Pre-Operative Versus Post-Operative Radiosurgery of Brain Metastases—Volumetric and Dosimetric Impact of Treatment Sequence and Margin Concept
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics and Performed Treatment
2.2. Survival and Local Control
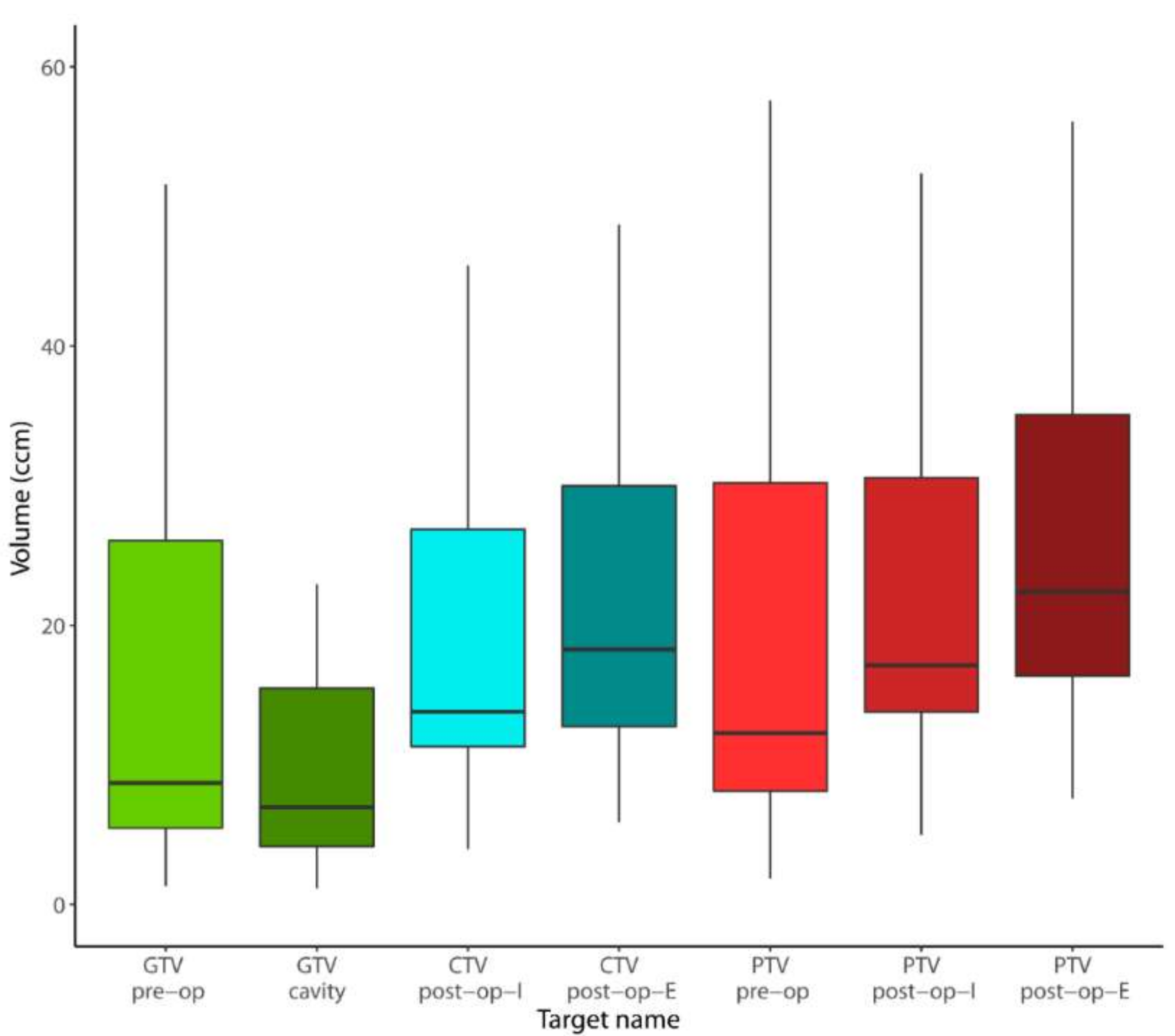
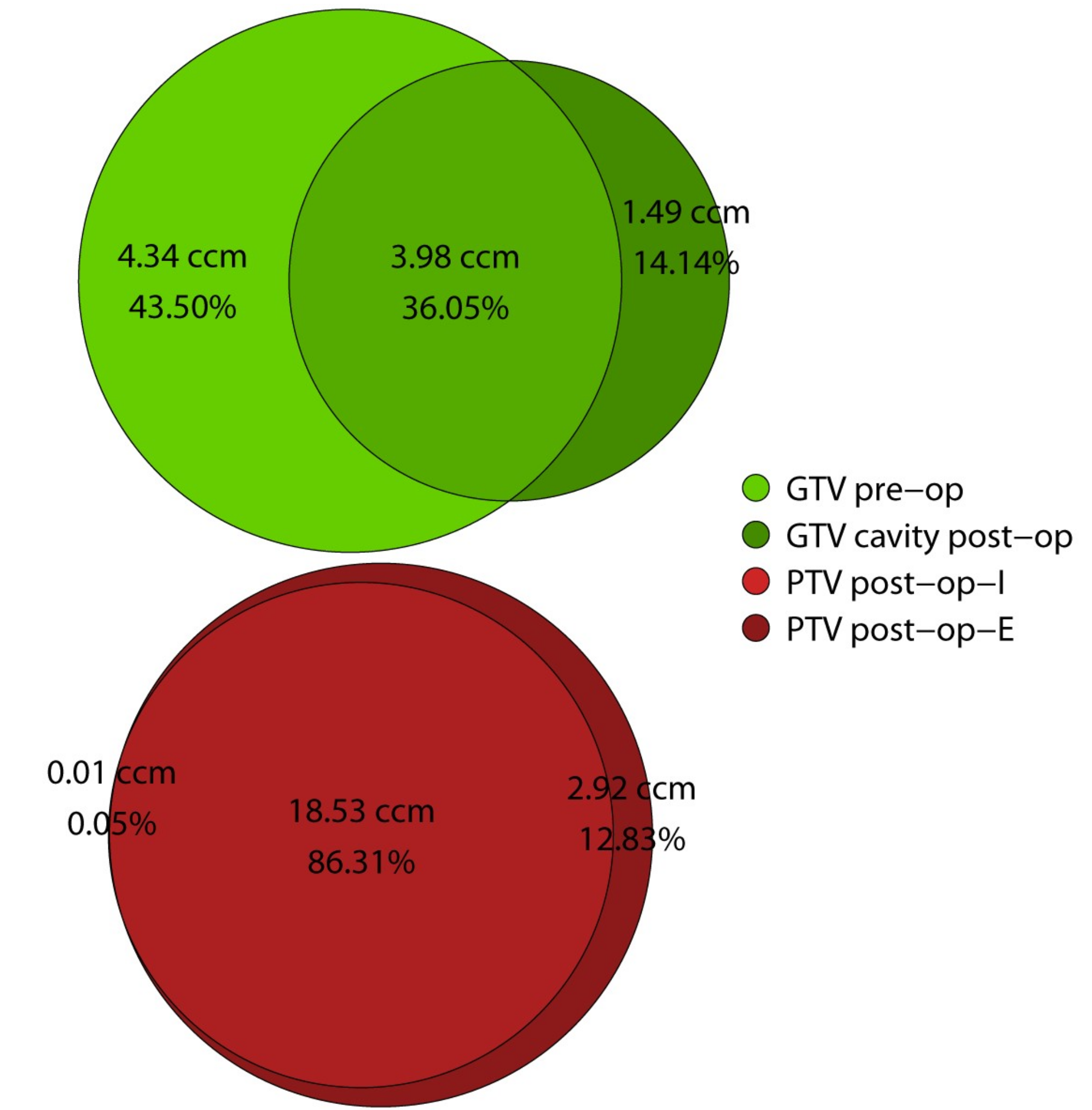
2.3. Volumetric Analysis
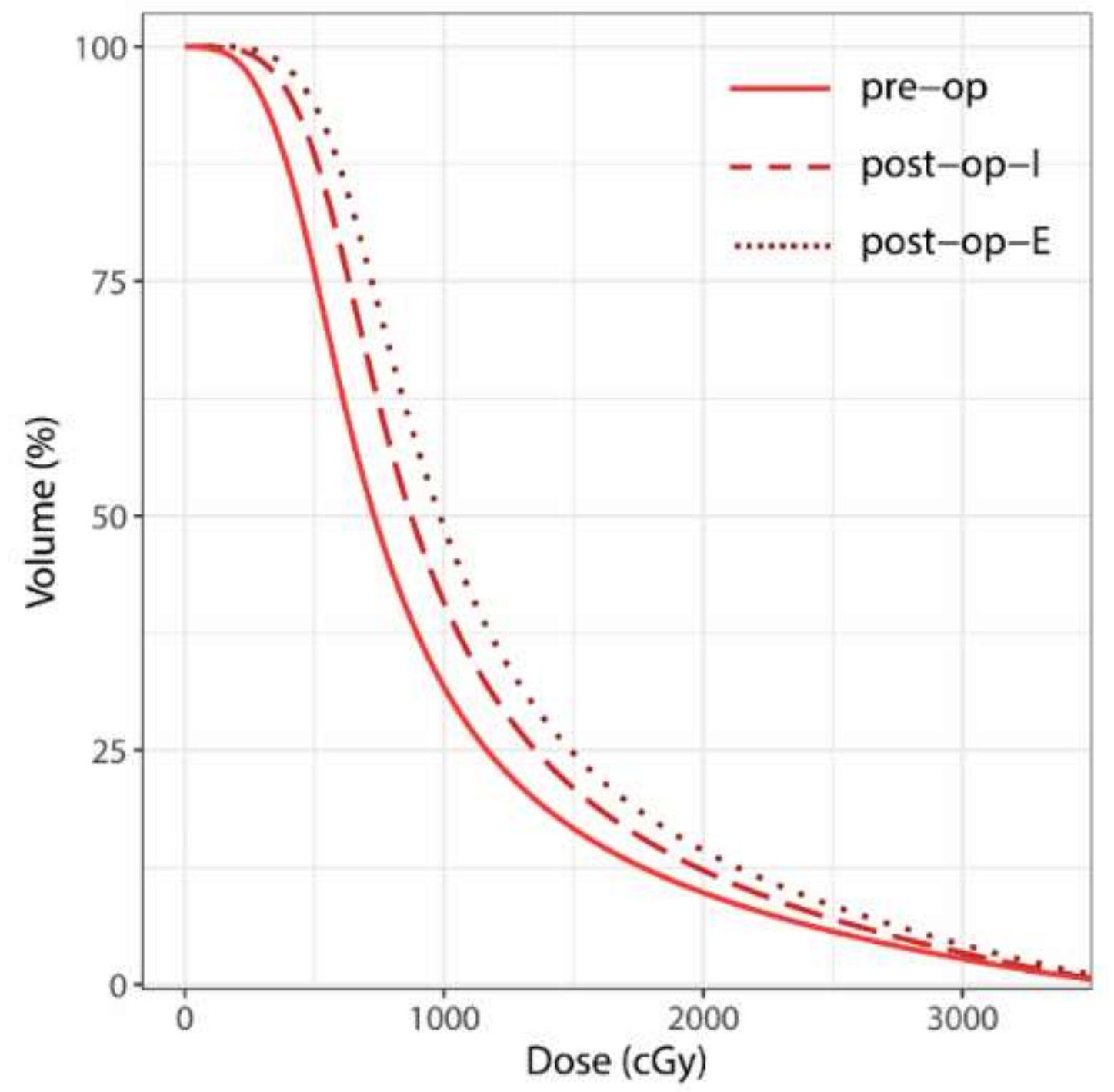
2.4. Dosimetric Analysis
3. Discussion
3.1. Local Control and Patterns of Failure
3.2. Volumetric and Dosimetric Comparison
4. Materials and Methods
4.1. Treatment Planning and Delivery for Performed SRS/FSRT
4.2. Treatment Planning for Comparative Analysis
4.2.1. Pre-Operative (pre-op)
4.2.2. Post-Operative Involved Field (post-op-I)
4.2.3. Post-Operative Extended Field (post-op-E)
4.3. Volumetric and Dosimetric Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Owonikoko, T.K.; Arbiser, J.; Zelnak, A.; Shu, H.-K.G.; Shim, H.; Robin, A.M.; Kalkanis, S.N.; Whitsett, T.G.; Salhia, B.; Tran, N.L.; et al. Current approaches to the treatment of metastatic brain tumours. Nat. Rev. Clin. Oncol. 2014, 11, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Network, N.C.C. Central Nervous System Cancers (Version 1.2017). Available online: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf (accessed on 24 February 2018).
