Recurrent or Refractory High-Grade Gliomas Treated by Convection-Enhanced Delivery of a TGFβ2-Targeting RNA Therapeutic: A Post-Hoc Analysis with Long-Term Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Investigational Medicinal Product and CED Drug Delivery System
2.2. Patient Characteristics and Execution of the Clinical Trial
2.3. Ethics Statement and Study Approval
2.4. Safety and Efficacy Measurements
2.5. Statistical Analyses
3. Results
3.1. Safety of the Drug Delivery System and Intratumoral OT101 Treatments in R/R HGG Patients
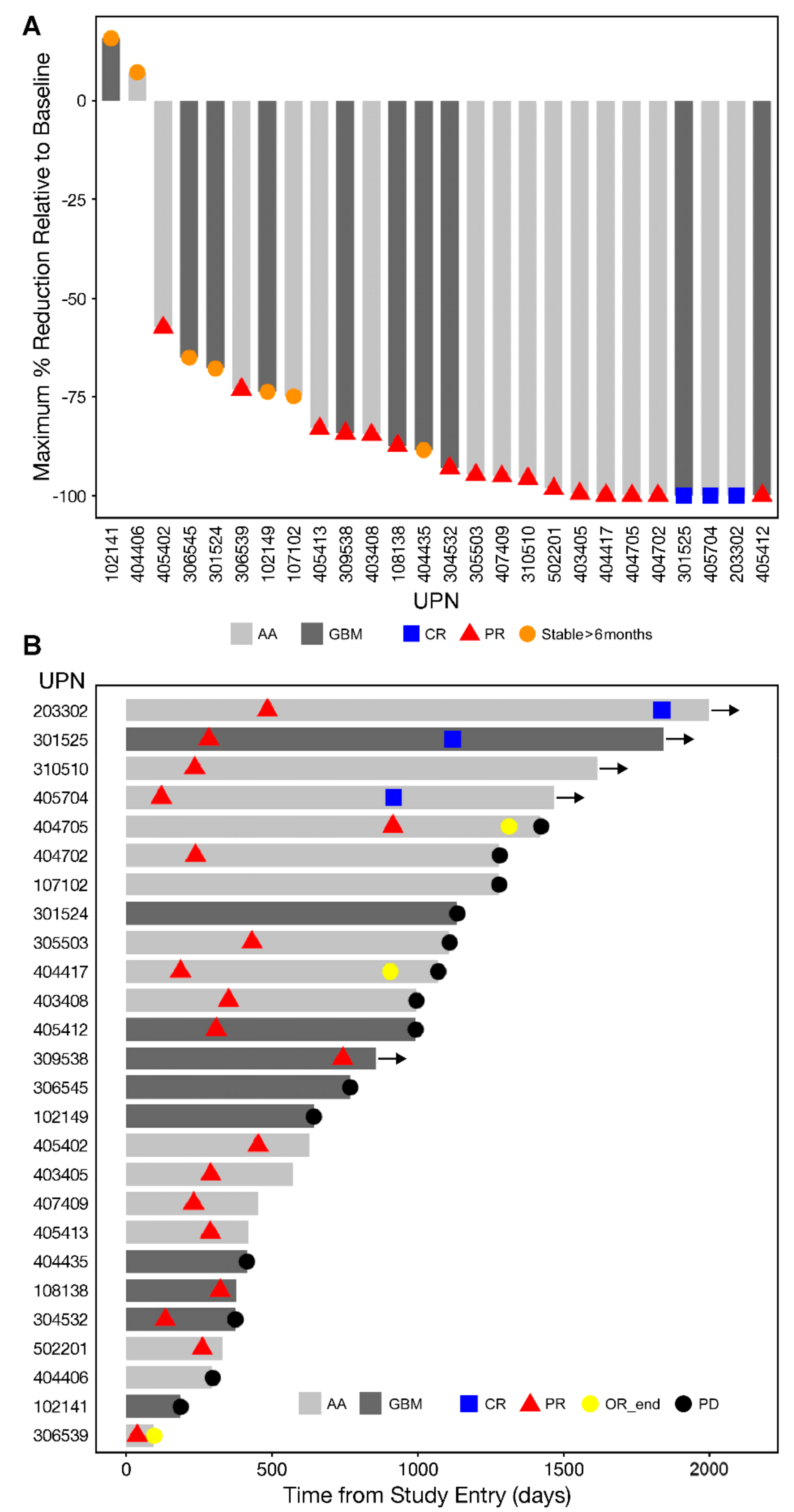
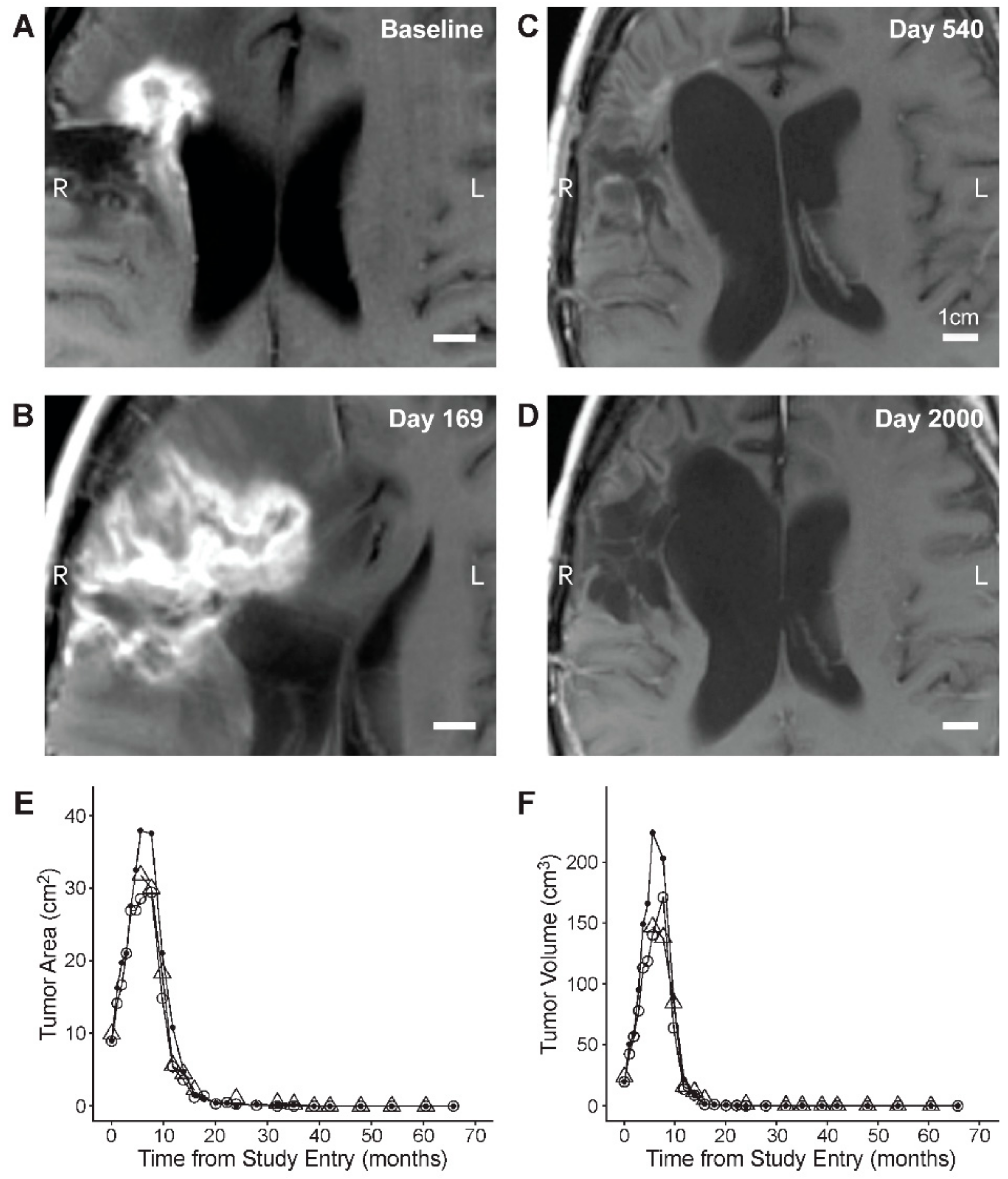
3.2. Single Agent Anti-HGG Activity of Intratumorally Delivered OT101 as Measured by Best Overall Response
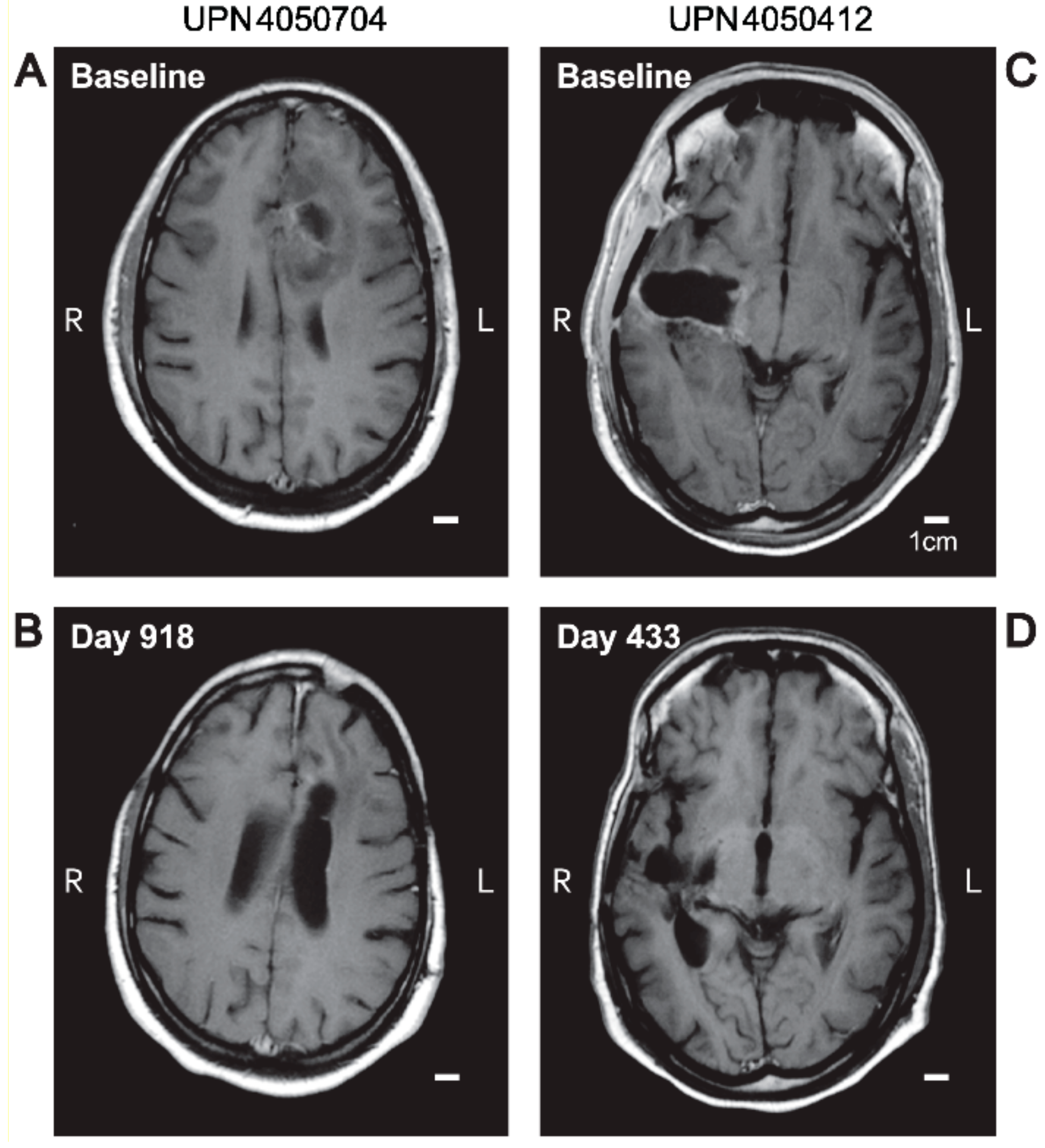
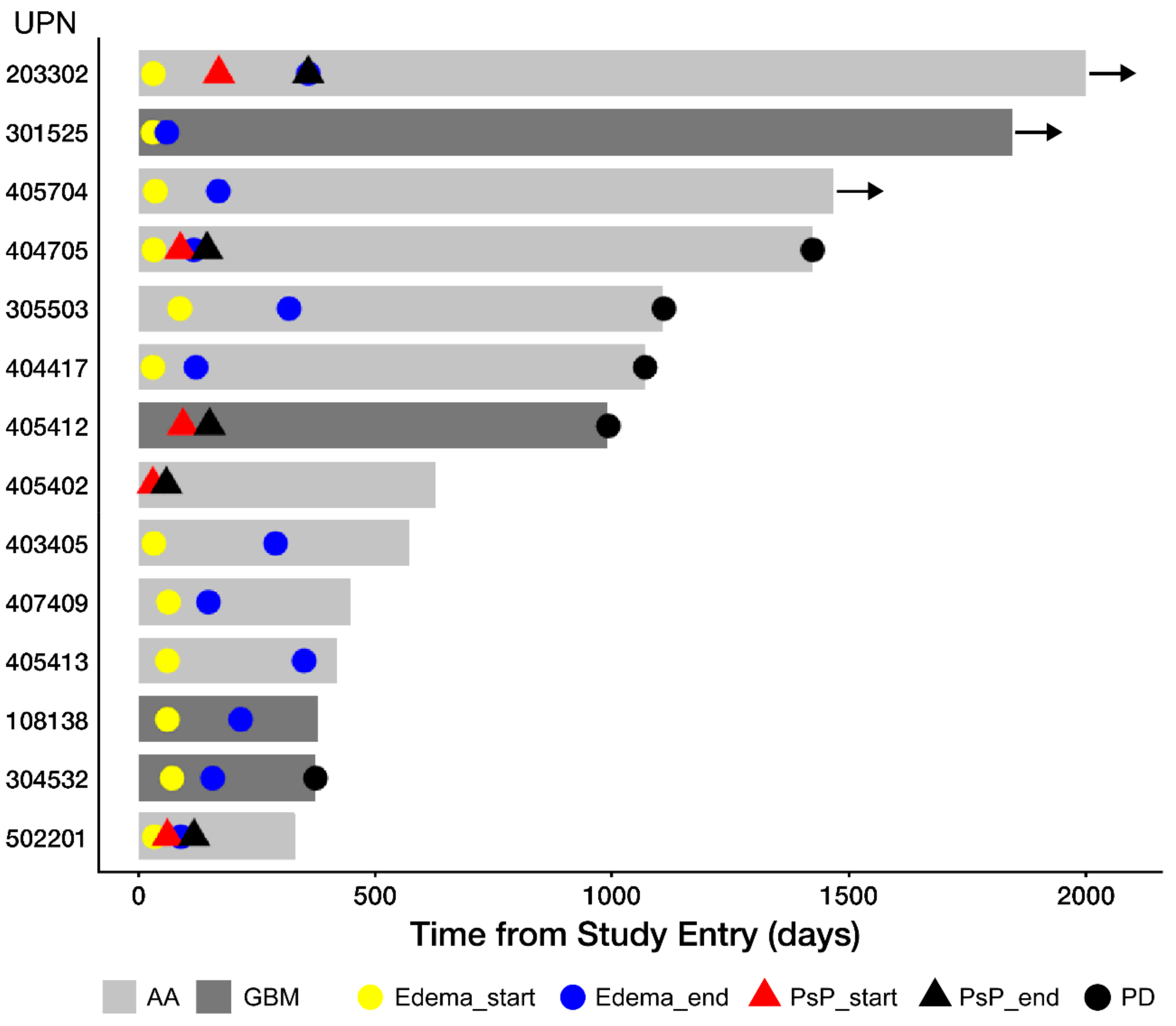
3.3. Edema and Pseudo-Progression in OT101-Yreated Target HGG Lesions
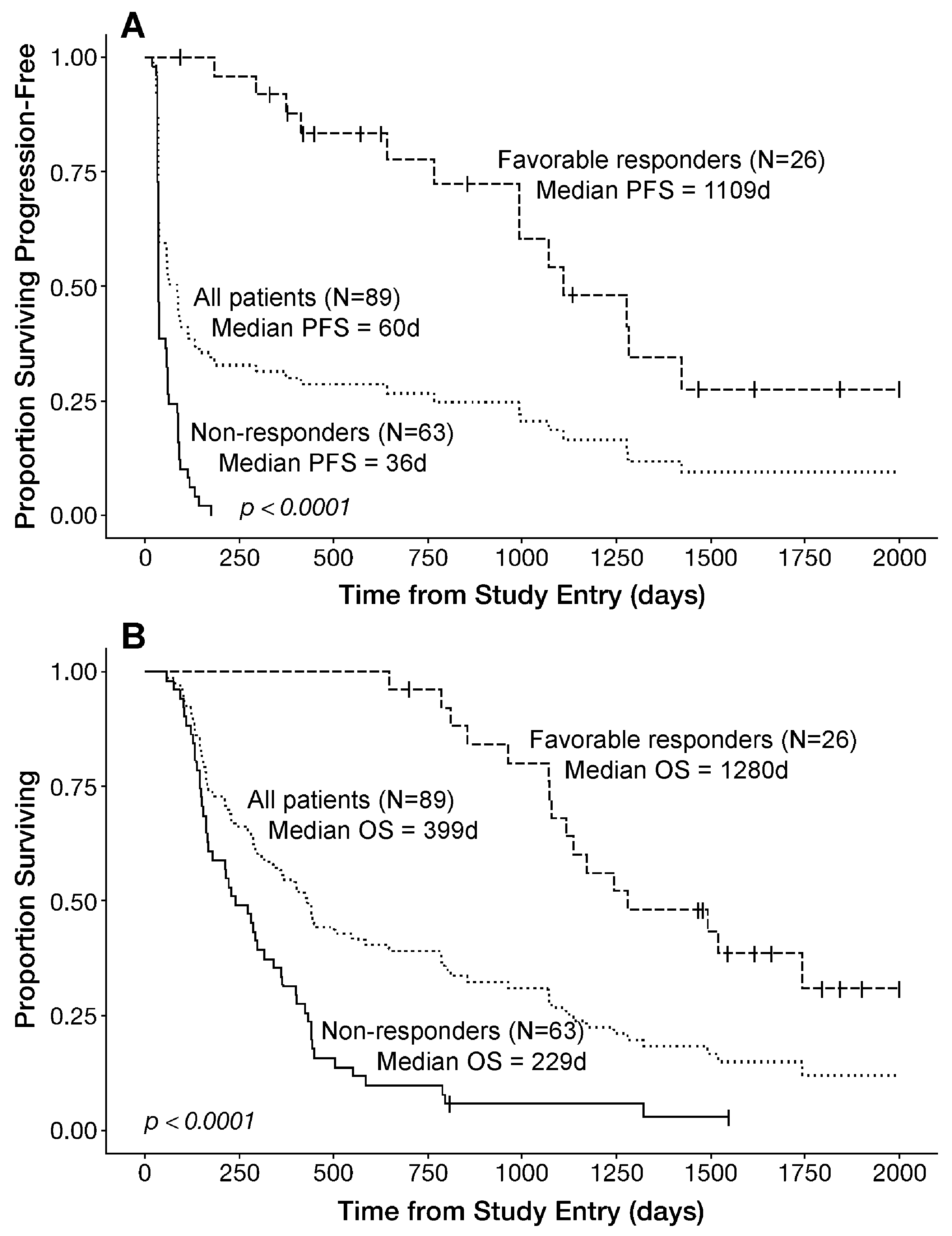
3.4. PFS and OS Outcomes After OT101 Therapy
3.5. Univariate and Multivariate Analysis of Potential Predictors of PFS and OS Outcome After OT101 Monotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taal, W.; Brandsma, D.; de Bruin, H.G.; Bromberg, J.E.; Swaak-Kragten, A.T.; Sillevis Smitt, P.A.; van Es, C.A.; van den Bent, M.J. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 2008, 113, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy with Tumor-Treating Fields Plus Temozolomide vs. Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef]
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Hau, P.; Kortmann, R.D.; Krex, D.; Grauer, O.; et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A randomised, open-label, phase 3 trial. Lancet 2019, 393, 678–688. [Google Scholar] [CrossRef]
- Jain, K.K. A critical overview of targeted therapies for glioblastoma. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef]
