.; Pastan, I. Topology of NGEP, a prostate-specific cell:cell junction protein widely expressed in many cancers of different grade level. Cancer Res. 2008, 68, 6306–6312. [Google Scholar] [CrossRef]
- Das, S.; Hahn, Y.; Nagata, S.; Willingham, M.C.; Bera, T.K.; Lee, B.; Pastan, I. NGEP, a prostate-specific plasma membrane protein that promotes the association of LNCaP cells. Cancer Res. 2007, 67, 1594–1601. [Google Scholar] [CrossRef]
- Bera, T.K.; Das, S.; Maeda, H.; Beers, R.; Wolfgang, C.D.; Kumar, V.; Hahn, Y.; Lee, B.; Pastan, I. NGEP, a gene encoding a membrane protein detected only in prostate cancer and normal prostate. Proc. Natl. Acad. Sci. USA 2004, 101, 3059–3064. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, X.; Vural, S.; Mishra, N.K.; Cowan, K.H.; Guda, C. Exome analysis reveals differentially mutated gene signatures of stage, grade and subtype in breast cancers. PLoS ONE 2015, 10, e0119383. [Google Scholar] [CrossRef]
- Jun, I.; Park, H.S.; Piao, H.; Han, J.W.; An, M.J.; Yun, B.G.; Zhang, X.; Cha, Y.H.; Shin, Y.K.; Yook, J.I.; et al. ANO9/TMEM16J promotes tumourigenesis via EGFR and is a novel therapeutic target for pancreatic cancer. Br. J. Cancer 2017. [Google Scholar] [CrossRef]
- Li, C.; Cai, S.; Wang, X.; Jiang, Z. Identification and characterization of ANO9 in stage II and III colorectal carcinoma. Oncotarget 2015, 6, 29324–29334. [Google Scholar] [CrossRef]
- Kunzelmann, K.; Tian, Y.; Martins, J.R.; Faria, D.; Kongsuphol, P.; Ousingsawat, J.; Thevenod, F.; Roussa, E.; Rock, J.R.; Schreiber, R. Anoctamins. Pflug. Arch. 2011, 462, 195–208. [Google Scholar] [CrossRef]
- Pedemonte, N.; Galietta, L.J. Structure and Function of TMEM16 Proteins (Anoctamins). Physiol. Rev. 2014, 94, 419–459. [Google Scholar] [CrossRef] [Green Version]
- Paulino, C.; Neldner, Y.; Lam, A.K.; Kalienkova, V.; Brunner, J.D.; Schenck, S.; Dutzler, R. Structural basis for anion conduction in the calcium-activated chloride channel TMEM16A. Elife 2017, 6, e26232. [Google Scholar] [CrossRef]
- Brunner, J.D.; Lim, N.K.; Schenck, S.; Duerst, A.; Dutzler, R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature 2014, 516, 207–212. [Google Scholar] [CrossRef]
- Lee, B.C.; Khelashvili, G.; Falzone, M.; Menon, A.K.; Weinstein, H.; Accardi, A. Gating mechanism of the extracellular entry to the lipid pathway in a TMEM16 scramblase. Nat. Commun. 2018, 9, 3251. [Google Scholar] [CrossRef]
- Schreiber, R.; Ousingsawat, J.; Wanitchakool, P.; Sirianant, L.; Benedetto, R.; Reiss, K.; Kunzelmann, K. Regulation of TMEM16A/ANO1 and TMEM16F/ANO6 ion currents and phospholipid scrambling by Ca2+ and plasma membrane lipid. J. Physiol. 2018, 596, 217–229. [Google Scholar] [CrossRef]
- Shimizu, T.; Iehara, T.; Sato, K.; Fujii, T.; Sakai, H.; Okada, Y. TMEM16F is a component of a Ca2+-activated Cl-channel but not a volume-sensitive outwardly rectifying Cl-channel. Am. J. Physiol. Cell Physiol.. 2013, 304, C748–C759. [Google Scholar] [CrossRef]
- Grubb, S.; Poulsen, K.A.; Juul, C.A.; Kyed, T.; Klausen, T.K.; Larsen, E.H.; Hoffmann, E.K. TMEM16F (Anoctamin 6), an anion channel of delayed Ca2+ activation. J. Gen. Physiol.. 2013, 141, 585–600. [Google Scholar] [CrossRef] [Green Version]
- Ousingsawat, J.; Wanitchakool, P.; Kmit, A.; Romao, A.M.; Jantarajit, W.; Schreiber, S.; Kunzelmann, K. Anoctamin 6 mediates effects essential for innate immunity downstream of P2X7-receptors in macrophages. Nat. Commun. 2015, 6, 6245. [Google Scholar] [CrossRef]
- Gyobu, S.; Ishihara, K.; Suzuki, J.; Segawa, K.; Nagata, S. Characterization of the scrambling domain of the TMEM16 family. Proc. Natl. Acad. Sci. USA 2017. [Google Scholar] [CrossRef]
- Watanabe, R.; Sakuragi, T.; Noji, H.; Nagata, S. Single-molecule analysis of phospholipid scrambling by TMEM16F. Proc. Natl. Acad. Sci. USA 2018. [Google Scholar] [CrossRef]
- Paulino, C.; Kalienkova, V.; Lam, A.K.M.; Neldner, Y.; Dutzler, R. Activation mechanism of the calcium-activated chloride channel TMEM16A revealed by cryo-EM. Nature 2017, 552, 421–425. [Google Scholar] [CrossRef]
- Tian, Y.; Schreiber, R.; Kunzelmann, K. Anoctamins are a family of Ca2+ activated Cl-channels. J. Cell Sci. 2012, 125, 4991–4998. [Google Scholar] [CrossRef]
- Whitlock, J.M.; Yu, K.; Cui, Y.Y.; Hartzell, H.C. Anoctamin 5/TMEM16E facilitates muscle precursor cell fusion. J. Gen. Physiol.. 2018. [Google Scholar] [CrossRef]
- He, Q.; Halm, S.T.; Zhang, J.; Halm, D.R. Activation of the basolateral membrane Cl conductance essential for electrogenic K secretion suppresses electrogenic Cl secretion. Exp. Physiol. 2011, 96, 305–316. [Google Scholar] [CrossRef]
- Yokoyama, T.; Takemoto, M.; Hirakawa, M.; Saino, T. Different immunohistochemical localization for TMEM16A and CFTR in acinar and ductal cells of rat major salivary glands and exocrine pancreas. Acta Histochem. 2018. [Google Scholar] [CrossRef]
- Schreiber, R.; Faria, D.; Skryabin, B.V.; Rock, J.R.; Kunzelmann, K. Anoctamins support calcium-dependent chloride secretion by facilitating calcium signaling in adult mouse intestine. Pflüg. Arch. 2014, 467, 1203–1213. [Google Scholar] [CrossRef]
- Benedetto, R.; Cabrita, I.; Schreiber, R.; Kunzelmann, K. TMEM16A is indispensable for basal mucus secretion in airways and intestine. FASEB J. 2019. [Google Scholar] [CrossRef]
- Wanitchakool, P.; Ousingsawat, J.; Sirianant, L.; Cabrita, I.; Faria, D.; Schreiber, R.; Kunzelmann, K. Cellular defects by deletion of ANO10 are due to deregulated local calcium signaling. Cell Signal. 2017, 30, 41–49. [Google Scholar] [CrossRef]
