Implication of Voltage-Gated Potassium Channels in Neoplastic Cell Proliferation
Abstract
1. Potassium Channels: Classification and Function
2. Potassium Channels in Cancer
3. Kv Channels and Cancer
3.1. Delayed Rectifier Channels (IDR)
3.2. A-Type Channels (IA)
3.3. Modifier/Silencer Subunits
3.4. Others
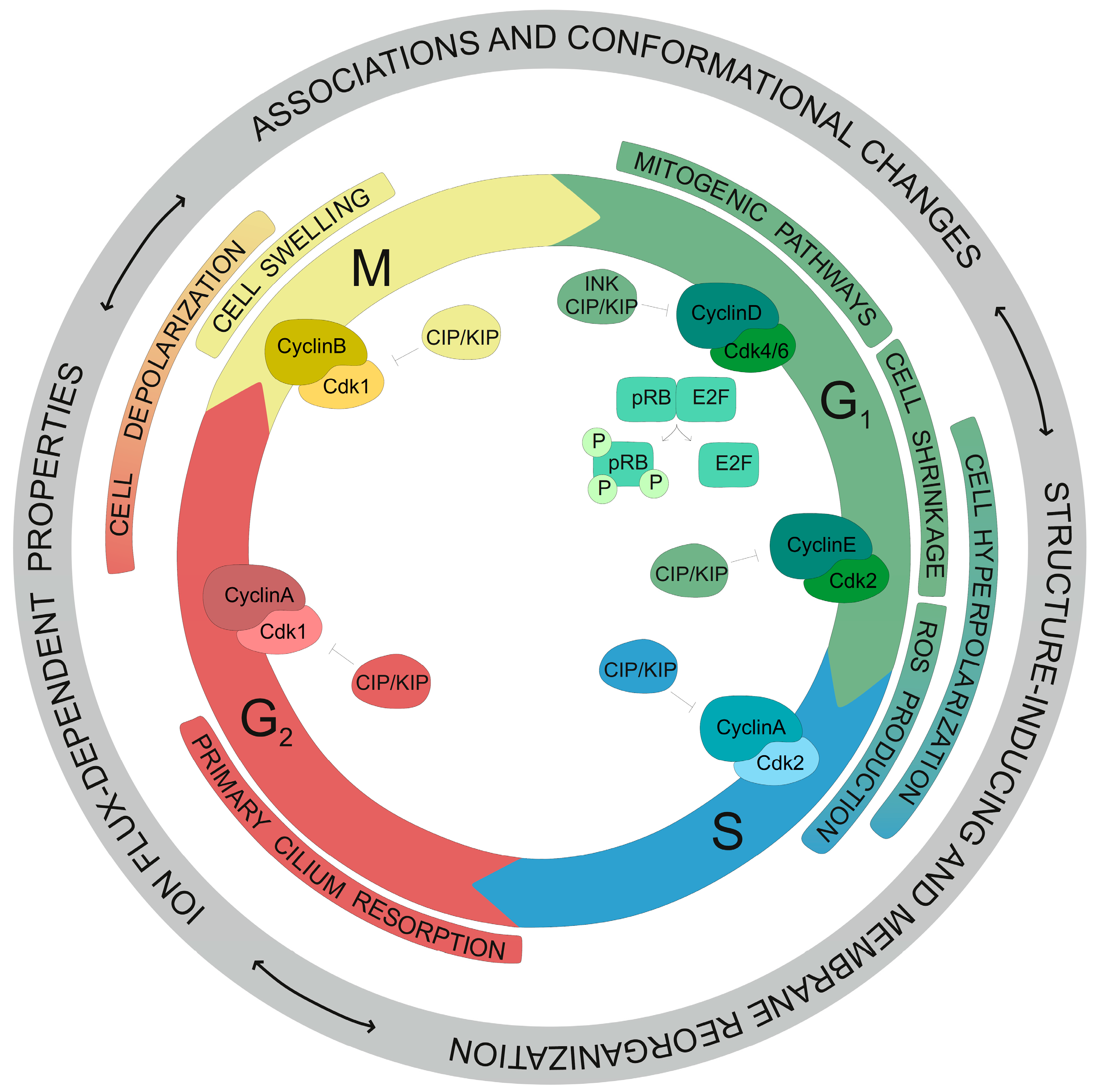
4. Regulation of Cell Cycle Progression by Kv Channels
5. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer: Sunderland, MA, USA, 2001. [Google Scholar]
- Kass, R.S. The Channelopathies: Novel Insights into Molecular and Genetic Mechanisms of Human Disease. J. Clin. Investig. 2005, 115, 1986–1989. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, F.M. Ion. Channels and Disease: Channelopathies; Academic: San Diego, CA, USA; London, UK, 2000. [Google Scholar]
- Yu, F.H.; Catterall, W.A. The Vgl-Chanome: A Protein Superfamily Specialized for Electrical Signaling and Ionic Homeostasis. Sci. Signal. 2004, 2004, re15. [Google Scholar] [CrossRef] [PubMed]
- Gutman, G.A.; Chandy, K.G.; Grissmer, S.; Lazdunski, M.; McKinnon, D.; Pardo, L.A.; Robertson, G.A.; Rudy, B.; Sanguinetti, M.C.; Stuhmer, W.; et al. International Union of Pharmacology. Liii. Nomenclature and Molecular Relationships of Voltage-Gated Potassium Channels. Pharmacol. Rev. 2005, 57, 473–508. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.; Mohler, P.J. Alternative Paradigms for Ion Channelopathies: Disorders of Ion Channel Membrane Trafficking and Posttranslational Modification. Annu. Rev. Physiol. 2015, 77, 505–524. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, K. Ion Channels and Cancer. J. Membr. Biol. 2005, 205, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Barkan, D.; Green, J.E.; Chambers, A.F. Extracellular Matrix: A Gatekeeper in the Transition from Dormancy to Metastatic Growth. Eur. J. Cancer 2010, 46, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Ghiso, J.A. Models, Mechanisms and Clinical Evidence for Cancer Dormancy. Nat. Rev. Cancer 2007, 7, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Felipe, A.; Vicente, R.; Villalonga, N.; Roura-Ferrer, M.; Martinez-Marmol, R.; Sole, L.; Ferreres, J.C.; Condom, E. Potassium Channels: New Targets in Cancer Therapy. Cancer Detect. Prev. 2006, 30, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Roles of K+ Channels in Regulating Tumour Cell Proliferation and Apoptosis. Pflugers Arch. 2004, 448, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.A. Voltage-Gated Potassium Channels in Cell Proliferation. Physiology 2004, 19, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Rouzaire-Dubois, B.; Dubois, J.M. K+ Channel Block-Induced Mammalian Neuroblastoma Cell Swelling: A Possible Mechanism to Influence Proliferation. J. Physiol. 1998, 510 Pt 1, 93–102. [Google Scholar] [CrossRef]
- Pardo, L.A.; Stuhmer, W. The Roles of K(+) Channels in Cancer. Nat. Rev. Cancer 2014, 14, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Escalada, A.; Coma, M.; Fuster, G.; Sanchez-Tillo, E.; Lopez-Iglesias, C.; Soler, C.; Solsona, C.; Celada, A.; Felipe, A. Differential Voltage-Dependent K+ Channel Responses During Proliferation and Activation in Macrophages. J. Biol. Chem. 2003, 278, 46307–46320. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Escalada, A.; Soler, C.; Grande, M.; Celada, A.; Tamkun, M.M.; Solsona, C.; Felipe, A. Pattern of Kv Beta Subunit Expression in Macrophages Depends Upon Proliferation and the Mode of Activation. J. Immunol. 2005, 174, 4736–4744. [Google Scholar] [CrossRef] [PubMed]
- Arcangeli, A.; Crociani, O.; Lastraioli, E.; Masi, A.; Pillozzi, S.; Becchetti, A. Targeting Ion Channels in Cancer: A Novel Frontier in Antineoplastic Therapy. Curr. Med. Chem. 2009, 16, 66–93. [Google Scholar] [CrossRef] [PubMed]
- Villalonga, N.; Ferreres, J.C.; Argiles, J.M.; Condom, E.; Felipe, A. Potassium Channels Are a New Target Field in Anticancer Drug Design. Recent Pat. Anticancer Drug Discov. 2007, 2, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Szabo, I.; Trentin, L.; Trimarco, V.; Semenzato, G.; Leanza, L. Biophysical Characterization and Expression Analysis of Kv1.3 Potassium Channel in Primary Human Leukemic B Cells. Cell. Physiol. Biochem. 2015, 37, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Aissaoui, D.; Mlayah-Bellalouna, S.; Jebali, J.; Abdelkafi-Koubaa, Z.; Souid, S.; Moslah, W.; Othman, H.; Luis, J.; ElAyeb, M.; Marrakchi, N.; et al. Functional Role of Kv1.1 and Kv1.3 Channels in the Neoplastic Progression Steps of Three Cancer Cell Lines, Elucidated by Scorpion Peptides. Int. J. Biol. Macromol. 2018, 111, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.; Stuhmer, W.; Martin, S.; Schell, J.; Reichmann, A.; Rohde, V.; Pardo, L. Analysis of the Expression of Kv10.1 Potassium Channel in Patients with Brain Metastases and Glioblastoma Multiforme: Impact on Survival. BMC Cancer 2015, 15, 839. [Google Scholar] [CrossRef] [PubMed]
