Imaging and Tissue Biomarkers of Choline Metabolism in Diffuse Adult Glioma: 18F-Fluoromethylcholine PET/CT, Magnetic Resonance Spectroscopy, and Choline Kinase α
Abstract
1. Introduction
2. Method
2.1. Patients
2.2. Image Acquisition
2.3. Tumour Biopsies
2.4. Tissue Analyses
2.5. Data Analyses
2.5.1. Venous Sampling
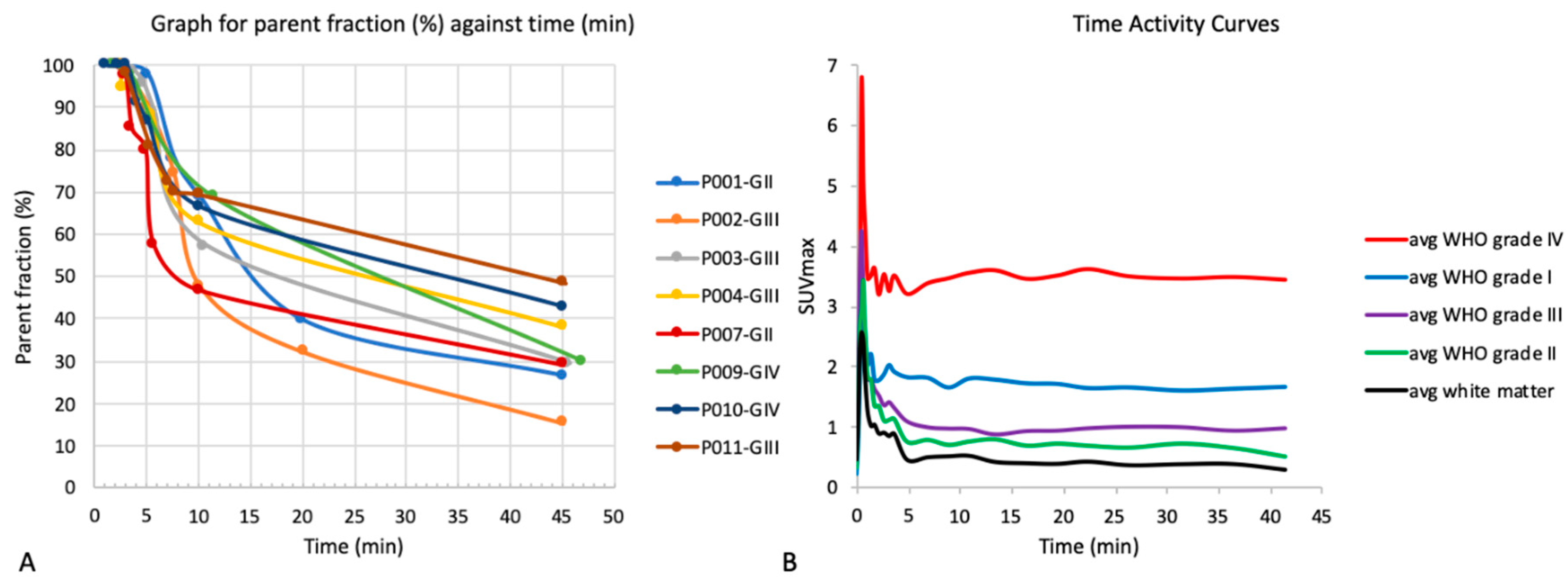
2.5.2. Dynamic Image Analysis
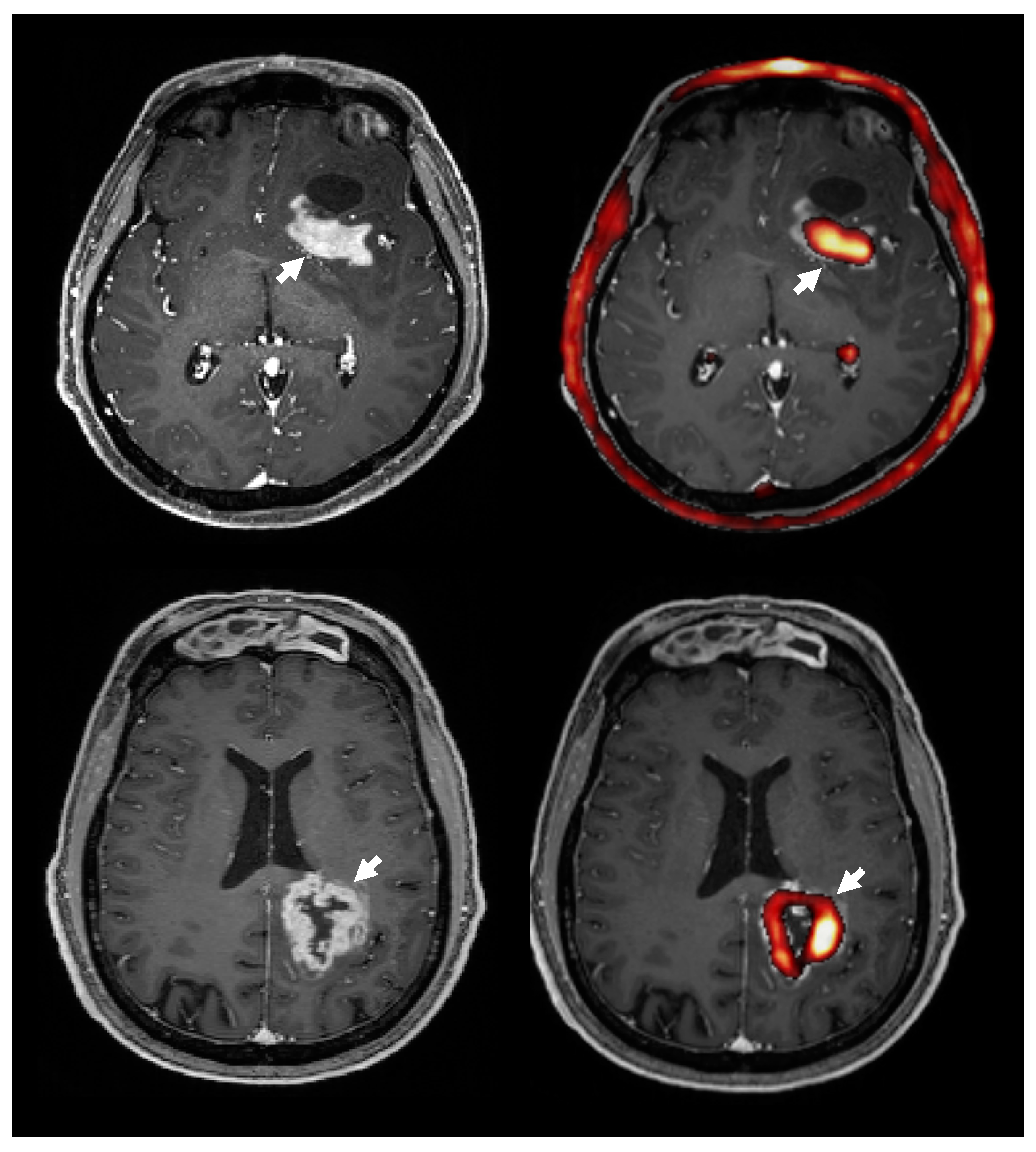
2.5.3. Static Image Analysis
2.5.4. MR Spectroscopy
2.5.5. Imaging vs. Tumour Grade
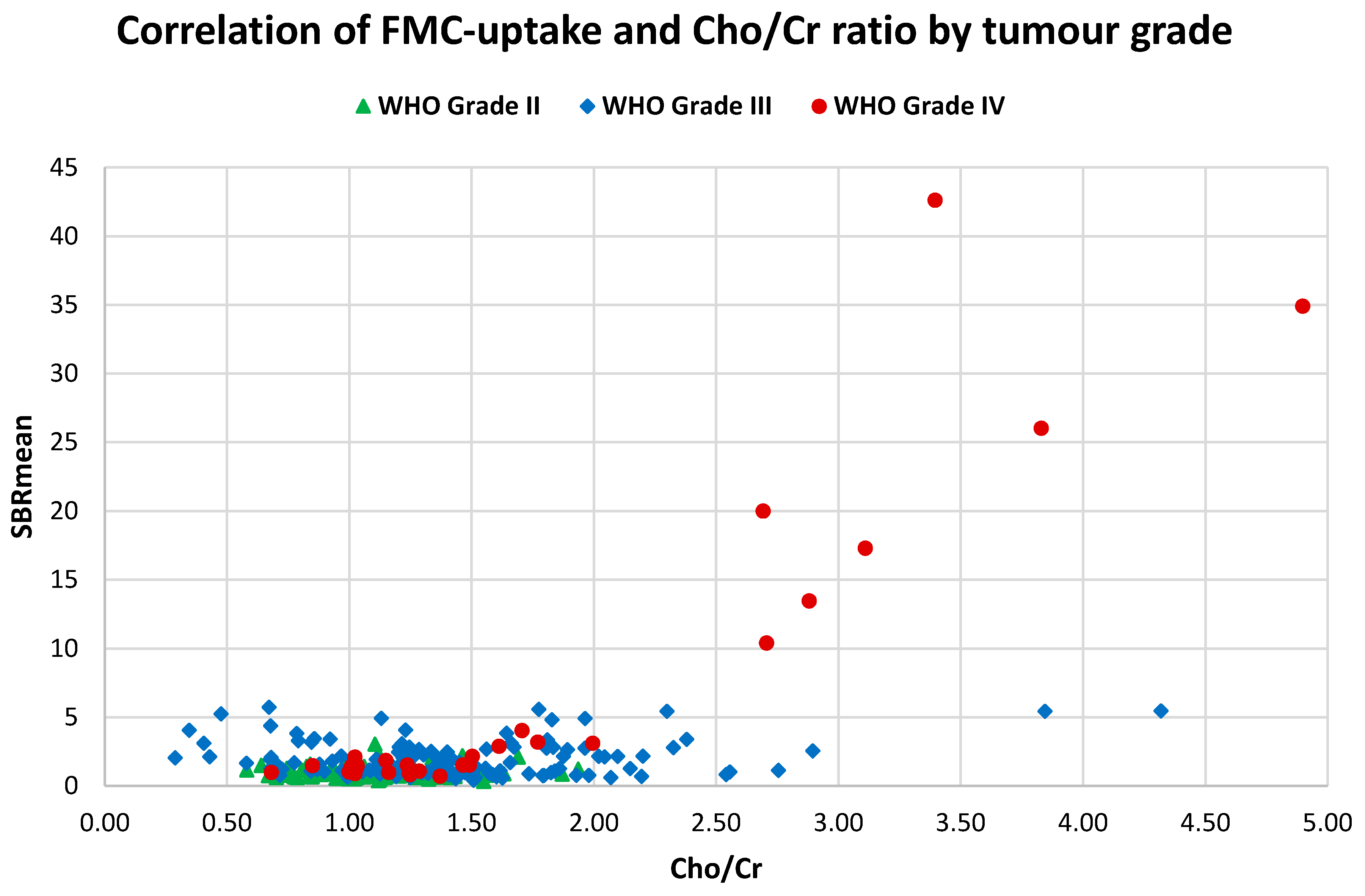
2.5.6. Correlating 18F-FMC PET Uptake and Cho/Cr Ratio
2.5.7. Choline Uptake and Contrast Enhancement on MRI
2.5.8. Correlation between Imaging and Tissue Parameters
3. Results
3.1. Neuropathology
3.2. Metabolite Analysis
3.3. Time Activity Curves
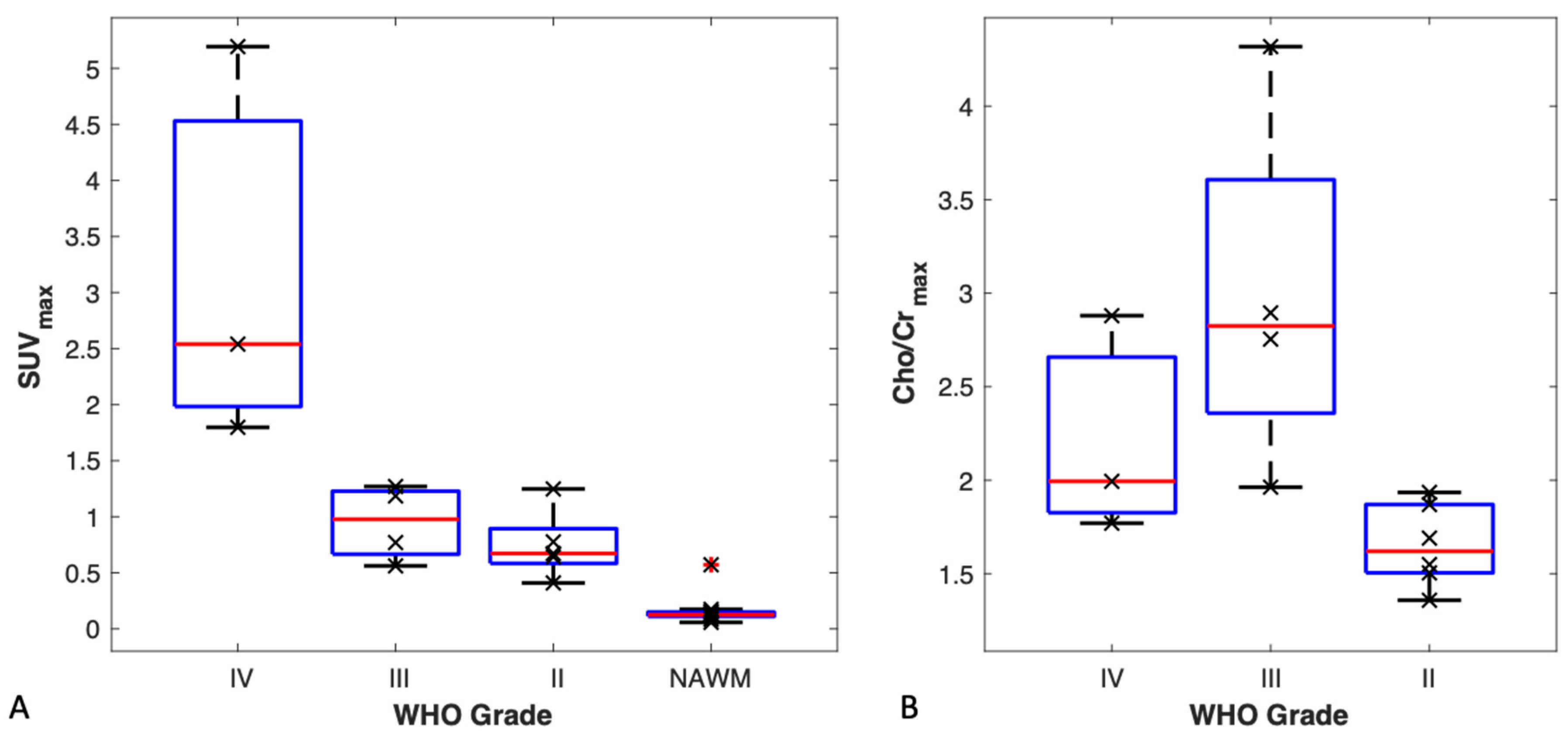
3.4. TBR and SUVmax vs. Tumour Grade
3.5. MR Spectroscopy vs. Tumour Grade
3.6. FMC-PET Uptake vs. MR Spectroscopy
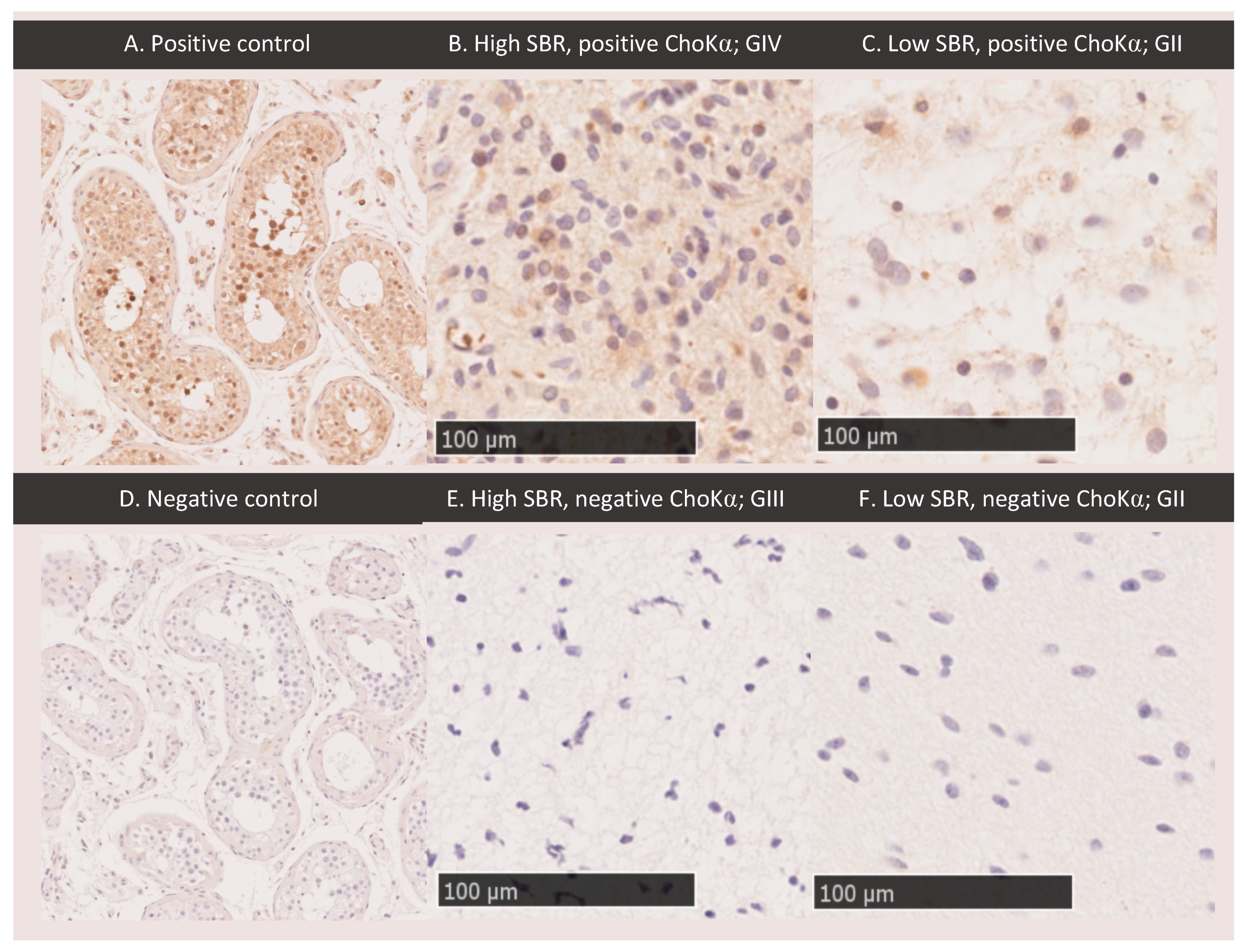
