Circulating Tumor Cell Detection in Lung Cancer: But to What End?
Abstract
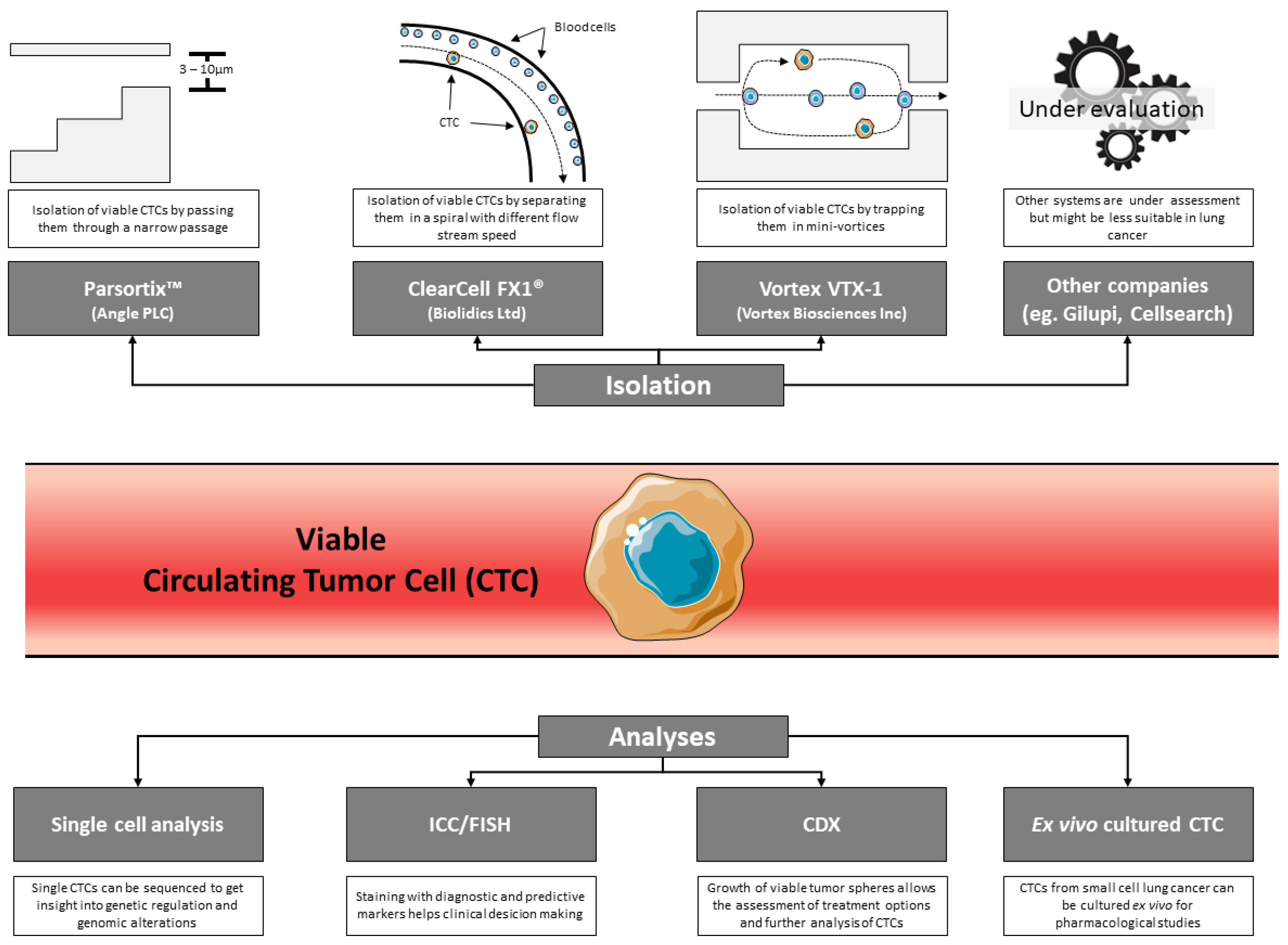
1. Introduction
2. Opportunities Offered by Studying CTCs in Thoracic Oncology
2.1. Developing Xenografts from Circulating Tumor Cells and Cells Cultured In Vitro
2.2. Single-Cell Analysis and Functional Studies
2.3. Correlation between Cytopathological and Molecular Phenotypic Analyses
3. What Are the Prospects?
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bracht, J.W.P.; Mayo-de-Las-Casas, C.; Berenguer, J.; Karachaliou, N.; Rosell, R. The Present and Future of Liquid Biopsies in Non-Small Cell Lung Cancer: Combining Four Biosources for Diagnosis, Prognosis, Prediction, and Disease Monitoring. Curr. Oncol. Rep. 2018, 20, 70. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P. Liquid Biopsy and Therapeutic Targets: Present and Future Issues in Thoracic Oncology. Cancers 2017, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Mader, S.; Pantel, K. Epithelial-mesenchymal plasticity in circulating tumor cells. J. Mol. Med. 2017, 95, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Bardelli, A.; Pantel, K. Liquid Biopsies, What We Do Not Know (Yet). Cancer Cell 2017, 31, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, H.; Zhao, Z.; Gao, H.; Liu, C.; Zhu, L.; Wang, C.; Yang, Y. Emerging Nanotechnologies for Liquid Biopsy: The Detection of Circulating Tumor Cells and Extracellular Vesicles. Adv. Mater. 2018, e1805344. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Sorber, L.; Zwaenepoel, K.; De Winne, K.; Van Casteren, K.; Augustus, E.; Jacobs, J.; Zhang, X.H.; Galdermans, D.; De Droogh, E.; Lefebure, A.; et al. A Multicenter Study to Assess EGFR Mutational Status in Plasma: Focus on an Optimized Workflow for Liquid Biopsy in a Clinical Setting. Cancers 2018, 10, 290. [Google Scholar] [CrossRef] [PubMed]
- Ntzifa, A.; Kroupis, C.; Haliassos, A.; Lianidou, E. A pilot plasma-ctDNA ring trial for the Cobas® EGFR Mutation Test in clinical diagnostic laboratories. Clin. Chem. Lab. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Mack, P.C.; Scagliotti, G.V.; Baas, P.; Barlesi, F.; Bivona, T.G.; Herbst, R.S.; Mok, T.S.; Peled, N.; Pirker, R.; et al. IASLC Statement Paper: Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC). J. Thorac. Oncol. 2018, 13, 1248–1268. [Google Scholar] [CrossRef] [PubMed]
- Sacher, A.G.; Alden, R.S.; Oxnard, G.R. Early Intervention in Lung Cancers With Rapid Plasma Genotyping for EGFR and KRAS Mutations-Reply. JAMA Oncol. 2016, 2, 1096–1097. [Google Scholar] [CrossRef] [PubMed]
- Reimers, N.; Pantel, K. Liquid biopsy: Novel technologies and clinical applications. Clin. Chem. Lab. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Afrifa, J.; Zhao, T.; Yu, J. Circulating mitochondria DNA, a non-invasive cancer diagnostic biomarker candidate. Mitochondrion 2018. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, S.; Babayan, A.; Pantel, K.; Calin, G.A. Clinical utility of circulating non-coding RNAs—An update. Nat. Rev. Clin. Oncol. 2018, 15, 541–563. [Google Scholar] [CrossRef] [PubMed]
- Integrative Analysis of Lung Cancer Etiology and Risk (INTEGRAL) Consortium for Early Detection of Lung Cancer; Guida, F.; Sun, N.; Bantis, L.E.; Muller, D.C.; Li, P.; Taguchi, A.; Dhillon, D.; Kundnani, D.L.; Patel, N.J.; et al. Assessment of Lung Cancer Risk on the Basis of a Biomarker Panel of Circulating Proteins. JAMA Oncol. 2018, 4, e182078. [Google Scholar] [CrossRef] [PubMed]