- Crociani, O.; Lastraioli, E.; Boni, L.; Pillozzi, S.; Romoli, M.R.; D’Amico, M.; Stefanini, M.; Crescioli, S.; Masi, A.; Taddei, A.; et al. Herg1 Channels Regulate Vegf-a Secretion in Human Gastric Cancer: Clinicopathological Correlations and Therapeutical Implications. Clin. Cancer Res. 2014, 20, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Comes, N.; Bielanska, J.; Vallejo-Gracia, A.; Serrano-Albarras, A.; Marruecos, L.; Gomez, D.; Soler, C.; Condom, E.; Ramon, Y.C.S.; Hernandez-Losa, J.; et al. The Voltage-Dependent K(+) Channels Kv1.3 and Kv1.5 in Human Cancer. Front. Physiol. 2013, 4, 283. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Gracia, A.; Bielanska, J.; Hernandez-Losa, J.; Castellvi, J.; Ruiz-Marcellan, M.C.; Cajal, S.R.y.; Condom, E.; Manils, J.; Soler, C.; Comes, N.; et al. Emerging Role for the Voltage-Dependent K+ Channel Kv1.5 in B-Lymphocyte Physiology: Expression Associated with Human Lymphoma Malignancy. J. Leukoc. Biol. 2013, 94, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Bielanska, J.; Hernandez-Losa, J.; Perez-Verdaguer, M.; Moline, T.; Somoza, R.; Ramon, Y.C.S.; Condom, E.; Ferreres, J.C.; Felipe, A. Voltage-Dependent Potassium Channels Kv1.3 and Kv1.5 in Human Cancer. Curr. Cancer Drug Targets 2009, 9, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Felipe, A.; Bielanska, J.; Comes, N.; Vallejo, A.; Roig, S.; Ramon, Y.C.S.; Condom, E.; Hernandez-Losa, J.; Ferreres, J.C. Targeting the Voltage-Dependent K(+) Channels Kv1.3 and Kv1.5 as Tumor Biomarkers for Cancer Detection and Prevention. Curr. Med. Chem. 2012, 19, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Szabo, I.; Bock, J.; Grassme, H.; Soddemann, M.; Wilker, B.; Lang, F.; Zoratti, M.; Gulbins, E. Mitochondrial Potassium Channel Kv1.3 Mediates Bax-Induced Apoptosis in Lymphocytes. Proc. Natl. Acad. Sci. USA 2008, 105, 14861–14866. [Google Scholar] [CrossRef] [PubMed]
- Bock, J.; Szabo, I.; Jekle, A.; Gulbins, E. Actinomycin D-Induced Apoptosis Involves the Potassium Channel Kv1.3. Biochem. Biophys. Res. Commun. 2002, 295, 526–531. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, D.; Wu, X.; Sha, M.; Kang, L.; Ding, Z. Voltage-Gated Potassium Channel Kv1.3 Is Highly Expressed in Human Osteosarcoma and Promotes Osteosarcoma Growth. Int. J. Mol. Sci. 2013, 14, 19245–19256. [Google Scholar] [CrossRef] [PubMed]
- Peruzzo, R.; Mattarei, A.; Romio, M.; Paradisi, C.; Zoratti, M.; Szabo, I.; Leanza, L. Regulation of Proliferation by a Mitochondrial Potassium Channel in Pancreatic Ductal Adenocarcinoma Cells. Front. Oncol. 2017, 7, 239. [Google Scholar] [CrossRef] [PubMed]
- Venturini, E.; Leanza, L.; Azzolini, M.; Kadow, S.; Mattarei, A.; Weller, M.; Tabatabai, G.; Edwards, M.J.; Zoratti, M.; Paradisi, C.; et al. Targeting the Potassium Channel Kv1.3 Kills Glioblastoma Cells. Neurosignals 2017, 25, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Huang, X.Q.; Zhao, L.Y.; Li, J.; Chen, J.W.; Xiao, Y.; Huang, Y.Y.; Liu, J.; Wang, G.L.; Guan, Y.Y. Involvement of Kv1.5 Protein in Oxidative Vascular Endothelial Cell Injury. PLoS ONE 2012, 7, e49758. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Shi, Y.; Han, Z.; Hao, Z.; Pan, Y.; Liu, N.; Guo, C.; Hong, L.; Wang, J.; Qiao, T.; et al. Expression of Delayed Rectifier Potassium Channels and Their Possible Roles in Proliferation of Human Gastric Cancer Cells. Cancer Biol. Ther. 2005, 4, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, Z.; Liu, Q.; Zeng, W.; Wu, X.; Lin, B. Silencing of Kv1.5 Gene Inhibits Proliferation and Induces Apoptosis of Osteosarcoma Cells. Int. J. Mol. Sci. 2015, 16, 26914–26926. [Google Scholar] [CrossRef] [PubMed]
- Bielanska, J.; Hernandez-Losa, J.; Moline, T.; Somoza, R.; Cajal, S.R.y.; Condom, E.; Ferreres, J.C.; Felipe, A. Differential Expression of Kv1.3 and Kv1.5 Voltage-Dependent K+ Channels in Human Skeletal Muscle Sarcomas. Cancer Investig. 2012, 30, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Bielanska, J.; Hernandez-Losa, J.; Moline, T.; Somoza, R.; Ramon, Y.C.S.; Condom, E.; Ferreres, J.C.; Felipe, A. Increased Voltage-Dependent K(+) Channel Kv1.3 and Kv1.5 Expression Correlates with Leiomyosarcoma Aggressiveness. Oncol. Lett. 2012, 4, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Preussat, K.; Beetz, C.; Schrey, M.; Kraft, R.; Wolfl, S.; Kalff, R.; Patt, S. Expression of Voltage-Gated Potassium Channels Kv1.3 and Kv1.5 in Human Gliomas. Neurosci. Lett. 2003, 346, 33–36. [Google Scholar] [CrossRef]