3.7. ChoKα Expression
3.8. Choline PET Uptake vs. Contrast Enhanced T1 MRI
4. Discussion
4.1. 18F-FMC Metabolism
4.2. 18F-FMC Uptake and Cho/Cr in Glioma Grading
4.3. 18F-FMC PET Uptake and Contrast Enhancement
4.4. 18F-FMC PET vs. MRS
4.5. Choline Kinase Alpha Expression
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fidler, M.M.; Gupta, S.; Soerjomataram, I.; Ferlay, J.; Steliarova-Foucher, E.; Bray, F. Cancer incidence and mortality among young adults aged 20-39 years worldwide in 2012: A population-based study. Lancet Oncol. 2017, 18, 1579–1589. [Google Scholar] [CrossRef]
- Ishidate, K. Choline/ethanolamine kinase from mammalian tissues. Biochim. Biophys. Acta 1997, 1348, 70–78. [Google Scholar] [CrossRef]
- Podo, F. Tumour phospholipid metabolism. NMR Biomed. 1999, 12, 413–439. [Google Scholar] [CrossRef]
- Aboagye, E.O.; Bhujwalla, Z.M. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res. 1999, 59, 80–84. [Google Scholar] [PubMed]
- Shimizu, H.; Kumabe, T.; Shirane, R.; Yoshimoto, T. Correlation between choline level measured by proton MR spectroscopy and Ki-67 labeling index in gliomas. Am. J. Neuroradiol. 2000, 21, 659–665. [Google Scholar] [PubMed]
- Matsumura, A.; Isobe, T.; Anno, I.; Takano, S.; Kawamura, H. Correlation between choline and MIB-1 index in human gliomas. A quantitative in proton MR spectroscopy study. J. Clin. Neurosci. 2005, 12, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, T.; Kinoshita, K.; Ono, Y.; Matsumoto, K.; Furuta, T.; Ohmoto, T. Proton magnetic resonance spectroscopy reflects cellular proliferative activity in astrocytomas. Neuroradiology 2000, 42, 333–338. [Google Scholar] [CrossRef]
- Guillevin, R.; Menuel, C.; Duffau, H.; Kujas, M.; Capelle, L.; Aubert, A.; Taillibert, S.; Idbaih, A.; Pallud, J.; Demarco, G.; et al. Proton magnetic resonance spectroscopy predicts proliferative activity in diffuse low-grade gliomas. J. Neurooncol. 2008, 87, 181–187. [Google Scholar] [CrossRef]
