Monitoring Patients with Metastatic Hormone-Sensitive and Metastatic Castration-Resistant Prostate Cancer: A Multidisciplinary Consensus Document
Abstract
1. Introduction
2. Results
2.1. When Should Clinical and Biochemical Assessments Be Scheduled in the Case of an mCSPC Patient Who Is a Candidate for Androgen Deprivation Therapy (ADT) Alone?
2.2. When Should Imaging Assessments Be Scheduled in the Case of an mCSPC Patient Who Is a Candidate for ADT Alone?
2.3. Are There Any Factors that Could Influence the Baseline Monitoring Plan of an mCSPC Patient Who Is a Candidate for ADT Alone?
2.4. Are There Any Factors that Could Change the Initially Defined Monitoring Schedule of an mCSPC Patient Being Treated with ADT Alone?
2.5. When Should Clinical and Biochemical Assessments Be Scheduled in the Case of of an mCSPC Patient Who Is a Candidate for Treatment with ADT + Docetaxel?
2.6. When Should Imaging Assessments Be Scheduled in the Case of an mCSPC Patient Who Is a Candidate for Treatment with ADT + Docetaxel?
2.7. Are There Any Factors that Could Influence the Baseline Monitoring Plan of an mCSPC Patient Who Is a Candidate for Treatment with ADT + Docetaxel?
2.8. Are There Any Factors that Could Change the Initially Defined Monitoring Schedule of an mCSPC Patient Being Treated with ADT + Docetaxel?
2.9. When Should Clinical and Biochemical Assessments Be Scheduled in the Case of an mCSPC Patient without Progressive Disease Who Has Concluded Docetaxel Treatment but Is Continuing ADT?
2.10. When Should Imaging Assessments Be Scheduled in the Case of an mCSPC Patient Who Has Concluded Docetaxel Treatment but Is Continuing ADT?
2.11. Are There Any Factors that Could Influence the Baseline Monitoring Plan of an mCSPC Patient Who Has Concluded Docetaxel Treatment but Is Continuing ADT in the Absence of Progressive Disease?
2.12. Are There Any Factors that Could Change the Initially Defined Monitoring Schedule of an mCSPC Patient Undergoing ADT Who Has Been Previously Treated with Docetaxel?
2.13. When Should Clinical Assessments Be Scheduled in the Case of an mCRPC Patient Who Is a Candidate for Chemotherapy?
2.14. When Should Biochemical Assessments Be Scheduled in the Case of an mCRPC Patient Who Is a Candidate for Chemotherapy?
2.15. When Should Imaging Assessments Be Scheduled in the Case of an mCRPC Patient Who Is a Candidate for Chemotherapy?
2.16. Are There Any Factors that Could Change the Initially Defined Monitoring Schedule of an mCRPC Patient during Docetaxel Treatment?
2.17. When Should Imaging Assessments Be Scheduled in the Case of an mCRPC Patient Who Has Completed Chemotherapy and Shows No Signs of Progression?
2.18. When Should Clinical and Biochemical Assessments Be Scheduled in the Case of an mCRPC Patient Who Is a Candidate for ARTA Treatment?
2.19. When Should Imaging Assessments Be Scheduled in the Case of an mCRPC Patient Who Is a Candidate for Treatment with an ARTA?
2.20. Are There Any Factors that Could Influence the Baseline Monitoring Plan of an mCRPC Patient Who Is a Candidate for ARTA Treatment?
2.21. Are There Any Factors that Could Change the Initially Defined Monitoring Schedule of an mCRPC Patient Undergoing ARTA Treatment?
2.22. When Should Testosterone Assessments other than the Baseline Assessment Be Scheduled in the Case of Patients with Advanced Prostate Cancer (mCSPC/mCRPC)?
2.23. When Should Bone Health Assessments Other than the Baseline Assessment Be Scheduled in the Case of Patients with Advanced Prostate Cancer (mCSPC/mCRPC)?
2.24. When Should Assessments of Metabolic Alterations Other than the Baseline Assessment Be Scheduled in the Case of Patients with Advanced Prostate Cancer (mCSPC/mCRPC) Treated with ADT?