- Merkel, A.; Soeldner, D.; Wendl, C.; Urkan, D.; Kuramatsu, J.B.; Seliger, C.; Proescholdt, M.; Eyupoglu, I.Y.; Hau, P.; Uhl, M. Early postoperative tumor progression predicts clinical outcome in glioblastoma—implication for clinical trials. J. Neurooncol. 2017, 132, 249–254. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Miller, J.J.; Wen, P.Y. Emerging targeted therapies for glioma. Expert Opin. Emerg. Drugs 2016, 21, 441–452. [Google Scholar] [CrossRef]
- Carlsson, S.K.; Brothers, S.P.; Wahlestedt, C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol. Med. 2014, 6, 1359–1370. [Google Scholar] [CrossRef]
- Lieberman, F. Glioblastoma update: Molecular biology, diagnosis, treatment, response assessment, and translational clinical trials. F1000Research 2017, 6, 1892. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Xu, J.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro Oncol. 2016, 18 (Suppl. 5), v1–v75. [Google Scholar] [CrossRef]
- Chang, S.M.; Theodosopoulos, P.; Lamborn, K.; Malec, M.; Rabbitt, J.; Page, M.; Prados, M.D. Temozolomide in the treatment of recurrent malignant glioma. Cancer 2004, 100, 605–611. [Google Scholar] [CrossRef]
- Desjardins, A.; Gromeier, M.; Herndon, J.E.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. New Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, V.; Bao, X.; Yu, X.; Parker, S.; McDowall, C.; Yu, Y.R.; Healy, P.; Desjardins, A.; Gunn, M.D.; Gromeier, M.; et al. Improved efficacy against malignant brain tumors with EGFRwt/EGFRvIII targeting immunotoxin and checkpoint inhibitor combinations. J. Immunother. Cancer 2019, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Alvarez-Breckenridge, C.A.; Wang, Q.E.; Yu, J. TGF-β signaling and its targeting for glioma treatment. Am. J. Cancer Res. 2015, 5, 945. [Google Scholar] [PubMed]
- Kjellman, C.; Olofsson, S.P.; Hansson, O.; Von Schantz, T.; Lindvall, M.; Nilsson, I.; Salford, L.G.; Sjögren, H.O.; Widegren, B. Expression of TGF-beta isoforms, TGF-beta receptors, and SMAD molecules at different stages of human glioma. Int. J. Cancer 2000, 89, 251–258. [Google Scholar] [CrossRef]
