A Novel Anti-PD-L1 Vaccine for Cancer Immunotherapy and Immunoprevention
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Cell Lines
2.2. Immunization of Bone Marrow (BM)-Derived Dendritic Cells (DC) and Mouse Models
2.3. Enzyme-Linked Immunospot (Elispot) Assay
2.4. Antibody and Cytokine ELISA
2.5. PD-1/PD-L1 Inhibition Assay
2.6. CTL Assay
2.7. Statistical Analysis
3. Results
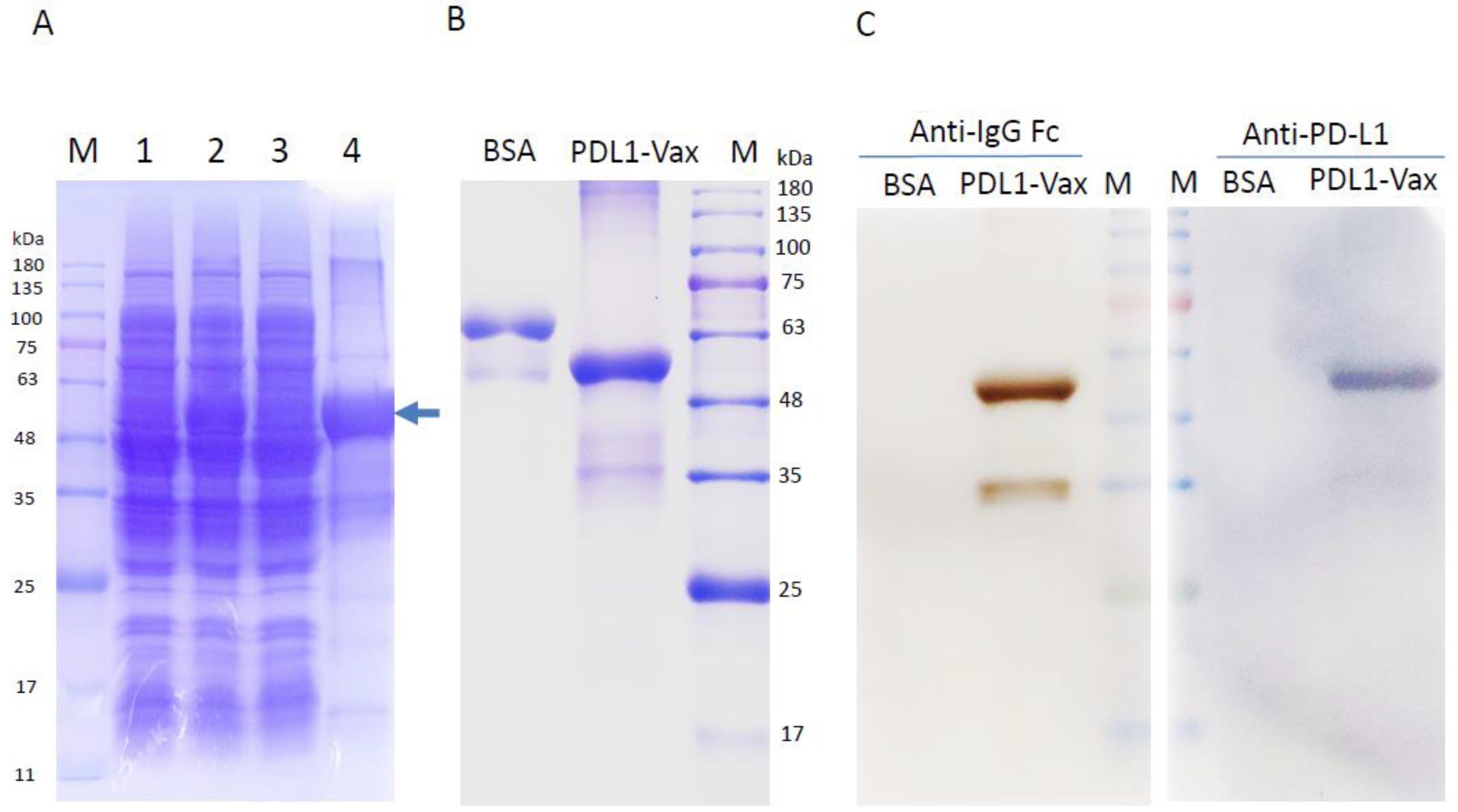
3.1. Production of Recombinant PD-L1 Immunogens (PDL1-Vax)
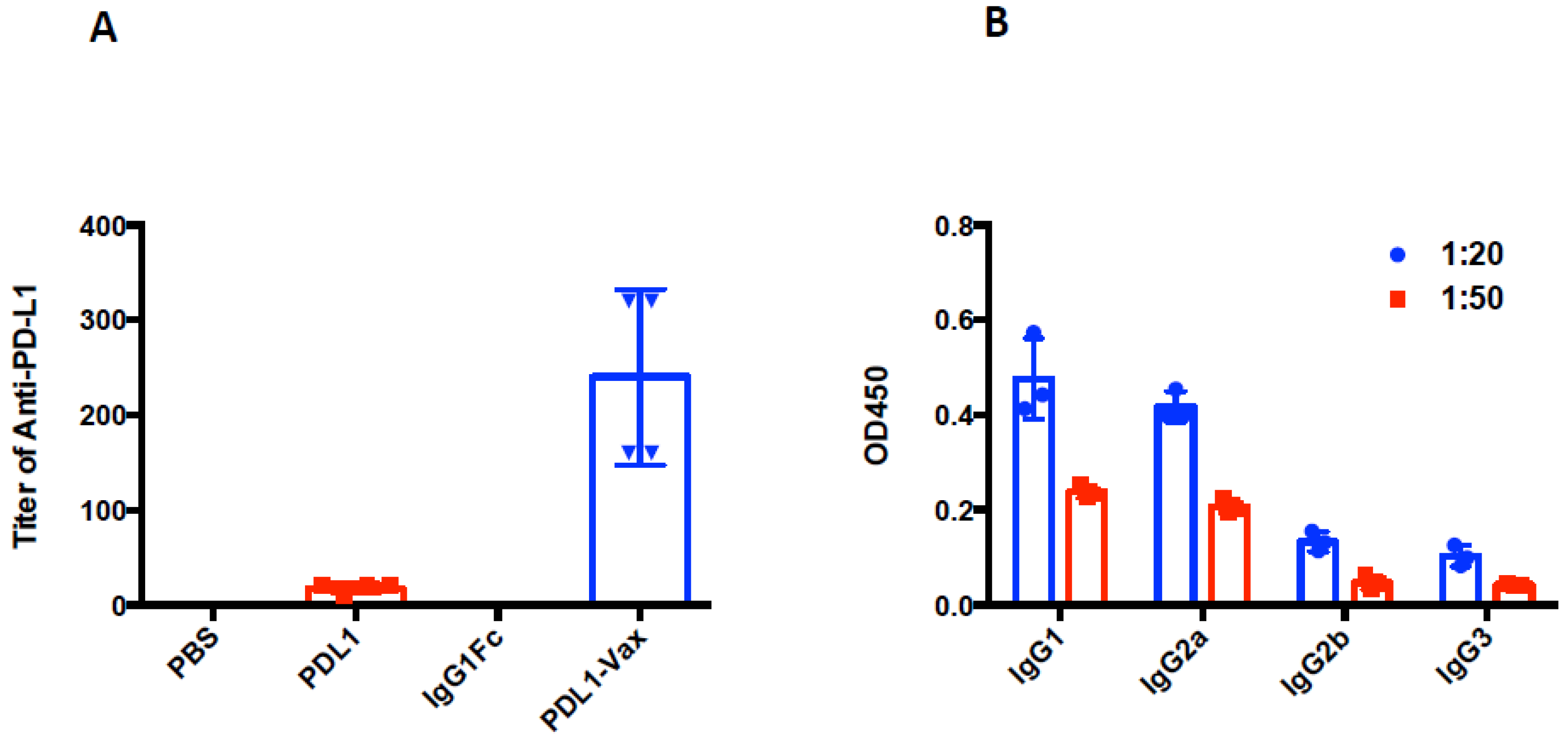
3.2. Induction of Anti-PD-L1 Antibody Response by PDL1-Vax-Loaded DC Vaccination
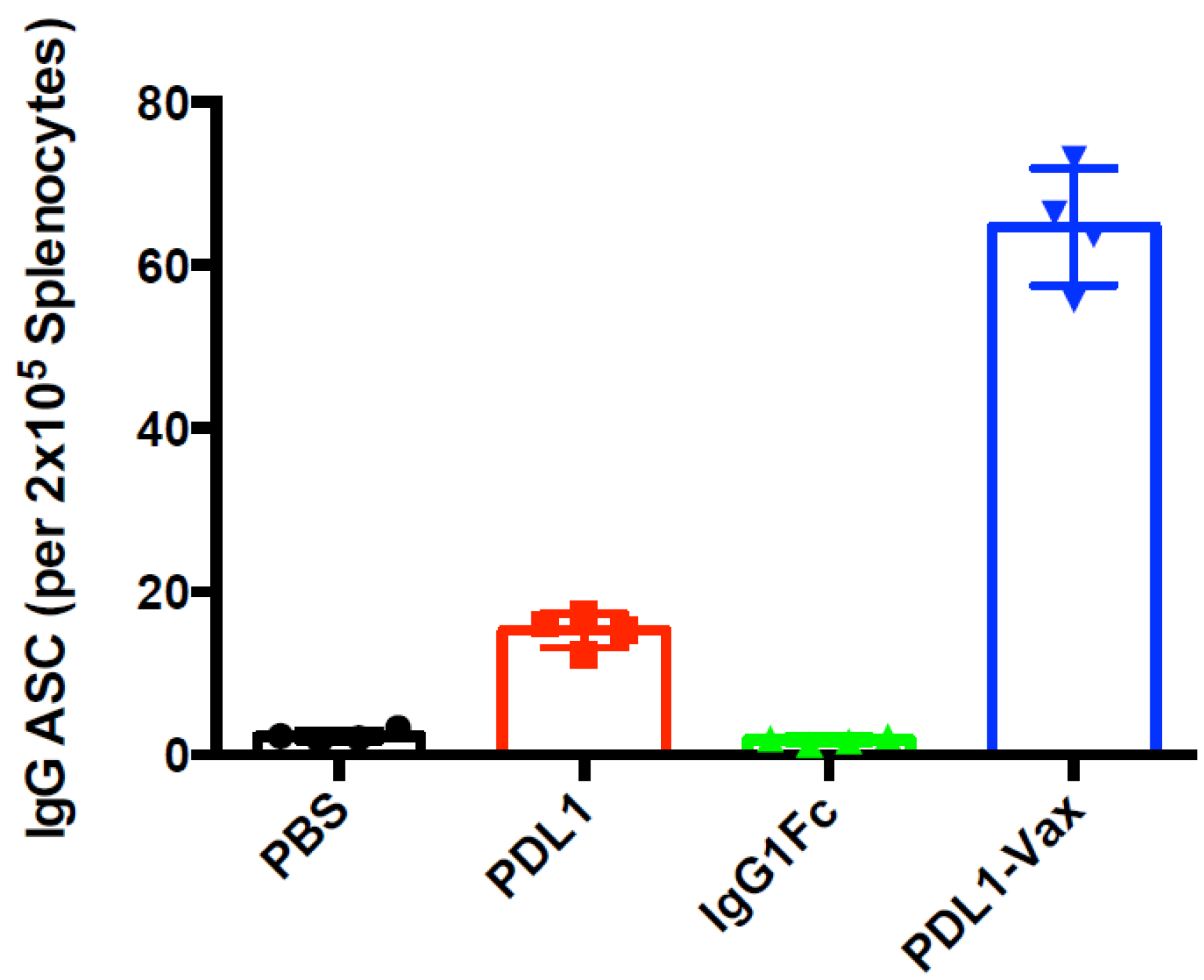
3.3. Induction of PD-L1-Specific T Cell Response by PDL1-Vax DC Vaccination
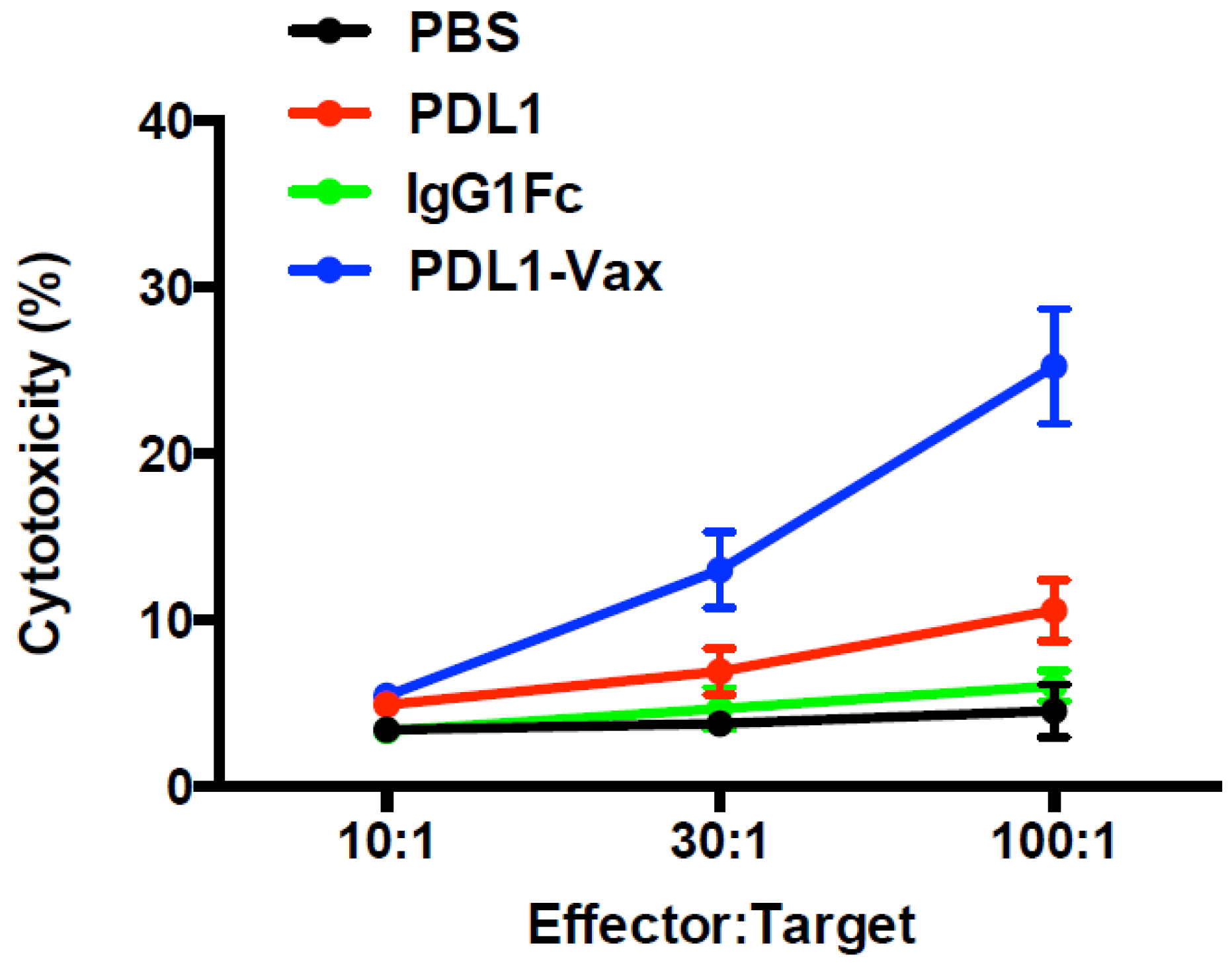
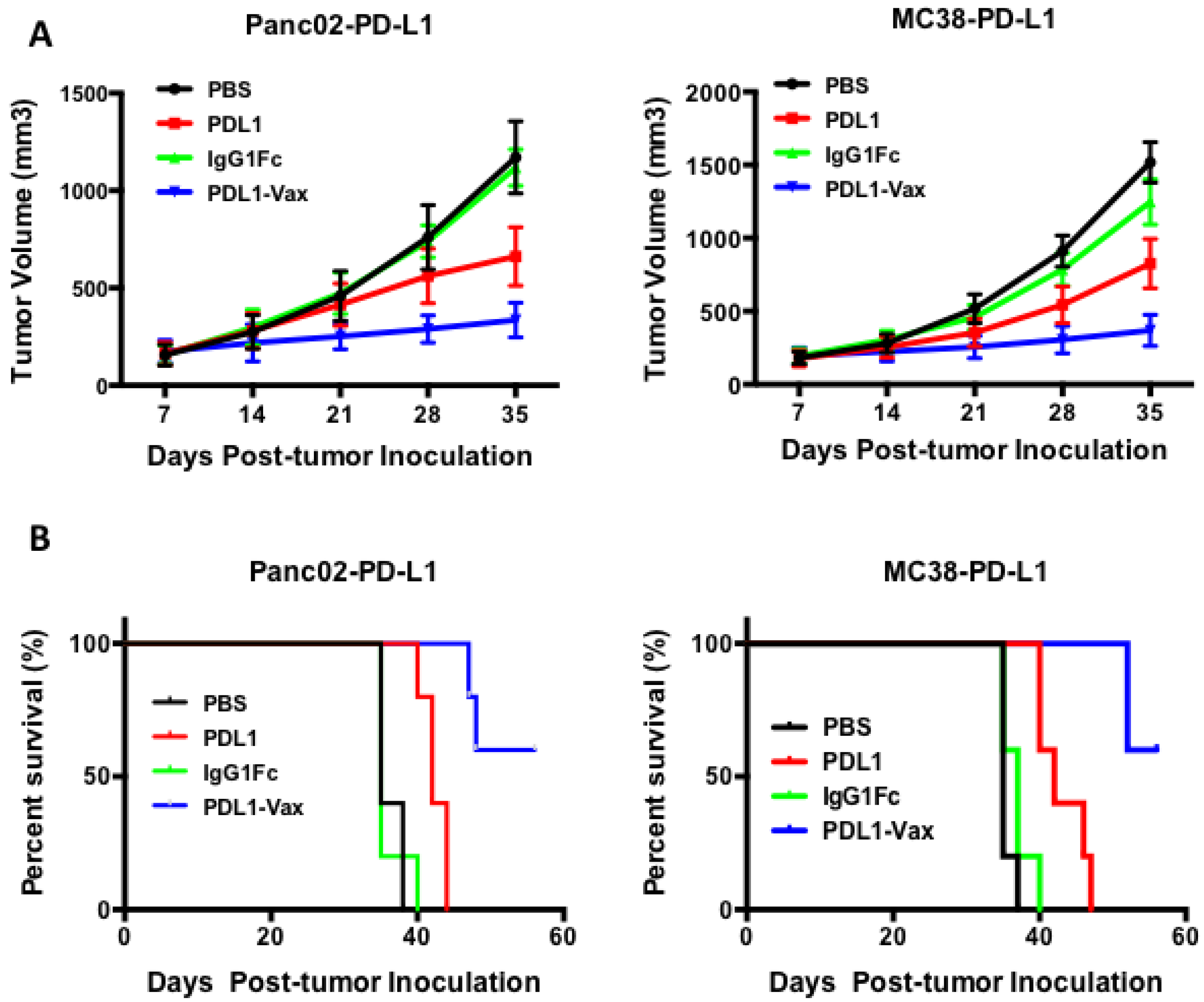
3.4. Inhibition of PD-L1+ Tumor Growth by PDL1-Vax DC Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APCs | Antigen-presenting cells |
| CTL | Cytotoxic T lymphocyte |
| DC | Dendritic cell |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| IFN | Interferon |
| IL | Interleukin |
| mAb | Monoclonal antibody |
| MHC | Major histocompatibility complex |
| PD-L1 | Programmed death ligand 1 |
| TAAs | Tumor-associated antigens |
| Th | T helper |
| TNF | Tumor necrosis factor |
References
- Steinman, R.M.; Banchereau, J. Taking dendritic cells into medicine. Nature 2007, 449, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, C.M.; Weiden, J.; Eggermont, L.J.; Figdor, C.G. Dendritic cells in cancer immunotherapy. Nat. Mater. 2018, 17, 474–475. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Evel-Kabler, K.; Strube, R.; Chen, S.Y. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat. Biotechnol. 