Hereditary Pancreatic Cancer: A Retrospective Single-Center Study of 5143 Italian Families with History of BRCA-Related Malignancies
Abstract
:1. Introduction
2. Results
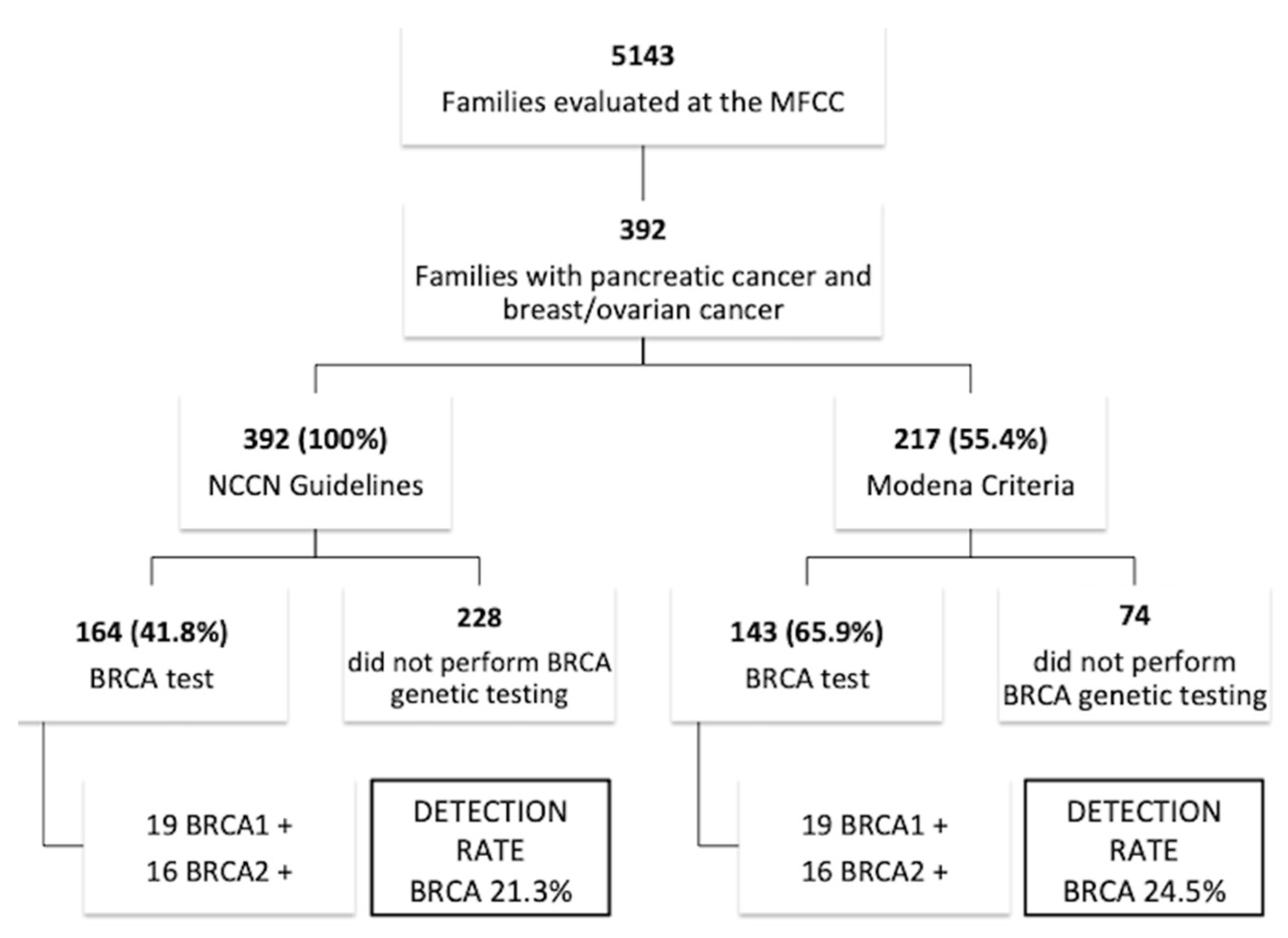
2.1. Family Characteristics
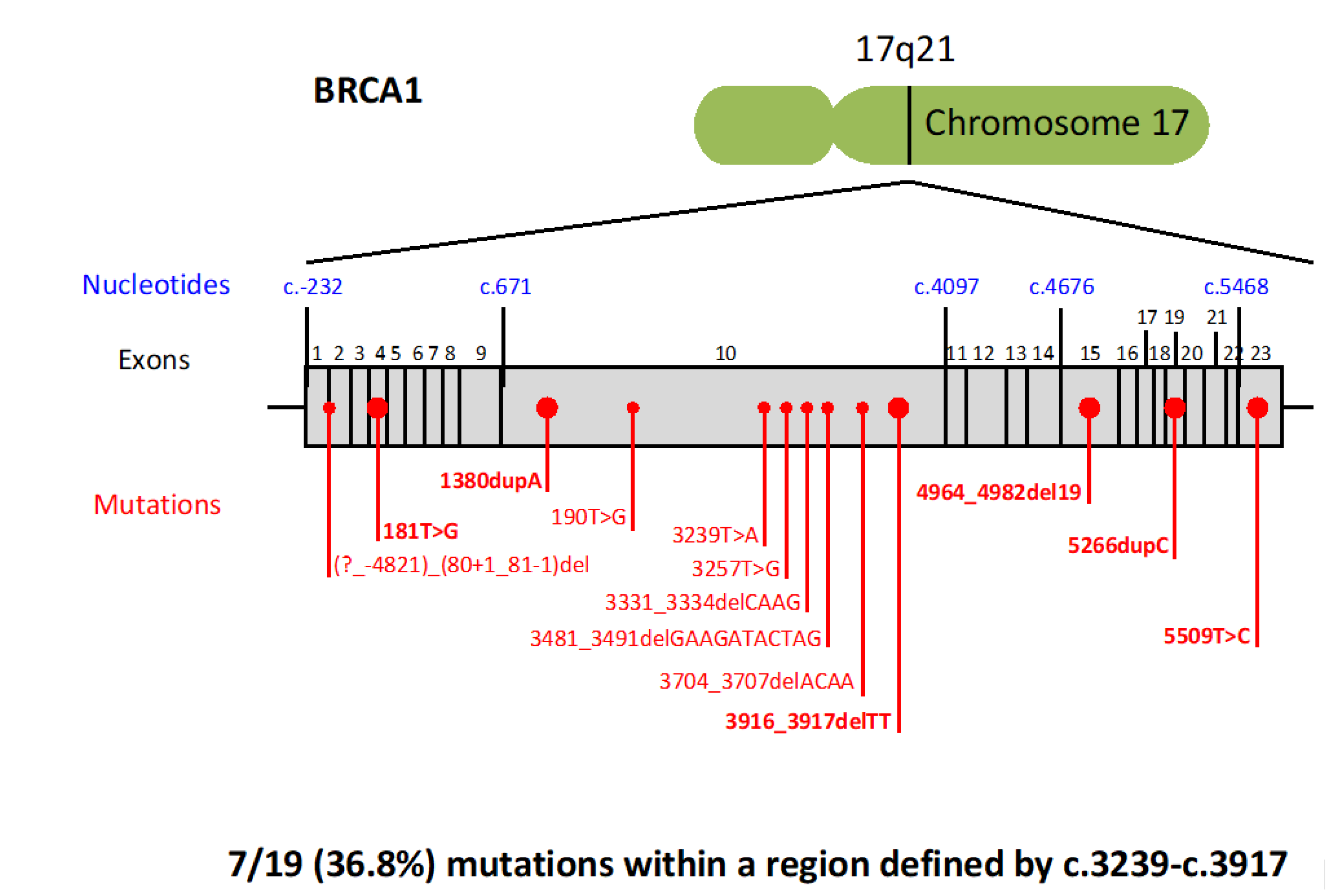
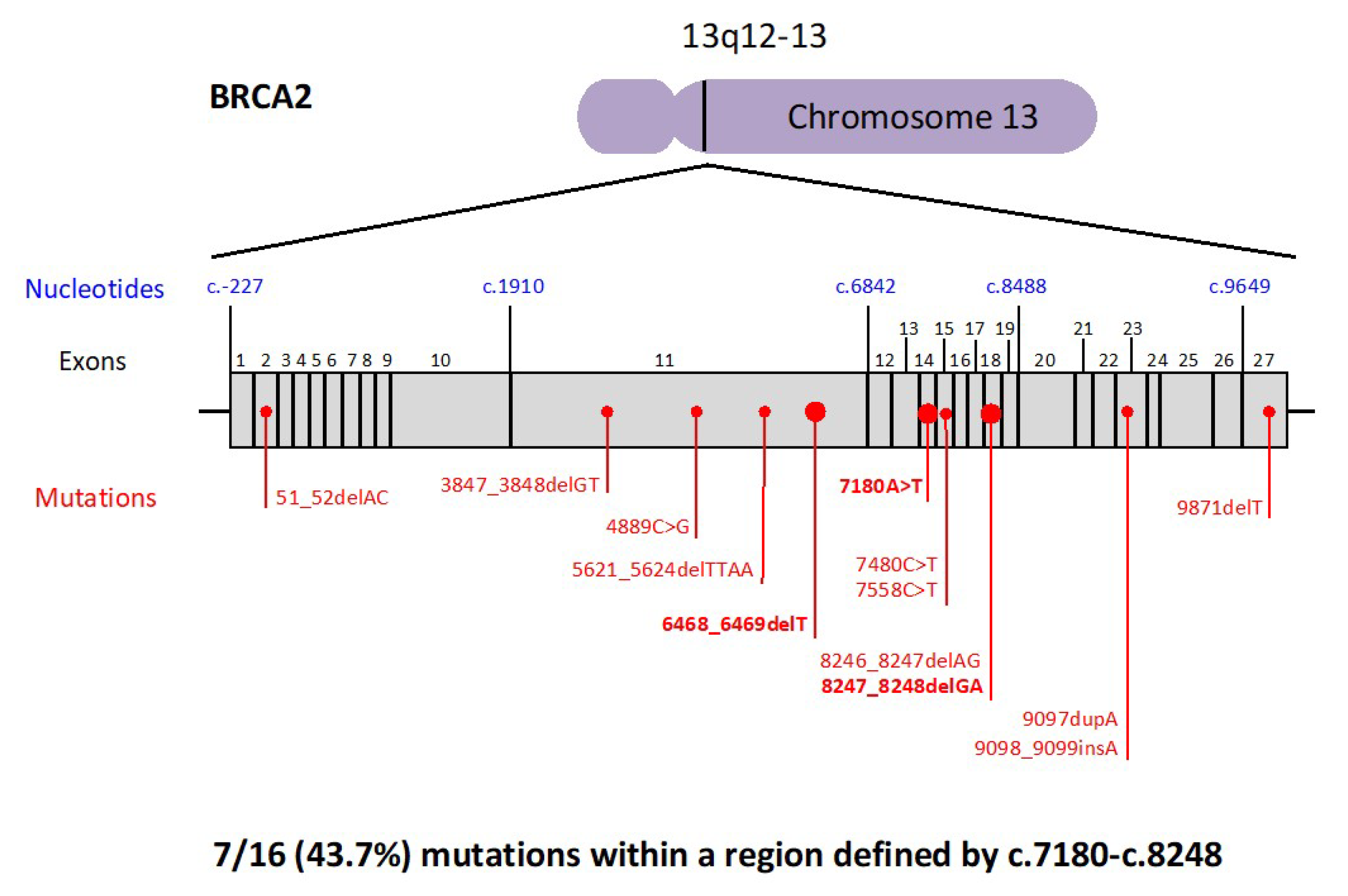
2.2. BRCA Pathogenic Variants and Pancreatic Cancer Risk
2.3. Characteristics of Pancreatic Cancers Developed in BRCA Families
3. Discussion
4. Material and Methods
4.1. Study Population and Design
4.2. BRCA Testing Procedures
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Toss, A.; Cortesi, L. Molecular mechanisms of PARP inhibitors in BRCA-related ovarian cancer. J. Cancer Sci. Ther. 2013, 5, 377–383. [Google Scholar] [CrossRef]
- Lancaster, J.M.; Powell, C.B.; Chen, L.M.; Richardson, D.L.; SGO Clinical Practice Committee, Society of Gynecologic Oncology. Statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol. Oncol. 2015, 136, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Shiovitz, S.; Korde, L.A. Genetics of breast cancer: A topic in evolution. Ann. Oncol. 2015, 26, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Moyer, V.A.; U.S. Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 160, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.; Pharoah, P.D.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, A.; et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Brose, M.S.; Rebbeck, T.R.; Calzone, K.A.; Stopfer, J.E.; Nathanson, K.L.; Weber, B.L. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J. Natl. Cancer Inst. 2002, 94, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J. Natl. Cancer Inst. 1999, 91, 1310–1316. [Google Scholar] [CrossRef]
- Ford, D.; Easton, D.F.; Stratton, M.; Narod, S.; Goldgar, D.; Devilee, P.; Bishop, D.T.; Weber, B.; Lenoir, G.; Chang-Claude, J.; et al. The Breast Cancer Linkage Consortium. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am. J. Hum. Genet. 1998, 62, 676–689. [Google Scholar] [CrossRef]
- BRCA1 and BRCA2 hereditary breast and ovarian cancer. In GeneReviews; Petrucelli, N.; Daly, M.; Pal, T. (Eds.) University of Washington: Seattle, WA, USA, 1993–2018. [Google Scholar]
- Grindedal, E.M.; Heramb, C.; Karsrud, I.; Ariansen, S.L.; Mæhle, L.; Undlien, D.E.; Norum, J.; Schlichting, S. Current guidelines for BRCA testing of breast cancer patients are insufficient to detect all mutation carriers. BMC Cancer 2017, 17, 438. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 2.2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf (accessed on 20 September 2018).
