Systematic Review of the Potential of MicroRNAs in Diffuse Large B Cell Lymphoma
Abstract
1. Introduction
2. Results
2.1. Tumor Tissue miRNAs as Biomarkers for Diagnosis in DLBCL
2.2. Tumor Tissue miRNAs as Biomarkers for DLBCL Subtype Classification
2.3. Tumor Tissue miRNAs as Biomarkers for Prediction of Treatment Response in DLBCL
2.4. Tumor Tissue miRNAs as Biomarkers for DLBCL Prognosis
2.5. Pathway Enrichment Analysis
3. Discussion
4. Materials and Methods
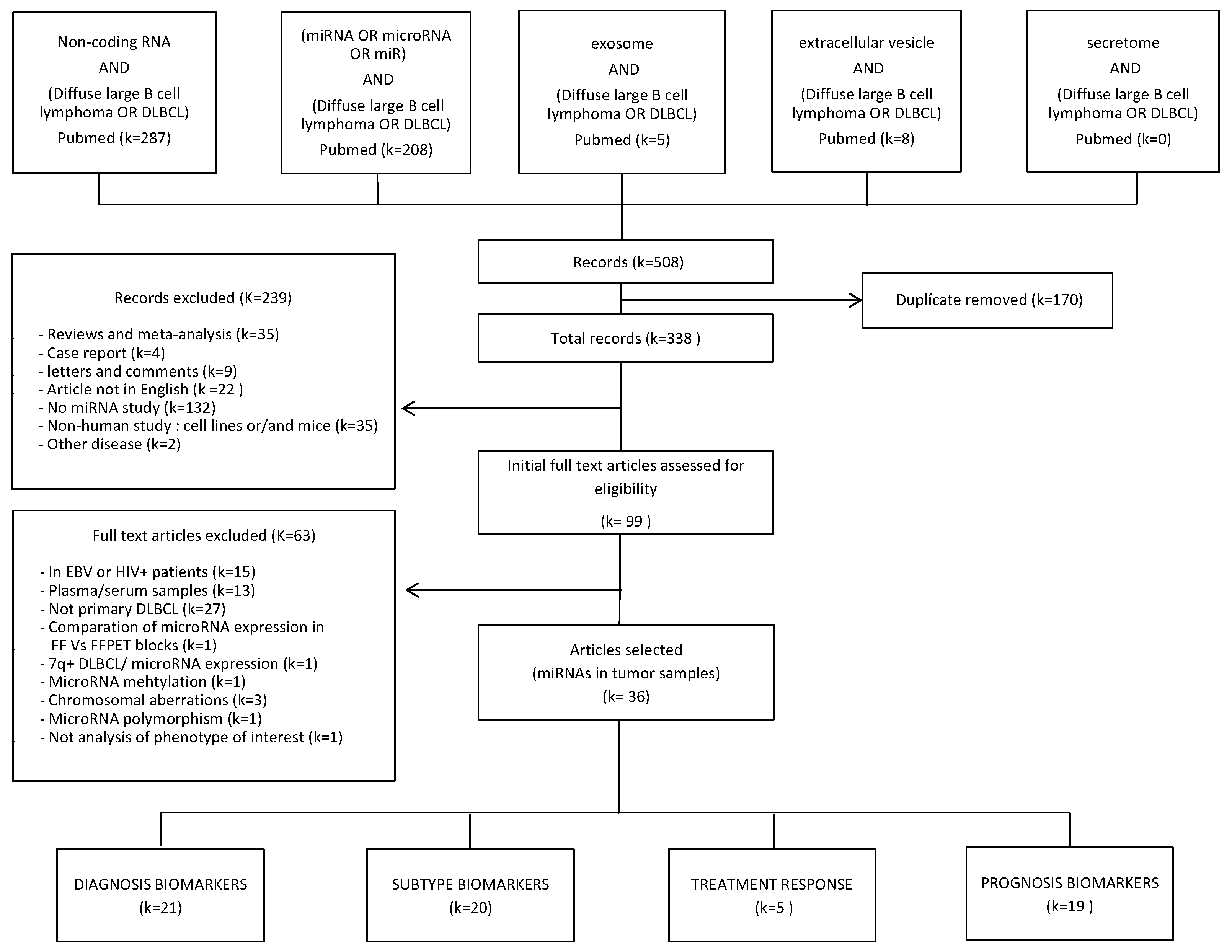
4.1. Systematic Review
4.1.1. Search Strategy
4.1.2. Inclusion and exclusion criteria
4.1.3. Data Extraction
4.2. Data Analysis
4.2.1. Target Genes Selection
4.2.2. Pathway Enrichment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Grubor, V.; Love, C.L.; Banerjee, A.; Richards, K.L.; Mieczkowski, P.A.; Dunphy, C.; Choi, W.; Au, W.Y.; Srivastava, G.; et al. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc. Natl. Acad. Sci. USA 2013, 110, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Coiffier, B.; Thieblemont, C.; Van Den Neste, E.; Lepeu, G.; Plantier, I.; Castaigne, S.; Lefort, S.; Marit, G.; Macro, M.; Sebban, C.; et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 2010, 116, 2040–2045. [Google Scholar] [CrossRef] [PubMed]
- Raut, L.S.; Chakrabarti, P.P. Management of relapsed-refractory diffuse large B cell lymphoma. South Asian J. Cancer 2014, 3, 66–70. [Google Scholar] [CrossRef] [PubMed]
- The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N. Engl. J. Med. 1993, 329, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Berry, B.; Chhanabhai, M.; Fitzgerald, C.; Gill, K.; Hoskins, P.; Klasa, R.; Savage, K.J.; Shenkier, T.; Sutherland, J.; et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 2007, 109, 1857–1861. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, A.; Wright, G.; Chan, W.C.; Connors, J.M.; Campo, E.; Fisher, R.I.; Gascoyne, R.D.; Muller-Hermelink, H.K.; Smeland, E.B.; Giltnane, J.M.; et al. The Use of Molecular Profiling to Predict Survival after Chemotherapy for Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2002, 346, 1937–1947. [Google Scholar] [CrossRef]
- Lopez-Santillan, M.; Larrabeiti-Etxebarria, A.; Arzuaga-Mendez, J.; Lopez-Lopez, E.; Garcia-Orad, A. Circulating miRNAs as biomarkers in diffuse large B-cell lymphoma: A systematic review. Oncotarget 2018, 9, 22850–22861. [Google Scholar] [CrossRef]
- Gutierrez-Camino, A.; Umerez, M.; Santos, B.; Martin-Guerrero, I.; García de Andoin, N.; Sastre, A.; Navajas, A.; Astigarraga, I.; Garcia-Orad, A. Pharmacoepigenetics in childhood acute lymphoblastic leukemia: Involvement of miRNA polymorphisms in hepatotoxicity. Epigenomics 2018, 10, 409–417. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hahne, J.C.; Valeri, N. Non-Coding RNAs and Resistance to Anticancer Drugs in Gastrointestinal Tumors. Front. Oncol. 2018, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Bica-Pop, C.; Cojocneanu-Petric, R.; Magdo, L.; Raduly, L.; Gulei, D.; Berindan-Neagoe, I. Overview upon miR-21 in lung cancer: Focus on NSCLC. Cell. Mol. Life Sci. 2018, 75, 3539–3551. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network; Linehan, W.M.; Spellman, P.T.; Ricketts, C.J.; Creighton, C.J.; Fei, S.S.; Davis, C.; Wheeler, D.A.; Murray, B.A.; Schmidt, L.; et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 374, 135–145. [Google Scholar] [PubMed]