2004, 22, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Song, X.T.; Evel-Kabler, K.; Shen, L.; Rollins, L.; Huang, X.F.; Chen, S.Y. A20 is an antigen presentation attenuator, and its inhibition overcomes regulatory T cell-mediated suppression. Nat. Med. 2008, 14, 258–265. [Google Scholar] [CrossRef]
- Evel-Kabler, K.; Song, X.T.; Aldrich, M.; Huang, X.F.; Chen, S.Y. SOCS1 restricts dendritic cells’ ability to break self tolerance and induce antitumor immunity by regulating IL-12 production and signaling. J. Clin. Investig. 2006, 116, 90–100. [Google Scholar] [CrossRef]
- You, Z.; Huang, X.; Hester, J.; Toh, H.C.; Chen, S.Y. Targeting dendritic cells to enhance DNA vaccine potency. Cancer Res. 2001, 61, 3704–3711. [Google Scholar]
- You, Z.; Huang, X.F.; Hester, J.; Rollins, L.; Rooney, C.; Chen, S.Y. Induction of vigorous helper and cytotoxic T cell as well as B cell responses by DCs expressing a modified antigen targeting receptor-mediated internalization pathway. J. Immunol. 2000, 165, 4581–4592. [Google Scholar] [CrossRef]
- Wang, L.; Rollins, L.; Gu, Q.; Chen, S.Y.; Huang, X.F. A Mage3/Heat Shock Protein70 DNA vaccine induces both innate and adaptive immune responses for the antitumor activity. Vaccine 2009, 28, 561–570. [Google Scholar] [CrossRef][Green Version]
- Hong, B.; Ren, W.; Song, X.T.; Evel-Kabler, K.; Chen, S.Y.; Huang, X.F. Human suppressor of cytokine signaling 1 controls immunostimulatory activity of monocyte-derived dendritic cells. Cancer Res. 2009, 69, 8076–8084. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’Andre, S.D.; et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar] [CrossRef]
