The Role of the Lymph Node Ratio in Advanced Gastric Cancer After Neoadjuvant Chemotherapy
Abstract
1. Introduction
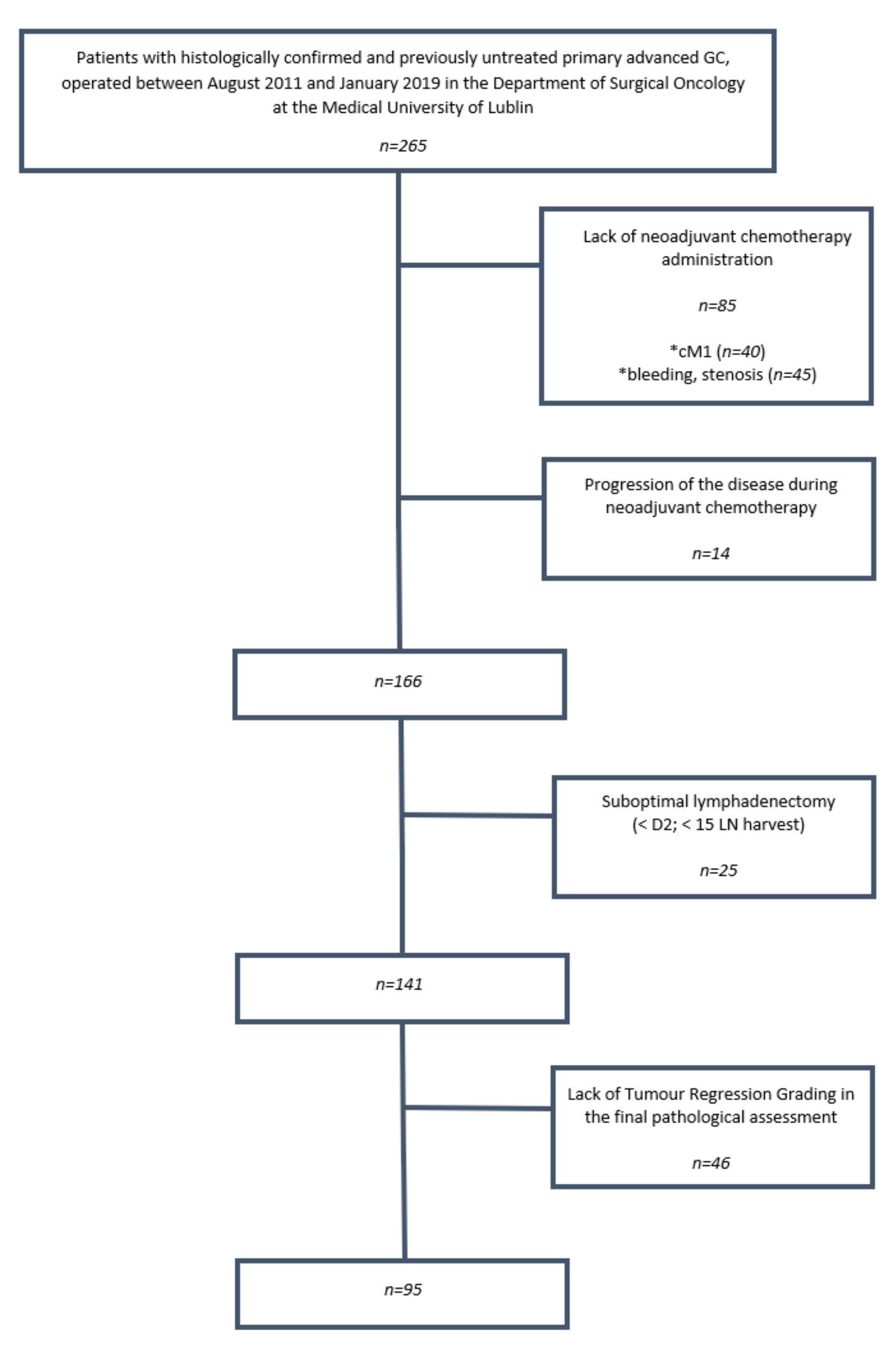
2. Materials and Methods
2.1. Study Subjects
2.2. Preoperative Staging
2.3. Neoadjuvant (Perioperative) Chemotherapy
2.4. Tumor Regression Grading after NAC
2.5. Statistical Analysis
2.6. Follow-Up
3. Results
3.1. ypLNR in Selected Subgroups
3.2. Correlation between ypLNR and Selected Clinicopathological Variables
3.3. Tumor Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Almhanna, K.; Bentrem, D.J.; Chao, J.; Das, P.; Denlinger, C.S.; Fanta, P.; Farjah, F.; Fuchs, C.S.; et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2016, 14, 1286–1312. [Google Scholar] [CrossRef]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D.; Committee, E.G. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef]
- Maruyama, K.; Sasako, M.; Kinoshita, T.; Sano, T.; Katai, H. Surgical treatment for gastric cancer: The Japanese approach. Semin. Oncol. 1996, 23, 360–368. [Google Scholar]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Illerhaus, G.; Martens, U.M.; Stoehlmacher, J.; Schmalenberg, H.; Luley, K.B.; Prasnikar, N.; Egger, M.; et al. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol. 2017, 3, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Fassan, M.; Cunningham, D.; Allum, W.H.; Okines, A.F.; Lampis, A.; Hahne, J.C.; Rugge, M.; Peckitt, C.; Nankivell, M.; et al. Effect of Pathologic Tumor Response and Nodal Status on Survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J. Clin. Oncol. 2016, 34, 2721–2727. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xue, Z.; Zhang, S.; Guo, X.; Zhai, L.; Shang, S.; Zhang, Y.; Lu, H. Integrated analysis of the prognostic role of the lymph node ratio in node-positive gastric cancer: A meta-analysis. Int. J. Surg. 2018, 57, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.R.; Myoteri, D.; Zylstra, J.; Baker, C.R.; Wulaningsih, W.; Van Hemelrijck, M.; Maisey, N.; Allum, W.H.; Smyth, E.; Gossage, J.A.; et al. Lymph node regression and survival following neoadjuvant chemotherapy in oesophageal adenocarcinoma. Br. J. Surg. 2018, 105, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Shibata, T.; Sasako, M.; Sano, T.; Tsuburaya, A.; Iwasaki, Y.; Fukuda, H. Validity of response assessment criteria in neoadjuvant chemotherapy for gastric cancer (JCOG0507-A). Gastric Cancer 2014, 17, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Oyama, K.; Fushida, S.; Kinoshita, J.; Makino, I.; Nakamura, K.; Hayashi, H.; Nakagawara, H.; Tajima, H.; Fujita, H.; Takamura, H.; et al. Efficacy of pre-operative chemotherapy with docetaxel, cisplatin, and S-1 (DCS therapy) and curative resection for gastric cancer with pathologically positive para-aortic lymph nodes. J. Surg. Oncol. 2012, 105, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Bu, Z.D.; Li, Z.Y.; Zhang, L.H.; Wu, X.J.; Wu, A.W.; Shan, F.; Ji, X.; Dong, Q.S.; Ji, J.F. Neoadjuvant chemoradiation therapy for resectable esophago-gastric adenocarcinoma: A meta-analysis of randomized clinical trials. BMC Cancer 2015, 15, 322. [Google Scholar] [CrossRef]