- Poptani, H.; Gupta, R.K.; Roy, R.; Pandey, R.; Jain, V.K.; Chhabra, D.K. Characterization of intracranial mass lesions with in vivo proton MR spectroscopy. AJNR Am. J. Neuroradiol. 1995, 16, 1593–1603. [Google Scholar]
- Tedeschi, G.; Lundbom, N.; Raman, R.; Bonavita, S.; Duyn, J.H.; Alger, J.R.; Di Chiro, G. Increased choline signal coinciding with malignant degeneration of cerebral gliomas: A serial proton magnetic resonance spectroscopy imaging study. J. Neurosurg. 1997, 87, 516–524. [Google Scholar] [CrossRef]
- DeGrado, T.R.; Baldwin, S.W.; Wang, S.; Orr, M.D.; Liao, R.P.; Friedman, H.S.; Reiman, R.; Price, D.T.; Coleman, R.E. Synthesis and evaluation of (18)F-labeled choline analogs as oncologic PET tracers. J. Nucl. Med. 2001, 42, 1805–1814. [Google Scholar] [PubMed]
- Hara, T.; Kondo, T.; Hara, T.; Kosaka, N. Use of 18F-choline and 11C-choline as contrast agents in positron emission tomography imaging-guided stereotactic biopsy sampling of gliomas. J. Neurosurg. 