- Federico, M.; Maiorana, A.; Mangone, L.; Turchetti, D.; Canossi, B.; Romagnoli, R.; Silingardi, V. Identification of families with hereditary breast and ovarian cancer for clinical and mammographic surveillance: The Modena Study Group proposal. Breast Cancer Res. Treat. 1999, 55, 213–221. [Google Scholar] [CrossRef]
- Cortesi, L.; Turchetti, D.; Marchi, I.; Fracca, A.; Canossi, B.; Rachele, B.; Silvia, R.; Rita, P.A.; Pietro, T.; Massimo, F. Breast cancer screening in women at increased risk according to different family histories: an update of the Modena Study Group experience. BMC Cancer 2006, 6, 210. [Google Scholar] [CrossRef] [PubMed]
- AIOM Guidelines 2018. Neoplasie Della Mammella. Available online: https://www.aiom.it/linee-guida/linee-guida-aiom-2018-neoplasie-della-mammella/ (accessed on 31 January 2019).
- Cortesi, L.; De Matteis, E.; Toss, A.; Marchi, I.; Medici, V.; Contu, G.; Xholli, A.; Grandi, G.; Cagnacci, A.; Federico, M. Evaluation of Transvaginal Ultrasound plus CA-125, Measurement and Prophylactic Salpingo-Oophorectomy