- Zhang, X.-L.; Pan, S.-H.; Yan, J.-J.; Xu, G. The prognostic value of microRNA-183 in human cancers: A meta-analysis. Medicine 2018, 97, e11213. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-D.; Li, X.-M.; Gu, J.-W.; Sun, X.-C. MiR-155 regulates lymphoma cell proliferation and apoptosis through targeting SOCS3/JAK-STAT3 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5153–5159. [Google Scholar] [PubMed]
- Jia, Y.J.; Liu, Z.B.; Wang, W.G.; Sun, C.B.; Wei, P.; Yang, Y.L.; You, M.J.; Yu, B.H.; Li, X.Q.; Zhou, X.Y. HDAC6 regulates microRNA-27b that suppresses proliferation, promotes apoptosis and target MET in diffuse large B-cell lymphoma. Leukemia 2017, 32, 703–711. [Google Scholar] [CrossRef]
- Liu, K.; Du, J.; Ruan, L. MicroRNA-21 regulates the viability and apoptosis of diffuse large B-cell lymphoma cells by upregulating B cell lymphoma-2. Exp. Ther. Med. 2017, 14, 4489–4496. [Google Scholar] [CrossRef]
- Wang, W.; Yang, C.; Han, X.; Wang, R.; Huang, Y.; Zi, Y.; Li, J. MicroRNA-23a expression in paraffin-embedded specimen correlates with overall survival of diffuse large B-cell lymphoma. Med. Oncol. 2014, 31, 919. [Google Scholar] [CrossRef]
- Wu, P.Y.; Zhang, X.D.; Zhu, J.; Guo, X.Y.; Wang, J.F. Low expression of microRNA-146b-5p and microRNA-320d predicts poor outcome of large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone. Hum. Pathol. 2014, 45, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Tamaddon, G.; Geramizadeh, B.; Karimi, M.H.; Mowla, S.J.; Abroun, S. miR-4284 and miR-4484 as Putative Biomarkers for Diffuse Large B-Cell Lymphoma. Iran. J. Med. Sci. 2016, 41, 334–339. [Google Scholar] [PubMed]
- Ni, H.; Wang, X.; Liu, H.; Tian, F.; Song, G. Low expression of miRNA-224 predicts poor clinical outcome in diffuse large B-cell lymphoma treated with R-CHOP. Biomarkers 2015, 20, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Huskova, H.; Korecka, K.; Karban, J.; Vargova, J.; Vargova, K.; Dusilkova, N.; Trneny, M.; Stopka, T. Oncogenic microRNA-155 and its target PU.1: An integrative gene expression study in six of the most prevalent lymphomas. Int. J. Hematol. 2015, 102, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Troppan, K.; Wenzl, K.; Pichler, M.; Pursche, B.; Schwarzenbacher, D.; Feichtinger, J.; Thallinger, G.G.; Beham-Schmid, C.; Neumeister, P.; Deutsch, A. miR-199a and miR-497 Are Associated with Better Overall Survival due to Increased Chemosensitivity in Diffuse Large B-Cell Lymphoma Patients. Int. J. Mol. Sci. 2015, 16, 18077–18095. [Google Scholar] [CrossRef] [PubMed]