- Patchell, R.A.; Tibbs, P.A.; Walsh, J.W.; Dempsey, R.J.; Maruyama, Y.; Kryscio, R.J.; Markesbery, W.R.; Macdonald, J.S.; Young, B. A Randomized Trial of Surgery in the Treatment of Single Metastases to the Brain. N. Engl. J. Med. 1990, 322, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J.; Mohiuddin, M.; Kryscio, R.J.; Markesbery, W.R.; Foon, K.A.; Young, B. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA 1998, 280, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Kocher, M.; Soffietti, R.; Abacioglu, U.; Villà, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.D.; Carrie, C.; et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J. Clin. Oncol. 2011, 29, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Ahmed, S.; McAleer, M.F.; Weinberg, J.S.; Li, J.; Brown, P.; Settle, S.; Prabhu, S.S.; Lang, F.F.; Levine, N.; et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: A single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1040–1048. [Google Scholar] [CrossRef]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef]
- Prabhu, R.S.; Patel, K.R.; Press, R.H.; Soltys, S.G.; Brown, P.D.; Mehta, M.P.; Asher, A.L.; Burri, S.H. Preoperative Vs Postoperative Radiosurgery For Resected Brain Metastases: A Review. Neurosurgery 2018, 84, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, L.A.; Simmons, N.E.; Bellerive, M.; Erkmen, K.; Eskey, C.J.; Gladstone, D.J.; Hug, E.B.; Roberts, D.W.; Hartford, A.C. Tumor bed dynamics after surgical resection of brain metastases: Implications for postoperative radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Clarke, E.; Lanzetta, G.; Osti, M.; Trasimeni, G.; Bozzao, A.; Romano, A.; Enrici, R. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat. Oncol. 2011, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Asher, A.L.; Burri, S.H.; Wiggins, W.F.; Kelly, R.P.; Boltes, M.O.; Mehrlich, M.; Norton, H.J.; Fraser, R.W. A new treatment paradigm: Neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Prabhu, R.S.; Kandula, S.; Oliver, D.E.; Kim, S.; Hadjipanayis, C.; Olson, J.J.; Oyesiku, N.; Curran, W.J.; Khan, M.K.; et al. Intracranial control and radiographic changes with adjuvant radiation therapy for resected brain metastases: Whole brain radiotherapy versus stereotactic radiosurgery alone. J. Neurooncol. 2014, 120, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Avkshtol, V.; Baschnagel, A.M.; Meyer, K.; Ye, H.; Grills, I.S.; Chen, P.Y.; Maitz, A.; Olson, R.E.; Pieper, D.R.; et al. Surgical Resection of Brain Metastases and the Risk of Leptomeningeal Recurrence in Patients Treated With Stereotactic Radiosurgery. Int. J. Radiat. Oncol. 2016, 94, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Atalar, B.; Modlin, L.A.; Choi, C.Y.H.; Adler, J.R.; Gibbs, I.C.; Chang, S.D.; Harsh IV, G.R.; Li, G.; Nagpal, S.; Hanlon, A.; et al. Risk of leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Ojerholm, E.; Lee, J.Y.K.; Thawani, J.P.; Miller, D.; O’Rourke, D.M.; Dorsey, J.F.; Geiger, G.A.; Nagda, S.; Kolker, J.D.; Lustig, R.A.; et al. Stereotactic radiosurgery to the resection bed for intracranial metastases and risk of leptomeningeal carcinomatosis. J. Neurosurg. 2014, 121 (Suppl. 2), 75–83. [Google Scholar] [CrossRef]