- Hammer, C.; Wanitchakool, P.; Sirianant, L.; Papiol, S.; Monnheimer, M.; Faria, D.; Ousingsawat, J.; Schramek, N.; Schmitt, C.; Margos, G.; et al. A coding variant of ANO10, affecting volume regulation of macrophages, is associated with Borrelia seropositivity. Mol. Med. 2015, 21, 26–37. [Google Scholar] [CrossRef]
- Cabrita, I.; Benedetto, R.; Fonseca, A.; Wanitchakool, P.; Sirianant, L.; Skryabin, B.V.; Schenk, L.K.; Pavenstadt, H.; Schreiber, R.; Kunzelmann, K. Differential effects of anoctamins on intracellular calcium signals. Faseb J. 2017, 31, 2123–2134. [Google Scholar] [CrossRef]
- Ruppersburg, C.C.; Hartzell, H.C. The Ca2+-activated Cl- channel ANO1/TMEM16A regulates primary ciliogenesis. Mol. Biol. Cell 2014, 25, 1793–1807. [Google Scholar] [CrossRef]
- Schreiber, R.; Kunzelmann, K. Expression of anoctamins in retinal pigment epithelium (RPE). Pflug. Arch. 2016, 468, 1921–1929. [Google Scholar] [CrossRef]
- Forschbach, V.; Goppelt-Struebe, M.; Kunzelmann, K.; Schreiber, R.; Piedagnel, R.; Kraus, A.; Eckardt, K.U.; Buchholz, B. Anoctamin 6 is localized in the primary cilium of renal tubular cells and is involved in apoptosis-dependent cyst lumen formation. Cell Death Dis. 2015, 6, e1899. [Google Scholar] [CrossRef]
- Buchholz, B.; Faria, D.; Schley, G.; Schreiber, R.; Eckardt, K.U.; Kunzelmann, K. Anoctamin 1 induces calcium-activated chloride secretion and tissue proliferation in polycystic kidney disease. Kidney Int. 2014, 85, 1058–1067. [Google Scholar] [CrossRef]
- Tian, Y.; Schreiber, R.; Wanitchakool, P.; Kongsuphol, P.; Sousa, M.; Uliyakina, I.; Palma, M.; Faria, D.; Traynor-Kaplen, A.E.; Fragata, J.I.; et al. Control of TMEM16A by INO-4995 and other inositolphosphates. Br. J. Pharmacol. 2012, 168, 253–265. [Google Scholar] [CrossRef] [Green Version]
- AlDehni, F.; Spitzner, M.; Martins, J.R.; Barro Soria, R.; Schreiber, R.; Kunzelmann, K. Role of bestrophin for proliferation and in epithelial to mesenchymal transition. J. Am. Soc. Nephrol. 2009, 20, 1556–1564. [Google Scholar] [CrossRef]
- Barro Soria, R.; Spitzner, M.; Schreiber, R.; Kunzelmann, K. Bestrophin 1 enables Ca2+ activated Cl-conductance in epithelia. J. Biol. Chem. 2009, 284, 29405–29412. [Google Scholar] [CrossRef]
- Schreiber, R.; Buchholz, B.; Kraus, A.; Schley, G.; Scholz, J.; Ousingsawat, J.; Kunzelmann, K. Lipid peroxidation drives renal cyst growth in vitro through activation of TMEM16A. J. Am. Soc. Nephrol. JASN 2019. [Google Scholar] [CrossRef]
- Schlatter, E.; Frobe, U.; Greger, R. Ion conductances of isolated cortical collecting duct cells. Pflug. Arch. 1992, 421, 381–387. [Google Scholar] [CrossRef]
- Morris, A.P.; Frizzell, R.A. Ca(2+)-dependent Cl-channels in undifferentiated human colonic cells (HT-29). I. Single-channel properties. Am. J. Physiol. 1993, 264, C968–C976. [Google Scholar] [CrossRef]
- Qu, Z.; Yao, W.; Yao, R.; Liu, X.; Yu, K.; Hartzell, H.C. The Ca -activated Cl channel, ANO1 (TMEM16A), is a double-edged sword in cell proliferation and tumorigenesis. Cancer Med. 2014, 3, 453–461. [Google Scholar] [CrossRef]
- Fujimoto, M.; Kito, H.; Kajikuri, J.; Ohya, S. Transcriptional repression of human epidermal growth factor receptor 2 by ClC-3 Cl(-) /H(+) transporter inhibition in human breast cancer cells. Cancer Sci. 2018, 109, 2781–2791. [Google Scholar] [CrossRef]
- Almaca, J.; Tian, Y.; AlDehni, F.; Ousingsawat, J.; Kongsuphol, P.; Rock, J.R.; Harfe, B.D.; Schreiber, R.; Kunzelmann, K. TMEM16 proteins produce volume regulated chloride currents that are reduced in mice lacking TMEM16A. J. Biol. Chem. 2009, 284, 28571–28578. [Google Scholar] [CrossRef]
- Wang, M.; Yang, H.; Zheng, L.Y.; Zhang, Z.; Tang, Y.B.; Wang, G.L.; Du, Y.H.; Lv, X.F.; Liu, J.; Zhou, J.G.; et al. Downregulation of TMEM16A Calcium-Activated Chloride Channel Contributes to Cerebrovascular Remodeling during Hypertension through Promoting Basilar Smooth Muscle Cell Proliferation. Circulation 2012, 125, 697–707. [Google Scholar] [CrossRef]
- Allawzi, A.M.; Vang, A.; Clements, R.T.; Jhun, B.S.; Kue, N.R.; Mancini, T.J.; Landi, A.K.; Terentyev, D.; O-Uchi, J.; Comhair, S.A.; et al. Activation of Anoctamin-1 Limits Pulmonary Endothelial Cell Proliferation via p38-MAPK-dependent Apoptosis. Am. J. Respir. Cell Mol. Biol. 2017. [Google Scholar] [CrossRef]
- Sui, Y.; Wu, F.; Lv, J.; Li, H.; Li, X.; Du, Z.; Sun, M.; Zheng, Y.; Yang, L.; Zhong, L.; et al. Identification of the Novel TMEM16A Inhibitor Dehydroandrographolide and Its Anticancer Activity on SW620 Cells. PLoS ONE 2015, 10, e0144715. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, J.; Chang, J.; Kim, S.S.; Namkung, W.; Kim, I. Synthesis and biological evaluation of novel Ani9 derivatives as potent and selective ANO1 inhibitors. Eur. J. Med. Chem. 2018, 160, 245–255. [Google Scholar] [CrossRef]
- Miner, K.; Labitzke, K.; Liu, B.; Elliot, R.; Wang, P.; Henckels, K.; Gaida, K.; Elliot, R.; Chen, J.J.; Liu, L.; et al. The Anthelminthic Niclosamide And Related Compounds Represent Potent Tmem16a Antagonists That Fully Relax Mouse And Human Airway Rings. Froniers Pharmacol. 2019. [Google Scholar] [CrossRef]
- Seo, Y.; Park, J.; Kim, M.; Lee, H.K.; Kim, J.H.; Jeong, J.H.; Namkung, W. Inhibition of ANO1/TMEM16A Chloride Channel by Idebenone and Its Cytotoxicity to Cancer Cell Lines. PLoS ONE 2015, 10, e0133656. [Google Scholar] [CrossRef]
- Wang, A.M.; Ku, H.H.; Liang, Y.C.; Chen, Y.C.; Hwu, Y.M.; Yeh, T.S. The autonomous notch signal pathway is activated by baicalin and baicalein but is suppressed by niclosamide in K562 cells. J. Cell Biochem. 2009, 106, 682–692. [Google Scholar] [CrossRef]