- Go, H.; Jang, J.-Y.; Kim, P.-J.; Kim, Y.-G.; Nam, S.J.; Paik, J.H.; Kim, T.M.; Heo, D.S.; Kim, C.-W.; Jeon, Y.K. MicroRNA-21 plays an oncogenic role by targeting FOXO1 and activating the PI3K/AKT pathway in diffuse large B-cell lymphoma. Oncotarget 2015, 6, 15035–15049. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.L.; Trinh, D.L.; Scott, D.W.; Chu, A.; Krzywinski, M.; Zhao, Y.; Robertson, A.G.; Mungall, A.J.; Schein, J.; Boyle, M.; et al. Comprehensive miRNA sequence analysis reveals survival differences in diffuse large B-cell lymphoma patients. Genome Biol. 2015, 16, 18. [Google Scholar] [CrossRef]
- Caramuta, S.; Lee, L.; Ozata, D.M.; Akçakaya, P.; Georgii-Hemming, P.; Xie, H.; Amini, R.-M.; Lawrie, C.H.; Enblad, G.; Larsson, C.; et al. Role of microRNAs and microRNA machinery in the pathogenesis of diffuse large B-cell lymphoma. Blood Cancer J. 2013, 3, e152. [Google Scholar] [CrossRef]
- Handal, B.; Enlow, R.; Lara, D.; Bailey, M.; Vega, F.; Hu, P.; Lennon, A. Investigating the Expression of Oncogenic and Tumor Suppressive MicroRNA in DLBCL. J. Assoc. Genet. Technol. 2013, 39, 14–20. [Google Scholar]
- Zhong, H.; Xu, L.; Zhong, J.-H.; Xiao, F.; Liu, Q.; Huang, H.-H.; Chen, F.-Y. Clinical and prognostic significance of miR-155 and miR-146a expression levels in formalin-fixed/paraffin-embedded tissue of patients with diffuse large B-cell lymphoma. Exp. Ther. Med. 2012, 3, 763–770. [Google Scholar] [CrossRef]
- Fassina, A.; Marino, F.; Siri, M.; Zambello, R.; Ventura, L.; Fassan, M.; Simonato, F.; Cappellesso, R. The miR-17-92 microRNA cluster: A novel diagnostic tool in large B-cell malignancies. Lab. Investig. 2012, 92, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Roehle, A.; Hoefig, K.P.; Repsilber, D.; Thorns, C.; Ziepert, M.; Wesche, K.O.; Thiere, M.; Loeffler, M.; Klapper, W.; Pfreundschuh, M.; et al. MicroRNA signatures characterize diffuse large B-cell lymphomas and follicular lymphomas. Br. J. Haematol. 2008, 142, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, C.H.; Soneji, S.; Marafioti, T.; Cooper, C.D.O.; Palazzo, S.; Paterson, J.C.; Cattan, H.; Enver, T.; Mager, R.; Boultwood, J.; et al. Microrna expression distinguishes between germinal center B cell-like and activated B cell-like subtypes of diffuse large B cell lymphoma. Int. J. Cancer 2007, 121, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Eis, P.S.; Tam, W.; Sun, L.; Chadburn, A.; Li, Z.; Gomez, M.F.; Lund, E.; Dahlberg, J.E. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc. Natl. Acad. Sci. USA 2005, 102, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Meng, X.; Liang, H.; Zhang, H.; Liu, X.; Li, L.; Li, W.; Sun, W.; Zhang, H.; Zen, K.; et al. miR-10a inhibits cell proliferation and promotes cell apoptosis by targeting BCL6 in diffuse large B-cell lymphoma. Protein Cell 2016, 7, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shao, Q.; Li, C.; Liu, H.; Li, J.; Wang, Y.; Song, W.; Li, L.; Wang, G.; Shao, Z.; et al. Effects of microRNA-21 on apoptosis by regulating the expression of PTEN in diffuse large B-cell lymphoma. Medicine 2017, 96, e7952. [Google Scholar] [CrossRef] [PubMed]