- Hau, P.; Jachimczak, P.; Bogdahn, U. Treatment of malignant gliomas with TGF-beta2 antisense oligonucleotides. Expert Rev. Anticancer Ther. 2009, 9, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Vallières, L. Trabedersen, a TGFβ2-specific antisense oligonucleotide for the treatment of malignant gliomas and other tumors overexpressing TGFβ2. IDrugs 2009, 12, 445–453. [Google Scholar]
- Fakhrai, H.; Dorigo, O.; Shawler, D.L.; Lin, H.; Mercola, D.; Black, K.L.; Royston, I.; Sobol, R.E. Eradication of established intracranial rat gliomas by transforming growth factor beta antisense gene therapy. Proc. Natl. Acad. Sci. USA 1996, 93, 2909–2914. [Google Scholar] [CrossRef]
- Frei, K.; Gramatzki, D.; Tritschler, I.; Schroeder, J.J.; Espinoza, L.; Rushing, E.J.; Weller, M. Transforming growth factor-β pathway activity in glioblastoma. Oncotarget 2015, 6, 5963–5977. [Google Scholar] [CrossRef]
- Roy, L.-O.; Poirier, M.-B.; Fortin, D. Transforming growth factor-beta and its implication in the malignancy of gliomas. Target. Oncol. 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Peñuelas, S.; Anido, J.; Prieto-Sánchez, R.M.; Folch, G.; Barba, I.; Cuartas, I.; García-Dorado, D.; Poca, M.A.; Sahuquillo, J.; Baselga, J.; et al. TGF-beta Increases Glioma-Initiating Cell Self-Renewal through the Induction of LIF in Human Glioblastoma. Cancer Cell 2009, 15, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Bruna, A.; Darken, R.S.; Rojo, F.; Ocaña, A.; Peñuelas, S.; Arias, A.; Paris, R.; Tortosa, A.; Mora, J.; Baselga, J.; et al. High TGFβ-Smad Activity Confers Poor Prognosis in Glioma Patients and Promotes Cell Proliferation Depending on the Methylation of the PDGF-B Gene. Cancer Cell 2007, 11, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Brooks, W.H.; Netsky, M.G.; Normansell, D.E.; Horwitz, D.A. Depressed cell-mediated immunity in patients with primary intracranial tumors. Characterization of a humoral immunosuppressive factor. J. Exp. Med. 1972, 136, 1631–1647. [Google Scholar] [CrossRef] [PubMed]