- Wang, D.; Huang, X.F.; Hong, B.; Song, X.T.; Hu, L.; Jiang, M.; Zhang, B.; Ning, H.; Li, Y.; Xu, C.; et al. Efficacy of intracellular immune checkpoint-silenced DC vaccine. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Kong, H.L.; Carpenter, H.; Torii, H.; Granstein, R.; Rafii, S.; Moore, M.A.; Crystal, R.G. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J. Exp. Med. 1997, 186, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Specht, J.M.; Wang, G.; Do, M.T.; Lam, J.S.; Royal, R.E.; Reeves, M.E.; Rosenberg, S.A.; Hwu, P. Dendritic cells retrovirally transduced with a model antigen gene are therapeutically effective against established pulmonary metastases. J. Exp. Med. 1997, 186, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A. Progress in human tumour immunology and immunotherapy. Nature 2001, 411, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Palucka, K.; Banchereau, J. Dendritic-Cell-Based Therapeutic Cancer Vaccines. Immunity 2013, 39, 38–48. [Google Scholar] [CrossRef]
- Gilboa, E. The promise of cancer vaccines. Nat. Rev. Cancer 2004, 4, 401–411. [Google Scholar] [CrossRef]
- Timmerman, J.M.; Levy, R. Dendritic cell vaccines for cancer immunotherapy. Annu. Rev. Med. 1999, 50, 507–529. [Google Scholar] [CrossRef]
- Josefowicz, S.Z.; Lu, L.F.; Rudensky, A.Y. Regulatory T Cells: Mechanisms of Differentiation and Function. Annu. Rev. Immunol. 2012, 30, 531–564. [Google Scholar] [CrossRef]
- Condamine, T.; Gabrilovich, D.I. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011, 32, 19–25. [Google Scholar] [CrossRef]