- Mader, S.; Pantel, K. Liquid Biopsy: Current Status and Future Perspectives. Oncol. Res. Treat. 2017, 40, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, R.; Soon, R.H.; Zhang, P.; Jiang, K.; Lim, C.T. Cancer diagnosis: From tumor to liquid biopsy and beyond. Lab Chip 2018, 19, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Leone, K.; Poggiana, C.; Zamarchi, R. The Interplay between Circulating Tumor Cells and the Immune System: From Immune Escape to Cancer Immunotherapy. Diagnostics 2018, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-W.; Rong, Y.; Liu, Q.; Luo, C.-L.; Zhang, Y.; Wang, F.-B. Integrative diagnosis of cancer by combining CTCs and associated peripheral blood cells in liquid biopsy. Clin. Transl. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cabanero, M.; Tsao, M.S. Circulating tumour DNA in EGFR-mutant non-small-cell lung cancer. Curr. Oncol. 2018, 25, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; O’Connell, A.; Sacher, A.G.; Feeney, N.; Alden, R.S.; Oxnard, G.R.; Paweletz, C.P. Monitoring of Response and Resistance in Plasma of EGFR-Mutant Lung Cancer Using Droplet Digital PCR. Methods Mol. Biol. 2018, 1768, 193–207. [Google Scholar] [PubMed]
- Sacher, A.G.; Komatsubara, K.M.; Oxnard, G.R. Application of Plasma Genotyping Technologies in Non-Small Cell Lung Cancer: A Practical Review. J. Thorac. Oncol. 2017, 12, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Schrock, A.B.; Welsh, A.; Chung, J.H.; Pavlick, D.; Bernicker, E.H.; Creelan, B.C.; Forcier, B.; Ross, J.S.; Stephens, P.J.; Ali, S.M.; et al. Hybrid Capture-Based Genomic Profiling of Circulating Tumor DNA from Patients with Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2019, 14, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Duréndez-Sáez, E.; Azkárate, A.; Meri, M.; Calabuig-Fariñas, S.; Aguilar-Gallardo, C.; Blasco, A.; Jantus-Lewintre, E.; Camps, C. New insights in non-small-cell lung cancer: Circulating tumor cells and cell-free DNA. J. Thorac. Dis. 2017, 9, S1332–S1345. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dumenil, C.; Julié, C.; Giraud, V.; Dumoulin, J.; Labrune, S.; Chinet, T.; Emile, J.-F.; He, B.; Leprieur, E.G. Molecular characterization of circulating tumor cells in lung cancer: Moving beyond enumeration. Oncotarget 2017, 8, 109818–109835. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K. Blood-Based Analysis of Circulating Cell-Free DNA and Tumor Cells for Early Cancer Detection. PLoS Med. 2016, 13, e1002205. [Google Scholar] [CrossRef] [PubMed]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Mu, Z.; Rademaker, A.W.; Austin, L.K.; Strickland, K.S.; Costa, R.L.B.; Nagy, R.J.; Zagonel, V.; Taxter, T.J.; Behdad, A.; et al. Cell-Free DNA and Circulating Tumor Cells: Comprehensive Liquid Biopsy Analysis in Advanced Breast Cancer. Clin. Cancer Res. 2018, 24, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Morbelli, S.; Alama, A.; Ferrarazzo, G.; Coco, S.; Genova, C.; Rijavec, E.; Bongioanni, F.; Biello, F.; Dal Bello, M.G.; Barletta, G.; et al. Circulating Tumor DNA Reflects Tumor Metabolism Rather Than Tumor Burden in Chemotherapy-Naive Patients with Advanced Non–Small Cell Lung Cancer: 18 F-FDG PET/CT Study. J. Nucl. Med. 2017, 58, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, T.K.; Sequist, L.V.; Heymach, J.V.; Riely, G.J.; Jänne, P.A.; Koch, W.H.; Sullivan, J.P.; Fox, D.B.; Maher, R.; Muzikansky, A.; et al. Detection of T790M, the acquired resistance EGFR mutation, by tumor biopsy versus noninvasive blood-based analyses. Clin. Cancer Res. 2016, 22, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.K.; Tellez-Gabriel, M.; Cartron, P.-F.; Vallette, F.M.; Heymann, M.-F.; Heymann, D. Characterization of circulating tumor cells as a reflection of the tumor heterogeneity: Myth or reality? Drug Discov. Today 2018. [Google Scholar] [CrossRef] [PubMed]
- Hofman, V.; Ilie, M.; Long, E.; Guibert, N.; Selva, E.; Washetine, K.; Mograbi, B.; Mouroux, J.; Vénissac, N.; Reverso-Meinietti, J.; et al. Detection of circulating tumor cells from lung cancer patients in the era of targeted therapy: Promises, drawbacks and pitfalls. Curr. Mol. Med. 2014, 14, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Farace, F.; Massard, C.; Vimond, N.; Drusch, F.; Jacques, N.; Billiot, F.; Laplanche, A.; Chauchereau, A.; Lacroix, L.; Planchard, D.; et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br. J. Cancer 2011, 105, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Hofman, V.; Ilie, M.I.; Long, E.; Selva, E.; Bonnetaud, C.; Molina, T.; Vénissac, N.; Mouroux, J.; Vielh, P.; Hofman, P. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: Comparison of the efficacy of the CellSearch AssayTM and the isolation by size of epithelial tumor cell method. Int. J. Cancer 2011, 129, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Fici, P. Cell-Free DNA in the Liquid Biopsy Context: Role and Differences Between ctDNA and CTC Marker in Cancer Management. Methods Mol. Biol. 2019, 1909, 47–73. [Google Scholar] [PubMed]