- Kuppner, M.C.; Hamou, M.F.; Sawamura, Y.; Bodmer, S.; De Tribolet, N. Inhibition of lymphocyte function by glioblastoma-derived transforming growth factor beta 2. J. Neurosurg. 1989, 71, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Hau, P.; Jachimczak, P.; Schlingensiepen, R.; Schulmeyer, F.; Jauch, T.; Steinbrecher, A.; Brawanski, A.; Proescholdt, M.; Schlaier, J.; Buchroithner, J.; et al. Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: From preclinical to phase I/II studies. Oligonucleotides 2007, 17, 201–212. [Google Scholar] [CrossRef]
- Krichevsky, A.M.; Uhlmann, E.J. Oligonucleotide Therapeutics as a New Class of Drugs for Malignant Brain Tumors: Targeting mRNAs, Regulatory RNAs, Mutations, Combinations, and Beyond. Neurotherapeutics 2019, 16, 319–347. [Google Scholar] [CrossRef]
- Schlingensiepen, R.; Goldbrunner, M.; Szyrach, M.N.; Stauder, G.; Jachimczak, P.; Bogdahn, U.; Schulmeyer, F.; Hau, P.; Schlingensiepen, K.H. Intracerebral and intrathecal infusion of the TGF-beta2-specific antisense phosphorothioate oligonucleotide AP 12009 in rabbits and primates: Toxicology and safety. Oligonucleotides 2005, 15, 94–104. [Google Scholar] [CrossRef]
- Bogdahn, U.; Hau, P.; Stockhammer, G.; Venkataramana, N.K.; Mahapatra, A.K.; Suri, A.A.; Balasubramaniam, A.; Nair, S.; Oliushine, V.; Parfenov, V.; et al. Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: Results of a randomized and controlled phase IIb study. Neuro-Oncol. 2011, 13, 132–142. [Google Scholar] [CrossRef]
- Tauriello, D.V.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Cañellas, A.; Hernando-Momblona, X.; et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018, 554, 538–543. [Google Scholar] [CrossRef]