in Women at Different Risk Levels of Ovarian Cancer: The Modena Study Group Cohort Study. Oncology 2017, 93, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Razzaboni, E.; Toss, A.; Cortesi, L.; Marchi, I.; Sebastiani, F.; De Matteis, E.; Federico, M. Acceptability and adherence in a chemoprevention trial among women at increased risk for breast cancer attending the Modena Familial Breast and Ovarian Cancer Center (Italy). Breast J. 2013, 19, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Tyrer, J.; Duffy, S.W.; Cuzick, J. A breast cancer prediction model incorporating familial and personal risk factors. Stat. Med. 2004, 23, 1111–1130. [Google Scholar] [CrossRef]
- Cortesi, L.; Razzaboni, E.; Toss, A.; De Matteis, E.; Marchi, I.; Medici, V.; Tazzioli, G.; Andreotti, A.; De Santis, G.; Pignatti, M.; et al. Rapid genetic counselling and testing in newly diagnosed breast cancer is associated with high rate of risk-reducing mastectomy in BRCA1/2-positive Italian women. Ann. Oncol. 2014, 25, 57–63. [Google Scholar] [CrossRef] [PubMed]
- SEER Cancer Statistics Review 18 2011–2015, National Cancer Institute. Bethesda. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 20 September 2018).