3. Discussion
4. Materials and Methods
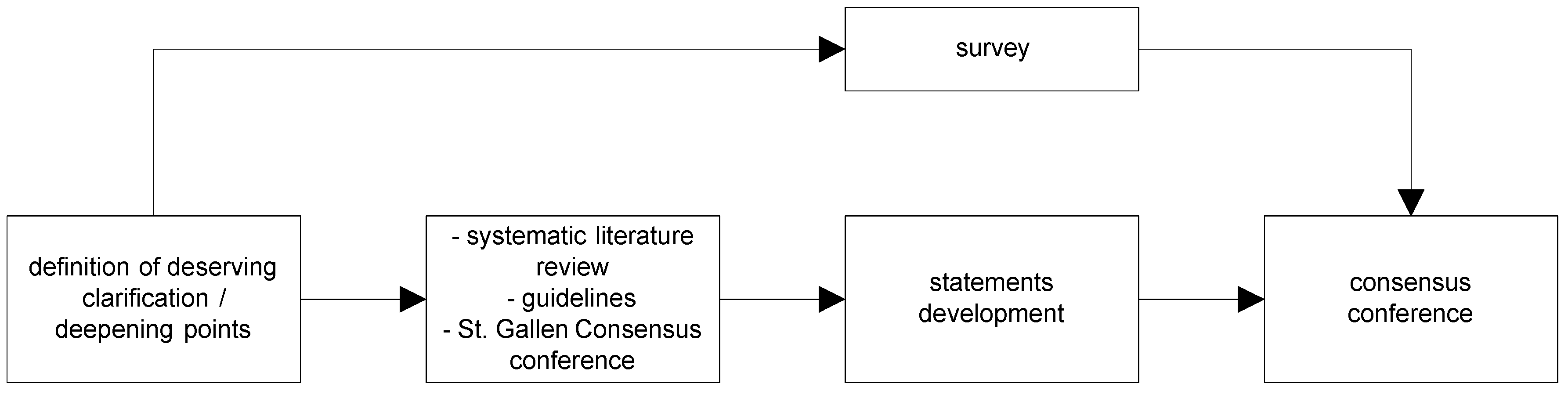
4.1. Definition of Questions Deserving Clarification/In-Depth Analysis
4.2. Review and Survey
4.3. Development of Statements
4.4. Consensus Conference
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; De Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.E.; Chen, Y.H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. 2018, 36, 1080–1087. [Google Scholar] [CrossRef]
- Chaumard-Billotey, N.; Chabaud, S.; Boyle, H.J.; Favier, B.; Devaux, Y.; Droz, J.-P.; Flechon, A. Impact of news drugs in the median overall survival of patients with metastatic castration resistant prostate cancer (mCRPC). J. Clin. Oncol. 2013, 31, e16096. [Google Scholar]
- Caffo, O.; Kinspergher, S.; Maines, F.; Macrini, S.; Veccia, A. Impact of new agents (NAs) on survival of metastatic castration-resistant prostate cancer (mCRPC) patients (pts): A single-Institution retrospective analysis. J. Clin. Oncol. 2018, 36, 323. [Google Scholar] [CrossRef]
- Francini, E.; Gray, K.P.; Shaw, G.K.; Evan, C.P.; Hamid, A.A.; Perry, C.E.; Kantoff, P.W.; Taplin, M.E.; Sweeney, C.J. Impact of new systemic therapies on overall survival of patients with metastatic castration-resistant prostate cancer in a hospital-based registry. Prostate Cancer Prostatic Dis. 2019, 22, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Koo, K.C.; Park, S.U.; Kim, K.H.; Rha, K.H.; Hong, S.J.; Yang, S.C.; Chung, B.H. Prognostic Impacts of Metastatic Site and Pain on Progression to Castrate Resistance and Mortality in Patients with Metastatic Prostate Cancer. Yonsei Med. J. 2015, 56, 1206–1212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Sella, A.; Sternberg, C.N.; Skoneczna, I.; Kovel, S. Prostate-specific antigen flare phenomenon with docetaxel-based chemotherapy in patients with androgen-independent prostate cancer. BJU Int. 2008, 102, 1607–1609. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; Fizazi, K.; Saad, F.; Mulders, P.F.; Sternberg, C.N.; Miller, K.; Logothetis, C.J.; Shore, N.D.; Small, E.J.; et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015, 16, 152–160. [Google Scholar] [CrossRef]
- Bryce, A.H.; Alumkal, J.J.; Armstrong, A.; Higano, C.S.; Iversen, P.; Sternberg, C.N.; Rathkopf, D.; Loriot, Y.; de Bono, J.; Tombal, B.; et al. Radiographic progression with nonrising PSA in metastatic castration-resistant prostate cancer: Post hoc analysis of PREVAIL. Prostate Cancer Prostatic Dis. 2017, 20, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Evans, C.P.; Kim, C.S.; Kimura, G.; et al. Enzalutamide in Men with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur. Urol. 2017, 71, 151–154. [Google Scholar] [CrossRef]
- Chi, K.N.; Kheoh, T.; Ryan, C.J.; Molina, A.; Bellmunt, J.; Vogelzang, N.J.; Rathkopf, D.E.; Fizazi, K.; Kantoff, P.W.; Li, J.; et al. A prognostic index model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel. Ann. Oncol. 2016, 27, 454–460. [Google Scholar] [CrossRef]
- Gillessen, S.; Attard, G.; Beer, T.M.; Beltran, H.; Bossi, A.; Bristow, R.; Carver, B.; Castellano, D.; Chung, B.H.; Clarke, N.; et al. Management of Patients with Advanced Prostate Cancer: The Report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur. Urol. 2018, 73, 178–211. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Unger, J.M.; Wright, J.D.; Ramsey, S.; Till, C.; Tangen, C.M.; Barlow, W.E.; Blanke, C.; Thompson, I.M.; Hussain, M. Adverse Health Events Following Intermittent and Continuous Androgen Deprivation in Patients With Metastatic Prostate Cancer. JAMA Oncol. 2016, 2, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Kupelian, V.; Page, S.T.; Araujo, A.B.; Travison, T.G.; Bremner, W.J.; McKinlay, J.B. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J. Clin. Endocrinol. Metab. 2006, 91, 843–850. [Google Scholar] [CrossRef]
- O’Farrell, S.; Garmo, H.; Holmberg, L.; Adolfsson, J.; Stattin, P.; Van Hemelrijck, M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J. Clin. Oncol. 2015, 33, 1243–1251. [Google Scholar] [CrossRef]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008, 26, 1148–1159. [Google Scholar] [CrossRef]