- Ganesh, K.; Massagué, J. TGF-β Inhibition and Immunotherapy: Checkmate. Immunity 2018, 48, 626–628. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel III, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.A.; Massagué, J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005, 8, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Kang, D.; Joo, Y.; Lee, J.; Oh, G.H.; Choi, S.; Ko, S.; Je, S.; Choi, H.J.; Song, J.J. TGF-β downregulation-induced cancer cell death is finely regulated by the SAPK signaling cascade. Exp. Mol. Med. 2018, 50, 162. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Wen, P.Y.; Cloughesy, T.F. Evidence and context of use for contrast enhancement as a surrogate of disease burden and treatment response in malignant glioma. Neuro-Oncol. 2017, 20, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Kruser, T.J.; Mehta, M.P.; Robins, H.I. Pseudoprogression after glioma therapy: A comprehensive review. Expert Rev. Neurother. 2013, 13, 389–403. [Google Scholar] [CrossRef]
- Macdonald, D.R.; Cascino, T.L.; Schold, S.C., Jr.; Cairncross, J.G. Response criteria for phase II studies of supratentorial malignant glioma. J. Clin. Oncol. 1990, 8, 1277–1280. [Google Scholar] [CrossRef]
- Huang, R.Y.; Rahman, R.; Ballman, K.V.; Felten, S.J.; Anderson, S.K.; Ellingson, B.M.; Nayak, L.; Lee, E.Q.; Abrey, L.E.; Galanis, E.; et al. The Impact of T2/FLAIR Evaluation per RANO Criteria on Response Assessment of Recurrent Glioblastoma Patients Treated with Bevacizumab. Clin. Cancer Res. 2016, 22, 575–581. [Google Scholar] [CrossRef]
- Jahangiri, A.J.; Chin, A.T.; Flanigan, P.M.; Chen, R.; Bankiewicz, K.; Aghi, M.K. Convection-enhanced delivery in glioblastoma: A review of preclinical and clinical studies. J. Neurosurg. 2017, 126, 191–200. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control Release 2018, 270, 290–303. [Google Scholar] [CrossRef]