2003, 99, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Kwee, S.A.; Ko, J.P.; Jiang, C.S.; Watters, M.R.; Coel, M.N. Solitary brain lesions enhancing at MR imaging: Evaluation with fluorine 18 fluorocholine PET. Radiology 2007, 244, 557–565. [Google Scholar] [CrossRef] [PubMed]
- De Molina, A.R.; Rodríguez-González, A.; Gutiérrez, R.; Martınez-Pineiro, L.; Sánchez, J.J.; Bonilla, F.; Rosell, R.; Lacal, J.C. Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochem. Biophys. Res. Commun. 2002, 296, 580–583. [Google Scholar] [CrossRef]
- Righi, V.; Roda, J.M.; Paz, J.; Mucci, A.; Tugnoli, V.; Rodriguez-Tarduchy, G.; Barrios, L.; Schenetti, L.; Cerdan, S.; García-Martín, M.L. 1H HR-MAS and genomic analysis of human tumor biopsies discriminate between high and low grade astrocytomas. NMR Biomed. 2009, 22, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Glunde, K.; Bhujwalla, Z.M.; Ronen, S.M. Choline metabolism in malignant transformation. Nat. Rev. Cancer 2011, 11, 835–848. [Google Scholar] [CrossRef]
- Grech-Sollars, M.; Vaqas, B.; Thompson, G.; Barwick, T.; Honeyfield, L.; O’Neill, K.; Waldman, A.D. An MRS and PET guided biopsy tool for intra-operative neuro-navigational systems. J. Neurosurg. 2017, 127, 812–818. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 1–18. [Google Scholar] [CrossRef]
- Mertens, K.; Ham, H.; Deblaere, K.; Kalala, J.P.; Van den Broecke, C.; Slaets, D.; De Vos, F.; Goethals, I. Distribution Patterns of 18F-Labelled Fluoromethylcholine in Normal Structures and Tumors of the Head. Clin. Nucl. Med. 2012, 37, e196–e203. [Google Scholar] [CrossRef]
- Wilson, M.; Reynolds, G.; Kauppinen, R.A.; Arvanitis, T.N.; Peet, A.C. A constrained least-squares approach to the automated quantitation of in vivo 1H magnetic resonance spectroscopy data. Magn. Reson. Med. 2011, 65, 1–12. [Google Scholar] [CrossRef]
- Protein Atlas: CKA in Testis. Available online: https://www.proteinatlas.org/ENSG00000110721-CHKA/tissue/testis#img (accessed on 6 December 2019).