- Roland, C.L.; Yang, A.D.; Katz, M.H.; Chatterjee, D.; Wang, H.; Lin, H.; Vauthey, J.N.; Pisters, P.W.; Varadhachary, G.R.; Wolff, R.A.; et al. Neoadjuvant therapy is associated with a reduced lymph node ratio in patients with potentially resectable pancreatic cancer. Ann. Surg. Oncol. 2015, 22, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Kelly, N.P.; Duff, G.P.; Condon, E.T.; Waldron, D.; Coffey, J.C. Neoadjuvant therapy does not affect lymph node ratio in rectal cancer. Surgeon 2016, 14, 270–273. [Google Scholar] [CrossRef]
- Tsai, J.; Bertoni, D.; Hernandez-Boussard, T.; Telli, M.L.; Wapnir, I.L. Lymph Node Ratio Analysis After Neoadjuvant Chemotherapy is Prognostic in Hormone Receptor-Positive and Triple-Negative Breast Cancer. Ann. Surg. Oncol. 2016, 23, 3310–3316. [Google Scholar] [CrossRef]
- Ronellenfitsch, U.; Schwarzbach, M.; Hofheinz, R.; Kienle, P.; Nowak, K.; Kieser, M.; Slanger, T.E.; Burmeister, B.; Kelsen, D.; Niedzwiecki, D.; et al. Predictors of overall and recurrence-free survival after neoadjuvant chemotherapy for gastroesophageal adenocarcinoma: Pooled analysis of individual patient data (IPD) from randomized controlled trials (RCTs). Eur. J. Surg. Oncol. 2017, 43, 1550–1558. [Google Scholar] [CrossRef]
- Claassen, Y.H.M.; de Steur, W.O.; Hartgrink, H.H.; Dikken, J.L.; van Sandick, J.W.; van Grieken, N.C.T.; Cats, A.; Trip, A.K.; Jansen, E.P.M.; Kranenbarg, W.M.M.; et al. Surgicopathological Quality Control and Protocol Adherence to Lymphadenectomy in the CRITICS Gastric Cancer Trial. Ann. Surg. 2018, 268, 1008–1013. [Google Scholar] [CrossRef]
- Becker, K.; Mueller, J.D.; Schulmacher, C.; Ott, K.; Fink, U.; Busch, R.; Bottcher, K.; Siewert, J.R.; Hofler, H. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003, 98, 1521–1530. [Google Scholar] [CrossRef]
- Tsekrekos, A.; Detlefsen, S.; Riddell, R.; Conner, J.; Mastracci, L.; Sheahan, K.; Shetye, J.; Lundell, L.; Vieth, M. Histopathologic tumor regression grading in patients with gastric carcinoma submitted to neoadjuvant treatment: Results of a Delphi survey. Hum. Pathol. 2019, 84, 26–34. [Google Scholar] [CrossRef]