- Patel, K.R.; Burri, S.H.; Asher, A.L.; Crocker, I.R.; Fraser, R.W.; Zhang, C.; Chen, Z.; Kandula, S.; Zhong, J.; Press, R.H.; et al. Comparing Preoperative With Postoperative Stereotactic Radiosurgery for Resectable Brain Metastases. Neurosurgery 2016, 79, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.J.; Huang, K.E.; Page, B.R.; Ayala-Peacock, D.N.; Lucas, J.T.; Lesser, G.J.; Laxton, A.W.; Tatter, S.B.; Chan, M.D. Risk factors for leptomeningeal carcinomatosis in patients with brain metastases who have previously undergone stereotactic radiosurgery. J. Neurooncol. 2014, 120, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Burri, S.H.; Boselli, D.; Symanowski, J.T.; Asher, A.L.; Sumrall, A.; Fraser, R.W.; Press, R.H.; Zhong, J.; Cassidy, R.J.; et al. Comparing pre-operative stereotactic radiosurgery (SRS) to post-operative whole brain radiation therapy (WBRT) for resectable brain metastases: A multi-institutional analysis. J. Neurooncol. 2017, 131, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.; Ruschin, M.; Angelov, L.; Brown, P.D.; Chiang, V.L.S.; Kirkpatrick, J.P.; Lo, S.S.; Mahajan, A.; Oh, K.S.; Sheehan, J.P.; et al. Consensus Contouring Guidelines for Postoperative Completely Resected Cavity Stereotactic Radiosurgery for Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2017, 100, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Serizawa, T.; Shuto, T.; Akabane, A.; Higuchi, Y.; Kawagishi, J.; Yamanaka, K.; Sato, Y.; Jokura, H.; Yomo, S.; et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014, 15, 387–395. [Google Scholar] [CrossRef]
- Choi, C.Y.H.; Chang, S.D.; Gibbs, I.C.; Adler, J.R.; Harsh, G.R.; Lieberson, R.E.; Soltys, S.G. Stereotactic Radiosurgery of the Postoperative Resection Cavity for Brain Metastases: Prospective Evaluation of Target Margin on Tumor Control. Int. J. Radiat. Oncol. 2012, 84, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Soltys, S.G.; Adler, J.R.; Lipani, J.D.; Jackson, P.S.; Choi, C.Y.H.; Puataweepong, P.; White, S.; Gibbs, I.C.; Chang, S.D. Stereotactic Radiosurgery of the Postoperative Resection Cavity for Brain Metastases. Int. J. Radiat. Oncol. 2008, 70, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.; Yang, T.J.; Hilden, P.; Zhang, Z.; Chan, K.; Yamada, Y.; Chan, T.A.; Lymberis, S.C.; Narayana, A.; Tabar, V.; et al. A Phase 2 Trial of Stereotactic Radiosurgery Boost After Surgical Resection for Brain Metastases. Int. J. Radiat. Oncol. 2014, 88, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Hamilton, J.; Colen, R.; Schellingerhout, D.; Vu, T.; Rao, G.; McAleer, M.F.; Mahajan, A. Change in postsurgical cavity size within the first 30 days correlates with extent of surrounding edema: Consequences for postoperative radiosurgery. J. Comput. Assist. Tomogr. 2014, 38, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Potrebko, P.S.; Keller, A.; All, S.; Sejpal, S.; Pepe, J.; Saigal, K.; Kandula, S.; Sensakovic, W.F.; Shridhar, R.; Poleszczuk, J.; et al. GammaKnife versus VMAT radiosurgery plan quality for many brain metastases. J. Appl. Clin. Med. Phys. 2018, 19, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Masi, L.; Doro, R.; Favuzza, V.; Cipressi, S.; Livi, L. Impact of plan parameters on the dosimetric accuracy of volumetric modulated arc therapy. Med. Phys. 2013, 40, 071718. [Google Scholar] [CrossRef] [PubMed]
- Ohira, S.; Ueda, Y.; Akino, Y.; Hashimoto, M.; Masaoka, A.; Hirata, T.; Miyazaki, M.; Koizumi, M.; Teshima, T. HyperArc VMAT planning for single and multiple brain metastases stereotactic radiosurgery: A new treatment planning approach. Radiat. Oncol. 2018, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Blonigen, B.J.; Steinmetz, R.D.; Levin, L.; Lamba, M.A.; Warnick, R.E.; Breneman, J.C. Irradiated Volume as a Predictor of Brain Radionecrosis After Linear Accelerator Stereotactic Radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Sneed, P.K.; Mendez, J.; Vemer-van den Hoek, J.G.M.; Seymour, Z.A.; Ma, L.; Molinaro, A.M.; Fogh, S.E.; Nakamura, J.L.; McDermott, M.W. Adverse radiation effect after stereotactic radiosurgery for brain metastases: Incidence, time course, and risk factors. J. Neurosurg. 