- Meurette, O.; Mehlen, P. Notch Signaling in the Tumor Microenvironment. Cancer Cell 2018, 34, 536–548. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kang, J.W.; Song, X.; Kim, B.K.; Yoo, Y.D.; Kwon, Y.T.; Lee, Y.J. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell Signal. 2013, 25, 961–969. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Lu, Z.; Ding, K.; Li, J.; Du, X.; Chen, C.; Sun, X.; Wu, Y.; Zhou, J.; Pan, J. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: Inactivation of the NF-kappaB pathway and generation of reactive oxygen species. Cancer Res. 2010, 70, 2516–2527. [Google Scholar] [CrossRef]
- Ren, X.; Duan, L.; He, Q.; Zhang, Z.; Zhou, Y.; Wu, D.; Pan, J.; Pei, D.; Ding, K. Identification of Niclosamide as a New Small-Molecule Inhibitor of the STAT3 Signaling Pathway. ACS Med. Chem. Lett. 2010, 1, 454–459. [Google Scholar] [CrossRef] [Green Version]
- Osada, T.; Chen, M.; Yang, X.Y.; Spasojevic, I.; Vandeusen, J.B.; Hsu, D.; Clary, B.M.; Clay, T.M.; Chen, W.; Morse, M.A.; et al. Antihelminth compound niclosamide downregulates Wnt signaling and elicits antitumor responses in tumors with activating APC mutations. Cancer Res. 2011, 71, 4172–4182. [Google Scholar] [CrossRef]
- Wang, L.H.; Xu, M.; Fu, L.Q.; Chen, X.Y.; Yang, F. The Antihelminthic Niclosamide Inhibits Cancer Stemness, Extracellular Matrix Remodeling, and Metastasis through Dysregulation of the Nuclear beta-catenin/c-Myc axis in OSCC. Sci. Rep. 2018, 8, 12776. [Google Scholar] [CrossRef]
- Arend, R.C.; Londono-Joshi, A.I.; Gangrade, A.; Katre, A.A.; Kurpad, C.; Li, Y.; Samant, R.S.; Li, P.K.; Landen, C.N.; Yang, E.S.; et al. Niclosamide and its analogs are potent inhibitors of Wnt/beta-catenin, mTOR and STAT3 signaling in ovarian cancer. Oncotarget 2016, 7, 86803–86815. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Yang, J.H.; Kim, N.H.; Lee, K.; Cha, Y.H.; Yun, J.S.; Kang, H.E.; Lee, Y.; Choi, J.; Kim, H.S.; et al. Anti-helminthic niclosamide inhibits Ras-driven oncogenic transformation via activation of GSK-3. Oncotarget 2017, 8, 31856–31863. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Wei, W.; Ma, L.; Yang, B.; Gill, R.M.; Chua, M.S.; Butte, A.J.; So, S. Computational Discovery of Niclosamide Ethanolamine, a Repurposed Drug Candidate That Reduces Growth of Hepatocellular Carcinoma Cells In Vitro and in Mice by Inhibiting Cell Division Cycle 37 Signaling. Gastroenterology 2017, 152, 2022–2036. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.K.; Roberts, M.J.; Arend, R.C.; Samant, R.S.; Buchsbaum, D.J. Multi-targeted therapy of cancer by niclosamide: A new application for an old drug. Cancer Lett. 2014, 349, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Lafkas, D.; Shelton, A.; Chiu, C.; de Leon, B.G.; Chen, Y.; Stawicki, S.S.; Siltanen, C.; Reichelt, M.; Zhou, M.; Wu, X.; et al. Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature 2015, 528, 127–131. [Google Scholar] [CrossRef]
- Danahay, H.; Pessotti, A.D.; Coote, J.; Montgomery, B.E.; Xia, D.; Wilson, A.; Yang, H.; Wang, Z.; Bevan, L.; Thomas, C.; et al. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep. 2015, 10, 239–252. [Google Scholar] [CrossRef]
- Kondo, M.; Tsuji, M.; Hara, K.; Arimura, K.; Yagi, O.; Tagaya, E.; Takeyama, K.; Tamaoki, J. Chloride ion transport and overexpression of TMEM16A in a guinea pig asthma model. Clin. Exp. Allergy 2017, 47, 795–804. [Google Scholar] [CrossRef]
- Kondo, M.; Nakata, J.; Arai, N.; Izumo, T.; Tagaya, E.; Takeyama, K.; Tamaoki, J.; Nagai, A. Niflumic acid inhibits goblet cell degranulation in a guinea pig asthma model. Allergol. Int. 2012, 61, 133–142. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Wu, Y.; Ren, Y.; Li, Z.; Yao, X.; Zhang, C.; Ye, N.; Jing, C.; Dong, J.; et al. Suppression of the Growth and Invasion of Human Head and Neck Squamous Cell Carcinomas via Regulating STAT3 Signaling and the miR-21/beta-catenin Axis with HJC0152. Mol. Cancer Ther. 2017, 16, 578–590. [Google Scholar] [CrossRef]
- Liu, C.; Lou, W.; Zhu, Y.; Nadiminty, N.; Schwartz, C.T.; Evans, C.P.; Gao, A.C. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin. Cancer Res. 2014, 20, 3198–3210. [Google Scholar] [CrossRef]
- Wieland, A.; Trageser, D.; Gogolok, S.; Reinartz, R.; Hofer, H.; Keller, M.; Leinhaas, A.; Schelle, R.; Normann, S.; Klaas, L.; et al. Anticancer effects of niclosamide in human glioblastoma. Clin. Cancer Res. 2013, 19, 4124–4136. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Haugk, K.; McKiernan, J.S.; Gulati, R.; Cheng, H.H.; Maes, J.L.; Dumpit, R.F.; Nelson, P.S.; Montgomery, B.; McCune, J.S.; et al. A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer. PLoS ONE 2018, 13, e0198389. [Google Scholar] [CrossRef]
- Burock, S.; Daum, S.; Keilholz, U.; Neumann, K.; Walther, W.; Stein, U. Phase II trial to investigate the safety and efficacy of orally applied niclosamide in patients with metachronous or sychronous metastases of a colorectal cancer progressing after therapy: The NIKOLO trial. BMC Cancer 2018, 18, 297. [Google Scholar] [CrossRef]
- Di Zanni, E.; Gradogna, A.; Scholz-Starke, J.; Boccaccio, A. Gain of function of TMEM16E/ANO5 scrambling activity caused by a mutation associated with gnathodiaphyseal dysplasia. Cell. Mol. Life Sci. 2017. [Google Scholar] [CrossRef]
- Mattheij, N.J.; Braun, A.; van Kruchten, R.; Castoldi, E.; Pircher, J.; Baaten, C.C.; Wulling, M.; Kuijpers, M.J.; Kohler, R.; Poole, A.W.; et al. Survival protein anoctamin-6 controls multiple platelet responses including phospholipid scrambling, swelling, and protein cleavage. FASEB J. 2015, 30, 727–737. [Google Scholar] [CrossRef]