- Ott, K.; Blank, S.; Ruspi, L.; Bauer, M.; Sisic, L.; Schmidt, T. Prognostic impact of nodal status and therapeutic implications. Transl. Gastroenterol. Hepatol. 2017, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.M.; Teng, R.Y.; Shen, J.G.; Xie, S.D.; Xu, C.Y.; Wang, L.B. Reduced lymph node harvest after neoadjuvant chemotherapy in gastric cancer. J. Int. Med. Res. 2011, 39, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Bu, Z.D.; Li, Z.Y.; Wu, A.W.; Zhang, L.H.; Zhang, J.; Wu, X.J.; Zong, X.L.; Li, S.X.; Shan, F.; et al. Prognostic significance of the total number of harvested lymph nodes for lymph node-negative gastric cancer patients. BMC Cancer 2017, 17, 558. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, J.; Wang, W.; Sun, Z.; Wang, Z.; Zhou, Z.; Xu, H.; Liang, H. Validation of clinical significance of examined lymph node count for accurate prognostic evaluation of gastric cancer for the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system. Chin. J. Cancer Res. 2018, 30, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Li, Y.; Xu, H.; Chen, J.; Wang, B.; Liu, C.; Lu, P.; Alatengbaolide. Lymph node ratio is an independent prognostic factor in gastric cancer after curative resection (R0) regardless of the examined number of lymph nodes. Am. J. Clin. Oncol. 2013, 36, 325–330. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Cao, S.; Li, Y. The evaluation of metastatic lymph node ratio staging system in gastric cancer. Gastric Cancer 2013, 16, 309–317. [Google Scholar] [CrossRef]
- Kutlu, O.C.; Watchell, M.; Dissanaike, S. Metastatic lymph node ratio successfully predicts prognosis in western gastric cancer patients. Surg. Oncol. 2015, 24, 84–88. [Google Scholar] [CrossRef]
- Marchet, A.; Mocellin, S.; Ambrosi, A.; Morgagni, P.; Garcea, D.; Marrelli, D.; Roviello, F.; de Manzoni, G.; Minicozzi, A.; Natalini, G.; et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: Results from an Italian multicentric study in 1853 patients. Ann. Surg. 2007, 245, 543–552. [Google Scholar] [CrossRef]
- Nelen, S.D.; van Steenbergen, L.N.; Dassen, A.E.; van der Wurff, A.A.; Lemmens, V.E.; Bosscha, K. The lymph node ratio as a prognostic factor for gastric cancer. Acta Oncol. 2013, 52, 1751–1759. [Google Scholar] [CrossRef]
- Kong, S.H.; Lee, H.J.; Ahn, H.S.; Kim, J.W.; Kim, W.H.; Lee, K.U.; Yang, H.K. Stage migration effect on survival in gastric cancer surgery with extended lymphadenectomy: The reappraisal of positive lymph node ratio as a proper N-staging. Ann. Surg. 2012, 255, 50–58. [Google Scholar] [CrossRef]
- Li, M.X.; Jin, Z.X.; Zhou, J.G.; Ying, J.M.; Liang, Z.Y.; Mao, X.X.; Bi, X.Y.; Zhao, J.J.; Li, Z.Y.; Huang, Z.; et al. Prognostic Value of Lymph Node Ratio in Patients Receiving Combined Surgical Resection for Gastric Cancer Liver Metastasis: Results from Two National Centers in China. Medicine 2016, 95, e3395. [Google Scholar] [CrossRef]