- He, T.; Wang, C.; Zhang, M.; Zhang, X.; Zheng, S.; Linghu, E.; Guo, M. Epigenetic Regulation of Voltage-Gated Potassium Ion Channel Molecule Kv1.3 in Mechanisms of Colorectal Cancer. Discov. Med. 2017, 23, 155–162. [Google Scholar] [PubMed]
- Ryland, K.E.; Hawkins, A.G.; Weisenberger, D.J.; Punj, V.; Borinstein, S.C.; Laird, P.W.; Martens, J.R.; Lawlor, R.E. Promoter Methylation Analysis Reveals That Kcna5 Ion Channel Silencing Supports Ewing Sarcoma Cell Proliferation. Mol. Cancer Res. 2016, 14, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Ryland, K.E.; Svoboda, L.K.; Vesely, E.D.; McIntyre, J.C.; Zhang, L.; Martens, J.R.; Lawlor, E.R. Polycomb-Dependent Repression of the Potassium Channel-Encoding Gene Kcna5 Promotes Cancer Cell Survival under Conditions of Stress. Oncogene 2015, 34, 4591–4600. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Villalonga, N.; Calvo, M.; Escalada, A.; Solsona, C.; Soler, C.; Tamkun, M.M.; Felipe, A. Kv1.5 Association Modifies Kv1.3 Traffic and Membrane Localization. J. Biol. Chem. 2008, 283, 8756–8764. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.; Costa, R.; Peruzzo, R.; Prosdocimi, E.; Checchetto, V.; Leanza, L. Targeting Mitochondrial Ion Channels to Fight Cancer. Int. J. Mol. Sci. 2018, 19, 2060. [Google Scholar] [CrossRef] [PubMed]
- Leanza, L.; Zoratti, M.; Gulbins, E.; Szabo, I. Mitochondrial Ion Channels as Oncological Targets. Oncogene 2014, 33, 5569–5581. [Google Scholar] [CrossRef] [PubMed]
- Zaccagnino, A.; Manago, A.; Leanza, L.; Gontarewitz, A.; Linder, B.; Azzolini, M.; Biasutto, L.; Zoratti, M.; Peruzzo, R.; Legler, K.; et al. Tumor-Reducing Effect of the Clinically Used Drug Clofazimine in a Scid Mouse Model of Pancreatic Ductal Adenocarcinoma. Oncotarget 2017, 8, 38276–38293. [Google Scholar] [CrossRef] [PubMed]
- Leanza, L.; Romio, M.; Becker, K.A.; Azzolini, M.; Trentin, L.; Manago, A.; Venturini, E.; Zaccagnino, A.; Mattarei, A.; Carraretto, L.; et al. Direct Pharmacological Targeting of a Mitochondrial Ion Channel Selectively Kills Tumor Cells in Vivo. Cancer Cell 2017, 31, 516–531 e10. [Google Scholar] [CrossRef] [PubMed]
- Lallet-Daher, H.; Wiel, C.; Gitenay, D.; Navaratnam, N.; Augert, A.; le Calve, B.; Verbeke, S.; Carling, D.; Aubert, S.; Vindrieux, D.; et al. Potassium Channel Kcna1 Modulates Oncogene-Induced Senescence and Transformation. Cancer Res. 2013, 73, 5253–5265. [Google Scholar] [CrossRef] [PubMed]
- Al-Owais, M.M.; Scragg, J.L.; Dallas, M.L.; Boycott, H.E.; Warburton, P.; Chakrabarty, A.; Boyle, J.P.; Peers, C. Carbon Monoxide Mediates the Anti-Apoptotic Effects of Heme Oxygenase-1 in Medulloblastoma Daoy Cells Via K+ Channel Inhibition. J. Biol. Chem. 2012, 287, 24754–24764. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Takimoto, K. Selective Expression of Herg and Kv2 Channels Influences Proliferation of Uterine Cancer Cells. Int. J. Oncol. 2004, 25, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Cobb, M.M.; Austin, D.C.; Sack, J.T.; Trimmer, J.S. Cell Cycle-Dependent Changes in Localization and Phosphorylation of the Plasma Membrane Kv2.1 K+ Channel Impact Endoplasmic Reticulum Membrane Contact Sites in Cos-1 Cells. J. Biol. Chem. 2015, 290, 29189–29201. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ramirez, M.; Moran-Zendejas, R.; Arechiga-Figueroa, I.A.; Toro-Castillo, C.; Ramirez-Martinez, J.F.; Rodriguez-Menchaca, A.A. Modulation of the Voltage-Gated Potassium Channel Kv2.1 by the Anti-Tumor Alkylphospholipid Perifosine. Pharmacol. Rep. 2016, 68, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Fujii, T.; Takahashi, Y.; Suzuki, T.; Ukai, M.; Tauchi, K.; Horikawa, N.; Tsukada, K.; Sakai, H. Up-Regulation of Kv7.1 Channels in Thromboxane A2-Induced Colonic Cancer Cell Proliferation. Pflugers Arch. 2014, 466, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Tsevi, I.; Vicente, R.; Grande, M.; Lopez-Iglesias, C.; Figueras, A.; Capella, G.; Condom, E.; Felipe, A. Kcnq1/Kcne1 Channels During Germ-Cell Differentiation in the Rat: Expression Associated with Testis Pathologies. J. Cell. Physiol. 2005, 202, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Roura-Ferrer, M.; Sole, L.; Martinez-Marmol, R.; Villalonga, N.; Felipe, A. Skeletal Muscle Kv7 (Kcnq) Channels in Myoblast Differentiation and Proliferation. Biochem. Biophys. Res. Commun. 2008, 369, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.A.; del Camino, D.; Sanchez, A.; Alves, F.; Bruggemann, A.; Beckh, S.; Stuhmer, W. Oncogenic Potential of Eag K(+) Channels. EMBO J. 1999, 18, 5540–5547. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, J.R.; Griesinger, F.; Stuhmer, W.; Pardo, L.A. The Potassium Channel Ether a Go-Go Is a Novel Prognostic Factor with Functional Relevance in Acute Myeloid Leukemia. Mol. Cancer 2010, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Heinemann, S.H. Characterization of an Eag-Like Potassium Channel in Human Neuroblastoma Cells. J. Physiol. 1998, 508 Pt 1, 49–56. [Google Scholar] [CrossRef]