- Sznol, M.; Chen, L. Antagonist Antibodies to PD-1 and B7-H1 (PD-L1) in the Treatment of Advanced Human Cancer. Clin. Cancer Res. 2013, 19, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Chikuma, S.; Iwai, Y.; Fagarasan, S.; Honjo, T. A rheostat for immune responses: The unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 2013, 14, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Page, D.B.; Postow, M.A.; Callahan, M.K.; Allison, J.P.; Wolchok, J.D. Immune modulation in cancer with antibodies. Annu. Rev. Med. 2014, 65, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.I.; Nagaraj, S.; Collazo, M.; Gabrilovich, D.I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 2008, 181, 5791–5802. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Diaz-Montero, C.M.; Finke, J.; Montero, A.J. Myeloid-Derived Suppressor Cells in Cancer: Therapeutic, Predictive, and Prognostic Implications. Semin. Oncol. 2014, 41, 174–184. [Google Scholar] [CrossRef]
- Mullard, A. New checkpoint inhibitors ride the immunotherapy tsunami. Nat. Rev. Drug Discov. 2013, 12, 489–492. [Google Scholar] [CrossRef]
- Couzin-Frankel, J. Cancer Immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Ren, W.; Rollins, L.; Pittman, P.; Shah, M.; Shen, L.; Gu, Q.; Strube, R.; Hu, F.; Chen, S.Y. A broadly applicable, personalized heat shock protein-mediated oncolytic tumor vaccine. Cancer Res. 2003, 63, 7321–7329. [Google Scholar] [PubMed]
- Ying, Z.; Huang, X.F.; Xiang, X.; Liu, Y.; Kang, X.; Song, Y.; Guo, X.; Liu, H.; Ding, N.; Zhang, T.; et al. A safe and potent anti-CD19 CAR T cell therapy. Nat. Med. 2019, 25, 947–953. [Google Scholar] [CrossRef]
- Ravetch, J.V.; Kinet, J.P. Fc receptors. Annu. Rev. Immunol. 1991, 9, 457–492. [Google Scholar] [CrossRef]
- Steinman, R.M.; Swanson, J. The endocytic activity of dendritic cells. J. Exp. Med. 1995, 182, 283–288. [Google Scholar] [CrossRef]