- AIRTUM (2011). I numeri del cancro in Italia 2011. Available online: www.registri-tumori.it/PDF/AIOM2011/I_numeri_del_cancro_2011.pdf (accessed on 20 September 2018).
- AIRTUM (2017). I numeri del cancro in Italia 2017. Available online: www.registri-tumori.it/PDF/AIOM2017/I_numeri_del_cancro_2017.pdf (accessed on 20 September 2018).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Zhen, D.B.; Rabe, K.G.; Gallinger, S.; Syngal, S.; Schwartz, A.G.; Goggins, M.G.; Hruban, R.; Cote, M.L.; McWilliams, R.R.; Roberts, N.J. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: A PACGENE study. Genet. Med. 2015, 17, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.J.; Norris, A.L.; Petersen, G.M.; Bondy, M.L.; Brand, R.; Gallinger, S.; Kurtz, R.C.; Olson, S.H.; Rustgi, A.K.; Schwartz, A.G.; et al. Whole Genome Sequencing Defines the Genetic Heterogeneity of Familial Pancreatic Cancer. Cancer Discov. 2016, 6, 166–175. [Google Scholar] [CrossRef]
- Holter, S.; Borgida, A.; Dodd, A.; Grant, R.; Dhani, N.; Narod, S.; Akbari, M.; Moore, M.; Gallinger, S.; Semotiuk, K.; et al. Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients with Pancreatic Adenocarcinoma. J. Clin. Oncol. 2015, 33, 3124–3129. [Google Scholar] [CrossRef]
- Bartsch, D.K.; Gress, T.M.; Langer, P. Familial pancreatic cancer-current knowledge. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 445. [Google Scholar] [CrossRef]
- Shindo, K.; Yu, J.; Suenaga, M.; Fesharakizadeh, S. Deleterious Germline Mutations in Patients With Apparently Sporadic Pancreatic Adenocarcinoma. J. Clin. Oncol. 2017, 35, 3382–3390. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.; Borazanci, E.; Speare, V.; Dudley, B.; Karloski, E.; Peters, M.L.B.; Stobie, L.; Bahary, N.; Zeh, H.; Zureikat, A.; et al. Prospective study of germline genetic testing in incident cases of pancreatic adenocarcinoma. Cancer 2018, 124, 3520–3527. [Google Scholar] [CrossRef]
- Hu, C.; Hart, S.N.; Polley, E.C.; Gnanaolivu, R.; Shimelis, H.; Lee, K.Y.; Lilyquist, J.; Na, J.; Moore, R.; Antwi, S.O.; et al. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA 2018, 319, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, E.M.; McKernin, S.E.; Brand, R.; Canto, M.; Goggins, M.; Moravek, C.; Nagarajan, A.; Petersen, G.M.; Simeone, D.M.; Yurgelun, M.; et al. Evaluating Susceptibility to Pancreatic Cancer: ASCO Provisional Clinical Opinion. J. Clin. Oncol. 2019, 37, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Vasen, H.; Ibrahim, I.; Ponce, C.G.; Slater, E.P.; Matthäi, E.; Carrato, A.; Earl, J.; Robbers, K.; van Mil, A.M.; Potjer, T. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J. Clin. Oncol. 2016, 34, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Early, D. Pancreatic Cancer Screening. Curr. Treat. Options Gastroenterol. 2017, 15, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, T.R.; Mitra, N.; Wan, F.; Sinilnikova, O.M.; Healey, S.; McGuffog, L.; Mazoyer, S.; Chenevix-Trench, G.; Easton, D.F.; Antoniou, A.C.; et al. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA 2015, 313, 1347–1361. [Google Scholar] [CrossRef]