- Hemmerlein, B.; Weseloh, R.M.; de Queiroz, F.M.; Knotgen, H.; Sanchez, A.; Rubio, M.E.; Martin, S.; Schliephacke, T.; Jenke, M.; Joachim, R.H.; et al. Overexpression of Eag1 Potassium Channels in Clinical Tumours. Mol. Cancer 2006, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Wang, T.; Guo, Q.; Zhang, Y.; Wang, Y.; Yuan, L.; Ling, R.; He, Y.; Wang, W. Positive Correlation between the Expression of Heag1 and Hif-1alpha in Breast Cancers: An Observational Study. BMJ Open 2014, 4, e005049. [Google Scholar] [CrossRef] [PubMed]
- Ousingsawat, J.; Spitzner, M.; Puntheeranurak, S.; Terracciano, L.; Tornillo, L.; Bubendorf, L.; Kunzelmann, K.; Schreiber, R. Expression of Voltage-Gated Potassium Channels in Human and Mouse Colonic Carcinoma. Clin. Cancer Res. 2007, 13, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.W.; Luo, H.S.; Jin, X.; Yan, J.J.; Ai, Y.W. Aberrant Expression of Eag1 Potassium Channels in Gastric Cancer Patients and Cell Lines. Med. Oncol. 2007, 24, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhong, D.; Lin, B.; Zhai, W.; Ding, Z.; Wu, J. P38 Mapk Regulates the Expression of Ether a Go-Go Potassium Channel in Human Osteosarcoma Cells. Radiol. Oncol. 2013, 47, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhong, D.; Fu, X.; Liu, Q.; Kang, L.; Ding, Z. Silencing of Ether a Go-Go 1 by Shrna Inhibits Osteosarcoma Growth and Cell Cycle Progression. Int. J. Mol. Sci. 2014, 15, 5570–5581. [Google Scholar] [CrossRef] [PubMed]
- Downie, B.R.; Sanchez, A.; Knotgen, H.; Contreras-Jurado, C.; Gymnopoulos, M.; Weber, C.; Stuhmer, W.; Pardo, L.A. Eag1 Expression Interferes with Hypoxia Homeostasis and Induces Angiogenesis in Tumors. J. Biol. Chem. 2008, 283, 36234–36240. [Google Scholar] [CrossRef] [PubMed]
- Toral, C.; Mendoza-Garrido, M.E.; Azorin, E.; Hernandez-Gallegos, E.; Gomora, J.C.; Delgadillo, D.M.; Solano-Agama, C.; Camacho, J. Effect of Extracellular Matrix on Adhesion, Viability, Actin Cytoskeleton and K+ Currents of Cells Expressing Human Ether a Go-Go Channels. Life Sci. 2007, 81, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, L.; Li, J.; Yu, B.; Su, L.; Chen, X.; Yu, Y.; Yan, M.; Liu, B.; Zhu, Z. Hypermethylated DNA as Potential Biomarkers for Gastric Cancer Diagnosis. Clin. Biochem. 2011, 44, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.W.; Yuan, T.C.; Fang, K.P.; Yang, F.S.; Liu, C.J.; Chang, C.S.; Lin, S.C. The Increase of Voltage-Gated Potassium Channel Kv3.4 Mrna Expression in Oral Squamous Cell Carcinoma. J. Oral Pathol. Med. 2003, 32, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Lew, T.S.; Chang, C.S.; Fang, K.P.; Chen, C.Y.; Chen, C.H.; Lin, S.C. The Involvement of K(V)3.4 Voltage-Gated Potassium Channel in the Growth of an Oral Squamous Cell Carcinoma Cell Line. J. Oral Pathol. Med. 2004, 33, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Menendez, S.T.; Rodrigo, J.P.; Allonca, E.; Garcia-Carracedo, D.; Alvarez-Alija, G.; Casado-Zapico, S.; Fresno, M.F.; Rodriguez, C.; Suarez, C.; Garcia-Pedrero, J.M. Expression and Clinical Significance of the Kv3.4 Potassium Channel Subunit in the Development and Progression of Head and Neck Squamous Cell Carcinomas. J. Pathol. 2010, 221, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Park, S.M.; Park, J.S.; Byun, J.H.; Jin, H.J.; Seo, S.H.; Ryu, P.D.; Lee, S.Y. Kv3.1 and Kv3.4, Are Involved in Cancer Cell Migration and Invasion. Int. J. Mol. Sci. 2018, 19, 1061. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Valle, A.; Rodrigo, J.P.; Garcia-Pedrero, J.M.; Rodriguez-Santamarta, T.; Allonca, E.; Lequerica-Fernandez, P.; de Vicente, J.C. Expression of the Voltage-Gated Potassium Channel Kv3.4 in Oral Leucoplakias and Oral Squamous Cell Carcinomas. Histopathology 2016, 69, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.Z.; Xu, Q.; Zhang, X.M.; Zhao, Z.Q.; Mei, Y.A.; Zhang, Y.Q. Targeting a-Type K(+) Channels in Primary Sensory Neurons for Bone Cancer Pain in a Rat Model. Pain 2012, 153, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jang, S.H.; Jeong, Y.A.; Ryu, P.D.; Kim, D.Y.; Lee, S.Y. Involvement of Kv4.1 K(+) Channels in Gastric Cancer Cell Proliferation. Biol. Pharm. Bull. 2010, 33, 1754–1757. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Choi, C.; Hong, S.G.; Yarishkin, O.V.; Bae, Y.M.; Kim, J.G.; O’Grady, S.M.; Yoon, K.A.; Kang, K.S.; Ryu, P.D.; et al. Silencing of Kv4.1 Potassium Channels Inhibits Cell Proliferation of Tumorigenic Human Mammary Epithelial Cells. Biochem. Biophys. Res. Commun. 2009, 384, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Bocksteins, E. Kv5, Kv6, Kv8, and Kv9 Subunits: No Simple Silent Bystanders. J. Gen. Physiol. 2016, 147, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, J.W.; Byun, J.K.; Kim, H.K.; Ryu, P.D.; Lee, S.Y.; Kim, D.Y. Silencing of Voltage-Gated Potassium Channel Kv9.3 Inhibits Proliferation in Human Colon and Lung Carcinoma Cells. Oncotarget 2015, 6, 8132–8143. [Google Scholar] [PubMed]
- Arcangeli, A.; Bianchi, L.; Becchetti, A.; Faravelli, L.; Coronnello, M.; Mini, E.; Olivotto, M.; Wanke, E. A Novel Inward-Rectifying K+ Current with a Cell-Cycle Dependence Governs the Resting Potential of Mammalian Neuroblastoma Cells. J. Physiol. 1995, 489 Pt 2, 455–471. [Google Scholar] [CrossRef]
- Lastraioli, E.; Guasti, L.; Crociani, O.; Polvani, S.; Hofmann, G.; Witchel, H.; Bencini, L.; Calistri, M.; Messerini, L.; Scatizzi, M.; et al. Herg1 Gene and Herg1 Protein Are Overexpressed in Colorectal Cancers and Regulate Cell Invasion of Tumor Cells. Cancer Res. 2004, 64, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, A.; Taddei, G.L.; Crociani, O.; Paglierani, M.; Buccoliero, A.M.; Fontana, L.; Noci, I.; Borri, P.; Borrani, E.; Giachi, M.; et al. Herg Potassium Channels Are More Frequently Expressed in Human Endometrial Cancer as Compared to Non-Cancerous Endometrium. Br. J. Cancer 2000, 83, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.A.; Tsui, H.W.; Newell, E.W.; Jiang, X.; Zhu, X.P.; Tsui, F.W.; Schlichter, L.C. Functional up-Regulation of Herg K+ Channels in Neoplastic Hematopoietic Cells. J. Biol. Chem. 2002, 277, 18528–18534. [Google Scholar] [CrossRef] [PubMed]
- Lastraioli, E.; Perrone, G.; Sette, A.; Fiore, A.; Crociani, O.; Manoli, S.; D’Amico, M.; Masselli, M.; Iorio, J.; Callea, M.; et al. Herg1 Channels Drive Tumour Malignancy and May Serve as Prognostic Factor in Pancreatic Ductal Adenocarcinoma. Br. J. Cancer 2015, 112, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.W.; Yang, W.B.; Gao, S.; Wang, W.; Li, Z.; Hu, W.M.; Li, J.J.; Luo, H.S. Prognostic Significance of Herg1 Expression in Gastric Cancer. Dig. Dis. Sci. 2010, 55, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Iorio, J.; Meattini, I.; Bianchi, S.; Bernini, M.; Maragna, V.; Dominici, L.; Casella, D.; Vezzosi, V.; Orzalesi, L.; Nori, J.; et al. Herg1 Channel Expression Associates with Molecular Subtypes and Prognosis in Breast Cancer. Cancer Cell Int. 2018, 18, 93. [Google Scholar] [CrossRef] [PubMed]
- Pillozzi, S.; Brizzi, M.F.; Bernabei, P.A.; Bartolozzi, B.; Caporale, R.; Basile, V.; Boddi, V.; Pegoraro, L.; Becchetti, A.; Arcangeli, A. Vegfr-1 (Flt-1), Beta1 Integrin, and Herg K+ Channel for a Macromolecular Signaling Complex in Acute Myeloid Leukemia: Role in Cell Migration and Clinical Outcome. Blood 2007, 110, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Crociani, O.; Zanieri, F.; Pillozzi, S.; Lastraioli, E.; Stefanini, M.; Fiore, A.; Fortunato, A.; D’Amico, M.; Masselli, M.; de Lorenzo, E.; et al. Herg1 Channels Modulate Integrin Signaling to Trigger Angiogenesis and Tumor Progression in Colorectal Cancer. Sci. Rep. 2013, 3, 3308. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Wible, B.; Arcangeli, A.; Taglialatela, M.; Morra, F.; Castaldo, P.; Crociani, O.; Rosati, B.; Faravelli, L.; Olivotto, M.; et al. Herg Encodes a K+ Current Highly Conserved in Tumors of Different Histogenesis: A Selective Advantage for Cancer Cells? Cancer Res. 1998, 58, 815–822. [Google Scholar] [PubMed]
- Tokuoka, S.; Moriok, H. The Membrane Potential of the Human Cancer and Related Cells. I. Gan 1957, 48, 353–354. [Google Scholar] [PubMed]
- Cone, C.D., Jr. Unified Theory on the Basic Mechanism of Normal Mitotic Control and Oncogenesis. J. Theor. Biol. 1971, 30, 151–181. [Google Scholar] [CrossRef]
- Blackiston, D.J.; McLaughlin, K.A.; Levin, M. Bioelectric Controls of Cell Proliferation: Ion Channels, Membrane Voltage and the Cell Cycle. Cell Cycle 2009, 8, 3527–3536. [Google Scholar] [CrossRef] [PubMed]
- Urrego, D.; Tomczak, A.P.; Zahed, F.; Stuhmer, W.; Pardo, L.A. Potassium Channels in Cell Cycle and Cell Proliferation. Philos Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130094. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Brackenbury, W.J. Membrane Potential and Cancer Progression. Front. Physiol. 2013, 4, 185. [Google Scholar] [CrossRef] [PubMed]