- Carpenter, G. The EGF receptor: A nexus for trafficking and signaling. Bioessays 2000, 22, 697–707. [Google Scholar] [CrossRef]
- Hodeify, R.; Yu, F.; Courjaret, R.; Nader, N.; Dib, M.; Sun, L.; Adap, E.; Hubrack, S.; Machaca, K. Regulation and Role of Store-Operated Ca(2+) Entry in Cellular Proliferation. In Calcium Entry Channels in Non-Excitable Cells; Kozak, J.A., Putney, J.W., Jr., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2018; pp. 215–240. [Google Scholar]
- Kunzelmann, K.; Cabrita, I.; Wanitchakool, P.; Ousingsawat, J.; Sirianant, L.; Benedetto, R.; Schreiber, R. Modulating Ca2+signals: A common theme for TMEM16, Ist2, and TMC. Pflüg. Arch. 2016, 468, 475–490. [Google Scholar] [CrossRef]
- Benedetto, R.; Ousingsawat, J.; Wanitchakool, P.; Zhang, Y.; Holtzman, M.J.; Amaral, M.; Rock, J.R.; Schreiber, R.; Kunzelmann, K. Epithelial Chloride Transport by CFTR Requires TMEM16A. Sci. Rep. 2017, 7, 12397. [Google Scholar] [CrossRef] [Green Version]
- Wolf, W.; Kilic, A.; Schrul, B.; Lorenz, H.; Schwappach, B.; Seedorf, M. Yeast Ist2 recruits the endoplasmic reticulum to the plasma membrane and creates a ribosome-free membrane microcompartment. PLoS ONE 2012, 7, e39703. [Google Scholar] [CrossRef]
- Jin, X.; Shah, S.; Liu, Y.; Zhang, H.; Lees, M.; Fu, Z.; Lippiat, J.D.; Beech, D.J.; Sivaprasadarao, A.; Baldwin, S.A.; et al. Activation of the Cl- Channel ANO1 by Localized Calcium Signals in Nociceptive Sensory Neurons Requires Coupling with the IP3 Receptor. Sci. Signal 2013, 6, ra73. [Google Scholar] [CrossRef]
- Jin, X.; Shah, S.; Du, X.; Zhang, H.; Gamper, N. Activation of Ca2+-activated Cl- channel ANO1 by localized Ca2+ signals. J. Physiol. 2016, 594, 19–30. [Google Scholar] [CrossRef]
- Franklin, B.M.; Voss, S.R.; Osborn, J.L. Ion channel signaling influences cellular proliferation and phagocyte activity during axolotl tail regeneration. Mech. Dev. 2017, 146, 42–54. [Google Scholar] [CrossRef]
- Kunzelmann, K. TMEM16, LRRC8A, bestrophin: Chloride channels controlled by Ca and cell volume. Trends Biochem. Sci. 2015, 40, 535–543. [Google Scholar] [CrossRef]
- Agell, N.; Bachs, O.; Rocamora, N.; Villalonga, P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell Signal. 2002, 14, 649–654. [Google Scholar] [CrossRef]
- Darling, N.J.; Cook, S.J. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta 2014, 1843, 2150–2163. [Google Scholar] [CrossRef] [Green Version]
- Gao da, Y.; Zhang, B.L.; Leung, M.C.; Au, S.C.; Wong, P.Y.; Shum, W.W. Coupling of TRPV6 and TMEM16A in epithelial principal cells of the rat epididymis. J. Gen. Physiol.. 2016, 148, 161–182. [Google Scholar] [CrossRef] [Green Version]
- Barro Soria, R.; AlDehni, F.; Almaca, J.; Witzgall, R.; Schreiber, R.; Kunzelmann, K. ER localized bestrophin1 acts as a counter-ion channel to activate Ca2+ dependent ion channels TMEM16A and SK4. Pflüg. Arch. 2009, 459, 485–497. [Google Scholar] [CrossRef]
- Martins, J.R.; Kongsuphol, P.; Sammels, E.; Daimène, S.; AlDehni, F.; Clarke, L.; Schreiber, R.; DeSmedt, H.; Amaral, M.D.; Kunzelmann, K. F508del-CFTR increases intracellular Ca2+ signaling that causes enhanced calcium-dependent Cl-conductance in cystic fibrosis. Biochim. Biophys. Acta 2011, 1812, 1385–1392. [Google Scholar] [CrossRef]
- Yang, H.; Kim, A.; David, T.; Palmer, D.; Jin, T.; Tien, J.; Huang, F.; Cheng, T.; Coughlin, S.R.; Jan, Y.N.; et al. TMEM16F Forms a Ca(2+)-Activated Cation Channel Required for Lipid Scrambling in Platelets during Blood Coagulation. Cell 2012, 151, 111–122. [Google Scholar] [CrossRef]
- Keckeis, S.; Wernecke, L.; Salchow, D.J.; Reichhart, N.; Strauss, O. Activation of a Ca2+-dependent cation conductance with properties of TRPM2 by reactive oxygen species in lens epithelial cells. Exp. Eye Res. 2017, 161, 61–70. [Google Scholar] [CrossRef]
- Keckeis, S.; Reichhart, N.; Roubeix, C.; Strauss, O. Anoctamin2 (TMEM16B) forms the Ca(2+)-activated Cl(-) channel in the retinal pigment epithelium. Exp. Eye Res. 2017, 154, 139–150. [Google Scholar] [CrossRef]
- Sirianant, L.; Ousingsawat, J.; Tian, Y.; Schreiber, R.; Kunzelmann, K. TMC8 (EVER2) attenuates intracellular signaling by Zn2+ and Ca2+ and suppresses activation of Cl-currents. Cell Signal. 2014, 26, 2826–2833. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Lee, J.; Lee, B.; Kim, H.R.; Jung, J.; Lee, M.O.; Oh, U. Anoctamin 9/TMEM16J is a cation channel activated by cAMP/PKA signal. Cell Calcium 2018, 71, 75–85. [Google Scholar] [CrossRef]
- Gyobu, S.; Miyata, H.; Ikawa, M.; Yamazaki, D.; Takeshima, H.; Suzuki, J.; Nagata, S. A role of TMEM16E carrying a scrambling domain in sperm motility. Mol. Cell. Biol. 2015, 36, 645–659. [Google Scholar] [CrossRef]
- Kunzelmann, K.; Schreiber, R. Chloride secretion, anoctamin 1 and Ca2+ signaling. Channels (Austin) 2014, 8, 387–388. [Google Scholar] [CrossRef]
- Zak, J.D.; Grimaud, J.; Li, R.C.; Lin, C.C.; Murthy, V.N. Calcium-activated chloride channels clamp odor-evoked spike activity in olfactory receptor neurons. Sci. Rep. 2018, 8, 10600. [Google Scholar] [CrossRef]
- Malysz, J.; Gibbons, S.J.; Saravanaperumal, S.A.; Du, P.; Eisenman, S.T.; Cao, C.; Oh, U.; Saur, D.; Klein, S.; Ordog, T.; et al. Conditional genetic deletion of Ano1 in interstitial cells of Cajal impairs Ca2+ transients and slow-waves in adult mouse small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2016. [Google Scholar] [CrossRef]