- Formicola, B.; Dal Magro, R.; Montefusco-Pereira, C.V.; Lehr, C.M.; Koch, M.; Russo, L.; Grasso, G.; Deriu, M.A.; Danani, A.; Bourdoulous, S.; et al. The synergistic effect of chlorotoxin-mApoE in boosting drug-loaded liposomes across the BBB. J. Nanobiotechnol. 2019, 17, 115. [Google Scholar] [CrossRef]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef]
- Asquier, N.; Bouchoux, G.; Canney, M.; Martin, C.; Law-Ye, B.; Leclercq, D.; Chapelon, J.Y.; Lafon, C.; Idbaih, A.; Carpentier, A. Blood-brain barrier disruption in humans using an implantable ultrasound device: Quantification with MR images and correlation with local acoustic pressure. J. Neurosurg. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Diagnosis | # (%) |
|---|---|
| AA (WHO Grade III) | 27 (30.3) |
| GBM (WHO Grade IV) | 62 (69.7) |
| Gender | # (%) |
| Female | 25 (28.1) |
| Male | 64 (71.9) |
| Race | # (%) |
| Caucasian | 58 (65.2) |
| Asian | 31 (34.8) |
| Black | 0 (0.0) |
| Age (Years) | |
| Median (Range) | 45 (19–73) |
| Mean ± SE | 46.3 ± 1.3 |
| KPS Score at Baseline | |
| Median (Range) | 90 (70–100) |
| Mean ± SE | 88 ± 1 |
| Size of Largest Target Tumor Lesion | |
| 2-D in cm2 – Median (Mean ± SE) | 8.6 (9.3 ± 0.5) |
| 3-D in cm3 – Median (Mean ± SE) | 21.5 (27.2 ± 2.5) |
| OT101 Dose Cohort | # (%) |
| Low (2.5 mg/cycle) | 40 (44.9) |
| High (19.8 mg/cycle) | 49 (55.1) |
| Number of OT101 Cycles | |
| Median (Range) | 6 (1–11) |
| Mean ± SE | 7.0 ± 0.3 |
| Total OT101 Dose (mg/m2) | |
| Median (Range) | 22.7 (1.1–152.1) |
| Mean ± SE | 45.2 ± 4.6 |
| Time from last cancer therapy | |
| Median (Mean ± SE) | 103 (248 ± 53) |
| Time from Diagnosis | |
| Median (Mean ± SE) | 229 (379 ± 59) |
| Previous antitumor therapy | # (%) |
| Surgery | 89 (100) |
| Radiotherapy | 81 (91.0) |
| Chemotherapy | 48 (53.9) |
| Other | 13 (14.6) |
| Number of previous surgeries | # (%) |
| 1 | 64 (71.9) |