- Berglund, M.; Hedström, G.; Amini, R.M.; Enblad, G.; Thunberg, U. High expression of microRNA-200c predicts poor clinical outcome in diffuse large B-cell lymphoma. Oncol. Rep. 2012, 29, 720–724. [Google Scholar] [CrossRef]
- Iqbal, J.; Shen, Y.; Huang, X.; Liu, Y.; Wake, L.; Liu, C.; Deffenbacher, K.; Lachel, C.M.; Wang, C.; Rohr, J.; et al. Global microRNA expression profiling uncovers molecular markers for classification and prognosis in aggressive B-cell lymphoma. Blood 2015, 125, 1137–1145. [Google Scholar] [CrossRef]
- Kim, S.-W.; Ramasamy, K.; Bouamar, H.; Lin, A.-P.; Jiang, D.; Aguiar, R.C.T. MicroRNAs miR-125a and miR-125b constitutively activate the NF-κB pathway by targeting the tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20). Proc. Natl. Acad. Sci. USA 2012, 109, 7865–7870. [Google Scholar] [CrossRef]
- Nie, K.; Zhang, T.; Allawi, H.; Gomez, M.; Liu, Y.; Chadburn, A.; Wang, Y.L.; Knowles, D.M.; Tam, W. Epigenetic down-regulation of the tumor suppressor gene PRDM1/Blimp-1 in diffuse large B cell lymphomas: A potential role of the microRNA let-7. Am. J. Pathol. 2010, 177, 1470–1479. [Google Scholar] [CrossRef]
- Lawrie, C.H.; Chi, J.; Taylor, S.; Tramonti, D.; Ballabio, E.; Palazzo, S.; Saunders, N.J.; Pezzella, F.; Boultwood, J.; Wainscoat, J.S.; et al. Expression of microRNAs in diffuse large B cell lymphoma is associated with immunophenotype, survival and transformation from follicular lymphoma. J. Cell. Mol. Med. 2009, 13, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.C.; Ranjbar, B.; Laursen, M.B.; Falgreen, S.; Bilgrau, A.E.; Bødker, J.S.; Jørgensen, L.K.; Primo, M.N.; Schmitz, A.; Ettrup, M.S.; et al. High miR-34a expression improves response to doxorubicin in diffuse large B-cell lymphoma. Exp. Hematol. 2016, 44, 238–246.e2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jima, D.D.; Jacobs, C.; Fischer, R.; Gottwein, E.; Huang, G.; Lugar, P.L.; Lagoo, A.S.; Rizzieri, D.A.; Friedman, D.R.; et al. Patterns of microRNA expression characterize stages of human B-cell differentiation. Blood 2009, 113, 4586–4594. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shen, Y.; Liu, M.; Bi, C.; Jiang, C.; Iqbal, J.; McKeithan, T.W.; Chan, W.C.; Ding, S.-J.; Fu, K. Quantitative proteomics reveals that miR-155 regulates the PI3K-AKT pathway in diffuse large B-cell lymphoma. Am. J. Pathol. 2012, 181, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Montes-Moreno, S.; Martinez, N.; Sanchez-Espiridión, B.; Díaz Uriarte, R.; Rodriguez, M.E.; Saez, A.; Montalbán, C.; Gomez, G.; Pisano, D.G.; García, J.F.; et al. miRNA expression in diffuse large B-cell lymphoma treated with chemoimmunotherapy. Blood 2011, 118, 1034. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zeng, L.; Tang, N.; Tang, Y.; Zhou, B.; Li, F.; Wu, W.; Zeng, X.; Peng, S. MicroRNA-155 Downregulation Promotes Cell Cycle Arrest and Apoptosis in Diffuse Large B-Cell Lymphoma. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2016, 24, 415–427. [Google Scholar] [CrossRef]