2015, 123, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Emami, B.; Lyman, J.; Brown, A.; Cola, L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Constine, L.S.; Deasy, J.O.; Eisbruch, A.; Jackson, A.; Marks, L.B.; Ten Haken, R.K.; Yorke, E.D. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): An Introduction to the Scientific Issues. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.M.S.; Miller, J.; Hoffer, S.A.; Mansur, D.B.; Coffey, M.; Lo, S.S.; Sloan, A.E.; Machtay, M. Postoperative hypofractionated stereotactic brain radiation (HSRT) for resected brain metastases: Improved local control with higher BED10. J. Neurooncol. 2018, 139, 449–454. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J. The linear quadratic model: Usage, interpretation and challenges. Phys. Med. Biol. 2018, 64, 01TR01. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, J.P.; Brenner, D.J.; Orton, C.G. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Med. Phys. 2009, 36, 3381–3384. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, R.; Shu, H.-K.; Hadjipanayis, C.; Dhabaan, A.; Hall, W.; Raore, B.; Olson, J.; Curran, W.; Oyesiku, N.; Crocker, I. Current dosing paradigm for stereotactic radiosurgery alone after surgical resection of brain metastases needs to be optimized for improved local control. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e61–e66. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.E.; Bilger, A.; Diehl, C.; Bretzinger, E.; Lorenz, H.; Oehlke, O.; Specht, H.M.; Kirstein, A.; Grosu, A.-L. Multicenter analysis of stereotactic radiotherapy of the resection cavity in patients with brain metastases. Cancer Med. 2018, 7, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Specht, H.M.; Kessel, K.A.; Oechsner, M.; Meyer, B.; Zimmer, C.; Combs, S.E. HFSRT of the resection cavity in patients with brain metastases. Strahlenther. Onkol. 2016, 192, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Ayas, A.W.; Grau, S.; Jablonska, K.; Ruess, D.; Ruge, M.; Marnitz, S.; Goldbrunner, R.; Kocher, M. Postoperative local fractionated radiotherapy for resected single brain metastases. Strahlentherapie Onkol. 2018, 194, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Broemme, J.; Abu-Isa, J.; Kottke, R.; Beck, J.; Wiest, R.; Malthaner, M.; Schmidhalter, D.; Raabe, A.; Aebersold, D.M.; Pica, A. Adjuvant therapy after resection of brain metastases. Strahlentherapie Onkol. 2013, 189, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Mintz, A.H.; Kestle, J.; Rathbone, M.P.; Gaspar, L.; Hugenholtz, H.; Fisher, B.; Duncan, G.; Skingley, P.; Foster, G.; Levine, M. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 1996, 78, 1470–1476. [Google Scholar] [CrossRef]
- Noordijk, E.M.; Vecht, C.J.; Haaxma-Reiche, H.; Padberg, G.W.; Voormolen, J.H.; Hoekstra, F.H.; Tans, J.T.; Lambooij, N.; Metsaars, J.A.; Wattendorff, A.R. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 711–717. [Google Scholar] [CrossRef]
- Dice, L.R. Measures of the Amount of Ecologic Association Between Species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Sørensen, T. A method of establishing groups of equal amplitude in plant sociology based on similarity. Kong. Dansk. vidensk. Selsk. Biol. Skr. 1948, 5, 1–34. [Google Scholar]
- Audet, C.; Poffenbarger, B.A.; Chang, P.; Jackson, P.S.; Lundahl, R.E.; Ryu, S.I.; Ray, G.R. Evaluation of volumetric modulated arc therapy for cranial radiosurgery using multiple noncoplanar arcs. Med. Phys. 2011, 38, 5863–5872. [Google Scholar] [CrossRef] [PubMed]