- He, M.; Ye, W.; Wang, W.J.; Sison, E.S.; Jan, Y.N.; Jan, L.Y. Cytoplasmic Cl(-) couples membrane remodeling to epithelial morphogenesis. Proc. Natl. Acad. Sci. USA 2017, 114, e11161–e11169. [Google Scholar] [CrossRef]
- Benedetto, R.; Sirianant, L.; Pankonien, I.; Wanitchakool, P.; Ousingsawat, J.; Cabrita, I.; Schreiber, R.; Amaral, M.; Kunzelmann, K. Relationship between TMEM16A/anoctamin 1 and LRRC8A. Pflug. Arch. 2016, 468, 1751–1763. [Google Scholar] [CrossRef]
- Schenk, L.K.; Buchholz, B.; Henke, S.F.; Michgehl, U.; Daniel, C.; Amann, K.; Kunzelmann, K.; Pavenstadt, H.J. Nephron-specific knockout of TMEM16A leads to reduced number of glomeruli and albuminuria. Am. J. Physiol. Renal Physiol. 2018. [Google Scholar] [CrossRef]
- Bricogne, C.; Fine, M.; Pereira, P.M.; Sung, J.; Tijani, M.; Wang, Y.; Henriques, R.; Collins, M.K.; Hilgemann, D. TMEM16F activation by Ca(2+) triggers plasma membrane expansion and directs PD-1 trafficking. Sci. Rep. 2019, 9, 619. [Google Scholar] [CrossRef]
- Faria, D.; Schlatter, E.; Witzgall, R.; Grahammer, F.; Bandulik, S.; Schweda, F.; Bierer, S.; Rock, J.R.; Heitzmann, D.; Kunzelmann, K.; et al. The calcium activated chloride channel Anoctamin 1 contributes to the regulation of renal function. Kindey Int. 2014, 85, 1369–1381. [Google Scholar] [CrossRef]
- Ousingsawat, J.; Wanitchakool, P.; Schreiber, R.; Wuelling, M.; Vortkamp, A.; Kunzelmann, K. Anoctamin 6 controls bone mineralization by activating the calcium transporter NCX1. J. Biol. Chem. 2015, 290, 6270–6280. [Google Scholar] [CrossRef]
- Benedetto, R.; Ousingsawat, J.; Cabrita, I.; Pinto, M.; Lérias, J.R.; Wanitchakool, P.; Schreiber, R.; Kunzelmann, K. Plasma membrane localized TMEM16 proteins are indispensable for expression of CFTR. J. Mol. Med. 2019. [Google Scholar] [CrossRef]
- Oheim, M.; Kirchhoff, F.; Stuhmer, W. Calcium microdomains in regulated exocytosis. Cell Calcium 2006, 40, 423–439. [Google Scholar] [CrossRef]
- Chieregatti, E.; Meldolesi, J. Regulated exocytosis: New organelles for non-secretory purposes. Nat. Rev. Mol. Cell Biol. 2005, 6, 181–187. [Google Scholar] [CrossRef]
- Jacobsen, K.S.; Zeeberg, K.; Poulsen, K.A.; Hoffmann, E.K.; Schwab, A. The role of TMEM16A (ANO1) and TMEM16F (ANO6) in cell migration. Pflug. Arch. 2013, 465, 1753–1762. [Google Scholar] [CrossRef] [Green Version]
- Veit, M.; Koyro, K.I.; Ahrens, B.; Bleibaum, F.; Munz, M.; Rövekamp, H.; Andrä, J.; Schreiber, R.; Kunzelmann, K.; Sommer, A.; et al. Anoctamin-6 regulates ADAM sheddase function. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1598–1610. [Google Scholar] [CrossRef]
- Bleibaum, F.; Sommer, A.; Veit, M.; Rabe, B.; Andrä, J.; Kunzelmann, K.; Nehls, C.; Correa, W.; Gutsmann, T.; Grötzinger, J.; et al. ADAM10 sheddase activation is controlled by cell membrane asymmetry. J. Mol. Cell. Biol. 2018. [Google Scholar] [CrossRef]
- Sommer, A.; Kordowski, F.; Buch, J.; Maretzky, T.; Evers, A.; Andra, J.; Dusterhoft, S.; Michalek, M.; Lorenzen, I.; Somasundaram, P.; et al. Phosphatidylserine exposure is required for ADAM17 sheddase function. Nat. Commun. 2016, 7, 11523. [Google Scholar] [CrossRef] [Green Version]
- Crutzen, R.; Virreira, M.; Markadieu, N.; Shlyonsky, V.; Sener, A.; Malaisse, W.J.; Beauwens, R.; Boom, A.; Golstein, P.E. Anoctamin 1 (Ano1) is required for glucose-induced membrane potential oscillations and insulin secretion by murine beta-cells. Pflug. Arch. 2015, 468, 573–591. [Google Scholar] [CrossRef]
- Edlund, A.; Esguerra, J.L.; Wendt, A.; Flodstrom-Tullberg, M.; Eliasson, L. CFTR and Anoctamin 1 (ANO1) contribute to cAMP amplified exocytosis and insulin secretion in human and murine pancreatic beta-cells. BMC Med. 2014, 12, 87–12. [Google Scholar] [CrossRef]
- Tian, Y.; Kongsuphol, P.; Hug, M.J.; Ousingsawat, J.; Witzgall, R.; Schreiber, R.; Kunzelmann, K. Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel TMEM16A. FASEB J. 2011, 25, 1058–1068. [Google Scholar] [CrossRef]
- Gupta, R.; Radicioni, G.; Abdelwahab, S.; Dang, H.; Carpenter, J.; Chua, M.; Mieczkowski, P.A.; Sheridan, J.; Randell, S.H.; Kesimer, M. Intercellular Communication between Airway Epithelial Cells is Mediated by Exosome-Like Vesicles. Am. J. Respir. Cell Mol. Biol. 2018. [Google Scholar] [CrossRef]
- Whitlock, J.M.; Hartzell, H.C. Anoctamins/TMEM16 Proteins: Chloride Channels Flirting with Lipids and Extracellular Vesicles. Annu. Rev. Physiol. 2016. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Han, T.W.; Ye, W.; Bethel, N.P.; Zubia, M.; Kim, A.; Li, K.H.; Burlingame, A.L.; Grabe, M.; Jan, Y.N.; Jan, L.Y. Chemically induced vesiculation as a platform for studying TMEM16F activity. Proc. Natl. Acad. Sci. USA 2019. [Google Scholar] [CrossRef]
- Svenningsen, P.; Nielsen, M.R.; Marcussen, N.; Walter, S.; Jensen, B.L. TMEM16A is a Ca -activated Cl channel expressed in the renal collecting duct. Acta Physiol. 2014, 212, 166–174. [Google Scholar] [CrossRef]
- Jimenez, A.J.; Maiuri, P.; Lafaurie-Janvore, J.; Divoux, S.; Piel, M.; Perez, F. ESCRT machinery is required for plasma membrane repair. Science 2014, 343, 1247136. [Google Scholar] [CrossRef]
- Gong, Y.N.; Guy, C.; Olauson, H.; Becker, J.U.; Yang, M.; Fitzgerald, P.; Linkermann, A.; Green, D.R. ESCRT-III Acts Downstream of MLKL to Regulate Necroptotic Cell Death and Its Consequences. Cell 2017, 169, 286–300.e216. [Google Scholar] [CrossRef]
- Bolduc, V.; Marlow, G.; Boycott, K.M.; Saleki, K.; Inoue, H.; Kroon, J.; Itakura, M.; Robitaille, Y.; Parent, L.; Baas, F.; et al. Recessive mutations in the putative calcium-activated chloride channel Anoctamin 5 cause proximal LGMD2L and distal MMD3 muscular dystrophies. Am. J. Hum. Genet. 2010, 86, 213–221. [Google Scholar] [CrossRef]
