Effect of Multiple Vaccinations with Tumor Cell-Based Vaccine with Codon-Modified GM-CSF on Tumor Growth in a Mouse Model
Abstract
1. Introduction
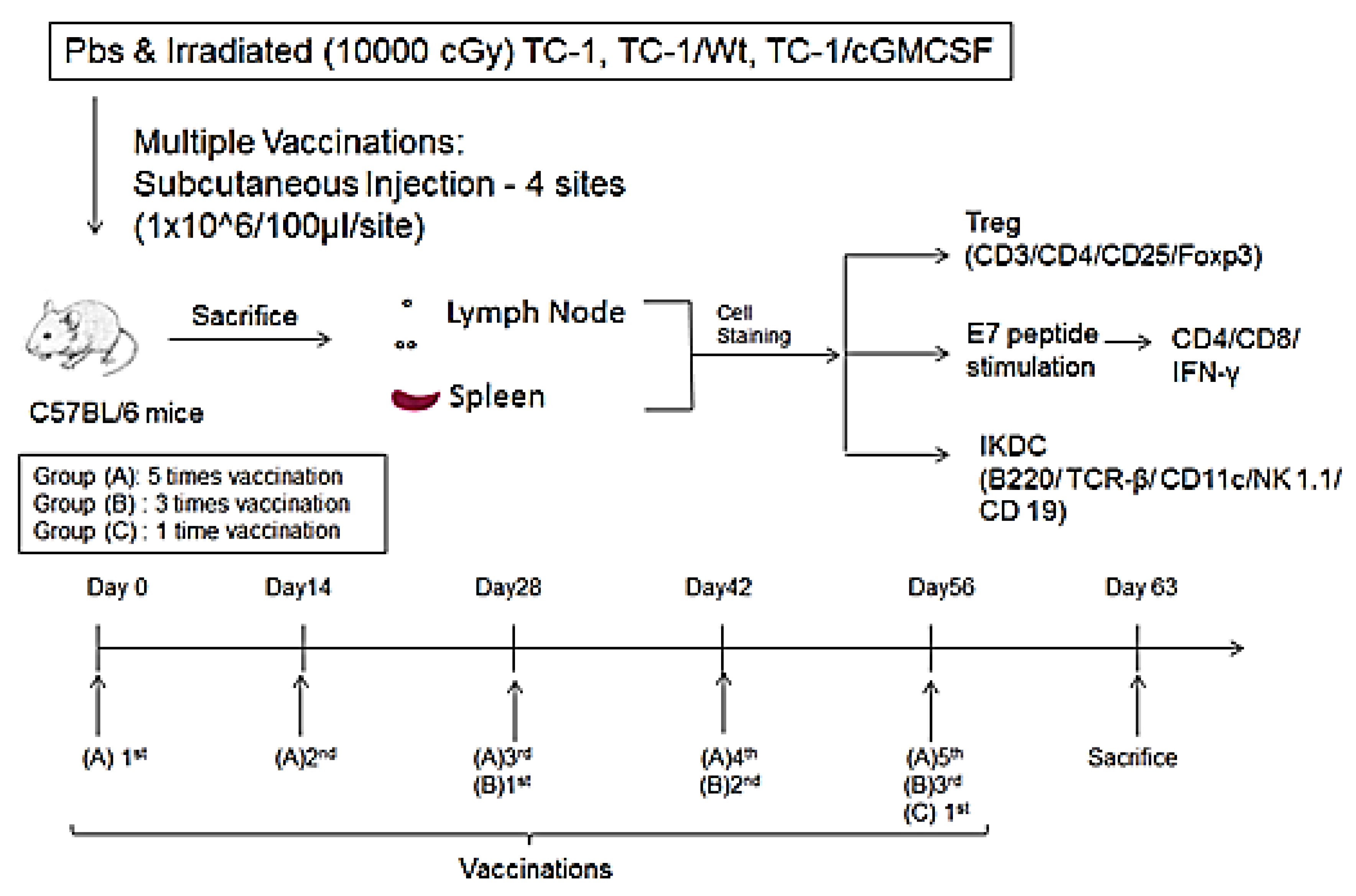
2. Materials and Methods
2.1. Mice
2.2. Cell Lines
2.3. Cytokine Secretion Measurement with ELISA
2.4. Tumor Model and Vaccination
2.5. Flow Cytometric Analysis
2.6. Statistical Analysis
3. Results
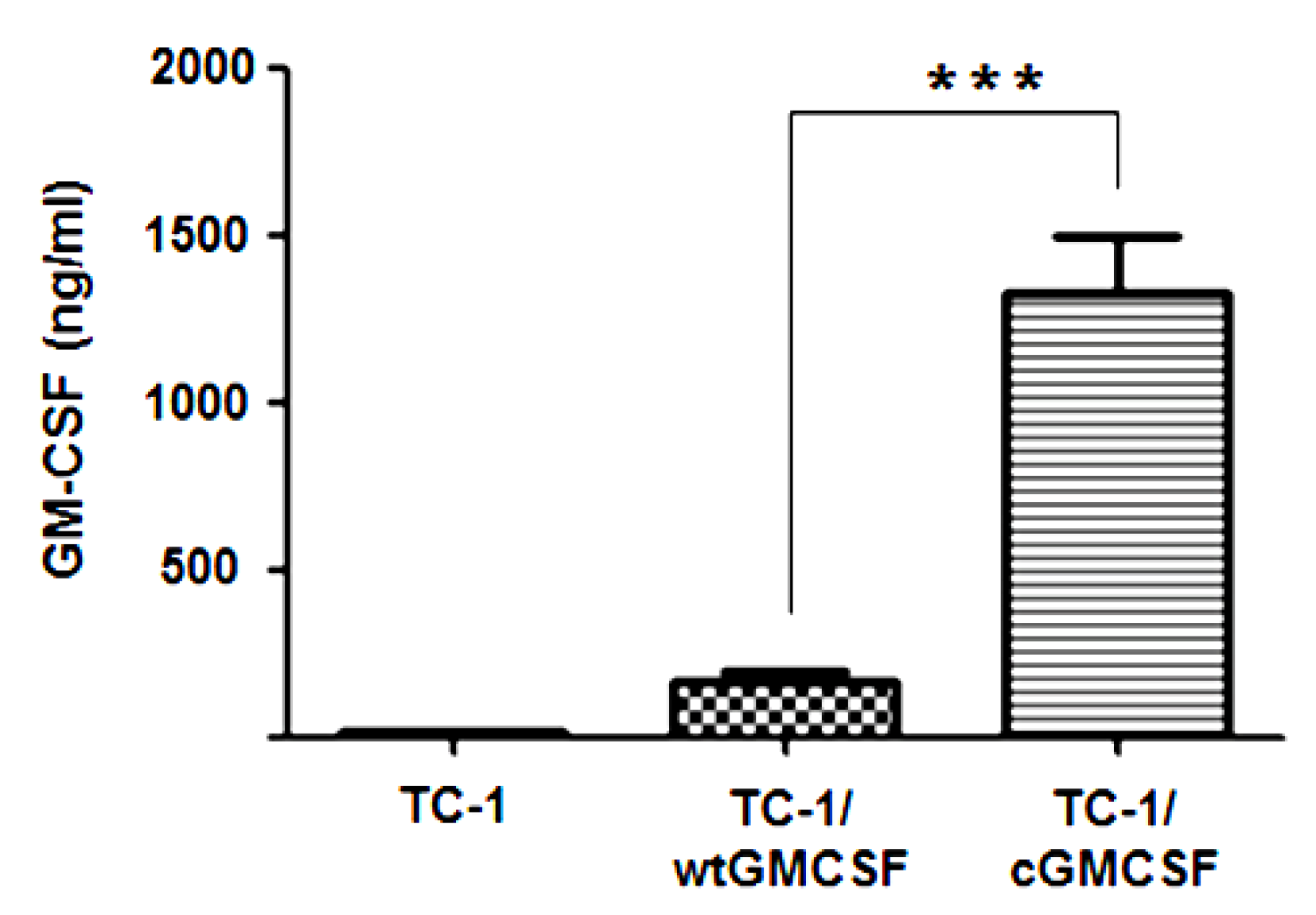
3.1. TC-1 Cells Transfected with LV-cGM-CSF(Lentiviral) (TC-1/cGM-CSF) Expressed Increased Levels of GM-CSF Compared with TC-1 Cells Transfected with LV-wtGM-CSF (TC-1/wtGM-CSF)
3.2. Mice Vaccinated with Three Doses of Irradiated TC-1/cGM-CSF Induced Enhanced Immunosurveillance Compared with Mice Vaccinated for TC-1 Tumor with One Dose and Five Doses
3.3. Generation of Antigen-Specific IFN-γ-Producing CD8+ and CD4+ T Cells
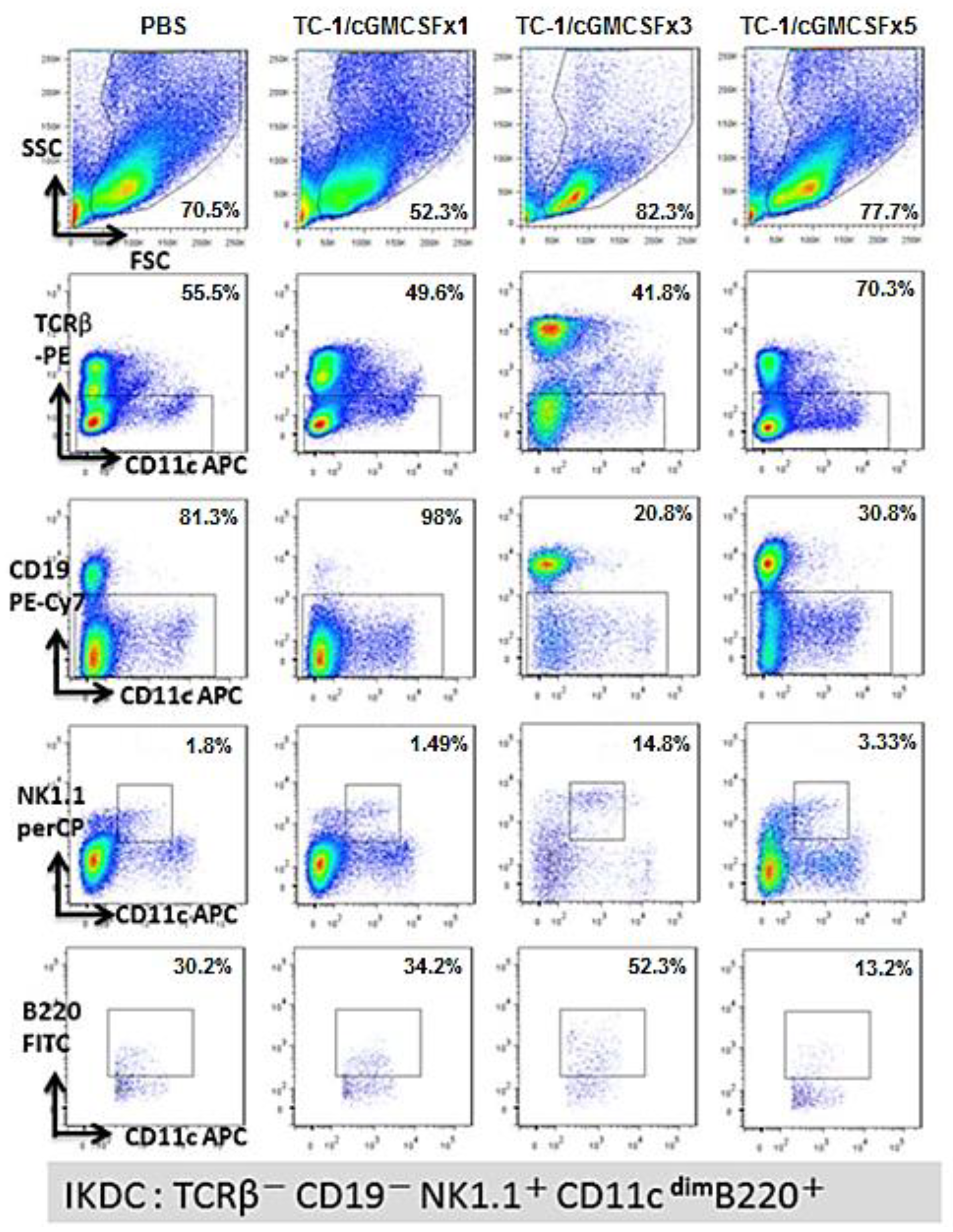
3.4. A Higher Percentage of B220+ NK1.1+ IKDC Were Produced in Mice Vaccinated with Three Doses of Irradiated TC-1/cGM-CSF Vaccine Compared with Those of Mice Vaccinated with One Dose and Five Doses of Irradiated TC-1/cGM-CSF Vaccine
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Daniyal, M.; Akhtar, N.; Ahmad, S.; Fatima, U.; Akram, M.; Asif, H.M. Update knowledge on cervical cancer incidence and prevalence in Asia. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 3617–3620. [Google Scholar] [CrossRef] [PubMed]
- de Sanjose, S.; Quint, W.G.V.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.-R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Catão Zampronha, R.; Freitas-Junior, R.; Murta, E.F.C.; Michelin, M.A.; Barbaresco, A.A.; Adad, S.J.; de Oliveira, A.M.; Rassi, A.B.; Oton, G.J.B. Human papillomavirus types 16 and 18 and the prognosis of patients with stage I cervical cancer. Clinics 2013, 68, 809–814. [Google Scholar] [CrossRef]
- Alam, M.S.; Ali, A.; Mehdi, S.J.; Alyasiri, N.S.; Kazim, Z.; Batra, S.; Mandal, A.K.; Rizvi, M.M. HPV typing and its relation with apoptosis in cervical carcinoma from Indian population. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2012, 33, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 2006, 118, 3030–3044. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Wallace, N.A.; Galloway, D.A. Novel Functions of the Human Papillomavirus E6 Oncoproteins. Annual Rev. Virol. 2015, 2, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Hoppe-Seyler, K.; Bossler, F.; Braun, J.A.; Herrmann, A.L.; Hoppe-Seyler, F. The HPV E6/E7 Oncogenes: Key Factors for Viral Carcinogenesis and Therapeutic Targets. Trends Microbiol. 2018, 26, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.-K.; Park, J.-S. The Role of HPV E6 and E7 Oncoproteins in HPV-associated Cervical Carcinogenesis. Cancer Res. Treat. 2005, 37, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.; Ehemann, V.; Waldeck, W.; Pipkorn, R.; Corban-Wilhelm, H.; Jenne, J.; Gissmann, L.; Debus, J. HPV18 E6 and E7 genes affect cell cycle, pRB and p53 of cervical tumor cells and represent prominent candidates for intervention by use peptide nucleic acids (PNAs). Cancer Lett. 2004, 209, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Stern, P.L.; van der Burg, S.H.; Hampson, I.N.; Broker, T.R.; Fiander, A.; Lacey, C.J.; Kitchener, H.C.; Einstein, M.H. Therapy of human papillomavirus-related disease. Vaccine 2012, 30 (Suppl. 5), F71–F82. [Google Scholar] [CrossRef]
- Tan, S.; de Vries, E.G.; van der Zee, A.G.; de Jong, S. Anticancer drugs aimed at E6 and E7 activity in HPV-positive cervical cancer. Curr. Cancer Drug Targets 2012, 12, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hodge, L.M.; Jones, H.P.; Tabor, L.; Simecka, J.W. Co-expression of granulocyte-macrophage colony-stimulating factor with antigen enhances humoral and tumor immunity after DNA vaccination. Vaccine 2002, 20, 1466–1474. [Google Scholar] [CrossRef]
- Toubaji, A.; Hill, S.; Terabe, M.; Qian, J.; Floyd, T.; Simpson, R.M.; Berzofsky, J.A.; Khleif, S.N. The combination of GM-CSF and IL-2 as local adjuvant shows synergy in enhancing peptide vaccines and provides long term tumor protection. Vaccine 2007, 25, 5882–5891. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Tsai, C.C.; Lee, J.M.; Fang, C.H.; Chang, K.S.; Wong, K.K.; Lin, C.T.; Qiu, J.T. The efficacy of a novel vaccine approach using tumor cells that ectopically express a codon-optimized murine GM-CSF in a murine tumor model. Vaccine 2016, 34, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Soiffer, R.; Hodi, F.S.; Haluska, F.; Jung, K.; Gillessen, S.; Singer, S.; Tanabe, K.; Duda, R.; Mentzer, S.; Jaklitsch, M.; et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 3343–3350. [Google Scholar] [CrossRef] [PubMed]