- Paddick, I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J. Neurosurg. 2000, 93 (Suppl. 3), 219–222. [Google Scholar] [CrossRef]
- Paddick, I.; Lippitz, B. A simple dose gradient measurement tool to complement the conformity index. J. Neurosurg. 2006, 105, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Stieler, F.; Fleckenstein, J.; Simeonova, A.; Wenz, F.; Lohr, F. Intensity modulated radiosurgery of brain metastases with flattening filter-free beams. Radiother. Oncol. 2013, 109, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Wollschlaeger, D.; Karle, H. DVHmetrics: Analyze Dose-Volume Histograms and Check Constraints; University Medical Center Mainz: Mainz, Germany, 2017. [Google Scholar]

| Characteristics | n | % |
|---|---|---|
| Age at Radiotherapy (years) | ||
| Median | 60 | |
| Q1-Q3 | 52–64 | |
| Gender | ||
| female | 16 | 66.67% |
| male | 8 | 33.33% |
| Additional Unresected Brain Metastases | ||
| 0 | 14 | 58.33% |
| 1 | 5 | 20.83% |
| 2 | 4 | 16.67% |
| 4 | 1 | 4.17% |
| Primary Histology | ||
| NSCLC | 9 | 37.5% |
| breast cancer | 7 | 29.17% |
| renal cell carcinoma | 3 | 12.5% |
| upper gastrointestinal tract | 2 | 8.33% |
| other | 3 | 12.5% |
| Location of Resected Brain Metastasis | ||
| supratentorial | 16 | 66.67% |
| infratentorial | 8 | 33.33% |
| Detailed Treatment | n | % |
|---|---|---|
| Interval Between Surgery and Radiotherapy (days) | ||
| Mean | 60 | |
| Median | 40 | |
| SD | 72 | |
| Q1–Q3 | 35–48 | |
| Min.–Max. | 23–377 | |
| treatment modality | ||
| HFSRT | 16 | 66.67% |
| SRS | 8 | 33.33% |
| width of safety margin (mm) | ||
| 1 | 6 | 25% |
| 2 | 3 | 12.5% |
| 3 | 15 | 62.5% |
| complete resection canal included in GTV | ||
| no | 13 | 54.17% |
| yes | 11 | 45.83% |
| physical dose for SRS (n = 8) | ||
| 18 | 4 | 50% |
| 20 | 2 | 25% |
| 12 | 1 | 12.5% |
| 17 | 1 | 12.5% |
| cumulative physical dose for HFSRT (n = 16) | ||
| Mean | 31 | |
| Median | 30 | |
| SD | NA | |
| Q1–Q3 | 30–31 | |
| Min.–Max. | 30–35 | |
| number of fractions for HFSRT (n = 16) | ||
| Mean | 6 | |
| Median | 6 | |
| SD | NA | |
| Q1–Q3 | 6–6 | |
| Min.–Max. | 6–7 | |
| biologically equivalent cumulative dose for SRS/HFSRT (α/β = 10) | ||
| Mean | 48 | |
| Median | 45 | |
| SD | 6 | |
| Q1–Q3 | 45–51 | |
| Min.–Max. | 26–60 | |
| prescription isodose for SRS/HFSRT (%) | ||
| Mean | 77 | |
| Median | 70 | |
| SD | 8 | |
| Q1–Q3 | 70–86 | |
| Min.–Max. | 65–88 | |
| dose exposure of the healthy brain for SRS (n = 16) | median (Q1–Q3) ccm | |
| V8 Gy | 23 (21–38) | |
| V10 Gy | 15 (14–23) | |
| V12 Gy | 10 (8–12) | |
| V14 Gy | 7 (5–8) | |
| V16 Gy | 4 (2–5) | |
| dose exposure of the healthy brain for HFSRT (n = 8) | median (Q1–Q3) ccm | |
| V15 Gy | 48 (35–56) | |
| V18 Gy | 33 (24–39) | |
| V21 Gy | 24 (17–27) | |
| V24 Gy | 16 (10–19) |
| Morphological Comparison | ccm | % (Relative to Sum) | ||
|---|---|---|---|---|
| median | Q1–Q3 | median | Q1–Q3 | |
| cavity morphology (pre-op vs. post-op) | ||||
| overlap GTV pre-op and cavity post-op | 3.98 | 2.31–13.69 | 36.05% | 29.46–46.26% |
| DSC GTV pre-op and cavity post-op | - | - | 53% | 46–63% |
| post-op not in pre-op | 1.49 | 0.84–3.28 | 14.14% | 5.85–27.89% |
| pre-op not in post-op | 4.34 | 2.77–11.52 | 43.50% | 24.14–51.35% |
| effect of added margins: PTV (post-op-I vs. post-op-E) | ||||
| overlap PTV post-op-I and post-op-E | 18.53 | 13.88–31.12 | 86.31% | 77.54–89.27% |
| DSC PTV post-op-I and post-op-E | - | - | 93% | 87–94% |
| post-op-I not in post-op-E | 0.01 | 0.00–0.07 | 0.05% | 0.00–0.27% |