- Ilie, M.; Hofman, V.; Long, E.; Bordone, O.; Selva, E.; Washetine, K.; Marquette, C.H.; Hofman, P. Current challenges for detection of circulating tumor cells and cell-free circulating nucleic acids, and their characterization in non-small cell lung carcinoma patients. What is the best blood substrate for personalized medicine? Ann. Transl. Med. 2014, 2, 107. [Google Scholar] [PubMed]
- Lianidou, E.; Pantel, K. Liquid Biopsies. Genes Chromosomes Cancer 2019, 58, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Kim, C.-J.; Sunkara, V.; Kim, M.-H.; Cho, Y.-K. Liquid Biopsy in Lung Cancer: Clinical Applications of Circulating Biomarkers (CTCs and ctDNA). Micromachines 2018, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Hofman, V.; Bonnetaud, C.; Ilie, M.I.; Vielh, P.; Vignaud, J.M.; Fléjou, J.F.; Lantuejoul, S.; Piaton, E.; Mourad, N.; Butori, C.; et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin. Cancer Res. 2011, 17, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.; Rothwell, D.G.; Mesquita, B.; Smowton, C.; Leong, H.S.; Fernandez-Gutierrez, F.; Li, Y.; Burt, D.J.; Antonello, J.; Morrow, C.J.; et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat. Med. 2017, 23, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Drapkin, B.J.; George, J.; Christensen, C.L.; Mino-Kenudson, M.; Dries, R.; Sundaresan, T.; Phat, S.; Myers, D.T.; Zhong, J.; Igo, P.; et al. Genomic and functional fidelity of small cell lung cancer patient-derived xenografts. Cancer Discov. 2018, 8, 600–615. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Lim, T.H.; Lim, T.K.; Tan, D.S.-W.; Chua, Y.W.; Ang, M.K.; Pang, B.; Lim, C.T.; Takano, A.; Lim, A.S.-T.; et al. Concordance of anaplastic lymphoma kinase (ALK) gene rearrangements between circulating tumor cells and tumor in non-small cell lung cancer. Oncotarget 2016, 7, 23251–23262. [Google Scholar] [CrossRef] [PubMed]
- Ilié, M.; Szafer-Glusman, E.; Hofman, V.; Chamorey, E.; Lalvée, S.; Selva, E.; Leroy, S.; Marquette, C.-H.; Kowanetz, M.; Hedge, P.; et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.L.; Adams, D.K.; He, J.; Kalhor, N.; Zhang, M.; Xu, T.; Gao, H.; Reuben, J.M.; Qiao, Y.; Komaki, R.; et al. Sequential Tracking of PD-L1 Expression and RAD50 Induction in Circulating Tumor and Stromal Cells of Lung Cancer Patients Undergoing Radiotherapy. Clin. Cancer Res. 2017, 23, 5948–5958. [Google Scholar] [CrossRef] [PubMed]
- Chudziak, J.; Burt, D.J.; Mohan, S.; Rothwell, D.G.; Mesquita, B.; Antonello, J.; Dalby, S.; Ayub, M.; Priest, L.; Carter, L.; et al. Clinical evaluation of a novel microfluidic device for epitope-independent enrichment of circulating tumour cells in patients with small cell lung cancer. Analyst 2016, 141, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.G.; Hou, J.-M.; Sloane, R.; Lancashire, L.; Priest, L.; Nonaka, D.; Ward, T.H.; Backen, A.; Clack, G.; Hughes, A.; et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J. Thorac. Oncol. 2012, 7, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Gorges, T.M.; Penkalla, N.; Schalk, T.; Joosse, S.A.; Riethdorf, S.; Tucholski, J.; Lücke, K.; Wikman, H.; Jackson, S.; Brychta, N.; et al. Enumeration and Molecular Characterization of Tumor Cells in Lung Cancer Patients Using a Novel In Vivo Device for Capturing Circulating Tumor Cells. Clin. Cancer Res. 2016, 22, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Bardia, A.; Aceto, N.; Bersani, F.; Madden, M.W.; Donaldson, M.C.; Desai, R.; Zhu, H.; Comaills, V.; Zheng, Z.; et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 2014, 345, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chu, G.C.Y.; Mrdenovic, S.; Annamalai, A.A.; Hendifar, A.E.; Nissen, N.N.; Tomlinson, J.S.; Lewis, M.; Palanisamy, N.; Tseng, H.-R.; et al. Cultured circulating tumor cells and their derived xenografts for personalized oncology. Asian J. Urol. 2016, 3, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Kulasinghe, A.; Perry, C.; Warkiani, M.E.; Blick, T.; Davies, A.; O’Byrne, K.; Thompson, E.W.; Nelson, C.C.; Vela, I.; Punyadeera, C. Short term ex-vivo expansion of circulating head and neck tumour cells. Oncotarget 2016, 7, 60101–60109. [Google Scholar] [CrossRef] [PubMed]
