Radiation and Stemness Phenotype May Influence Individual Breast Cancer Outcomes: The Crucial Role of MMPs and Microenvironment
Abstract
:1. Introduction
2. Results
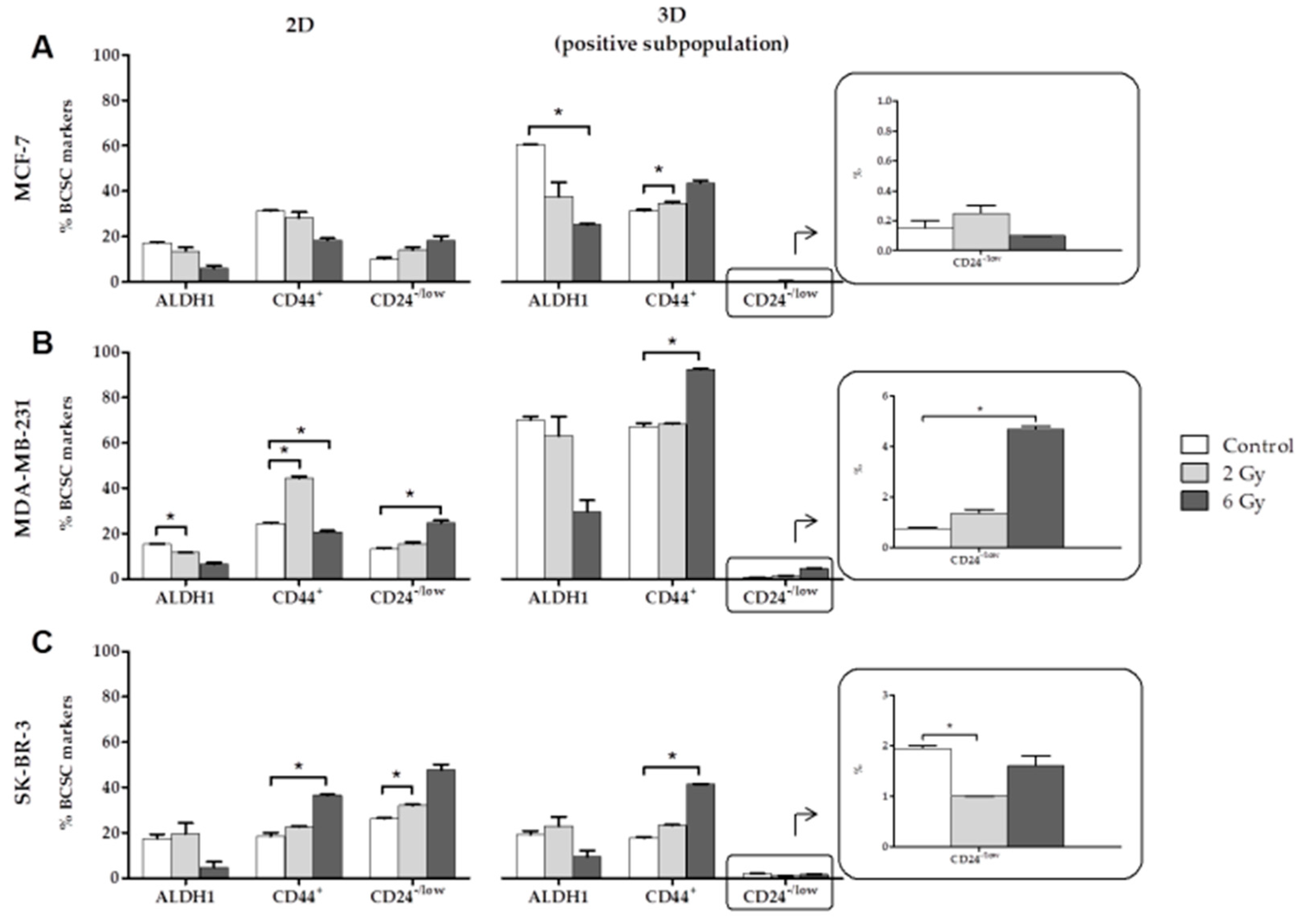
2.1. Effects of Ionizing Radiation (IR) on CSC Characterization
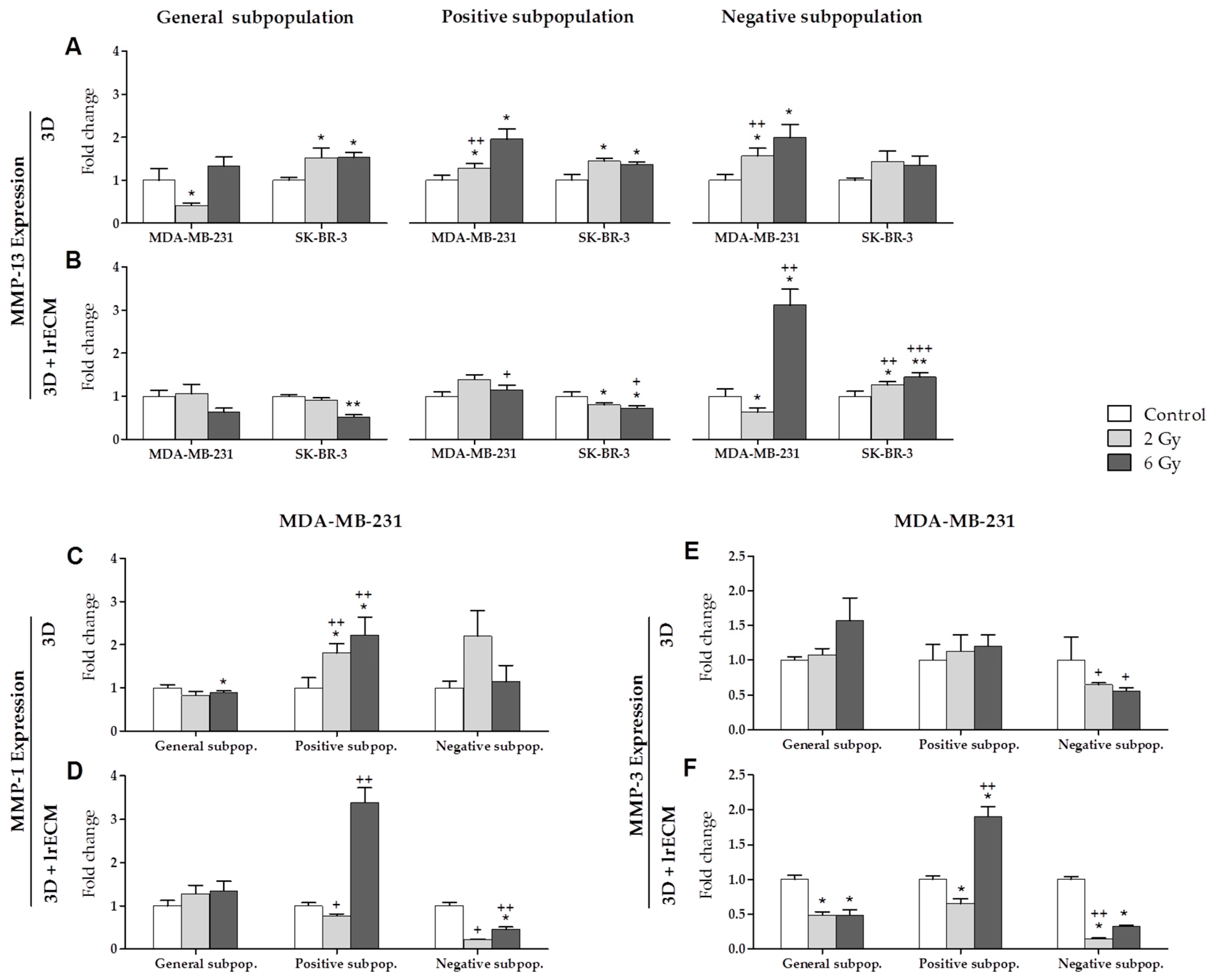
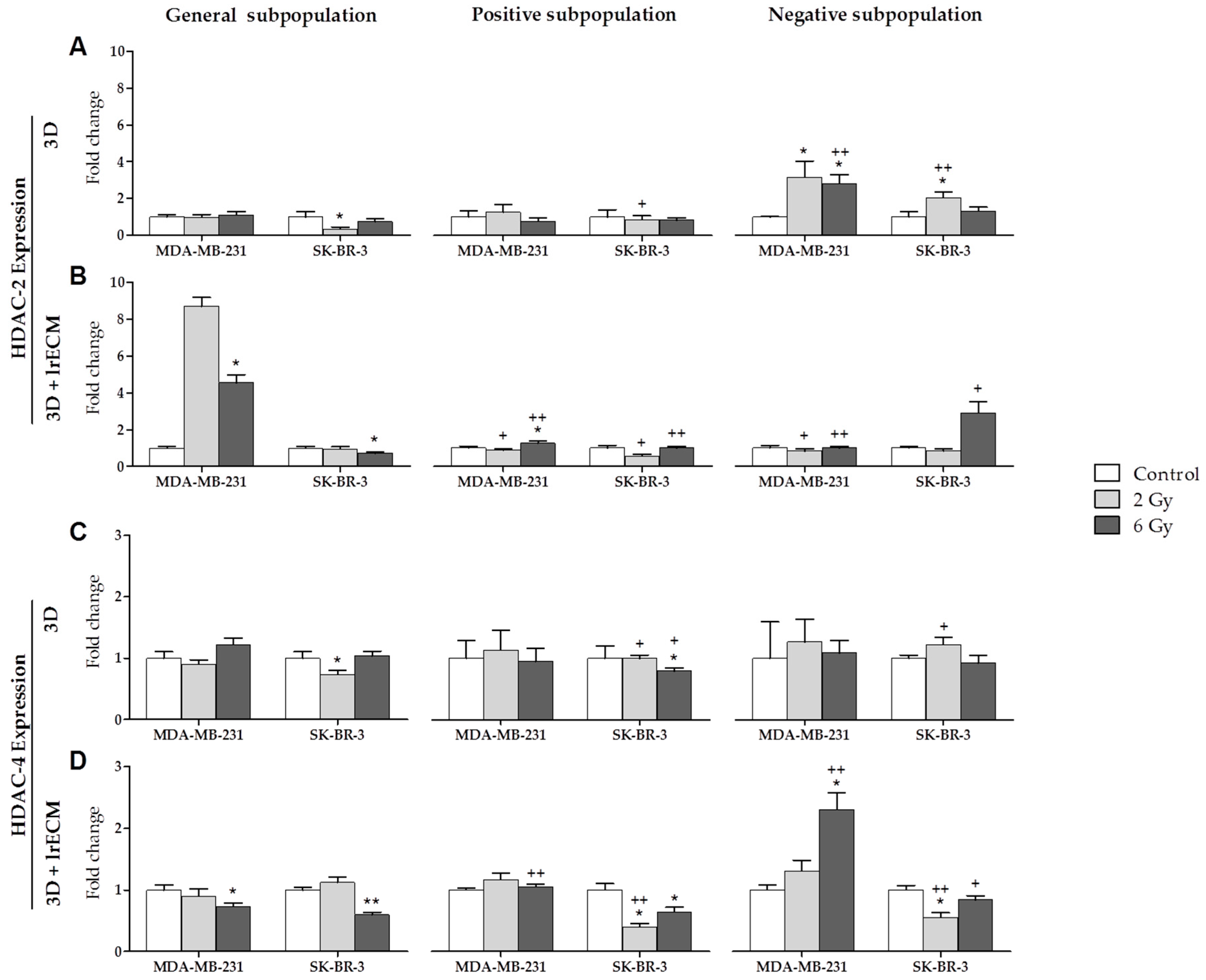
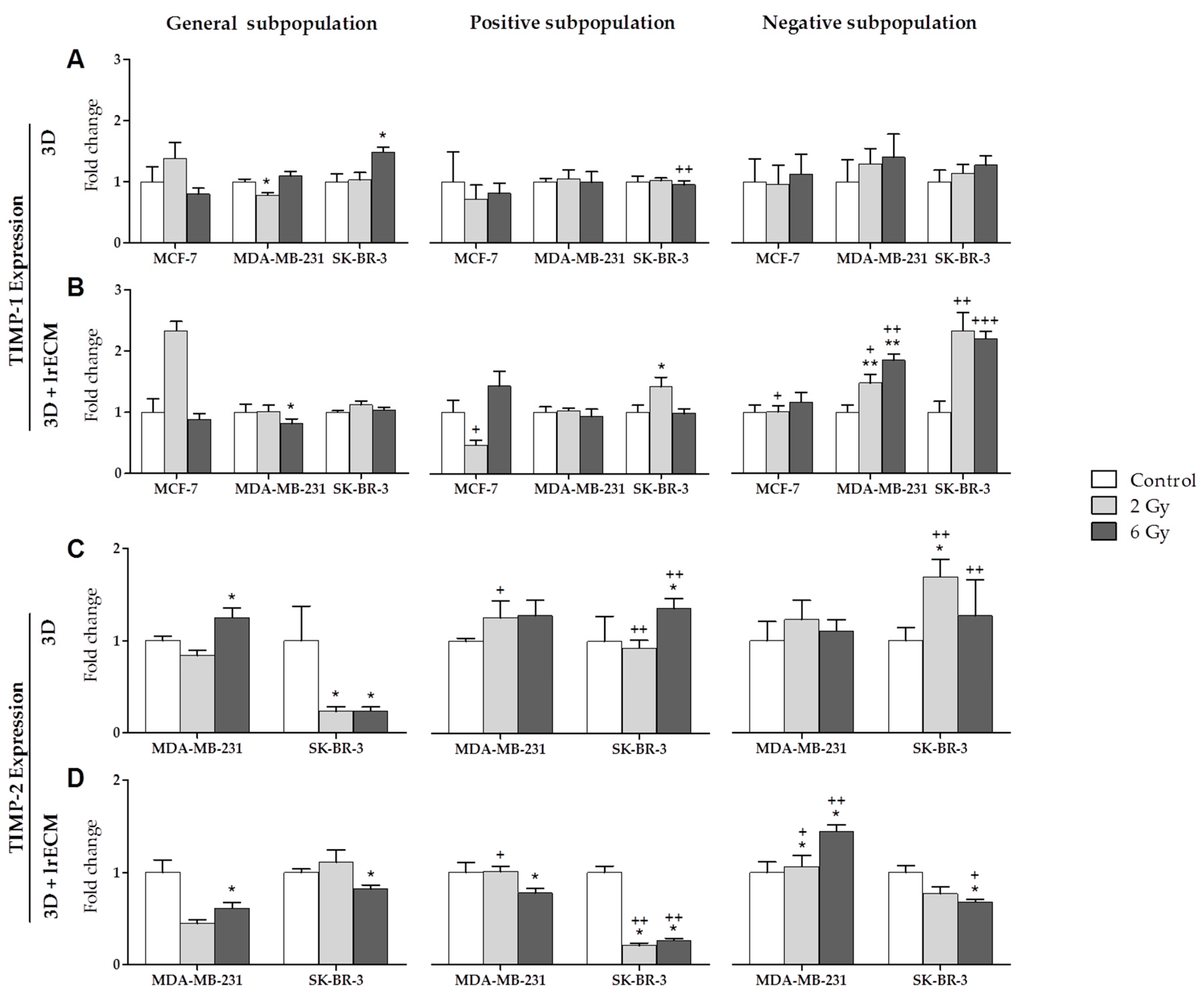
2.2. Effects of Ionizing Radiation on In Vitro Gene Expression
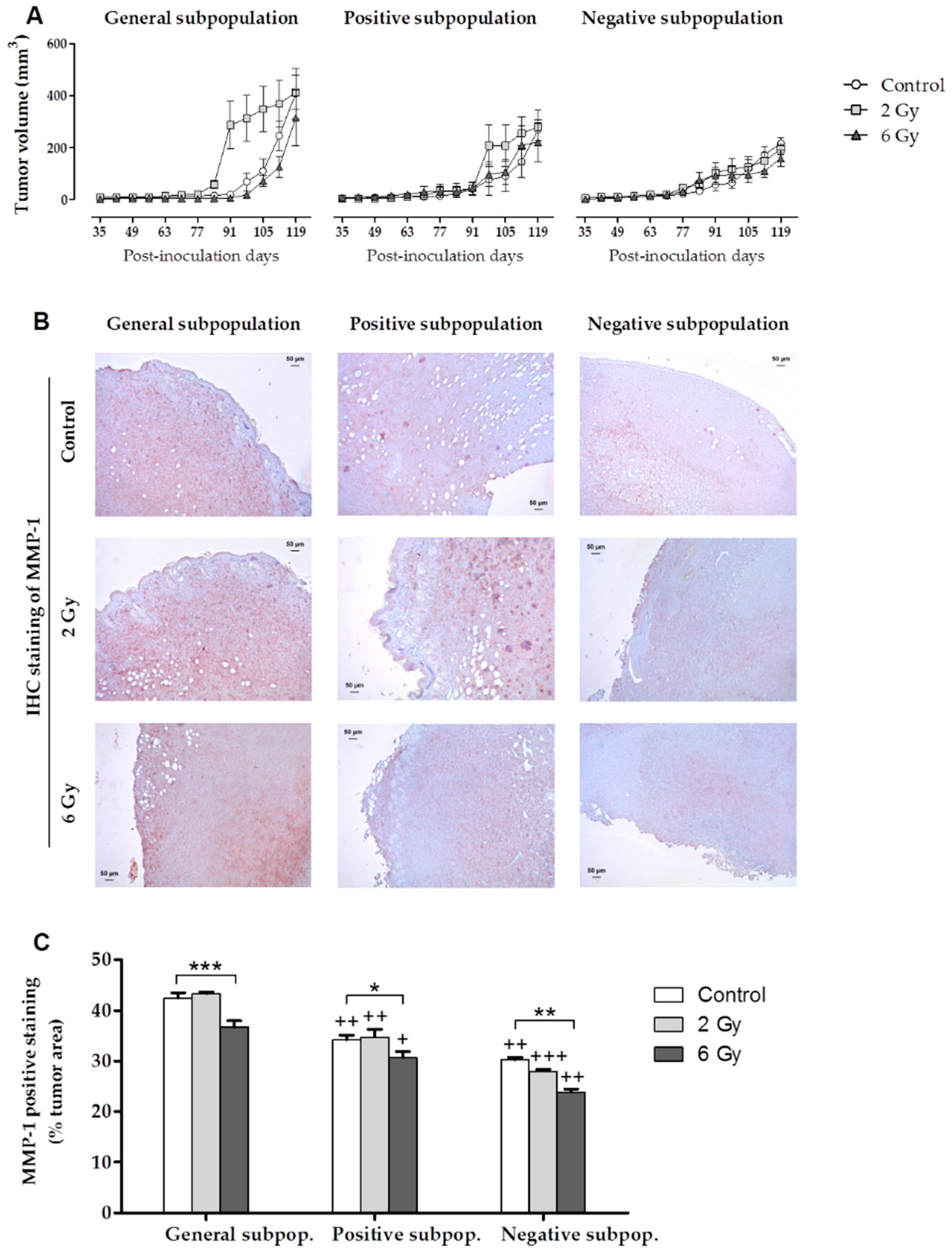
2.3. IR Effects on In Vivo Orthotopic Assay
2.3.1. Tumor Growth Monitoring