- Ousingsawat, J.; Cabrita, I.; Wanitchakool, P.; Sirianant, L.; Krautwald, S.; Linkermann, A.; Schreiber, R.; Kunzelmann, K. Ca2+ signals, cell membrane disintegration, and activation of TMEM16F during necroptosis. Cell. Mol. Life Sci. 2016. [Google Scholar] [CrossRef]
- Sirianant, L.; Ousingsawat, J.; Wanitchakool, P.; Schreiber, R.; Kunzelmann, K. Cellular Volume regulation by Anoctamin 6:Ca2+, phospholipase A2,osmosensing. Pflüg. Arch. 2015, 468, 335–349. [Google Scholar] [CrossRef]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Pfitzner, E.; Kliem, S.; Baus, D.; Litterst, C.M. The role of STATs in inflammation and inflammatory diseases. Curr. Pharm. Des. 2004, 10, 2839–2850. [Google Scholar] [CrossRef]
- Nussinov, R.; Tsai, C.J.; Jang, H. Oncogenic Ras Isoforms Signaling Specificity at the Membrane. Cancer Res. 2018, 78, 593–602. [Google Scholar] [CrossRef]
- Galietta, L.J.; Pagesy, P.; Folli, C.; Caci, E.; Romio, L.; Costes, B.; Nicolis, E.; Cabrini, G.; Goossens, M.; Ravazzolo, R.; et al. IL-4 Is a Potent Modulator of Ion Transport in the Human Bronchial Epithelium In Vitro. J. Immunol. 2002, 168, 839–845. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Zhang, H.; Wu, M.; Yang, H.; Kudo, M.; Peters, C.J.; Woodruff, P.G.; Solberg, O.D.; Donne, M.L.; Huang, X.; et al. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc. Natl. Acad. Sci. USA 2012, 109, 16354–16359. [Google Scholar] [CrossRef] [Green Version]
- Caci, E.; Scudieri, P.; Di Carlo, E.; Morelli, P.; Bruno, S.; De, F.I.; Bragonzi, A.; Gianotti, A.; Sondo, E.; Ferrera, L.; et al. Upregulation of TMEM16A Protein in Bronchial Epithelial Cells by Bacterial Pyocyanin. PLoS ONE 2015, 10, e0131775. [Google Scholar] [CrossRef]
- Scudieri, P.; Caci, E.; Bruno, S.; Ferrera, L.; Schiavon, M.; Sondo, E.; Tomati, V.; Gianotti, A.; Zegarra-Moran, O.; Pedemonte, N.; et al. Association of TMEM16A chloride channel overexpression with airway goblet cells metaplasia. J. Physiol. 2012, 590, 6141–6155. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Lang, F.; Hoffmann, E.K. CrossTalk proposal: Cell volume changes are an essential step in the cell death machinery. J. Physiol. 2013, 591, 6119–6121. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, E.K.; Lambert, I.H.; Pedersen, S.F. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009, 89, 193–277. [Google Scholar] [CrossRef]
- Lee, E.L.; Shimizu, T.; Ise, T.; Numata, T.; Kohno, K.; Okada, Y. Impaired activity of volume-sensitive Cl- channel is involved in cisplatin resistance of cancer cells. J. Cell. Physiol. 2007, 211, 513–521. [Google Scholar] [CrossRef]
- Okada, Y.; Shimizu, T.; Maeno, E.; Tanabe, S.; Wang, X.; Takahashi, N. Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. J. Membr. Biol. 2006, 209, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Lee, E.L.; Ise, T.; Okada, Y. Volume-sensitive Cl(-) channel as a regulator of acquired cisplatin resistance. Anticancer Res. 2008, 28, 75–83. [Google Scholar] [PubMed]
- Lemonnier, L.; Shuba, Y.; Crepin, A.; Roudbaraki, M.; Slomianny, C.; Mauroy, B.; Nilius, B.; Prevarskaya, N.; Skryma, R. Bcl-2-dependent modulation of swelling-activated Cl- current and ClC-3 expression in human prostate cancer epithelial cells. Cancer Res. 2004, 64, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- Planells-Cases, R.; Lutter, D.; Guyader, C.; Gerhards, N.M.; Ullrich, F.; Elger, D.A.; Kucukosmanoglu, A.; Xu, G.; Voss, F.K.; Reincke, S.M.; et al. Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J. 2015, 34, 2993–3008. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.F.; Okada, Y.; Nilius, B. Biophysics and Physiology of the Volume-Regulated Anion Channel (VRAC)/Volume-Sensitive Outwardly Rectifying Anion Channel (VSOR). Pflug. Arch. 2016, 468, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Foller, M.; Lang, K.; Lang, P.; Ritter, M.; Vereninov, A.; Szabo, I.; Huber, S.M.; Gulbins, E. Cell volume regulatory ion channels in cell proliferation and cell death. Methods Enzymol. 2007, 428, 209–225. [Google Scholar] [PubMed]
- Juul, C.A.; Grubb, S.; Poulsen, K.A.; Kyed, T.; Hashem, N.; Lambert, I.H.; Larsen, E.H.; Hoffmann, E.K. Anoctamin 6 differs from VRAC and VSOAC but is involved in apoptosis and supports volume regulation in the presence of Ca. Pflug. Arch. 2014, 466, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Dubin, A.E.; Mathur, J.; Tu, B.; Reddy, K.; Miraglia, L.J.; Reinhardt, J.; Orth, A.P.; Patapoutian, A. SWELL1, a Plasma Membrane Protein, Is an Essential Component of Volume-Regulated Anion Channel. Cell 2014, 157, 447–458. [Google Scholar] [CrossRef] [Green Version]
- Voss, F.K.; Ullrich, F.; Munch, J.; Lazarow, K.; Lutter, D.; Mah, N.; Andrade-Navarro, M.A.; von Kries, J.P.; Stauber, T.; Jentsch, T.J. Identification of LRRC8 Heteromers as an Essential Component of the Volume-Regulated Anion Channel VRAC. Science 2014, 344, 634–638. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, S.F.; Klausen, T.K.; Nilius, B. The identification of VRAC (Volume Regulated Anion Channel):An amazing Odyssey. Acta Physiol. 2015, 213, 868–881. [Google Scholar] [CrossRef]
- Deneka, D.; Sawicka, M.; Lam, A.K.M.; Paulino, C.; Dutzler, R. Structure of a volume-regulated anion channel of the LRRC8 family. Nature 2018, 558, 254–259. [Google Scholar] [CrossRef]