- Villalonga, N.; Martinez-Marmol, R.; Roura-Ferrer, M.; David, M.; Valenzuela, C.; Soler, C.; Felipe, A. Cell Cycle-Dependent Expression of Kv1.5 Is Involved in Myoblast Proliferation. Biochim. Biophys. Acta 2008, 1783, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Ghiani, C.A.; Yuan, X.; Eisen, A.M.; Knutson, P.L.; DePinho, R.A.; McBain, C.J.; Gallo, V. Voltage-Activated K+ Channels and Membrane Depolarization Regulate Accumulation of the Cyclin-Dependent Kinase Inhibitors P27(Kip1) and P21(Cip1) in Glial Progenitor Cells. J. Neurosci. 1999, 19, 5380–5392. [Google Scholar] [CrossRef] [PubMed]
- Chittajallu, R.; Chen, Y.; Wang, H.; Yuan, X.; Ghiani, C.A.; Heckman, T.; McBain, C.J.; Gallo, V. Regulation of Kv1 Subunit Expression in Oligodendrocyte Progenitor Cells and Their Role in G1/S Phase Progression of the Cell Cycle. Proc. Natl. Acad. Sci. USA 2002, 99, 2350–2355. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.L.; Lau, C.P.; Lai, K.; Cheung, K.F.; Lau, G.K.; Li, G.R. Cell Cycle-Dependent Expression of Potassium Channels and Cell Proliferation in Rat Mesenchymal Stem Cells from Bone Marrow. Cell Prolif. 2007, 40, 656–670. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, S.N.; Sontheimer, H. Changes in Ion Channel Expression Accompany Cell Cycle Progression of Spinal Cord Astrocytes. Glia 2000, 30, 39–48. [Google Scholar] [CrossRef]
- Fox, P.D.; Haberkorn, C.J.; Akin, E.J.; Seel, P.J.; Krapf, D.; Tamkun, M.M. Induction of Stable Er-Plasma-Membrane Junctions by Kv2.1 Potassium Channels. J. Cell Sci. 2015, 128, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Urrego, D.; Pardo, L.A. Cyclic Expression of the Voltage-Gated Potassium Channel Kv10.1 Promotes Disassembly of the Primary Cilium. EMBO Rep. 2016, 17, 708–723. [Google Scholar] [CrossRef] [PubMed]
- Urrego, D.; Sanchez, A.; Tomczak, A.P.; Pardo, L.A. The Electric Fence to Cell-Cycle Progression: Do Local Changes in Membrane Potential Facilitate Disassembly of the Primary Cilium? Timely and Localized Expression of a Potassium Channel May Set the Conditions That Allow Retraction of the Primary Cilium. Bioessays 2017, 39, 1600190. [Google Scholar] [CrossRef] [PubMed]
- Urrego, D.; Movsisyan, N.; Ufartes, R.; Pardo, L.A. Periodic Expression of Kv10.1 Driven by Prb/E2f1 Contributes to G2/M Progression of Cancer and Non-Transformed Cells. Cell Cycle 2016, 15, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.M.; Rouzaire-Dubois, B. The Influence of Cell Volume Changes on Tumour Cell Proliferation. Eur. Biophys. J. 2004, 33, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Abdelhady, S.; Kitambi, S.S.; Lundin, V.; Aufschnaiter, R.; Sekyrova, P.; Sinha, I.; Lundgren, K.T.; Castelo-Branco, G.; Linnarsson, S.; Wedlich-Soldner, R.; et al. Erg Channel Is Critical in Controlling Cell Volume During Cell Cycle in Embryonic Stem Cells. PLoS ONE 2013, 8, e72409. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Foller, M.; Lang, K.; Lang, P.; Ritter, M.; Vereninov, A.; Szabo, I.; Huber, S.M.; Gulbins, E. Cell Volume Regulatory Ion Channels in Cell Proliferation and Cell Death. Methods Enzymol. 2007, 428, 209–225. [Google Scholar] [PubMed]
- Cidad, P.; Jimenez-Perez, L.; Garcia-Arribas, D.; Miguel-Velado, E.; Tajada, S.; Ruiz-McDavitt, C.; Lopez-Lopez, J.R.; Perez-Garcia, M.T. Kv1.3 Channels Can Modulate Cell Proliferation During Phenotypic Switch by an Ion-Flux Independent Mechanism. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Perez, L.; Cidad, P.; Alvarez-Miguel, I.; Santos-Hipolito, A.; Torres-Merino, R.; Alonso, E.; de la Fuente, M.A.; Lopez-Lopez, J.R.; Perez-Garcia, M.T. Molecular Determinants of Kv1.3 Potassium Channels-Induced Proliferation. J. Biol. Chem. 2016, 291, 3569–3580. [Google Scholar] [CrossRef] [PubMed]
- Hegle, A.P.; Marble, D.D.; Wilson, G.F. A Voltage-Driven Switch for Ion-Independent Signaling by Ether-a-Go-Go K+ Channels. Proc. Natl. Acad. Sci. USA 2006, 103, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Leanza, L.; Trentin, L.; Becker, K.A.; Frezzato, F.; Zoratti, M.; Semenzato, G.; Gulbins, E.; Szabo, I. Clofazimine, Psora-4 and Pap-1, Inhibitors of the Potassium Channel Kv1.3, as a New and Selective Therapeutic Strategy in Chronic Lymphocytic Leukemia. Leukemia 2013, 27, 1782–1785. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Kang, K.S.; Ryu, P.D.; Lee, S.Y. Kv1.3 Voltage-Gated K(+) Channel Subunit as a Potential Diagnostic Marker and Therapeutic Target for Breast Cancer. BMB Rep. 2009, 42, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Brevet, M.; Haren, N.; Sevestre, H.; Merviel, P.; Ouadid-Ahidouch, H. DNA Methylation of K(V)1.3 Potassium Channel Gene Promoter Is Associated with Poorly Differentiated Breast Adenocarcinoma. Cell. Physiol. Biochem. 2009, 24, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Abdul, M.; Hoosein, N. Expression and Activity of Potassium Ion Channels in Human Prostate Cancer. Cancer Lett. 2002, 186, 99–105. [Google Scholar] [CrossRef]