- Watts, C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu. Rev. Immunol. 1997, 15, 821–850. [Google Scholar] [CrossRef]
- Regnault, A.; Lankar, D.; Lacabanne, V.; Rodriguez, A.; Thery, C.; Rescigno, M.; Saito, T.; Verbeek, S.; Bonnerot, C.; Ricciardi-Castagnoli, P.; et al. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 1999, 189, 371–380. [Google Scholar] [CrossRef]
- Song, X.T.; Evel-Kabler, K.; Rollins, L.; Aldrich, M.; Gao, F.; Huang, X.F.; Chen, S.Y. An alternative and effective HIV vaccination approach based on inhibition of antigen presentation attenuators in dendritic cells. PLoS Med. 2006, 3, e11. [Google Scholar] [CrossRef]
- Hong, B.; Lee, S.H.; Song, X.T.; Jones, L.; Machida, K.; Huang, X.F.; Chen, S.Y. A super TLR agonist to improve efficacy of dendritic cell vaccine in induction of anti-HCV immunity. PLoS ONE 2012, 7, e48614. [Google Scholar] [CrossRef]
- Overdijk, M.B.; Verploegen, S.; Ortiz Buijsse, A.; Vink, T.; Leusen, J.H.; Bleeker, W.K.; Parren, P.W. Crosstalk between human IgG isotypes and murine effector cells. J. Immunol. 2012, 189, 3430–3438. [Google Scholar] [CrossRef] [PubMed]
- Bruhns, P.; Jonsson, F. Mouse and human FcR effector functions. Immunol. Rev. 2015, 268, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takeuchi, O.; Mikamo-Satoh, E.; Hirai, R.; Kawai, T.; Matsushita, K.; Hiiragi, A.; Dermody, T.S.; Fujita, T.; Akira, S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 2008, 205, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, L.; Barchet, W.; Gilfillan, S.; Cella, M.; Beutler, B.; Flavell, R.A.; Diamond, M.S.; Colonna, M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 2006, 103, 8459–8464. [Google Scholar] [CrossRef]