| 2 | 19 (21.3) |
| > 2 | 6 (6.7) |
| Number of previous radiotherapies | # (%) |
| 0 | 8 (9.0) |
| 1 | 73 (82.0) |
| 2 | 8 (9.0) |
| Number of previous chemotherapies | |
| 0 | 41 (46.1) |
| 1 | 48 (53.9) |
| Number of previous other therapies | # (%) |
| 0 | 76 (85.3) |
| 1 | 11 (12.4) |
| 2 | 2 (2.2) |
| Number of measurable enhancing lesions* | # (%) |
| 1 | 68 (78.2) |
| 2 | 14 (16.1) |
| 3 | 5 (5.7) |
| Number of NMEL* | # (%) |
| 0 | 67 (77.0) |
| 1 | 12 (13.8) |
| 2 | 5 (5.7) |
| 3 | 3 (3.4) |
| Parameter | mITT | mITT, <4 Cycles | Efficacy/EP 4–11 Cycles | Objective Responders * | Favorable Responders * | EP, Non-Responder | mITT, Non-Responder |
|---|---|---|---|---|---|---|---|
| PFS (d) | (N = 89) | (N = 12) | (N = 77) | (N = 19) | (N = 26) | (N = 51) | (N = 63) |
| Median | 60 | 32 | 86 | 1281 | 1109 | 37 | 36 |
| 95% CI | 39–93 | 32–NA | 40–134 | 1070–NA | 992–NA | 35–59 | 35–54 |
| OS (d) | |||||||
| Median | 399 | 128 | 432 | 1243 | 1280 | 240 | 229 |
| 95% CI | 283–503 | 93–NA | 299–788 | 1079–NA | 1116–NA | 169–361 | 163–318 |
| Risk Factor | PFS (Days) | χ2 | p-Value | Z-Stat/p-Value | OS (Days) | χ2 | p-Value | Z-Stat/p-Value |
|---|---|---|---|---|---|---|---|---|
| Median (95% CI) | Median (95% CI) | |||||||
| Diagnosis | ||||||||
| GBM (N = 62) | 38 (35–61) | 17.5 | 0.00003 | 2.4/0.018 | 274 (180–399) | 16.1 | 0.00006 | 2.0/0.04 |
| AA (N = 27) | 994 (118–1423) | 1136 (811–1743) | ||||||
| Age (Years) | ||||||||
| 53–73 (N = 30) | 36 (35–86) | 6.0 | 0.014 | 0.5/0.6 | 213 (137–341) | 11.6 | 0.0007 | 0.3/0.7 |
| 19–41 (N = 30) | 101 (59–1109) | 803 (365–1243) | ||||||
| KPS Score | ||||||||
| 70–80 (N = 25) | 40 (36–67) | 6.3 | 0.012 | 0.8/0.4 | 162 (131–341) | 16.1 | 0.00006 | 2.1/0.03 |
| 90–100 (N = 64) | 88 (40–295) | 445 (399–1069) | ||||||
| OT101 Cycles | ||||||||
| 1–3 (N = 12) | 32 (32–NA) | 16.1 | 0.00006 | 0.6/0.5 | 128 (93–NA) | 19.5 | 0.00001 | 2.1/0.03 |