- Kamel, D.; Gray, C.; Walia, J.S.; Kumar, V. PARP Inhibitor Drugs in the Treatment of Breast, Ovarian, Prostate and Pancreatic Cancers: An Update of Clinical Trials. Curr Drug Targets. 2018, 19, 21–37. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Lee, J.W.; Lowery, M.A.; Capanu, M.; Stadler, Z.K.; Moore, M.J.; Dhani, N.; Kindler, H.; Estrella, H.; Maynard, H.; et al. Phase 1 trial evaluating cisplatin, gemcitabine, and veliparib in 2 patient cohorts: Germline BRCA mutation carriers and wild-type BRCA pancreatic ductal adenocarcinoma. Cancer 2018, 124, 1374–1382. [Google Scholar] [CrossRef]
- Peters, M.L.; Tseng, J.F.; Miksad, R.A. Genetic Testing in Pancreatic Ductal Adenocarcinoma: Implications for Prevention and Treatment. Clin. Ther. 2016, 38, 1622–1635. [Google Scholar] [CrossRef]
- Golan, T.; Kanji, Z.S.; Epelbaum, R.; Katz, M.H.; Devaud, N.; Dagan, E.; Holter, S.; Aderka, D.; Paluch-Shimon, S.; Kaufman, B.; et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br. J. Cancer 2014, 111, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Blair, A.B.; Groot, V.P.; Gemenetzis, G.; Wei, J.; Cameron, J.L.; Weiss, M.J.; Goggins, M.; Wolfgang, C.L.; Yu, J.; He, J. BRCA1/BRCA2 Germline Mutation Carriers and Sporadic Pancreatic Ductal Adenocarcinoma. J. Am. Coll. Surg. 2018, 226, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Doi, M.; Ikari, N.; Yamamoto, M.; Furukawa, T. Mutations in BRCA1, BRCA2, and PALB2, and a panel of 50 cancer-associated genes in pancreatic ductal adenocarcinoma. Sci. Rep. 2018, 8, 8105. [Google Scholar] [CrossRef] [PubMed]
- Copson, I.; Maishman, T.; Tapper, W.; Cutress, R.I.; Greville-Heygate, S.; Altman, D.G.; Eccles, B.; Gerty, S.; Durcan, L.T.; Jones, L.; et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): A prospective cohort study. Lancet Oncol. 2018, 19, 169–180. [Google Scholar] [CrossRef]
- Bolton, K.L.; Chenevix-Trench, G.; Goh, C.; Sadetzki, S.; Ramus, S.J.; Karlan, B.Y.; Lambrechts, D.; Despierre, E.; Barrowdale, D.; McGuffog, L.; et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA 2012, 307, 382–390. [Google Scholar] [CrossRef]
- Artusi, V.; Chiesi, L.; Bernardis, I.; Tenedini, E.; Artuso, L.; Cavallini, G.M.; Percesepe, A.; Marigo, V.; Tagliafico, E. A Next Generation Sequencing amplicon-based strategy to explore inherited Retinal Degeneration complexity. Eur. J. Hum. Gen. 2015, 23, 1. [Google Scholar]
- Tenedini, E.; Artuso, L.; Bernardis, I.; Artusi, V.; Percesepe, A.; De Rosa, L.; Contini, R.; Manfredini, R.; Pellacani, G.; Pagani, J. Amplicon-based next-generation sequencing: An effective approach for the molecular diagnosis of epidermolysis bullosa. Br. J. Dermatol. 2015, 173, 731–738. [Google Scholar] [CrossRef]
| Breast Cancer Diagnosed ≤ 45 Years |
| OC, fallopian tube or primary peritoneal cancer at any age. |
| Male breast cancer. |
| Triple negative BC diagnosed ≤ 60 years. |
| BC diagnosed 46–50 years with a second BC primary at any age. |
| BC diagnosed 46–50 years with ≥ 1 close relative with BC or prostate cancer (GS ≥ 7) or with unknown or limited family history. |
| BC diagnosed at any age with ≥ 1 close relative with BC ≤ 50 years or OC or male BC or metastatic prostate cancer or pancreatic cancer. |
| BC diagnosed at any age with ≥ 2 additional diagnosis of BC at any age in patient and/or in close blood relatives. |
| Personal history of BC or prostate cancer (GS ≥ 7) with Ashkenazi Jewish ancestry. |
| Pancreatic cancer. |
| Metastatic prostate cancer. |