- Bilici, A.; Selcukbiricik, F.; Seker, M.; Oven, B.B.; Olmez, O.F.; Yildiz, O.; Olmuscelik, O.; Hamdard, J.; Acikgoz, O.; Cakir, A.; et al. Prognostic Significance of Metastatic Lymph Node Ratio in Patients with pN3 Gastric Cancer Who Underwent Curative Gastrectomy. Oncol. Res. Treat. 2019, 42, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xing, X.M.; Ma, L.N.; Liu, L.; Hao, J.; Feng, L.X.; Yu, Z. Metastatic lymph node ratio and Lauren classification are independent prognostic markers for survival rates of patients with gastric cancer. Oncol. Lett. 2018, 15, 8853–8862. [Google Scholar] [CrossRef] [PubMed]
- Jimenez Fonseca, P.; Carmona-Bayonas, A.; Hernandez, R.; Custodio, A.; Cano, J.M.; Lacalle, A.; Echavarria, I.; Macias, I.; Mangas, M.; Visa, L.; et al. Lauren subtypes of advanced gastric cancer influence survival and response to chemotherapy: Real-world data from the AGAMENON National Cancer Registry. Br. J. Cancer 2017, 117, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, G.; Zhang, T.; Zhao, Y.; Zheng, Z. Is pathologic tumor regression grade after neo-adjuvant chemotherapy a promising prognostic indicator for patients with locally advanced gastric cancer? A cohort study evaluating tumor regression response. Cancer Chemother. Pharmacol. 2019, 84, 635–646. [Google Scholar] [CrossRef]
- Blackham, A.U.; Greenleaf, E.; Yamamoto, M.; Hollenbeak, C.; Gusani, N.; Coppola, D.; Pimiento, J.M.; Wong, J. Tumor regression grade in gastric cancer: Predictors and impact on outcome. J. Surg. Oncol. 2016, 114, 434–439. [Google Scholar] [CrossRef]
- Mansour, J.C.; Tang, L.; Shah, M.; Bentrem, D.; Klimstra, D.S.; Gonen, M.; Kelsen, D.P.; Brennan, M.F.; Coit, D.G. Does graded histologic response after neoadjuvant chemotherapy predict survival for completely resected gastric cancer? Ann. Surg. Oncol. 2007, 14, 3412–3418. [Google Scholar] [CrossRef]
- Foo, M.; Leong, T. Adjuvant therapy for gastric cancer: Current and future directions. World J. Gastroenterol. 2014, 20, 13718–13727. [Google Scholar] [CrossRef]
- Yamashita, K.; Hosoda, K.; Ema, A.; Watanabe, M. Lymph node ratio as a novel and simple prognostic factor in advanced gastric cancer. Eur. J. Surg. Oncol. 2016, 42, 1253–1260. [Google Scholar] [CrossRef]
- Ema, A.; Waraya, M.; Yamashita, K.; Kokubo, K.; Kobayashi, H.; Hoshi, K.; Shinkai, Y.; Kawamata, H.; Nakamura, K.; Nishimiya, H.; et al. Identification of EGFR expression status association with metastatic lymph node density (ND) by expression microarray analysis of advanced gastric cancer. Cancer Med. 2015, 4, 90–100. [Google Scholar] [CrossRef]
| Variables | No. of Patients n = 95 (%) |
|---|---|
| Sex | |
| Male | 54 (56.8%) |
| Female | 41 (43.2%) |
| Age (years) | |
| Average | 57.37 |
| Standard deviation (±) | 10.90 |
| Median (min-max) | 57 (31–77) |
| Tumor maximal diameter (cm) | |
| Average | 4.2 |
| Standard deviation (±) | 2.7 |
| Median (min-max) | 3.5 (1–15) |