- Leyton, J.; Smith, G.; Zhao, Y.; Perumal, M.; Nguyen, Q.D.; Robins, E.; Årstad, E.; Aboagye, E.O. [18F]fluoromethyl-[1,2-2H4]-choline: A novel radiotracer for imaging choline metabolism in tumors by positron emission tomography. Cancer Res. 2009, 69, 7721–7728. [Google Scholar] [CrossRef] [PubMed]
- Herholz, K. Brain Tumors: An Update on Clinical PET Research in Gliomas. Semin. Nucl. Med. 2017, 47, 5–17. [Google Scholar] [CrossRef] [PubMed]
| Subject | Tumour Type, WHO Grade | Molecular | Sample 1 | Sample 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IDH1 | 1p/19q | ATRX | SBR (PET) | Cho/Cr (MRS) | ChoKα | SBR (PET) | Cho/Cr (MRS) | ChoKα | ||
| P001 | Astrocytoma, II | M | R | M | 1.6 | 1.05 | - | 1 | 1.86 | - |
| P002 | Oligodendroglioma, III | M | U | WT | 3.4 | 1.8 | - | 2.2 | 0.42 | - |
| P003 | Oligodendroglioma, III | M | CD | WT | 4.4 | 1.65 | - | 3.6 | 1.68 | weak |
| P004 | Astrocytoma, III | WT | R | M | 0.8 | 0.9 | - | 0.8 | 1.59 | - |
| P005 | Oligodendroglioma, II | M | R | M | 1 | 0.96 | - | 1.1 | 1.23 | weak |
| P006 | Oligodendroglioma, II | M | U | WT | unrecorded location | weak | unrecorded location | weak | ||
| P007 | Astrocytoma, II | surgery at external institution | ||||||||
| P008 | GBM | M | R | M | 5.6 | 1.02 | weak | no second sample | ||
| P009 | GBM | WT | R | M | 9.3 | N/A | weak | 7.3 | 1.77 | weak |
| P010 | GBM | WT | R | U | 43 | 2.88 | strong | 20 | 2.7 | - |
| P011 | Astrocytoma, III | M | R | M | 3.1 | 1.89 | - | 3.9 | 2.91 | weak |
| P012 | Astrocytoma, II | M | R | M | 1.3 | 1.53 | - | 1.2 | 0.75 | weak |
| P013 | DNET, I | excluded from analyses | ||||||||
| P014 | Astrocytoma, II | M | R | M | 2.7 | 0.84 | - | no second sample | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grech-Sollars, M.; Ordidge, K.L.; Vaqas, B.; Davies, C.; Vaja, V.; Honeyfield, L.; Camp, S.; Towey, D.; Mayers, H.; Peterson, D.; et al. Imaging and Tissue Biomarkers of Choline Metabolism in Diffuse Adult Glioma: 18F-Fluoromethylcholine PET/CT, Magnetic Resonance Spectroscopy, and Choline Kinase α. Cancers 2019, 11, 1969. https://doi.org/10.3390/cancers11121969
Grech-Sollars M, Ordidge KL, Vaqas B, Davies C, Vaja V, Honeyfield L, Camp S, Towey D, Mayers H, Peterson D, et al. Imaging and Tissue Biomarkers of Choline Metabolism in Diffuse Adult Glioma: 18F-Fluoromethylcholine PET/CT, Magnetic Resonance Spectroscopy, and Choline Kinase α. Cancers. 2019; 11(12):1969. https://doi.org/10.3390/cancers11121969
Chicago/Turabian StyleGrech-Sollars, Matthew, Katherine L Ordidge, Babar Vaqas, Claire Davies, Vijay Vaja, Lesley Honeyfield, Sophie Camp, David Towey, Helen Mayers, David Peterson, and et al. 2019. "Imaging and Tissue Biomarkers of Choline Metabolism in Diffuse Adult Glioma: 18F-Fluoromethylcholine PET/CT, Magnetic Resonance Spectroscopy, and Choline Kinase α" Cancers 11, no. 12: 1969. https://doi.org/10.3390/cancers11121969
APA StyleGrech-Sollars, M., Ordidge, K. L., Vaqas, B., Davies, C., Vaja, V., Honeyfield, L., Camp, S., Towey, D., Mayers, H., Peterson, D., O’Neill, K., Roncaroli, F., Barwick, T. D., & Waldman, A. D. (2019). Imaging and Tissue Biomarkers of Choline Metabolism in Diffuse Adult Glioma: 18F-Fluoromethylcholine PET/CT, Magnetic Resonance Spectroscopy, and Choline Kinase α. Cancers, 11(12), 1969. https://doi.org/10.3390/cancers11121969