- Soiffer, R.; Lynch, T.; Mihm, M.; Jung, K.; Rhuda, C.; Schmollinger, J.C.; Hodi, F.S.; Liebster, L.; Lam, P.; Mentzer, S.; et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte–macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc. Natl. Acad. Sci. USA 1998, 95, 13141. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Ruby, C.E.; Hughes, T.; Slingluff, C.L. Current status of granulocyte–macrophage colony-stimulating factor in the immunotherapy of melanoma. J. Immunother. Cancer 2014, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M. Prophylactic HPV vaccines. J. Clin. Pathol. 2007, 60, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Jit, M.; Chapman, R.; Hughes, O.; Choi, Y.H. Comparing bivalent and quadrivalent human papillomavirus vaccines: Economic evaluation based on transmission model. BMJ 2011, 343, d5775. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.A.; Edwards, J.; Castle, P.E.; Harro, C.D.; Lowy, D.R.; Schiller, J.T.; Wallace, D.; Kopp, W.; Adelsberger, J.W.; Baseler, M.W.; et al. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J. Infect. Dis. 2003, 188, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Moscicki, A.B.; Tsang, L.; Brockman, A.; Nakagawa, M. Memory T cell specific for novel human papillomavirus type 16 (HPV16) E6 epitopes in women whose HPV16 infection become undetectable. Clin. Vaccine Immunol. 2008, 15, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, K.; Yong, A.S.; Mielke, S.; Jafarpour, B.; Savani, B.N.; Le, R.Q.; Eniafe, R.; Musse, L.; Boss, C.; Kurlander, R.; et al. Repeated PR1 and WT1 peptide vaccination in Montanide-adjuvant fails to induce sustained high-avidity, epitope-specific CD8+ T cells in myeloid malignancies. Haematologica 2011, 96, 432–440. [Google Scholar] [CrossRef] [PubMed]