- Bernice, B.B. Delphi Process: A Methodology Used for the Elicitation of Opinions of Experts; RAND Corporation: Santa Monica, CA, USA, 1968. [Google Scholar]
- Delbecq, A.L.; Van de Ven, A.H. A Group Process Model for Problem Identification and Program Planning. J. Appl. Behav. Sci. 1971, 7, 26. [Google Scholar] [CrossRef]
- Rohrbaugh, J. Improving the quality of group judgment: Social judgment analysis and the nominal group technique. Organ. Behav. Hum. Perform. 1981, 28, 272–288. [Google Scholar] [CrossRef]
- Miner, F.C. A Comparative Analysis of Three Diverse Group Decision Making Approaches. Acad. Manag. J. 1979, 22, 81–93. [Google Scholar]
- Gustafson, D.H.; Shukla, R.K.; Delbecq, A.; Walster, G.W. A comparative study of differences in subjective likelihood estimates made by individuals, interacting groups, Delphi groups, and nominal groups. Organ. Behav. Hum. Perform. 1973, 9, 280–291. [Google Scholar] [CrossRef]
| Statement | Timing | Factors |
|---|---|---|
| When should clinical and biochemical assessments be scheduled in the case of an mCSPC patient who is a candidate for ADT alone? | Every 12 weeks for the first 12 months, and every 24 weeks thereafter | |
| When should imaging assessments be scheduled in the case of an mCSPC patient who is a candidates for ADT alone? | In the case of a biochemical and/or clinical relapse (preferably CT and BS). | |
| Are there any factors that could influence the baseline monitoring plan of an mCSPC patient who is a candidate for ADT alone? | Age at the time of diagnosis, Gleason score, symptoms, the number and site(s) of metastases, the time of onset of metastases, time interval between radical local treatment and the onset of metastases. | |
| Are there any factors that could change the initially defined monitoring schedule of an mCSPC patient being treated with ADT alone? | Trend of PSA levels and disease-related symptoms, (worsening in performance status, occurrence of a skeletal event, change in analgesic treatment). | |
| When should clinical and biochemical assessments be scheduled in the case of of an mCSPC patient who is a candidate for treatment with ADT + docetaxel? | Every treatment cycle (clinical), at least at the third and sixth treatment cycle (biochemical). | |
| When should imaging assessments be scheduled in the case of an mCSPC patient who is a candidate for treatment with ADT + docetaxel? | At the end of docetaxel treatment using the same methods as those used at the time of the initial evaluation (preferably CT and BS). | |
| Are there any factors that could influence the baseline monitoring plan of an mCSPC patient who is a candidate for treatment with ADT + docetaxel? | No factor. | |
| Are there any factors that could change the initially defined monitoring schedule of an mCSPC patient being treated with ADT + docetaxel? | Increasing PSA levels and worsening disease-related symptoms (worsening performance status, occurrence of a skeletal event, increased analgesic treatment). | |
| When should clinical and biochemical assessments be scheduled in the case of an mCSPC patient without progressive disease who has concluded docetaxel treatment but is continuing ADT? | At least every 12 weeks. | |
| When should imaging assessments be scheduled in the case of an mCSPC patient who has concluded docetaxel treatment but is continuing ADT? | Only in the case of clinical and/or biochemical progression (preferably CT and BS). | |
| Are there any factors that could influence the baseline monitoring plan of an mCSPC patient who has concluded docetaxel treatment but is continuing ADT in the absence of progressive disease? | PSA level, appearance of symptoms, biological/clinical aggressiveness of the disease. | |
| Are there any factors that could change the initially defined monitoring schedule of an mCSPC patient undergoing ADT who has been previously treated with docetaxel? | Increase in PSA levels and/or the onset or worsening of disease-related symptoms (worsening performance status, occurrence of a skeletal event, increase in pain therapy). | |
| When should clinical assessments be scheduled in the case of an mCRPC patient who is a candidate for chemotherapy? | Every cycle. | |