- Grillet, F.; Bayet, E.; Villeronce, O.; Zappia, L.; Lagerqvist, E.L.; Lunke, S.; Charafe-Jauffret, E.; Pham, K.; Molck, C.; Rolland, N.; et al. Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture. Gut 2017, 66, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Foy, V.; Fernandez-Gutierrez, F.; Faivre-Finn, C.; Dive, C.; Blackhall, F. The clinical utility of circulating tumour cells in patients with small cell lung cancer. Transl. Lung Cancer Res. 2017, 6, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, C.L.; Morrow, C.J.; Li, Y.; Metcalf, R.L.; Rothwell, D.G.; Trapani, F.; Polanski, R.; Burt, D.J.; Simpson, K.L.; Morris, K.; et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat. Med. 2014, 20, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Gabriel, M.; Cochonneau, D.; Cadé, M.; Jubellin, C.; Heymann, M.-F.; Heymann, D. Circulating Tumor Cell-Derived Pre-Clinical Models for Personalized Medicine. Cancers 2018, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Lallo, A.; Gulati, S.; Schenk, M.W.; Khandelwal, G.; Berglund, U.W.; Pateras, I.S.; Chester, C.P.E.; Pham, T.M.; Kalderen, C.; Frese, K.K.; et al. Ex vivo culture of cells derived from circulating tumour cell xenograft to support small cell lung cancer research and experimental therapeutics. Br. J. Pharmacol. 2019, 176, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Pantel, K. Characterization of single circulating tumor cells. FEBS Lett. 2017, 591, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Heymann, D.; Téllez-Gabriel, M. Circulating Tumor Cells: The Importance of Single Cell Analysis. Adv. Exp. Med. Biol. 2018, 1068, 45–58. [Google Scholar] [PubMed]
- Miyamoto, D.T.; Ting, D.T.; Toner, M.; Maheswaran, S.; Haber, D.A. Single-Cell Analysis of Circulating Tumor Cells as a Window into Tumor Heterogeneity. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Palmirotta, R.; Lovero, D.; Silvestris, E.; Felici, C.; Quaresmini, D.; Cafforio, P.; Silvestris, F. Next-generation Sequencing (NGS) Analysis on Single Circulating Tumor Cells (CTCs) with No Need of Whole-genome Amplification (WGA). Cancer Genomics Proteomics 2017, 14, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.-M.; Krebs, M.G.; Lancashire, L.; Sloane, R.; Backen, A.; Swain, R.K.; Priest, L.J.C.; Greystoke, A.; Zhou, C.; Morris, K.; et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 2012, 30, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.C. Circulating Tumor Cells: Applications in Cytopathology. Surg. Pathol. Clin. 2018, 11, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Sundling, K.E.; Lowe, A.C. Circulating Tumor Cells: Overview and Opportunities in Cytology. Adv. Anat. Pathol. 2019, 26, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Catelain, C.; Pailler, E.; Oulhen, M.; Faugeroux, V.; Pommier, A.-L.; Farace, F. Detection of Gene Rearrangements in Circulating Tumor Cells: Examples of ALK-, ROS1-, RET-Rearrangements in Non-Small-Cell Lung Cancer and ERG-Rearrangements in Prostate Cancer. Adv. Exp. Med. Biol. 2017, 994, 169–179. [Google Scholar] [PubMed]
- Ilie, M.; Long, E.; Butori, C.; Hofman, V.; Coelle, C.; Mauro, V.; Zahaf, K.; Marquette, C.H.; Mouroux, J.; Paterlini-Bréchot, P.; et al. ALK-gene rearrangement: A comparative analysis on circulating tumour cells and tumour tissue from patients with lung adenocarcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 2907–2913. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-T.; Kim, Y.J.; Lee, T.H.; Cho, Y.-H.; Chang, H.J.; Lee, H.-M. Cytopathological Study of the Circulating Tumor Cells filtered from the Cancer Patients’ Blood using Hydrogel-based Cell Block Formation. Sci. Rep. 2018, 8, 15218. [Google Scholar] [CrossRef] [PubMed]