- De Vincenzo, R.; Conte, C.; Ricci, C.; Scambia, G.; Capelli, G. Long-term efficacy and safety of human papillomavirus vaccination. Int. J. Women’s Health 2014, 6, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.M.; DeMars, L.R. HPV vaccines—A review of the first decade. Gynecol. Oncol. 2017, 146, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Roteli-Martins, C.M.; Naud, P.; De Borba, P.; Teixeira, J.C.; De Carvalho, N.S.; Zahaf, T.; Sanchez, N.; Geeraerts, B.; Descamps, D. Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: Up to 8.4 years of follow-up. Hum. Vaccines Immunother. 2012, 8, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Einstein, M.H.; Takacs, P.; Chatterjee, A.; Sperling, R.S.; Chakhtoura, N.; Blatter, M.M.; Lalezari, J.; David, M.P.; Lin, L.; Struyf, F.; et al. Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: End-of-study analysis of a Phase III randomized trial. Hum. Vaccines Immunother. 2014, 10, 3435–3445. [Google Scholar] [CrossRef] [PubMed]
- Einstein, M.H.; Levin, M.J.; Chatterjee, A.; Chakhtoura, N.; Takacs, P.; Catteau, G.; Dessy, F.J.; Moris, P.; Lin, L.; Struyf, F.; et al. Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: Follow-up through Month 48 in a Phase III randomized study. Hum. Vaccines Immunother. 2014, 10, 3455–3465. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.; Guarnieri, F.G.; Staveley-O’Carroll, K.F.; Levitsky, H.I.; August, J.T.; Pardoll, D.M.; Wu, T.C. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996, 56, 21–26. [Google Scholar] [PubMed]
- Chang, L.J.; Zaiss, A.K. Self-inactivating lentiviral vectors and a sensitive Cre-loxP reporter system. Methods Mol. Med. 2003, 76, 367–382. [Google Scholar] [PubMed]
- Zaiss, A.K.; Son, S.; Chang, L.J. RNA 3′ readthrough of oncoretrovirus and lentivirus: Implications for vector safety and efficacy. J. Virol. 2002, 76, 7209–7219. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.J.; Urlacher, V.; Iwakuma, T.; Cui, Y.; Zucali, J. Efficacy and safety analyses of a recombinant human immunodeficiency virus type 1 derived vector system. Gene Ther. 1999, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Voskoboinik, I.; Smyth, M.J.; Trapani, J.A. Perforin-mediated target-cell death and immune homeostasis. Nat. Rev. Immunol. 