| post-op-E not in post-op-I | 2.92 | 2.14–4.84 | 12.83% | 10.34–21.68% |
| Detailed Results | pre-op | post-op-I | post-op-E | post-op-I vs. pre-op | post-op-E vs. pre-op | post-op-I vs. post-op-E |
|---|---|---|---|---|---|---|
| median (Q1–Q3) | median (Q1–Q3) | median (Q1–Q3) | p | p | p | |
| volume (ccm) | ||||||
| GTV | 8.71 (5.47–26.08) | 6.96 (4.16–15.50) | 6.96 (4.16–15.50) | 0.019 | - | - |
| CTV | - | 15.29 (11.32–27.69) | 18.96 (12.86–31.75) | - | - | <0.001 |
| PTV | 12.29 (8.13–30.20) | 18.58 (13.88–31.30) | 22.89 (16.37–37.21) | 0.016 | 0.002 | <0.001 |
| volume increase | ||||||
| GTV to PTV (absolute (ccm)) | 2.47 (1.70–4.35) | 12.14 (9.31–16.57) | 15.9 (11.52–22.87) | <0.001 | <0.001 | <0.001 |
| GTV to PTV (relative to GTV (%)) | 23.75% (15.87–30.36%) | 175.91% (107.25–221.50%) | 224.41% (139.49–290.27%) | <0.001 | <0.001 | <0.001 |
| dose to the healthy brain (median volume in ccm) | ||||||
| V28 Gy | 6.79 (4.28–12.79) | 8.91 (6.01–11.08) | 10.79 (7.01–13.54) | 0.345 | 0.005 | <0.001 |
| V25 Gy | 10.14 (6.25–18.73) | 13.39 (8.68–18.96) | 15.38 (9.94–19.93) | 0.229 | 0.004 | <0.001 |
| V20 Gy | 18.07 (10.76–31.79) | 23.21 (14.71–32.86) | 26.69 (16.43–34.08) | 0.188 | 0.006 | <0.001 |
| V15 Gy | 31.24 (18.09–53.53) | 39.25 (25.61–56.81) | 46.07 (27.61–58.68) | 0.178 | 0.005 | 0.001 |
| V10 Gy | 61.42 (33.60–105.51) | 77 (51.45–109.33) | 88.64 (52.93–118.15) | 0.114 | 0.003 | <0.001 |
| V5 Gy | 174.35 (91.39–270.16) | 203 (142.62–291.11) | 227.78 (139.79–313.24) | 0.107 | 0.003 | <0.001 |
| V2 Gy | 543.38 (370.01–738.26) | 637.6 (485.45–778.40) | 669.7 (489.51–856.66) | 0.011 | <0.001 | <0.001 |
| V1 Gy | 858.48 (646.83–927.82) | 875.18 (745.86–1007.22) | 974.05 (850.44–1071.30) | 0.009 | <0.001 | <0.001 |
| gradient and conformity indices | ||||||
| GIhigh | 2.52 (2.22–2.89) | 2.38 (2.26–2.58) | 2.43 (2.22–2.56) | 0.010 | 0.065 | 0.229 |
| GIlow | 2.6 (2.48–2.71) | 2.46 (2.38–2.57) | 2.42 (2.35–2.50) | 0.025 | 0.001 | 0.021 |
| nCI (PTV) | 1.12 (1.10–1.19) | 1.13 (1.09–1.14) | 1.14 (1.13–1.18) | 0.039 | 0.004 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Shafie, R.A.; Tonndorf-Martini, E.; Schmitt, D.; Weber, D.; Celik, A.; Dresel, T.; Bernhardt, D.; Lang, K.; Hoegen, P.; Adeberg, S.; et al. Pre-Operative Versus Post-Operative Radiosurgery of Brain Metastases—Volumetric and Dosimetric Impact of Treatment Sequence and Margin Concept. Cancers 2019, 11, 294. https://doi.org/10.3390/cancers11030294
El Shafie RA, Tonndorf-Martini E, Schmitt D, Weber D, Celik A, Dresel T, Bernhardt D, Lang K, Hoegen P, Adeberg S, et al. Pre-Operative Versus Post-Operative Radiosurgery of Brain Metastases—Volumetric and Dosimetric Impact of Treatment Sequence and Margin Concept. Cancers. 2019; 11(3):294. https://doi.org/10.3390/cancers11030294
Chicago/Turabian StyleEl Shafie, Rami A., Eric Tonndorf-Martini, Daniela Schmitt, Dorothea Weber, Aylin Celik, Thorsten Dresel, Denise Bernhardt, Kristin Lang, Philipp Hoegen, Sebastian Adeberg, and et al. 2019. "Pre-Operative Versus Post-Operative Radiosurgery of Brain Metastases—Volumetric and Dosimetric Impact of Treatment Sequence and Margin Concept" Cancers 11, no. 3: 294. https://doi.org/10.3390/cancers11030294
APA StyleEl Shafie, R. A., Tonndorf-Martini, E., Schmitt, D., Weber, D., Celik, A., Dresel, T., Bernhardt, D., Lang, K., Hoegen, P., Adeberg, S., Paul, A., Debus, J., & Rieken, S. (2019). Pre-Operative Versus Post-Operative Radiosurgery of Brain Metastases—Volumetric and Dosimetric Impact of Treatment Sequence and Margin Concept. Cancers, 11(3), 294. https://doi.org/10.3390/cancers11030294