| When should biochemical assessments be scheduled in the case of an mCRPC patient who is a candidate for chemotherapy? | At least every 6–8 weeks. | |
| When should imaging assessments be scheduled in the case of an mCRPC patient who is a candidate for chemotherapy? | After about 12 weeks using the same methods as those used for the baseline assessment (preferably CT and BS). | |
| Are there any factors that could change the initially defined monitoring schedule of an mCRPC patient during docetaxel treatment? | Increase in PSA levels and the onset or worsening of disease-related symptoms (worsening performance status, occurrence of a skeletal event, increase in pain therapy). | |
| When should imaging assessments be scheduled In the case of an mCRPC patient who has completed chemotherapy and shows no signs of progression? | Depend on the results of clinical/biochemical assessments by using the same methods as those used for the baseline assessment (preferably CT and BS). | |
| When should clinical and biochemical assessments be scheduled in the case of an mCRPC patient who is a candidate for ARTA treatment? | PSA assessment every 12 weeks clinical evaluation every four weeks. | |
| When should imaging assessments be scheduled in the case of an mCRPC patient who is a candidate for treatment with an ARTA? | Should be based on the findings of clinical/biochemical assessments. | |
| Are there any factors that could influence the baseline monitoring plan of an mCRPC patient who is a candidate for ARTA treatment? | Site of metastases and disease-related symptoms. | |
| Are there any factors that could change the initially defined monitoring schedule of an mCRPC patient undergoing ARTA treatment? | Trend of PSA levels and the onset of disease-related symptoms. | |
| When should testosterone assessments other than the baseline assessment be scheduled in the case of patients with advanced prostate cancer (mCSPC/mCRPC)? | Testosterone evaluation every time there is an increase in PSA levels. | |
| When should bone health assessments other than the baseline assessment be scheduled in the case of patients with advanced prostate cancer (mCSPC/mCRPC)? | Standard monitoring plan should include regular bone health assessments. | |
| When should assessments of metabolic alterations other than the baseline assessment be scheduled in the case of patients with advanced prostate cancer (mCSPC/mCRPC) treated with ADT? | Regular metabolic assessments, particularly in presence of cardiovascular risk. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapini, A.; Caffo, O.; Pappagallo, G.; Iacovelli, R.; D’Angelillo, R.M.; Vavassori, V.; Ceccarelli, R.; Bracarda, S.; Jereczek-Fossa, B.A.; Da Pozzo, L.; et al. Monitoring Patients with Metastatic Hormone-Sensitive and Metastatic Castration-Resistant Prostate Cancer: A Multidisciplinary Consensus Document. Cancers 2019, 11, 1908. https://doi.org/10.3390/cancers11121908
Lapini A, Caffo O, Pappagallo G, Iacovelli R, D’Angelillo RM, Vavassori V, Ceccarelli R, Bracarda S, Jereczek-Fossa BA, Da Pozzo L, et al. Monitoring Patients with Metastatic Hormone-Sensitive and Metastatic Castration-Resistant Prostate Cancer: A Multidisciplinary Consensus Document. Cancers. 2019; 11(12):1908. https://doi.org/10.3390/cancers11121908
Chicago/Turabian StyleLapini, Alberto, Orazio Caffo, Giovanni Pappagallo, Roberto Iacovelli, Rolando Maria D’Angelillo, Vittorio Vavassori, Roberta Ceccarelli, Sergio Bracarda, Barbara Alicja Jereczek-Fossa, Luigi Da Pozzo, and et al. 2019. "Monitoring Patients with Metastatic Hormone-Sensitive and Metastatic Castration-Resistant Prostate Cancer: A Multidisciplinary Consensus Document" Cancers 11, no. 12: 1908. https://doi.org/10.3390/cancers11121908
APA StyleLapini, A., Caffo, O., Pappagallo, G., Iacovelli, R., D’Angelillo, R. M., Vavassori, V., Ceccarelli, R., Bracarda, S., Jereczek-Fossa, B. A., Da Pozzo, L., & Conti, G. N. (2019). Monitoring Patients with Metastatic Hormone-Sensitive and Metastatic Castration-Resistant Prostate Cancer: A Multidisciplinary Consensus Document. Cancers, 11(12), 1908. https://doi.org/10.3390/cancers11121908