2006, 6, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, E.; Bonmort, M.; Mignot, G.; Chaput, N.; Taieb, J.; Ménard, C.; Viaud, S.; Tursz, T.; Kroemer, G.; Zitvogel, L. Therapy-Induced Tumor Immunosurveillance Involves IFN-Producing Killer Dendritic Cells. Cancer Res. 2007, 67, 851. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Struyf, F.; Del Rosario-Raymundo, M.R.; Hildesheim, A.; Skinner, S.R.; Wacholder, S.; Garland, S.M.; Herrero, R.; David, M.P.; Wheeler, C.M.; et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: Combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncol. 2015, 16, 775–786. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Herrero, R.; Sampson, J.N.; Porras, C.; Lowy, D.R.; Schiller, J.T.; Schiffman, M.; Rodriguez, A.C.; Chanock, S.; Jimenez, S.; et al. Evidence for single-dose protection by the bivalent HPV vaccine-Review of the Costa Rica HPV vaccine trial and future research studies. Vaccine 2018, 36, 4774–4782. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, R.H.M. Comparing bivalent and quadrivalent HPV vaccines. BMJ 2011, 343. [Google Scholar] [CrossRef] [PubMed]
- Floros, T.; Tarhini, A.A. Anticancer Cytokines: Biology and Clinical Effects of IFN-α2, IL-2, IL-15, IL-21, and IL-12. Semin. Oncol. 2015, 42, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Margolin, K. Cytokines in Cancer Immunotherapy. Cancers 2011, 3, 3856–3893. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.R.; Merlino, G. The Two Faces of Interferon-γ in cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 6118–6124. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Old, L.J.; Schreiber, R.D. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002, 13, 95–109. [Google Scholar] [CrossRef]
- Antonio, M.; Luis, S.; Veronica, N.; Carles, J.C.; Francisco, D.; David, H.; Maria, J.H.; Salvador, F.A. Silencing of Foxp3 enhances the antitumor efficacy of GM-CSF genetically modified tumor cell line against B16 melanoma. Onco. Targets Ther. 2017, 10, 503–514. [Google Scholar]
- Greg, T.M.; George, C. Deciphering and reversing tumor immune suppression. Immunity 2013, 39, 61–73. [Google Scholar]