- Skok, J.; Poudrier, J.; Gray, D. Dendritic cell-derived IL-12 promotes B cell induction of Th2 differentiation: A feedback regulation of Th1 development. J. Immunol. 1999, 163, 4284–4291. [Google Scholar]
- Inaba, K.; Witmer, M.D.; Steinman, R.M. Clustering of dendritic cells, helper T lymphocytes, and histocompatible B cells during primary antibody responses in vitro. J. Exp. Med. 1984, 160, 858–876. [Google Scholar] [CrossRef]
- Inaba, K.; Steinman, R.M.; Van Voorhis, W.C.; Muramatsu, S. Dendritic cells are critical accessory cells for thymus-dependent antibody responses in mouse and in man. Proc. Natl. Acad. Sci. USA 1983, 80, 6041–6045. [Google Scholar] [CrossRef]
- Huang, N.N.; Han, S.B.; Hwang, I.Y.; Kehrl, J.H. B Cells Productively Engage Soluble Antigen-Pulsed Dendritic Cells: Visualization of Live-Cell Dynamics of B Cell-Dendritic Cell Interactions. J. Immunol. 2005, 175, 7125–7134. [Google Scholar] [CrossRef]
- Ruprecht, C.R.; Lanzavecchia, A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur. J. Immunol. 2006, 36, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Flamand, V.; Sornasse, T.; Thielemans, K.; Demanet, C.; Bakkus, M.; Bazin, H.; Tielemans, F.; Leo, O.; Urbain, J.; Moser, M. Murine dendritic cells pulsed in vitro with tumor antigen induce tumor resistance in vivo. Eur. J. Immunol. 1994, 24, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Balazs, M.; Martin, F.; Zhou, T.; Kearney, J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 2002, 17, 341–352. [Google Scholar] [CrossRef]