- Pailler, E.; Adam, J.; Barthélémy, A.; Oulhen, M.; Auger, N.; Valent, A.; Borget, I.; Planchard, D.; Taylor, M.; André, F.; et al. Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non-small-cell lung cancer. J. Clin. Oncol. 2013, 31, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Pailler, E.; Auger, N.; Lindsay, C.R.; Vielh, P.; Islas-Morris-Hernandez, A.; Borget, I.; Ngo-Camus, M.; Planchard, D.; Soria, J.-C.; Besse, B.; et al. High level of chromosomal instability in circulating tumor cells of ROS1-rearranged non-small-cell lung cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Pailler, E.; Faugeroux, V.; Oulhen, M.; Catelain, C.; Farace, F. Routine clinical use of circulating tumor cells for diagnosis of mutations and chromosomal rearrangements in non-small cell lung cancer-ready for prime-time? Transl. lung cancer Res. 2017, 6, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Nicolazzo, C.; Gradilone, A.; Carpino, G.; Gazzaniga, P.; Raimondi, C. Molecular Characterization of Circulating Tumor Cells to Study Cancer Immunoevasion. Methods Mol. Biol. 2019, 1884, 247–258. [Google Scholar] [PubMed]
- Hofman, P. Liquid biopsy for early detection of lung cancer. Curr. Opin. Oncol. 2017, 29, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.; Hofman, V.; Long-Mira, E.; Selva, E.; Vignaud, J.-M.; Padovani, B.; Mouroux, J.; Marquette, C.-H.; Hofman, P. “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS ONE 2014, 9, e111597. [Google Scholar] [CrossRef] [PubMed]
- Leroy, S.; Benzaquen, J.; Mazzetta, A.; Marchand-Adam, S.; Padovani, B.; Israel-Biet, D.; Pison, C.; Chanez, P.; Cadranel, J.; Mazières, J.; et al. Circulating tumour cells as a potential screening tool for lung cancer (the AIR study): Protocol of a prospective multicentre cohort study in France. BMJ Open 2017, 7, e018884. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Guan, G.; Bhagat, A.A. ClearCell® FX, a label-free microfluidics technology for enrichment of viable circulating tumor cells. Cytometry A 2018, 93, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Robinson, P.S.; Wagner, C.; O’Shannessy, D.J. The ParsortixTM Cell Separation System-A versatile liquid biopsy platform. Cytometry A 2018, 93, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Sollier-Christen, E.; Renier, C.; Kaplan, T.; Kfir, E.; Crouse, S.C. VTX-1 Liquid Biopsy System for Fully-Automated and Label-Free Isolation of Circulating Tumor Cells with Automated Enumeration by BioView Platform. Cytometry A 2018, 93, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.C.; Metcalf, R.L.; Trapani, F.; Mohan, S.; Antonello, J.; Abbott, B.; Leong, H.S.; Chester, C.P.E.; Simms, N.; Polanski, R.; et al. Vasculogenic mimicry in small cell lung cancer. Nat. Commun. 2016, 7, 13322. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, G.; Miller, C. Improved PDX and CDX Data Processing-Response. Mol. Cancer Res. 2018, 16, 1814. [Google Scholar] [CrossRef] [PubMed]
- Lallo, A.; Schenk, M.W.; Frese, K.K.; Blackhall, F.; Dive, C. Circulating tumor cells and CDX models as a tool for preclinical drug development. Transl. Lung Cancer Res. 2017, 6, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Lallo, A.; Frese, K.K.; Morrow, C.J.; Sloane, R.; Gulati, S.; Schenk, M.W.; Trapani, F.; Simms, N.; Galvin, M.; Brown, S.; et al. The Combination of the PARP Inhibitor Olaparib and the WEE1 Inhibitor AZD1775 as a New Therapeutic Option for Small Cell Lung Cancer. Clin. Cancer Res. 2018, 24, 5153–5164. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Zhuang, R.; Long, M.; Pavlovic, M.; Kang, Y.; Ilyas, A.; Asghar, W. Circulating tumor cell isolation, culture, and downstream molecular analysis. Biotechnol. Adv. 2018, 36, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Makarova, O.V.; Adams, D.L.; Divan, R.; Rosenmann, D.; Zhu, P.; Li, S.; Amstutz, P.; Tang, C.