- Shepshelovich, D.; Ram, R.; Uziel, O.; Kushnir, M.; Lithwick-Yanai, G.; Hoshen, M.; Feinmesser, M.; Bairey, O.; Lahav, M. MicroRNA signature is indicative of long term prognosis in diffuse large B-cell lymphoma. Leuk. Res. 2015, 39, 632–637. [Google Scholar] [CrossRef]
- Alencar, A.J.; Malumbres, R.; Kozloski, G.A.; Advani, R.; Talreja, N.; Chinichian, S.; Briones, J.; Natkunam, Y.; Sehn, L.H.; Gascoyne, R.D.; et al. MicroRNAs Are Independent Predictors of Outcome in Diffuse Large B-Cell Lymphoma Patients Treated with R-CHOP. Clin. Cancer Res. 2011, 17, 4125–4135. [Google Scholar] [CrossRef]
- Malumbres, R.; Sarosiek, K.A.; Cubedo, E.; Ruiz, J.W.; Jiang, X.; Gascoyne, R.D.; Tibshirani, R.; Lossos, I.S. Differentiation stage–specific expression of microRNAs in B lymphocytes and diffuse large B-cell lymphomas. Blood 2009, 113, 3754–3764. [Google Scholar] [CrossRef]
- Testa, U.; Pelosi, E.; Castelli, G.; Labbaye, C. miR-146 and miR-155: Two Key Modulators of Immune Response and Tumor Development. Noncoding RNA 2017, 3, 22. [Google Scholar] [CrossRef]
- McInnes, N.; Sadlon, T.J.; Brown, C.Y.; Pederson, S.; Beyer, M.; Schultze, J.L.; McColl, S.; Goodall, G.J.; Barry, S.C. FOXP3 and FOXP3-regulated microRNAs suppress SATB1 in breast cancer cells. Oncogene 2011, 31, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Wang, X.-X.; Fu, H.; Tang, Y.-C.; Meng, B.-Q.; Chen, C.-H. Early diagnostic role of PSA combined miR-155 detection in prostate cancer. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1615–1621. [Google Scholar] [PubMed]
- Pedersen, I.M.; Otero, D.; Kao, E.; Miletic, A.V.; Hother, C.; Ralfkiaer, E.; Rickert, R.C.; Gronbaek, K.; David, M. Onco-miR-155 targets SHIP1 to promote TNFalpha-dependent growth of B cell lymphomas. EMBO Mol. Med. 2009, 1, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhang, X.; Min, M.; Zou, L.; Shen, P.; Zhu, Y. The clinical role of microRNA-21 as a promising biomarker in the diagnosis and prognosis of colorectal cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 44893–44909. [Google Scholar] [CrossRef] [PubMed]
- Musilova, K.; Mraz, M. MicroRNAs in B-cell lymphomas: How a complex biology gets more complex. Leukemia 2014, 29, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Stramucci, L.; Pranteda, A.; Bossi, G. Insights of Crosstalk between p53 Protein and the MKK3/MKK6/p38 MAPK Signaling Pathway in Cancer. Cancers 2018, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Anders, P.; Bhende, P.M.; Foote, M.; Dittmer, D.P.; Park, S.I.; Damania, B. Dual inhibition of phosphatidylinositol 3-kinase/mammalian target of rapamycin and mitogen activated protein kinase pathways in non-Hodgkin lymphoma. Leuk. Lymphoma 2015, 56, 263–266. [Google Scholar] [CrossRef]
- Dweep, H.; Gretz, N. miRWalk2.0: A comprehensive atlas of microRNA-target interactions. Nat. Methods 2015, 12, 697. [Google Scholar] [CrossRef]