- Abdul, M.; Hoosein, N. Reduced Kv1.3 Potassium Channel Expression in Human Prostate Cancer. J. Membr. Biol. 2006, 214, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Brevet, M.; Fucks, D.; Chatelain, D.; Regimbeau, J.M.; Delcenserie, R.; Sevestre, H.; Ouadid-Ahidouch, H. Deregulation of 2 Potassium Channels in Pancreas Adenocarcinomas: Implication of Kv1.3 Gene Promoter Methylation. Pancreas 2009, 38, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hu, Y.; Liao, Q.; Niu, Z.; Xing, X.; Xia, W.; Zhao, Y. Gene Identification of Potential Malignant Parathyroid Tumors Phenotype in Chinese Population. Endocr. J. 2014, 61, 597–605. [Google Scholar] [CrossRef] [PubMed]
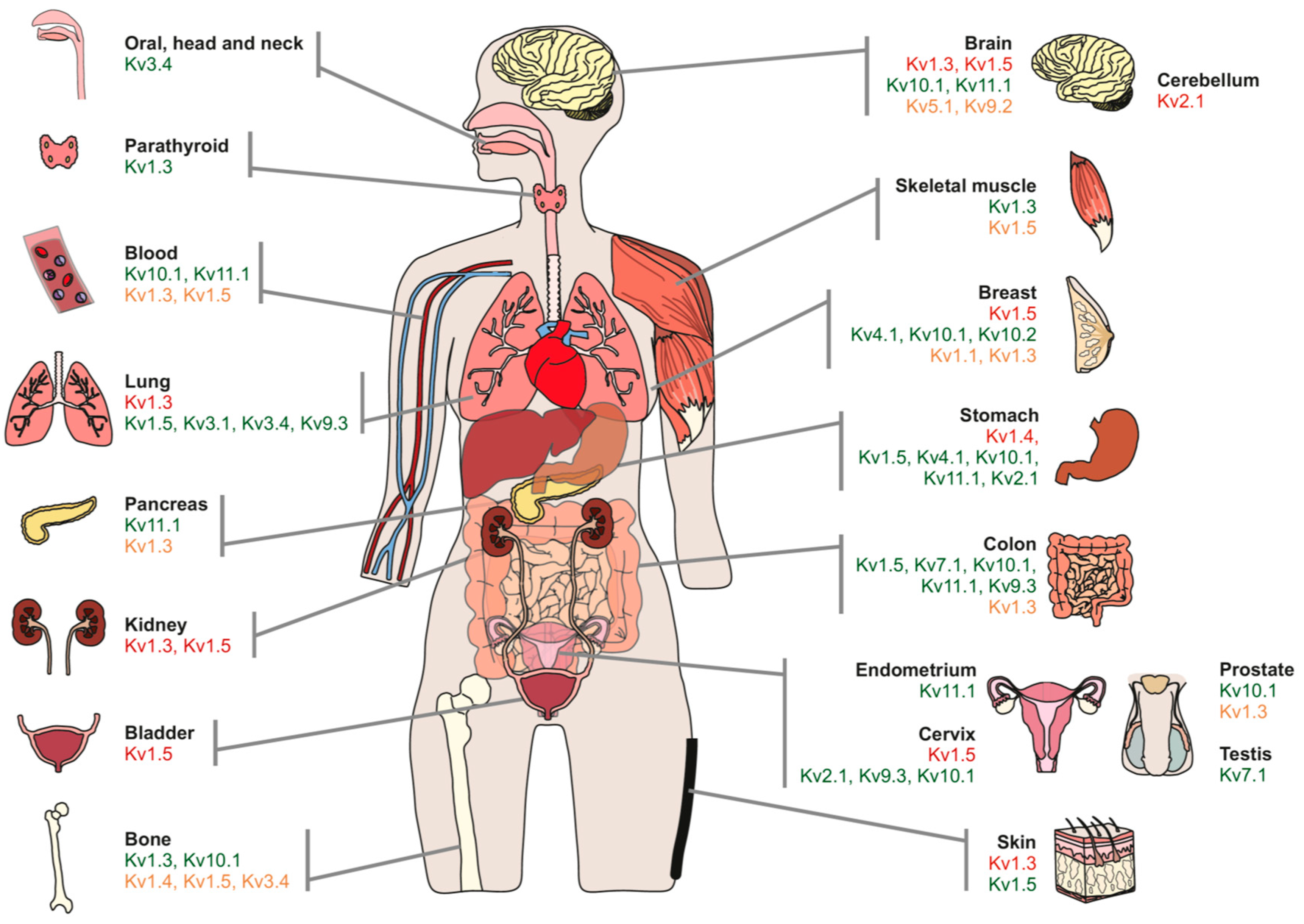

| Channel | Tissue | Modulation | Highlights | References |
|---|---|---|---|---|
| Kv1.3 | Blood | ↓ | MitoKv1.3 is downstream of a pro-apoptotic signaling pathway. Kv1.3 inhibition promotes cell survival. Considered a tumor suppressor. | [25,26,27] |
| ↑ | Upregulation of Kv1.3 in B lymphocytes is related to B-RAF signaling. Kv1.3 membrane-permeable inhibitors (clofazimine) induce apoptosis of B-CLL cells in the presence of mesenchymal stromal cells (anti-apoptotic). | [19,106] | ||
| ↓ | No relation with tumor malignancy. Tumor suppressor. Role in apoptosis. | [19,26,28] | ||
| Colon | ↓ | LS174 colon adenocarcinoma cell line. Methylation of the Kv1.3 promoter. | [38] | |
| ↑ | Kv1.3 modulates cell migration and adhesion but not apoptosis and proliferation. | [20] | ||
| Brain | ↓ | Kv1.3 is downregulated in the plasma membrane of glioblastoma cell lines. MitoKv1.3-directed membrane-permeable drugs induce apoptosis in cell lines. | [31] | |
| ↑ | U87 Glioblastoma cell line. Kv1.3 modulates cell migration and adhesion but not apoptosis and proliferation. | [20] | ||
| Breast | ↑ | MDA-MB-231 breast cancer cell line. Kv1.3 modulates cell migration and adhesion but not apoptosis and proliferation. | [20] | |
| ↑ | Breast cancer and tumorigenic human mammary epithelial cells. Kv blockers suppress tumorigenic cell proliferation. | [107] | ||
| ↓ | Breast carcinoma samples and the MCF-7 cell line. Methylation of the Kv1.3 promoter increases in grade III tumors and cells. Related to poorly differentiated tumors and young patients. | [108] | ||
| Prostate | ↑↓ | Protein levels vary from high to low expression in different primary prostate cancer patients. Low channel expression may correlate with the increased probability of metastatic disease. | [109,110] | |
| Pancreas | ↑ | Mito Kv1.3. Very aggressive and highly metastatic tumor. Correlated with high levels of anti-apoptotic Bcl-xL. | [30] | |
| ↓ | Methylation of the gene promoter. | [111] | ||
| ↓ | Decreased expression in ductal adenocarcinoma grade II. | [25] | ||
| Bones | ↑ | Osteosarcoma samples and derived cell lines. | [29] | |
| Skeletal muscle | ↑ | Increased expression in skeletal muscle carcinogenesis but no clear relationship with malignancy. | [35] | |
| Parathyroid | ↑ | DNA and protein overexpression of Kv1.3. Potential marker to distinguish carcinoma or adenoma. | [112] | |
| Kv1.5 | Blood | ↓ | Inversely correlates with aggressiveness in non-Hodgkin’s lymphomas. | [24] |
| Skeletal muscle | ↑ | Increased expression in skeletal muscle carcinogenesis. Correlation with the degree of malignancy. | [35] | |
| Breast | ↓ | Absent or low expression in mammary duct carcinoma samples. | [25] | |
| Brain | ↓ | Kv1.5 inversely correlates with glioma malignancy. High in astrocytoma, moderate in oligodendroglioma, and low in glioblastoma. | [37] | |
| Skin | ↑ | High expression in squamous skin cell carcinoma. | [25] | |
| Colon | ↑ | Overexpression in colon adenocarcinoma. | [25] | |
| Stomach | ↑ | Kv1.5 may be involved in tumor cell proliferation by controlling calcium entry. | [33] | |
| Bone | ↓ | Promoters of ion channels are highly methylated in Ewing Sarcoma. Inhibiting CpG islands, cancer cells are sensitive to death. Kv1.5 would act as a tumor suppressor. | [39,40] | |
| ↑ | Osteosarcoma samples and cell lines. Silencing Kv1.5 impairs osteosarcoma cell proliferation and induces cell cycle arrest (G0/G1) and apoptosis. | [34] | ||