-M. Polymer microfilters with nanostructured surfaces for the culture of circulating cancer cells. Mater. Sci. Eng. C 2016, 66, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, F.; Carloni, S.; Zoli, W.; Ulivi, P.; Gallerani, G.; Fici, P.; Chiadini, E.; Passardi, A.; Frassineti, G.L.; Ragazzini, A.; et al. Detection and recovery of circulating colon cancer cells using a dielectrophoresis-based device: KRAS mutation status in pure CTCs. Cancer Lett. 2013, 335, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.V.; Bingham, C.; Fittipaldi, P.; Austin, L.; Palazzo, J.; Palmer, G.; Alpaugh, K.; Cristofanilli, M. TP53 mutations detected in circulating tumor cells present in the blood of metastatic triple negative breast cancer patients. Breast Cancer Res. 2014, 16, 445. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; Wong, D.J.; Ooi, C.C.; Kurtz, D.M.; Vermesh, O.; Aalipour, A.; Suh, S.; Pian, K.L.; Chabon, J.J.; Lee, S.H.; et al. Molecular profiling of single circulating tumor cells from lung cancer patients. Proc. Natl. Acad. Sci. USA 2016, 113, E8379–E8386. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.T.; Wittner, B.S.; Ligorio, M.; Vincent Jordan, N.; Shah, A.M.; Miyamoto, D.T.; Aceto, N.; Bersani, F.; Brannigan, B.W.; Xega, K.; et al. Single-Cell RNA Sequencing Identifies Extracellular Matrix Gene Expression by Pancreatic Circulating Tumor Cells. Cell Rep. 2014, 8, 1905–1918. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, D.T.; Lee, R.J.; Kalinich, M.; LiCausi, J.; Zheng, Y.; Chen, T.; Milner, J.D.; Emmons, E.; Ho, U.; Broderick, K.; et al. An RNA-based digital circulating tumor cell signature is predictive of drug response and early dissemination in prostate cancer. Cancer Discov. 2018, 8, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Marjanovic, N.D.; Ng, S.R.; Regev, A.; Jacks, T. Abstract A24: Using single-cell RNA-seq approaches to decipher heterogeneity in autochthonous mouse models of small cell lung cancer. In Proceedings of the Fifth AACR-IASLC International Joint Conference: Lung Cancer Translational Science from the Bench to the Clinic, San Diego, CA, USA, 8–11 January 2018; American Association for Cancer Research: Philadelphia, PA, USA, 2018; p. A24. [Google Scholar]
- Hofman, V.J.; Ilie, M.; Hofman, P.M. Detection and characterization of circulating tumor cells in lung cancer: Why and how? Cancer Cytopathol. 2016, 124, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.L.; Alpaugh, R.K.; Tsai, S.; Tang, C.-M.; Stefansson, S. Multi-Phenotypic subtyping of circulating tumor cells using sequential fluorescent quenching and restaining. Sci. Rep. 2016, 6, 33488. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.G.; Zhu, P.; Siddappa, C.M.; Adams, D.L.; Li, S.; Makarova, O.V.; Amstutz, P.; Nunley, R.; Tang, C.-M.; Watson, M.A.; et al. Enrichment and Molecular Analysis of Breast Cancer Disseminated Tumor Cells from Bone Marrow Using Microfiltration. PLoS ONE 2017, 12, e0170761. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-M.; Zhu, P.; Li, S.; Makarova, O.V.; Amstutz, P.T.; Adams, D.L. Filtration and Analysis of Circulating Cancer Associated Cells from the Blood of Cancer Patients. Methods Mol. Biol. 2017, 1572, 511–524. [Google Scholar] [PubMed]
- Tang, C.; Zhu, P.; Li, S.; Makarova, O.V.; Amstutz, P.T.; Adams, D.L. Blood-based biopsies—clinical utility beyond circulating tumor cells. Cytom. Part A 2018, 93, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Milano, A.; Mazzetta, F.; Valente, S.; Ranieri, D.; Leone, L.; Botticelli, A.; Onesti, C.E.; Lauro, S.; Raffa, S.; Torrisi, M.R.; et al. Molecular Detection of EMT Markers in Circulating Tumor Cells from Metastatic Non-Small Cell Lung Cancer Patients: Potential Role in Clinical Practice. Anal. Cell. Pathol. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Q.; Li, H.; Wu, Y.; Feng, J.; Yan, Y. Mesenchymal circulating tumor cells (CTCs) and OCT4 mRNA expression in CTCs for prognosis prediction in patients with non-small-cell lung cancer. Clin. Transl. Oncol. 2017, 19, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, L.; Li, J.; Zheng, J.; Zhang, S.; Zhou, J. Epithelial-mesenchymal transition phenotype of circulating tumor cells is associated with distant metastasis in patients with NSCLC. Mol. Med. Rep. 2018, 19, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-C.; Zhang, X.-F.; Peng, J.; Li, X.-F.; Wang, A.-L.; Bie, Y.-Q.; Shi, L.-H.; Lin, M.-B.; Zhang, X.-F. Survival Mechanisms and Influence Factors of Circulating Tumor Cells. Biomed. Res. Int. 2018, 2018, 6304701. [Google Scholar] [CrossRef] [PubMed]