- Kamburov, A.; Stelzl, U.; Lehrach, H.; Herwig, R. The ConsensusPathDB interaction database: 2013 update. Nucleic Acids Res. 2013, 41, D793–D800. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Fabregat, A.; Sidiropoulos, K.; Garapati, P.; Gillespie, M.; Hausmann, K.; Haw, R.; Jassal, B.; Jupe, S.; Korninger, F.; McKay, S.; et al. The Reactome pathway Knowledgebase. Nucleic Acids Res. 2016, 44, D481–D487. [Google Scholar] [CrossRef]
| Significant miRNAs | Result | n DLBCL | n Control | Sample Source | Method | n miRNAs | Reference |
|---|---|---|---|---|---|---|---|
| miR-155-5p | up | 29 | 32 (RLH) | Tissue | qRT-PCR | 1 | Li et al. 2017 [17] |
| 22 | 6 (NLN) | Biopsie | qRT-PCR | 1 | Huskova et al. 2015 [24] | ||
| 200 | 11 (NT) | FFPE | qRT-PCR | 3 | Go et al. 2015 [26] | ||
| 45 (DC);75 (VC) | 10 (DC);6 (VC) (NLN) | FF and FFPE | qRT-PCR/array | 177 | Caramuta et al. 2013 [28] | ||
| 90 | 31 (RLN) | FFPE | qRT-PCR | 2 | Zhong et al. 2012 [30] | ||
| 58 | 7 (NLN) | FFPE | qRT-PCR | 157 | Roehle et al. 2008 [32] | ||
| 48 | 6 (NBC) | FF and FFPE | qRT-PCR | 3 | Lawrie et al. 2007 [33] | ||
| 23 | 2 | FF | Semi RT-PCR | 1 | Eis et al. 2005 [34] | ||
| 24 | 14 (NLN) | FFPE | array | 3100 probes | Tamaddon et al. 2016 [22] | ||
| NS | 92 | 15 | FF | sequencing | miRNAome | Lim 2015 et al. [27] | |
| 12 | 7 | FFPE | qRT-PCR | 4 | Handal et al. 2013 [29] | ||
| miR-21-5p | up | 55 | 20 (NLN) | FF and FFPE | qRT-PCR | 1 | Liu et al. 2017 [19] |
| 26 | 10 (NLN) | FFPE | qRT-PCR | 1 | Song et al. 2017 [36] | ||
| 200 | 11 (NT) | FFPE | qRT-PCR | 3 | Go et al. 2015 [26] | ||
| 45 (DC);75 (VC) | 10 (DC);6 (VC)(NLN) | FF and FFPE | qRT-PCR/array | 177 | Caramuta et al. 2013 [28] | ||
| 48 | 6 (NBC) | FF and FFPE | qRT-PCR | 3 | Lawrie et al. 2007 [33] | ||
| 24 | 14 (NLN) | FFPE | array | 3100 probes | Tamaddon et al. 2016 [22] | ||
| NS | 92 | 15 | FF | sequencing | miRNAome | Lim et al. 2015 [27] | |
| 58 | 7 (NLN) | FFPE | qRT-PCR | 157 | Roehle et al. 2008 [32] | ||
| miR-150-5p | up | 12 | 7 | FFPE | qRT-PCR | 4 | Handal et al. 2013 [29] |
| down | 45 (DC);75 (VC) | 10 (DC);6 (VC)(NLN) | FF and FFPE | qRT-PCR/array | 177 | Caramuta et al. 2013 [28] | |
| 36 | 5 (NLN) | Tissue | qRT-PCR | 8 | Fassina et al. 2012 [31] | ||
| 58 | 7 (NLN) | FFPE | qRT-PCR | 157 | Roehle et al. 2008 [32] | ||
| 5 | 4 (RLH) | Tissue | nanostring | 800 | Jia et al. 2017 [18] | ||
| NS | 92 | 15 | FF | sequencing | miRNAome | Lim et al. 2015 [27] | |
| miR-146b-5p | down | 106 | 30 (RLH) | FFPE | qRT-PCR | 939 | Wu et al. 2014 [21] |
| NS | 45 (DC);75 (VC) | 10 (DC);6 (VC)(NLN) | FF and FFPE | qRT-PCR/array | 177 | Caramuta et al. 2013 [28] | |
| up | 92 | 15 | FF | sequencing | miRNAome | Lim et al. 2015 [27] | |
| 24 | 14 (NLN) | FFPE | array | 3100 probes | Tamaddon et al. 2016 [22] | ||
| miR-146a-5p | 90 | 31 (RLN) | FFPE | qRT-PCR | 2 | Zhong et al. 2012 [30] | |
| 24 | 14 (NLN) | FFPE | array | 3100 probes | Tamaddon et al. 2016 [22] | ||