| 4–11 (N = 77) | 86 (40–134) | 432 (299–788) | ||||||
| Total OT101 Dose (mg/m2) | ||||||||
| 1.1–14 (N = 30) | 36 (35–60) | 2.0 | 0.16 | 2.7/0.006 | 222 (152–406) | 3.5 | 0.06 | 2.3/0.02 |
| 53–152 (N = 30) | 88 (40–176) | 447 (402–1079) | ||||||
| Dexamethasone Use | ||||||||
| Extensive (N = 25) | 36 (35–86) | 25.6 | <0.00001 | 1.7/0.08 | 273 (152–432) | 23.3 | <0.00001 | 2.3/0.02 |
| No or Minimal (N = 28) | 643 (295–NA) | 1172(963–NA) | ||||||
| Best Overall Response (BOR) | ||||||||
| Non-responders— (N = 63) | 36 (35–54) | 72.6 | <0.00001 | 16.5/<0.00001 | 229 (163–318) | 51.6 | <0.00001 | 5.8/<0.00001 |
| Favorable responder—PR, CR or SD ≥ 6 months (N = 26) | 1109 (992–NA) | 1280 (1116–NA) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uckun, F.M.; Qazi, S.; Hwang, L.; Trieu, V.N. Recurrent or Refractory High-Grade Gliomas Treated by Convection-Enhanced Delivery of a TGFβ2-Targeting RNA Therapeutic: A Post-Hoc Analysis with Long-Term Follow-Up. Cancers 2019, 11, 1892. https://doi.org/10.3390/cancers11121892
Uckun FM, Qazi S, Hwang L, Trieu VN. Recurrent or Refractory High-Grade Gliomas Treated by Convection-Enhanced Delivery of a TGFβ2-Targeting RNA Therapeutic: A Post-Hoc Analysis with Long-Term Follow-Up. Cancers. 2019; 11(12):1892. https://doi.org/10.3390/cancers11121892
Chicago/Turabian StyleUckun, Fatih M., Sanjive Qazi, Larn Hwang, and Vuong N. Trieu. 2019. "Recurrent or Refractory High-Grade Gliomas Treated by Convection-Enhanced Delivery of a TGFβ2-Targeting RNA Therapeutic: A Post-Hoc Analysis with Long-Term Follow-Up" Cancers 11, no. 12: 1892. https://doi.org/10.3390/cancers11121892
APA StyleUckun, F. M., Qazi, S., Hwang, L., & Trieu, V. N. (2019). Recurrent or Refractory High-Grade Gliomas Treated by Convection-Enhanced Delivery of a TGFβ2-Targeting RNA Therapeutic: A Post-Hoc Analysis with Long-Term Follow-Up. Cancers, 11(12), 1892. https://doi.org/10.3390/cancers11121892