- Seijo, L.M.; Peled, N.; Ajona, D.; Boeri, M.; Field, J.K.; Sozzi, G.; Pio, R.; Zulueta, J.J.; Spira, A.; Massion, P.P.; et al. Biomarkers in lung cancer screening: Achievements, promises and challenges. J. Thorac. Oncol. 2019, 14, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Di Trapani, M.; Manaresi, N.; Medoro, G. DEPArrayTM system: An automatic image-based sorter for isolation of pure circulating tumor cells. Cytometry A 2018, 93, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Blackhall, F.; Frese, K.K.; Simpson, K.; Kilgour, E.; Brady, G.; Dive, C. Will liquid biopsies improve outcomes for patients with small-cell lung cancer? Lancet Oncol. 2018, 19, e470–e481. [Google Scholar] [CrossRef]
- Kapeleris, J.; Kulasinghe, A.; Warkiani, M.E.; Vela, I.; Kenny, L.; O’Byrne, K.; Punyadeera, C. The Prognostic Role of Circulating Tumor Cells (CTCs) in Lung Cancer. Front. Oncol. 2018, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Kulasinghe, A.; Kapeleris, J.; Kimberley, R.; Mattarollo, S.R.; Thompson, E.W.; Thiery, J.-P.; Kenny, L.; O’Byrne, K.; Punyadeera, C. The prognostic significance of circulating tumor cells in head and neck and non-small-cell lung cancer. Cancer Med. 2018, 7, 5910–5919. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.R.; Faugeroux, V.; Michiels, S.; Pailler, E.; Facchinetti, F.; Ou, D.; Bluthgen, M.V.; Pannet, C.; Ngo-Camus, M.; Bescher, G.; et al. A prospective examination of circulating tumor cell profiles in non-small-cell lung cancer molecular subgroups. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Morrow, C.J.; Trapani, F.; Metcalf, R.L.; Bertolini, G.; Hodgkinson, C.L.; Khandelwal, G.; Kelly, P.; Galvin, M.; Carter, L.; Simpson, K.L.; et al. Tumourigenic non-small-cell lung cancer mesenchymal circulating tumour cells: A clinical case study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- De Wit, S.; Rossi, E.; Weber, S.; Tamminga, M.; Manicone, M.; Swennenhuis, J.F.; Groothuis-Oudshoorn, C.G.M.; Vidotto, R.; Facchinetti, A.; Zeune, L.L.; et al. Single tube liquid biopsy for advanced non-small cell lung cancer. Int. J. Cancer 2018. [Google Scholar] [CrossRef]
- Rothwell, D.G.; Smith, N.; Morris, D.; Leong, H.S.; Li, Y.; Hollebecque, A.; Ayub, M.; Carter, L.; Antonello, J.; Franklin, L.; et al. Genetic profiling of tumours using both circulating free DNA and circulating tumour cells isolated from the same preserved whole blood sample. Mol. Oncol. 2016, 10, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, V.; Yu, M. Analyzing Circulating Tumor Cells One at a Time. Trends Cell Biol. 2018, 28, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Salvianti, F.; Pazzagli, M.; Pinzani, P. Single circulating tumor cell sequencing as an advanced tool in cancer management. Expert Rev. Mol. Diagn. 2016, 16, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Baldassano, S.N.; Loh, P.-L.; Kording, K.; Litt, B.; Issadore, D. Machine learning to detect signatures of disease in liquid biopsies—A user’s guide. Lab Chip 2018, 18, 395–405. [Google Scholar] [CrossRef] [PubMed]
- La Porta, C.A.M.; Zapperi, S. Explaining the dynamics of tumor aggressiveness: At the crossroads between biology, artificial intelligence and complex systems. Semin. Cancer Biol. 2018, 53, 42–47. [Google Scholar] [CrossRef] [PubMed]
| Study | Histology | Approach | Method | Results | Ref |
|---|---|---|---|---|---|