| Tumor location | |
| Upper 1/3 | 29 (31%) |
| Middle 1/3 | 27 (28%) |
| Distal 1/3 | 39 (41%) |
| Tumor depth | |
| Mucosa | 2 (2%) |
| Submucosa | 7 (7%) |
| Muscularis Propria | 35 (37%) |
| Subserosa | 14 (15%) |
| Serosa | 37 (39%) |
| Lauren histological subtype | |
| Intestinal | 45 (47.4%) |
| Diffuse | 29 (30.5%) |
| Mixed | 21 (22.1%) |
| Grading | |
| G1 | 6 (8%) |
| G2 | 27 (9.3%) |
| G3 | 62 (82.7%) |
| No. of NAC cycles | |
| 1 | 2 (2%) |
| 2 | 8 (8%) |
| 3 | 56 (60%) |
| 4 | 20 (21%) |
| 6 | 6 (6.38%) |
| 8 | 2 (2.13%) |
| NAC regimen | |
| EOX | 83 (87.2%) |
| FLOT | 12 (12.8%) |
| Tumor regression grading (TRG) (Classification of response) | |
| Complete (Grade 1) | 10 (10.5%) |
| Subtotal (Grade 2) | 9 (9.4%) |
| Partial (Grade 3) | 32 (33.7%) |
| Minimal/No regression (Grade 4) | 44 (46.4%) |
| ypT | |
| T0 | 6 (6.3%) |
| T1 | 6 (6.3%) |
| T2 | 20 (21%) |
| T3 | 40 (42%) |
| T4 | 23 (24.6%) |
| ypN | |
| N0 | 42 (44.2%) |
| N1 | 7 (7.4%) |
| N2 | 14 (14.4%) |
| N3 | 32 (34%) |
| ypM | |
| M0 | 76 (80%) |
| M1 | 19 (20%) |
| No. of examined lymph nodes | |
| Mean | 32 |
| Standard deviation (±) | 14 |
| Median (min-max) | 28 (16–81) |
| Variable: | N1 | N2 | N1 + N2 (D2) | |||
|---|---|---|---|---|---|---|
| Me | p | Me | p | Me | p | |
| Sex ¥ | ||||||
| Male | 0.08 | 0.64 | 0.00 | 0.56 | 0.00 | 0.41 |
| Female | 0.00 | 0.00 | 0.07 | |||
| Age (years) ¥ | ||||||
| <57 | 0.08 | 0.54 | 0.00 | 0.83 | 0.09 | 0.40 |
| ≥57 | 0.02 | 0.00 | 0.04 | |||
| Maximal tumor dimension (cm) ¥ | ||||||
| <3.5 | 0.00 | 0.0003 | 0.00 | 0.009 | 0.00 | 0.0005 |
| ≥3.5 cm | 0.38 | 0.00 | 0.31 | |||
| Tumor location # | ||||||
| Upper 1/3 | 0.00 | 0.09 | 0.00 | 0.28 | 0.02 | 0.09 |
| Middle 1/3 | 0.30 | 0.00 | 0.06 | |||
| Lower 1/3 | 0.03 | 0.00 | 0.22 | |||
| Laurén histological subtype # | ||||||
| Intestinal | 0.00 | 0.0005 | 0.00 | 0.001 | 0.00 | 0.0005 |
| Diffuse | 0.45 | 0.00 | 0.30 | |||
| Mixed | 0.30 | 0.14 | 0.22 | |||
| Grading # | ||||||
| G1 | 0.01 | 0.27 | 0.00 | 0.73 | 0.01 | 0.46 |
| G2 | 0.00 | 0.00 | 0.07 | |||
| G3 | 0.10 | 0.00 | 0.09 | |||
| Response to NAC (TRG)¥ | ||||||
| Response to NAC (TRG 1–3) | 0.00 | <0.0001 | 0.00 | 0.0011 | 0.00 | <0.0001 |
| No response to NAC (TRG 4) | 0.40 | 0.07 | 0.30 | |||
| ypT ¥ | ||||||
| ypT0-T3 | 0.00 | 0.001 | 0.00 | 0.06 | 0.00 | 0.002 |
| ypT4 | 0.50 | 0.08 | 0.31 | |||
| ypN ¥ | ||||||
| N0 | 0.00 | <0.0001 | 0.00 | <0.0001 | 0.00 | <0.0001 |
| N1-N3b | 0.48 | 0.24 | 0.36 | |||
| ypM ¥ | ||||||
| M0 | 0.00 | 0.0001 | 0.00 | 0.04 | 0.00 | 0.001 |
| M1 | 0.53 | 0.08 | 0.30 | |||
| Variable (n = 95) | ypLNR | |||||
|---|---|---|---|---|---|---|
| N1 | N2 | N1 + N2 (D2) | ||||
| R (Spearman) | p | R (Spearman) | p | R (Spearman) | p | |
| Age | −0.015 | 0.88 | 0.005 | 0.96 | −0.024 | 0.82 |