| NS | 45 (DC);75 (VC) | 10 (DC);6 (VC)(NLN) | FF and FFPE | qRT-PCR/array | 177 | Caramuta et al. 2013 [28] | |
| 92 | 15 | FF | sequencing | miRNAome | Lim et al. 2015 [27] |
| Significant miRNAs | Result | n GCB | n ABC | Sample Source | Method | n miRNAs | Reference |
|---|---|---|---|---|---|---|---|
| miR-155-5p | Down GCB | 53 | 95 | FFPE | qRT-PCR | 8 | Go et al. 2015 [26] |
| 32 | 27 | FFPE | qRT-PCR/array | 377 | Iqbal et al. 2015 [38] | ||
| 20 | 34 | FF and FFPE | qRT-PCR/array | 177 | Caramuta et al. 2013 [28] | ||
| 36 | 31 | FF | qRT-PCR | 1 | Huang et al. 2012 [44] | ||
| 21 | 69 | FFPE | qRT-PCR | 2 | Zhong et al. 2012 [30] | ||
| 32 | 28 | FFPE | Array | 464 | Lawrie et al. 2009 [41] | ||
| 16 | 18 | FF and FFPE | qRT-PCR | 3 | Lawrie et al. 2007 [33] | ||
| 4 | 19 | FF | Semiq. RT-PCR | 1 | Eis et al. 2005 [34] | ||
| 41 | 30 | FF | sequencing | miRNAome | Lim et al. 2015 [27] | ||
| NA | 25 | 25 | FFPE | qRT-PCR | 157 | Roehle et al. 2008 [32] | |
| miR-221-3p | Down GCB | 11 | 18 | FFPE | qRT-PCR/array | 470 | Montes-Moreno et al. 2011 [45] |
| 32 | 28 | FFPE | Array | 464 | Lawrie et al. 2009 [41] | ||
| 16 | 18 | FF and FFPE | qRT-PCR | 3 | Lawrie et al. 2007 [33] | ||
| 41 | 30 | FF | sequencing | miRNAome | Lim et al. 2015 [27] | ||
| NS | 20 | 20 | Tissue | Array | 113 | Zhang et al. 2009 [43] | |
| 20 | 34 | FF and FFPE | qRT-PCR/array | 177 | Caramuta et al. 2013 [28] | ||
| miR-222-3p | Down GCB | 11 | 18 | FFPE | qRT-PCR/array | 470 | Montes-Moreno et al. 2011 [45] |
| 32 | 28 | FFPE | Array | 464 | Lawrie et al. 2009 [41] | ||
| 41 | 30 | FF | sequencing | miRNAome | Lim et al. 2015 [27] | ||
| NS | 25 | 25 | FFPE | qRT-PCR | 157 | Roehle et al. 2008 [32] | |
| 20 | 20 | Tissue | Array | 113 | Zhang et al. 2009 [43] | ||
| 32 | 27 | FFPE | qRT-PCR/array | 377 | Iqbal et al. 2015 [38] | ||
| 20 | 34 | FF and FFPE | qRT-PCR/array | 177 | Caramuta et al. 2013 [28] | ||
| miR-146a-5p | Down GCB | 20 | 34 | FF and FFPE | qRT-PCR/array | 177 | Caramuta et al. 2013 [28] |
| 21 | 69 | FFPE | qRT-PCR | 2 | Zhong et al. 2012 [30] | ||
| NS | 41 | 30 | FF | sequencing | miRNAome | Lim et al. 2015 [27] | |
| 20 | 20 | Tissue | Array | 113 | Zhang et al. 2009 [43] | ||
| miR-146b-5p | Down GCB | 32 | 28 | FFPE | Array | 464 | Lawrie et al. 2009 [41] |
| NS | 32 | 27 | FFPE | qRT-PCR/array | 377 | Iqbal et al. 2015 [38] | |
| 41 | 30 | FF | sequencing | miRNAome | Lim et al. 2015 [27] | ||
| 20 | 20 | Tissue | Array | 113 | Zhang et al. 2009 [43] | ||
| 47 | 59 | FFPE | qRT-PCR | 2 | Wu et al. 2014 [21] | ||
| 20 | 34 | FF and FFPE | qRT-PCR/array | 177 | Caramuta et al. 2013 [28] | ||
| miR-28-5p | Up GCB | 11 | 18 | FFPE | qRT-PCR/array | 470 | Montes-Moreno et al. 2011 [45] |
| 32 | 27 | FFPE | qRT-PCR/array | 377 | Iqbal et al. 2015 [38] | ||
| 41 | 30 | FF | sequencing | miRNAome | Lim et al. 2015 [27] | ||
| 20 | 20 | Tissue | Array | 113 | Zhang et al. 2009 [43] | ||