- Kefauver, J.M.; Saotome, K.; Dubin, A.E.; Pallesen, J.; Cottrell, C.A.; Cahalan, S.M.; Qiu, Z.; Hong, G.; Crowley, C.S.; Whitwam, T.; et al. Structure of the human volume regulated anion channel. Elife 2018, 7, e38461. [Google Scholar] [CrossRef]
- Kasuya, G.; Nakane, T.; Yokoyama, T.; Jia, Y.; Inoue, M.; Watanabe, K.; Nakamura, R.; Nishizawa, T.; Kusakizako, T.; Tsutsumi, A.; et al. Cryo-EM structures of the human volume-regulated anion channel LRRC8. Nat. Struct. Mol. Biol. 2018, 25, 797–804. [Google Scholar] [CrossRef]
- Sirianant, L.; Wanitchakool, P.; Ousingsawat, J.; Benedetto, R.; Zormpa, A.; Cabrita, I.; Schreiber, R.; Kunzelmann, K. Non-essential contribution of LRRC8A to volume regulation. Pflüg. Arch. 2016, 468, 1789–1796. [Google Scholar] [CrossRef]
- Milenkovic, A.; Brandl, C.; Milenkovic, V.M.; Jendrike, T.; Sirianant, L.; Wanitchakool, P.; Zimmermann, S.; Reif, C.M.; Horling, F.; Schrewe, H.; et al. Bestrophin1 is the volume-regulated anion channel in mouse sperm and human retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2015, 112, E2630–E2639. [Google Scholar] [CrossRef]
- Wanitchakool, P.; Ousingsawat, J.; Sirianant, L.; MacAulay, N.; Schreiber, R.; Kunzelmann, K. Cl-channels in apoptosis. Eur. Biophys. J. 2016, 45, 599–610. [Google Scholar] [CrossRef]
- Kumar, L.; Chou, J.; Yee, C.S.; Borzutzky, A.; Vollmann, E.H.; von Andrian, U.H.; Park, S.Y.; Hollander, G.; Manis, J.P.; Poliani, P.L.; et al. Leucine-rich repeat containing 8A (LRRC8A) is essential for T lymphocyte development and function. J. Exp. Med. 2014, 211, 929–942. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Wondergem, R.; Morrison, R.; Yin, V.P.; Strange, K. Leucine-rich repeat containing protein LRRC8A is essential for swelling-activated Cl-currents and embryonic development in zebrafish. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef]
- Sawada, A.; Takihara, Y.; Kim, J.Y.; Matsuda-Hashii, Y.; Tokimasa, S.; Fujisaki, H.; Kubota, K.; Endo, H.; Onodera, T.; Ohta, H.; et al. A congenital mutation of the novel gene LRRC8 causes agammaglobulinemia in humans. J. Clin. Investig. 2003, 112, 1707–1713. [Google Scholar] [CrossRef] [Green Version]
- Bao, J.; Perez, C.J.; Kim, J.; Zhang, H.; Murphy, C.J.; Hamidi, T.; Jaubert, J.; Platt, C.D.; Chou, J.; Deng, M.; et al. Deficient LRRC8A-dependent volume-regulated anion channel activity is associated with male infertility in mice. JCI Insight 2018, 3, e99767. [Google Scholar] [CrossRef]
- Lemonnier, L.; Prevarskaya, N.; Shuba, Y.; Vanden Abeele, F.; Nilius, B.; Mazurier, J.; Skryma, R. Ca2+ modulation of volume-regulated anion channels: Evidence for colocalization with store-operated channels. FASEB J. 2002, 16, 222–224. [Google Scholar] [CrossRef]
- Zholos, A.; Beck, B.; Sydorenko, V.; Lemonnier, L.; Bordat, P.; Prevarskaya, N.; Skryma, R. Ca(2+)- and volume-sensitive chloride currents are differentially regulated by agonists and store-operated Ca2+ entry. J. Gen. Physiol. 2005, 125, 197–211. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, Z.; Zhang, D.; Li, H.; Zhang, L.; Niu, J.; Zuo, W.; Fu, R.; Fan, L.; Ye, J.H.; et al. High expression of leucinerich repeatcontaining 8A is indicative of a worse outcome of colon cancer patients by enhancing cancer cell growth and metastasis. Oncol. Rep. 2018, 40, 1275–1286. [Google Scholar]
- Sorensen, B.H.; Nielsen, D.; Thorsteinsdottir, U.A.; Hoffmann, E.K.; Lambert, I.H. Downregulation of LRRC8A protects human ovarian and alveolar carcinoma cells against Cisplatin-induced expression of p53, MDM2, p21Waf1/Cip1, and Caspase-9/-3 activation. Am. J. Physiol. Cell Physiol. 2016, 310, C857–C873. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Khandelwal, N.; Kumar, A.; Bera, A.K. Leucine-rich repeat-containing 8B protein is associated with the endoplasmic reticulum Ca2+ leak in HEK293 cells. J. Cell Sci. 2017, 130, 3818–3828. [Google Scholar] [CrossRef]
- Borahay, M.A.; Kilic, G.S.; Yallampalli, C.; Snyder, R.R.; Hankins, G.D.; Al-Hendy, A.; Boehning, D. Simvastatin potently induces calcium-dependent apoptosis of human leiomyoma cells. J. Biol. Chem. 2014, 289, 35075–35086. [Google Scholar] [CrossRef]
- Jackisch, C.; Hahm, H.A.; Tombal, B.; McCloskey, D.; Butash, K.; Davidson, N.E.; Denmeade, S.R. Delayed micromolar elevation in intracellular calcium precedes induction of apoptosis in thapsigargin-treated breast cancer cells. Clin. Cancer Res. 2000, 6, 2844–2850. [Google Scholar]
- Martins, J.R.; Faria, D.; Kongsuphol, P.; Reisch, B.; Schreiber, R.; Kunzelmann, K. Anoctamin 6 is an essential component of the outwardly rectifying chloride channel. Proc. Natl. Acad. Sci. USA 2011, 108, 18168–18172. [Google Scholar] [CrossRef] [Green Version]
- Szabo, I.; Lepple-Wienhues, A.; Kaba, K.N.; Zoratti, M.; Gulbins, E.; Lang, F. Tyrosine kinase-dependent activation of a chloride channel in CD95-induced apoptosis in T lymphocytes. Proc. Natl. Acad. Sci. USA 1998, 95, 6169–6174. [Google Scholar] [CrossRef] [Green Version]
- Lepple-Wienhues, A.; Wieland, U.; Laun, T.; Heil, L.; Stern, M.; Lang, F. A src-like kinase activates outwardly rectifying chloride channels in CFTR-defective lymphocytes. FASEB J. 2001, 15, 927–931. [Google Scholar] [CrossRef]
- Lian, H.; Cheng, Y.; Wu, X. TMEM16A exacerbates renal injury by activating P38/JNK signaling pathway to promote podocyte apoptosis in diabetic nephropathy mice. Biochem. Biophys. Res. Commun. 2017. [Google Scholar] [CrossRef]