| cT | 0.344 | 0.0006 | 0.192 | 0.06 | 0.308 | 0.002 |
| Tumor max. diameter | 0.455 | <0.0001 | 0.246 | 0.01 | 0.420 | <0.0001 |
| Grading | 0.166 | 0.10 | 0.068 | 0.51 | 0.126 | 0.22 |
| Laurén histological subtype | 0.399 | 0.0001 | 0.0179 | 0.8632 | 0.387 | 0.0001 |
| Response to NAC (TRG) | 0.528 | <0.0001 | 0.335 | 0.0009 | 0.503 | <0.0001 |
| No. of NAC cycles | 0.120 | 0.24 | 0.187 | 0.07 | 0.151 | 0.14 |
| ypT | 0.436 | <0.0001 | 0.270 | 0.008 | 0.422 | <0.0001 |
| ypN | 0.903 | <0.0001 | 0.744 | <0.0001 | 0.953 | <0.0001 |
| ypM | 0.405 | <0.0001 | 0.213 | 0.03 | 0.330 | 0.001 |
| Variable | Univariate | Multivariate | |
|---|---|---|---|
| Months | HR (95%CI) p | HR (95%CI) p | |
| Intestinal-type GC | |||
| ypLNR > median (0.00) | 11 | 2.69 (1.09–6.64) | 2.87 (1.02–8.06) * |
| ypLNR ≤ median (0.00) | 37 | 0.0114 | 0.0465 |
| Diffuse-type GC | |||
| ypLNR > median (0.30) | 15 | 2.99 (1.18–7.60) | 2.28 (0.60–8.47) |
| ypLNR ≤ median (0.30) | 39 | 0.0008 | 0.3488 |
| Mixed-type GC | |||
| ypLNR > median (0.22) | 10 | 1.13 (0.41–3.14) | 0.48 (0.07–3.17) |
| ypLNR ≤ median (0.22) | 15 | 0.8150 | 0.4453 |
| Response to NAC (TRG 1–3) | |||
| ypLNR > median (0.00) | 14 | 2.18 (0.97–4.91) | 2.38 (0.94–6.03) |
| ypLNR ≤ median (0.00) | 39 | 0.0162 | 0.0683 |
| No response to NAC (TRG 4) | |||
| ypLNR > median (0.30) | 11 | 2.29 (1.15–4.55) | 2.46 (1.01–5.99) ** |
| ypLNR ≤ median (0.30) | 34 | 0.0097 | 0.0483 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rawicz-Pruszyński, K.; Ciseł, B.; Mlak, R.; Mielko, J.; Skórzewska, M.; Kwietniewska, M.; Pikuła, A.; Gęca, K.; Sędłak, K.; Kurylcio, A.; et al. The Role of the Lymph Node Ratio in Advanced Gastric Cancer After Neoadjuvant Chemotherapy. Cancers 2019, 11, 1914. https://doi.org/10.3390/cancers11121914
Rawicz-Pruszyński K, Ciseł B, Mlak R, Mielko J, Skórzewska M, Kwietniewska M, Pikuła A, Gęca K, Sędłak K, Kurylcio A, et al. The Role of the Lymph Node Ratio in Advanced Gastric Cancer After Neoadjuvant Chemotherapy. Cancers. 2019; 11(12):1914. https://doi.org/10.3390/cancers11121914
Chicago/Turabian StyleRawicz-Pruszyński, Karol, Bogumiła Ciseł, Radosław Mlak, Jerzy Mielko, Magdalena Skórzewska, Magdalena Kwietniewska, Agnieszka Pikuła, Katarzyna Gęca, Katarzyna Sędłak, Andrzej Kurylcio, and et al. 2019. "The Role of the Lymph Node Ratio in Advanced Gastric Cancer After Neoadjuvant Chemotherapy" Cancers 11, no. 12: 1914. https://doi.org/10.3390/cancers11121914
APA StyleRawicz-Pruszyński, K., Ciseł, B., Mlak, R., Mielko, J., Skórzewska, M., Kwietniewska, M., Pikuła, A., Gęca, K., Sędłak, K., Kurylcio, A., & Polkowski, W. P. (2019). The Role of the Lymph Node Ratio in Advanced Gastric Cancer After Neoadjuvant Chemotherapy. Cancers, 11(12), 1914. https://doi.org/10.3390/cancers11121914