| NS | 20 | 34 | FF and FFPE | qRT-PCR/array | 177 | Caramuta et al. 2013 [28] | |
| 32 | 28 | FFPE | Array | 464 | Lawrie et al. 2009 [41] |
| Significant miRNAs | Result | n DLBCL | Sample Source | Method | n miRNAs | Reference |
|---|---|---|---|---|---|---|
| miR-222-3p | Up: ↓OS | 176 | FFPE | qRT-PCR | 11 | Alencar et al. 2011 [48] |
| Up: ↓PFS and OS | 36/240 | FFPE | qRT-PCR/array | 470/9 | Montes-Moreno et al. 2011 [45] | |
| Up: ↓OS and PFS | 106 | FFPE | qRT-PCR | 3 | Malumbres et al. 2009 [49] | |
| NS | 64 | FFPE | Array | 464 | Lawrie et al. 2009 [41] | |
| 92 | FF | sequencing | miRNAome | Lim et al. 2015 [27] | ||
| 58 | Biopsie | qRT-PCR | 157 | Roehle et al. 2008 [32] | ||
| 83 | FFPE | qRT-PCR/array | ±900 | Shepshelovich et al. 2015 [47] | ||
| miR-155-5p | Up: ↓survival | 118 | FF | qRT-PCR | 1 | Zhu et al. 2016 [46] |
| Up: ↓OS | 79 | FFPE | qRT-PCR | 8 | Iqbal et al. 2015 [38] | |
| Down: ↑PFS | 90 | FFPE | qRT-PCR | 2 | Zhong et al. 2012 [30] | |
| NS | 176 | FFPE | qRT-PCR | 11 | Alencar et al. 2017 [48] | |
| 200 | FFPE | qRT-PCR | 3 | Go et al. 2015 [26] | ||
| 35 | FF and FFPE | qRT-PCR | 3 | Lawrie et al. 2007 [33] | ||
| 64 | FFPE | Array | 464 | Lawrie et al. 2009 [41] | ||
| 92 | FF | sequencing | miRNAome | Lim et al. 2015 [27] | ||
| 106 | FFPE | qRT-PCR | 3 | Malumbres et al. 2009 [49] | ||
| 58 | Biopsie | qRT-PCR | 157 | Roehle et al. 2008 [32] | ||
| 83 | FFPE | qRT-PCR/array | ±900 | Shepshelovich et al. 2015 [47] |
| Phenotype | Pathway Name | miRNA | p-value | FDR | n Target Genes | Coverage |
|---|---|---|---|---|---|---|
| Num. of Genes | ||||||
| Database | ||||||
| DLBCL Diagnosis | MAPK signaling pathway (Homo sapiens) 295 genes (KEGG) | miR-155-5p miR-21-5p | 3.42 × 10−07 | 0.000293 | 73 | 24.7% |
| DLBCL Subtype | Signaling by Receptor Tyrosine Kinases 422 genes (Reactome) | miR-155-5p miR-221-3p | 1.09 ×10−07 | 6.25 × 10−05 | 103 | 24.4% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larrabeiti-Etxebarria, A.; Lopez-Santillan, M.; Santos-Zorrozua, B.; Lopez-Lopez, E.; Garcia-Orad, A. Systematic Review of the Potential of MicroRNAs in Diffuse Large B Cell Lymphoma. Cancers 2019, 11, 144. https://doi.org/10.3390/cancers11020144
Larrabeiti-Etxebarria A, Lopez-Santillan M, Santos-Zorrozua B, Lopez-Lopez E, Garcia-Orad A. Systematic Review of the Potential of MicroRNAs in Diffuse Large B Cell Lymphoma. Cancers. 2019; 11(2):144. https://doi.org/10.3390/cancers11020144
Chicago/Turabian StyleLarrabeiti-Etxebarria, Ane, Maria Lopez-Santillan, Borja Santos-Zorrozua, Elixabet Lopez-Lopez, and Africa Garcia-Orad. 2019. "Systematic Review of the Potential of MicroRNAs in Diffuse Large B Cell Lymphoma" Cancers 11, no. 2: 144. https://doi.org/10.3390/cancers11020144
APA StyleLarrabeiti-Etxebarria, A., Lopez-Santillan, M., Santos-Zorrozua, B., Lopez-Lopez, E., & Garcia-Orad, A. (2019). Systematic Review of the Potential of MicroRNAs in Diffuse Large B Cell Lymphoma. Cancers, 11(2), 144. https://doi.org/10.3390/cancers11020144