- Chan, C.W.; Crafton, E.; Fan, H.N.; Flook, J.; Yoshimura, K.; Skarica, M.; Brockstedt, D.; Dubensky, T.W.; Stins, M.F.; Lanier, L.L.; et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat. Med. 2006, 12, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Taieb, J.; Chaput, N.; Menard, C.; Apetoh, L.; Ullrich, E.; Bonmort, M.; Pequignot, M.; Casares, N.; Terme, M.; Flament, C.; et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat. Med. 2006, 12, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Welner, R.S.; Pelayo, R.; Garrett, K.P.; Chen, X.; Perry, S.S.; Sun, X.-H.; Kee, B.L.; Kincade, P.W. Interferon-producing killer dendritic cells (IKDCs) arise via a unique differentiation pathway from primitive c-kit(Hi)CD62L(+) lymphoid progenitors. Blood 2007, 109, 4825–4931. [Google Scholar] [CrossRef] [PubMed]
- Guimont-Desrochers, F.; Lesage, S. Revisiting the Prominent Anti-Tumoral Potential of Pre-mNK Cells. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.T.; Alson, D.; Lee, T.-H.; Tsai, C.-C.; Yu, T.-W.; Chen, Y.-S.; Cheng, Y.-F.; Lin, C.-C.; Schuyler, S.C. Effect of Multiple Vaccinations with Tumor Cell-Based Vaccine with Codon-Modified GM-CSF on Tumor Growth in a Mouse Model. Cancers 2019, 11, 368. https://doi.org/10.3390/cancers11030368
Qiu JT, Alson D, Lee T-H, Tsai C-C, Yu T-W, Chen Y-S, Cheng Y-F, Lin C-C, Schuyler SC. Effect of Multiple Vaccinations with Tumor Cell-Based Vaccine with Codon-Modified GM-CSF on Tumor Growth in a Mouse Model. Cancers. 2019; 11(3):368. https://doi.org/10.3390/cancers11030368
Chicago/Turabian StyleQiu, Jiantai Timothy, Donia Alson, Ta-Hsien Lee, Ching-Chou Tsai, Ting-Wei Yu, Yu-Sing Chen, Ya-Fang Cheng, Chu-Chi Lin, and Scott C. Schuyler. 2019. "Effect of Multiple Vaccinations with Tumor Cell-Based Vaccine with Codon-Modified GM-CSF on Tumor Growth in a Mouse Model" Cancers 11, no. 3: 368. https://doi.org/10.3390/cancers11030368
APA StyleQiu, J. T., Alson, D., Lee, T.-H., Tsai, C.-C., Yu, T.-W., Chen, Y.-S., Cheng, Y.-F., Lin, C.-C., & Schuyler, S. C. (2019). Effect of Multiple Vaccinations with Tumor Cell-Based Vaccine with Codon-Modified GM-CSF on Tumor Growth in a Mouse Model. Cancers, 11(3), 368. https://doi.org/10.3390/cancers11030368