| Hofman et al. | NSCLC | Analysis of preoperative CTCs to predict relapse in early stage NSCLC patients. | ISET™ (Rarecells, Paris, France) | Circulating non-hematologic cells were detected in 102/208 patients with patients with >50 cells having worse prognosis | [38] |
| Hofman et al. | NSCLC | Assessment of CTCs before radical surgery as prognostic factor. | ISET (Rarecells) and CellSearch™ (Menarini Silicon Biosystems, Bologna, Italy) | CTCs were detected in 69% (144/210) of patients but only in 20% (42/210) of patients with both ISET and CellSearch. Patients where CTCs were detected with both methods had worse prognosis | [33] |
| Carter et al. | SCLC | Assessment of copy number alterations in CTCs to distinguish chemosensitive from chemorefractory patients | CellSearch™ (Menarini Silicon Biosystems) | 31 patients tested. 27–20,815 CTCs per 7.5 mL of blood (median, 836). 83.3% correctly classified cases | [39] |
| Drapkin et al. | SCLC | Generation of CTC-derived Xenografts. | CTC-iChipneg device ‡ | CDX could be obtained with an efficiency of 38% | [40] |
| Tan et al. | NSCLC | Comparison of EML4-ALK FISH in CTCs and tumor tissues | ClearCell FX™ (ClearBridge Biomedics, Singapore, Singapore) | >90% of concordance. More CTCs in EML4-ALK positive patients (3–15/1.88 mL blood) than in negative patients (0–2). | [41] |
| Ilie et al. | NSCLC | Analysis of PD-L1 expression on CTCs and white blood cells compared to tumor tissue. | ISET™ (Rarecells) | PD-L1 in CTCs can be detected at 93% concordance to tumor tissue and 73% in white blood cells | [42] |
| Adams et al. | NSCLC | Sequential analysis of PD-L1 and RAD50 expression in patient undergoing radiotherapy. | CellSieve™ (Creatv MicroTech, Rockville, MD, USA) | CTCs and cancer-associated macrophage-like cells (CAMLs) were detected in up to 100% of 41 patients and presence increased during treatment. RAD50 and PD-L1 expression also increased over time | [43] |
| Chudziak et al. | SCLC | Comparison of Parsortix™ and CellSearch™ devices for clinical evaluation. | Parsortix™ (Angle PLC. Guildford, UK) and CellSearch™ (Menarini Silicon Biosystems) | 1–3780 CTCs per 7.5 mL of blood in CellSearch™ (10/12 samples) and 20–1474 using Parsortix™ (12/12 patients) | [44] |
| Krebs et al. | NSCLC | Comparison of ISET™ with CellSearch™ | ISET (Rarecells) and CellSearch™ (Menarini Silicon Biosystems) | 80% positive patients using ISET™ (0–1045, mean = 71 cells) compared to 23% in CellSearch™ (0–78, mean = 4 cells) | [45] |
| Gorges et al. | NSCLC | Comparison of CellSearch™ with GILUPI CellCollector™ | GILUPI CellCollector™ (GILUPI, Potsdam, Germany) and CellSearch™ (Menarini Silicon Biosystems) | 58% positive patients with GILUPI™ (1–56, median = 5 cells) compared to 27% with CellSearch™ (1–300 cells) | [46] |
| Approaches | Interests | Issues | Ref |
|---|---|---|---|
| CTCs cultured ex vivo |
|
| [47,48,49,50] |
| CDX |
|
| [40,51,52,53] |
| CTC-derived explant |
|
| [54] |
| Single-cell analyses |
|
| [55,56,57,58] |
| Microemboli tumor cells |
|
| [59] |
| CTCs & circulating immune cells interaction |
|
| [17] |
| Cytomorphological assessment |
|
| [42,60,61,62,63,64,65,66,67,68] |
| CTCs quantification at baseline and monitoring |
|
| [69,70,71] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofman, V.; Heeke, S.; Marquette, C.-H.; Ilié, M.; Hofman, P. Circulating Tumor Cell Detection in Lung Cancer: But to What End? Cancers 2019, 11, 262. https://doi.org/10.3390/cancers11020262
Hofman V, Heeke S, Marquette C-H, Ilié M, Hofman P. Circulating Tumor Cell Detection in Lung Cancer: But to What End? Cancers. 2019; 11(2):262. https://doi.org/10.3390/cancers11020262
Chicago/Turabian StyleHofman, Véronique, Simon Heeke, Charles-Hugo Marquette, Marius Ilié, and Paul Hofman. 2019. "Circulating Tumor Cell Detection in Lung Cancer: But to What End?" Cancers 11, no. 2: 262. https://doi.org/10.3390/cancers11020262
APA StyleHofman, V., Heeke, S., Marquette, C.-H., Ilié, M., & Hofman, P. (2019). Circulating Tumor Cell Detection in Lung Cancer: But to What End? Cancers, 11(2), 262. https://doi.org/10.3390/cancers11020262