- Litinskiy, M.B.; Nardelli, B.; Hilbert, D.M.; He, B.; Schaffer, A.; Casali, P.; Cerutti, A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 2002, 3, 822–829. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, I.; Vinuesa, C. Dendritic cells, BAFF, and APRIL: Innate players in adaptive antibody responses. Immunity 2002, 17, 235–238. [Google Scholar] [CrossRef]
- Dubois, B.; Massacrier, C.; Vanbervliet, B.; Fayette, J.; Briere, F.; Banchereau, J.; Caux, C. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J. Immunol. 1998, 161, 2223–2231. [Google Scholar]
- Munir, S.; Andersen, G.H.; Woetmann, A.; Odum, N.; Becker, J.C.; Andersen, M.H. Cutaneous T cell lymphoma cells are targets for immune checkpoint ligand PD-L1-specific, cytotoxic T cells. Leukemia 2013, 27, 2251–2253. [Google Scholar] [CrossRef]
- Munir, S.; Andersen, G.H.; Met, O.; Donia, M.; Frosig, T.M.; Larsen, S.K.; Klausen, T.W.; Svane, I.M.; Andersen, M.H. HLA-restricted CTL that are specific for the immune checkpoint ligand PD-L1 occur with high frequency in cancer patients. Cancer Res. 2013, 73, 1764–1776. [Google Scholar] [CrossRef]
- Ahmad, S.M.; Larsen, S.K.; Svane, I.M.; Andersen, M.H. Harnessing PD-L1-specific cytotoxic T cells for anti-leukemia immunotherapy to defeat mechanisms of immune escape mediated by the PD-1 pathway. Leukemia 2014, 28, 236–238. [Google Scholar] [CrossRef]
- Dong, H.; Strome, S.E.; Matteson, E.L.; Moder, K.G.; Flies, D.B.; Zhu, G.; Tamura, H.; Driscoll, C.L.; Chen, L. Costimulating aberrant T cell responses by B7-H1 autoantibodies in rheumatoid arthritis. J. Clin. Investig. 2003, 111, 363–370. [Google Scholar] [CrossRef]