| Kv1.1 | Breast | ↑ | Implicated in MDA-MB-231 breast cancer cell line migration and tumorigenesis via EGFR. | [20] |
| ↓ | Tumor suppressor in primary mammary epithelia cancer samples and cell lines. Delocalization of Kv1.1 affects cellular senescence and transformation processes. | [46] | ||
| Kv2.1 | Gastric | ↑ | Several gastric cancer cell lines. | [33] |
| Cerebellum | ↓ | Medulloblastoma samples. Tumor suppressor. Heme Oxygenase-1 affects apoptosis via CO-mediated Inhibition of Kv2.1. Tumor cells become resistant to apoptosis. | [47] | |
| Cervix (uterus) | ↑ | Kv2.1/Kv9.3 participates in cell cycle regulation in cervical adenocarcinoma cells. | [48] | |
| Kv7.1 | Germinal | ↑ | High levels of KCNQ1/KCNE1 in human seminoma samples, characterized by the proliferation of undifferentiated germ cells. | [52] |
| Colon | ↑ | Upregulated in human colorectal cancer and cell lines. Involved in TXA2-induced cancer cell proliferation. | [51] | |
| Kv10.1 | Brain | ↑ | Overexpression in primary brain tumor and metastases correlates with a poor prognosis. Antidepressants blocking Kv10.1 improve the survival rate in patients with moderate Kv10.1 expression. | [21] |
| ↑ | Cell cycle-dependent expression in neuroblastoma cells. | [56] | ||
| Colon | ↑ | Malignant colorectal adenocarcinomas. Enhanced function in carcinogenesis. | [59] | |
| Gastric | ↑ | Aberrant expression in gastric cancer tissues and cell lines. Role in proliferation in association with lymph node metastasis and cancer stage. | [60] | |
| Breast | ↑ | Kv10.1 expression induces cancer progression in several human cancer cell lines. | [54,57] | |
| ↑ | Correlation with the overexpression of HIF-1α in invasive ductal carcinoma samples. Close correlation with the clinical parameters of tumors. Interference with hypoxia homeostasis of the early stage of tumor progression. | [58] | ||
| Bone | ↑ | Kv10.1 silencing inhibited cancer cell proliferation and colony formation via G1 phase arrest in the MG-63 osteosarcoma cell line. | [61,62] | |
| Kv3.4 | Lung | ↑ | Cell density- and hypoxia-dependent overexpression in A549 lung adenocarcinoma cell lines. Migration and invasion are affected in aggressive tumors. | [69] |
| Oral, head and neck | ↑ | Leukoplakia and oral squamous cell carcinoma samples. Role in tumorigenesis, malignant transformation migration, and invasion. | [68,70] | |
| Kv4.1 | Gastric | ↑ | MKN-45 and SNU-638 gastric cancer cell lines. Inhibition of Kv4.1 impairs cell proliferation and cell cycle distribution. | [72] |
| Breast | ↑ | M13SV1 mammary epithelial cells and breast cancer samples. Kv4.1 positively correlates with malignant stages. | [73] | |
| Kv9.3 | Cervix (uterus) | ↑ | Kv2.1/Kv9.3 participates in cell cycle regulation in cervical adenocarcinoma cells. | [48] |
| Colon | ↑ | Kv2.1-independent role in cancer progression. Kv9.3 blockade halts tumor cell proliferation by arresting the cell cycle at G0/G1. | [75] | |
| Lung | ↑ | Kv2.1-independent role in cancer progression. Kv9.3 blockade halts tumor cell proliferation by arresting the cell cycle at G0/G1. | [75] | |
| Kv11.1 | Gastric | ↑ | Crucial in the P13K/Akt-dependent pathway that induces HIF and VEGF to promote tumor progression. Blocking Kv11.1 inhibits cell growth, angiogenesis and metastasis. | [22] |
| Pancreas | ↑ | High levels in primary PDAC samples and related to EGFR. Channel blockade impairs PDAC cell line growth and migration. | [80] | |
| Breast | ↑ | Present in all breast cancers. Kv11.1 is associated with a better prognosis and lower metastasis rate. | [82] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano-Novillo, C.; Capera, J.; Colomer-Molera, M.; Condom, E.; Ferreres, J.C.; Felipe, A. Implication of Voltage-Gated Potassium Channels in Neoplastic Cell Proliferation. Cancers 2019, 11, 287. https://doi.org/10.3390/cancers11030287
Serrano-Novillo C, Capera J, Colomer-Molera M, Condom E, Ferreres JC, Felipe A. Implication of Voltage-Gated Potassium Channels in Neoplastic Cell Proliferation. Cancers. 2019; 11(3):287. https://doi.org/10.3390/cancers11030287
Chicago/Turabian StyleSerrano-Novillo, Clara, Jesusa Capera, Magalí Colomer-Molera, Enric Condom, Joan Carles Ferreres, and Antonio Felipe. 2019. "Implication of Voltage-Gated Potassium Channels in Neoplastic Cell Proliferation" Cancers 11, no. 3: 287. https://doi.org/10.3390/cancers11030287
APA StyleSerrano-Novillo, C., Capera, J., Colomer-Molera, M., Condom, E., Ferreres, J. C., & Felipe, A. (2019). Implication of Voltage-Gated Potassium Channels in Neoplastic Cell Proliferation. Cancers, 11(3), 287. https://doi.org/10.3390/cancers11030287