| Prostate cancer (GS ≥ 7) at any age with ≥1 close blood relative with OC at any age or pancreatic cancer or metastatic prostate cancer or BC ≤ 50 years. |
| Prostate cancer (GS ≥ 7) at any age with ≥ 2 close blood relatives with BC or prostate cancer (any grade). |
| BRCA 1/2 pathogenic/likely pathogenic mutation detected by tumor profiling of any tumor type in the absence of germline pathogenic/likely pathogenic variant analysis. |
| Regardless of family history, some individuals with a BRCA-related cancer may benefit from genetic testing to determinate eligibility for targeted treatment. |
| An individual who does not meet the other criteria but with ≥ 1 first- or second-degree blood relative meeting any of the above criteria. The significant limitations of interpreting test results for an unaffected individual should be discussed. |
| BC and OC Diagnosed in The Same Patient |
| OC, fallopian tube or primary peritoneal cancer (excluded mucinous and borderline) at any age. |
| Male Breast Cancer. |
| Triple negative BC diagnosed ≤60 years. |
| BC diagnosed ≤ 35 years. |
| At least 1 BC and at least 1 OC. |
| At least 2 first-degree blood relative with BC, at least one diagnosed ≤ 40 years or bilateral. |
| Healthy individuals with an estimated risk of carrying a BRCA mutation ≥ 40%, calculated with the BRCAPro risk calculator (Version CaGene6) |
| Overall Study Population (392 patients) | BRCA1 Families (19 patients) | BRCA2 families (16 patients) | General Population (Cancer Registries) | |
|---|---|---|---|---|
| Age at Pancreatic Cancer Diagnosis | 65.8 (20–94) (31 unknown) | 65.2 (46–85) (3 unknown) | 66.3 (49–80) (1 unknown) | 70 [18] |
| one-year OS | 42% (59 unknown) | 42.8% (5 unknown) | 61.5% (3 unknown) | 23% [19] |
| 5-year OS | 6.6% (59 unknown) | 7.1% (5 unknown) | 0% (3 unknown) | 8.1% [20] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toss, A.; Venturelli, M.; Molinaro, E.; Pipitone, S.; Barbieri, E.; Marchi, I.; Tenedini, E.; Artuso, L.; Castellano, S.; Marino, M.; et al. Hereditary Pancreatic Cancer: A Retrospective Single-Center Study of 5143 Italian Families with History of BRCA-Related Malignancies. Cancers 2019, 11, 193. https://doi.org/10.3390/cancers11020193
Toss A, Venturelli M, Molinaro E, Pipitone S, Barbieri E, Marchi I, Tenedini E, Artuso L, Castellano S, Marino M, et al. Hereditary Pancreatic Cancer: A Retrospective Single-Center Study of 5143 Italian Families with History of BRCA-Related Malignancies. Cancers. 2019; 11(2):193. https://doi.org/10.3390/cancers11020193
Chicago/Turabian StyleToss, Angela, Marta Venturelli, Eleonora Molinaro, Stefania Pipitone, Elena Barbieri, Isabella Marchi, Elena Tenedini, Lucia Artuso, Sara Castellano, Marco Marino, and et al. 2019. "Hereditary Pancreatic Cancer: A Retrospective Single-Center Study of 5143 Italian Families with History of BRCA-Related Malignancies" Cancers 11, no. 2: 193. https://doi.org/10.3390/cancers11020193
APA StyleToss, A., Venturelli, M., Molinaro, E., Pipitone, S., Barbieri, E., Marchi, I., Tenedini, E., Artuso, L., Castellano, S., Marino, M., Tagliafico, E., Razzaboni, E., De Matteis, E., Cascinu, S., & Cortesi, L. (2019). Hereditary Pancreatic Cancer: A Retrospective Single-Center Study of 5143 Italian Families with History of BRCA-Related Malignancies. Cancers, 11(2), 193. https://doi.org/10.3390/cancers11020193