- Spiotto, M.; Fu, Y.X.; Weichselbaum, R.R. The intersection of radiotherapy and immunotherapy: Mechanisms and clinical implications. Sci. Immunol. 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Ngiow, S.F.; Ribas, A.; Teng, M.W. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 2016, 13, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Middleton, G. The interplay of immunotherapy and chemotherapy: Harnessing potential synergies. Cancer Immunol. Res. 2015, 3, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Mascaux, C.; Angelova, M.; Vasaturo, A.; Beane, J.; Hijazi, K.; Anthoine, G.; Buttard, B.; Rothe, F.; Willard-Gallo, K.; Haller, A.; et al. Immune evasion before tumour invasion in early lung squamous carcinogenesis. Nature 2019. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Liu, H.; Jehng, T.; Li, Y.; Chen, Z.; Lee, K.-D.; Shen, H.-T.; Jones, L.; Huang, X.F.; Chen, S.-Y. A Novel Anti-PD-L1 Vaccine for Cancer Immunotherapy and Immunoprevention. Cancers 2019, 11, 1909. https://doi.org/10.3390/cancers11121909
Chen J, Liu H, Jehng T, Li Y, Chen Z, Lee K-D, Shen H-T, Jones L, Huang XF, Chen S-Y. A Novel Anti-PD-L1 Vaccine for Cancer Immunotherapy and Immunoprevention. Cancers. 2019; 11(12):1909. https://doi.org/10.3390/cancers11121909
Chicago/Turabian StyleChen, Jie, Hui Liu, Tiffany Jehng, Yanqing Li, Zhoushi Chen, Kuan-Der Lee, Hsieh-Tsung Shen, Lindsey Jones, Xue F. Huang, and Si-Yi Chen. 2019. "A Novel Anti-PD-L1 Vaccine for Cancer Immunotherapy and Immunoprevention" Cancers 11, no. 12: 1909. https://doi.org/10.3390/cancers11121909
APA StyleChen, J., Liu, H., Jehng, T., Li, Y., Chen, Z., Lee, K.-D., Shen, H.-T., Jones, L., Huang, X. F., & Chen, S.-Y. (2019). A Novel Anti-PD-L1 Vaccine for Cancer Immunotherapy and Immunoprevention. Cancers, 11(12), 1909. https://doi.org/10.3390/cancers11121909