- Zeng, J.W.; Chen, B.Y.; Lv, X.F.; Sun, L.; Zeng, X.L.; Zheng, H.Q.; Du, Y.H.; Wang, G.L.; Ma, M.M.; Guan, Y.Y. TMEM16A Participates in Hydrogen Peroxide-Induced Apoptosis by Facilitating Mitochondria-Dependent Pathway in Vascular Smooth Muscle Cells. Br. J. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Henkel, B.; Drose, D.R.; Ackels, T.; Oberland, S.; Spehr, M.; Neuhaus, E.M. Co-expression of Anoctamins in Cilia of Olfactory Sensory Neurons. Chem. Sens. 2015, 40, 73–87. [Google Scholar] [CrossRef]
- Linkermann, A.; Stockwell, B.R.; Krautwald, S.; Anders, H.J. Regulated cell death and inflammation: An auto-amplification loop causes organ failure. Nat. Rev. Immunol. 2014, 14, 759–767. [Google Scholar] [CrossRef]
- Ousingsawat, J.; Wanitchakool, P.; Schreiber, R.; Kunzelmann, K. Contribution of TMEM16F to pyroptotic cell death. Cell Death Dis. 2018, 9, 300. [Google Scholar] [CrossRef]
- Simoes, F.; Ousingsawat, J.; Wanitchakool, P.; Fonseca, A.; Cabrita, I.; Benedetto, R.; Schreiber, R.; Kunzelmann, K. CFTR supports cell death through ROS-dependent activation of TMEM16F (anoctamin 6). Pflug. Arch. 2018, 470, 305–314. [Google Scholar] [CrossRef]
- Kunzelmann, K. Ion channels in regulated cell death. Cell. Mol. Life Sci. 2016, 73, 2387–2403. [Google Scholar] [CrossRef]
- Orsolic, N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef]
- Zheng, J.; Lee, H.L.; Ham, Y.W.; Song, H.S.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom on colon cancer cell growth by activation of death receptors and inhibition of nuclear factor kappa B. Oncotarget 2015, 6, 44437–44451. [Google Scholar] [CrossRef] [Green Version]
- Park, M.H.; Choi, M.S.; Kwak, D.H.; Oh, K.W.; Yoon, D.Y.; Han, S.B.; Song, H.S.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom in prostate cancer cells through activation of caspase pathway via inactivation of NF-kappaB. Prostate 2011, 71, 801–812. [Google Scholar] [CrossRef]
- Dar, H.H.; Tyurina, Y.Y.; Mikulska-Ruminska, K.; Shrivastava, I.; Ting, H.C.; Tyurin, V.A.; Krieger, J.; St Croix, C.M.; Watkins, S.; Bayir, E.; et al. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J. Clin. Investig. 2018. [Google Scholar] [CrossRef]
- Gueraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010, 44, 1098–1124. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Florean, C.; Song, S.; Dicato, M.; Diederich, M. Redox biology of regulated cell death in cancer: A focus on necroptosis and ferroptosis. Free Radic. Biol. Med. 2019. [Google Scholar] [CrossRef]
- Moreira, J.D.; Hamraz, M.; Abolhassani, M.; Bigan, E.; Peres, S.; Pauleve, L.; Nogueira, M.L.; Steyaert, J.M.; Schwartz, L. The Redox Status of Cancer Cells Supports Mechanisms behind the Warburg Effect. Metabolites 2016, 6, 33. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef]
- Pei, S.; Minhajuddin, M.; Callahan, K.P.; Balys, M.; Ashton, J.M.; Neering, S.J.; Lagadinou, E.D.; Corbett, C.; Ye, H.; Liesveld, J.L.; et al. Targeting aberrant glutathione metabolism to eradicate human acute myelogenous leukemia cells. J. Biol. Chem. 2013, 288, 33542–33558. [Google Scholar] [CrossRef]
- Trachootham, D.; Zhou, Y.; Zhang, H.; Demizu, Y.; Chen, Z.; Pelicano, H.; Chiao, P.J.; Achanta, G.; Arlinghaus, R.B.; Liu, J.; et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 2006, 10, 241–252. [Google Scholar] [CrossRef]
- Takeshita, N.; Evangelinos, M.; Zhou, L.; Serizawa, T.; Somera-Fajardo, R.A.; Lu, L.; Takaya, N.; Nienhaus, G.U.; Fischer, R. Pulses of Ca2+ coordinate actin assembly and exocytosis for stepwise cell extension. Proc. Natl. Acad. Sci. USA 2017, 114, 5701–5706. [Google Scholar] [CrossRef] [Green Version]
- Dolma, S.; Lessnick, S.L.; Hahn, W.C.; Stockwell, B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 2003, 3, 285–296. [Google Scholar] [CrossRef] [Green Version]

| Anoctamin Paralogue | References |
|---|---|
| Anoctamin 1, TMEM16A | |
| GIST, squamous carcinoma, head and neck cancer | [12,13,14,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] |
| Pancreatic cancer | [42,43,44] |
| Prostate cancer | [45,46,47] |
| Breast cancer | [48,49,50,51,52,53] |
| Colorectal carcinoma | [54,55] |
| Gastric cancer | [56,57] |
| Glioma, Glioblastoma | [58,59] |
| Esophageal cancer | [60] |
| Lung cancer | [61,62,63] |
| Hepatocellular carcinoma | [64] |
| Ovarian cancer | |
| Liposarcoma | [65] |
| Leimyosarcoma | [66] |
| Salivary gland cancer | [67] |
| Chondroblastoma | [68] |
| General role in cancer and proliferation | [14,69,70,71,72,73,74,75,76] |
| Anoctamin 5, TMEM16E | |
| Colorectal cancer | [77,78] |
| Thyroid cancer | [79] |
| Anoctamin 6, TMEM16F | |
| Myoblast proliferation | [80] |
| Anoctamin 7, TMEM16G | |
| Prostate cancer | [81,82,83,84,85,86] |
| Breast cancer | [87] |
| Anoctamin 9, TMEM16J | |
| Pancreatic cancer | [88] |
| Colorectal carcinoma | [89] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunzelmann, K.; Ousingsawat, J.; Benedetto, R.; Cabrita, I.; Schreiber, R. Contribution of Anoctamins to Cell Survival and Cell Death. Cancers 2019, 11, 382. https://doi.org/10.3390/cancers11030382
Kunzelmann K, Ousingsawat J, Benedetto R, Cabrita I, Schreiber R. Contribution of Anoctamins to Cell Survival and Cell Death. Cancers. 2019; 11(3):382. https://doi.org/10.3390/cancers11030382
Chicago/Turabian StyleKunzelmann, Karl, Jiraporn Ousingsawat, Roberta Benedetto, Ines Cabrita, and Rainer Schreiber. 2019. "Contribution of Anoctamins to Cell Survival and Cell Death" Cancers 11, no. 3: 382. https://doi.org/10.3390/cancers11030382
APA StyleKunzelmann, K., Ousingsawat, J., Benedetto, R., Cabrita, I., & Schreiber, R. (2019). Contribution of Anoctamins to Cell Survival and Cell Death. Cancers, 11(3), 382. https://doi.org/10.3390/cancers11030382