2.3.2. Histological and Immunohistochemical (IHC) Staining
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Flow Cytometry Analysis
4.2.1. BCSC Characterization
4.2.2. Apoptotic Cell Identification
4.3. In Vitro Measurements of Gene Expression
4.3.1. Separation of Cell Subpopulations
3D Culture (Mammospheres in Suspension)
3D+lrECM Culture (3D Laminin-Rich ECM)
4.3.2. Irradiation Protocol
4.3.3. cDNA Amplification and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.4. In Vivo Orthotopic Xenotransplant Assays
4.4.1. Inoculation of Cells in Matrigel and Monitoring of Tumor Growth
4.4.2. Histological and Immunohistochemical (IHC) Assays
4.5. Statistical Analysis
5. Conclusions
- -
- The expression of the BCSCs (ALDH1, CD44+ and CD24−/low) varies with the dose of radiation administered. In the positive subpopulation (CSCs), high doses of radiation (6 Gy) increases CD44+, which is associated with EMT and a poor prognosis in BC.
- -
- In the in vitro 3D and 3D+lrECM studies, the expression of MMPs, TIMPs and HDACs varies depending on the tumor cell line (MCF-7, MDA-MB-231 and SK-BR-3), the radiation dose (0, 2 and 6 Gy), the cell subpopulation (general, positive or CSCs, and negative or non-CSCs) and the 3D culture model (spheres suspended or embedded in Matrigel).
- -
- MMP-1 expression was increased in both 3D culture models after radiation in the positive subpopulation (CSCs) of the MDA-MB-231 triple-negative cell line. This fact suggests the importance of MMP-1 in the process of invasion and metastasis.
- -
- In the in vivo study, tumors derived from cells irradiated at 2 Gy have been of higher volume with respect to the control in the general and positive subpopulations. This fact shows that low doses of radiation would be insufficient to eradicate CSCs due to their radioresistance, contributing to repopulation and the tumor’s growth.
- -
- In tumor development in vivo, tumors derived from cells irradiated at 6 Gy were the smallest within each cell subpopulation. This result suggests that high doses of radiation (6 Gy) counteract repopulation by reducing the tumor growth rate and the final volume of the tumor.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Cancer Observatory. Available online: https://www.uicc.org/new-global-cancer-data-globocan-2018 (accessed on 10 April 2019).
- Yeo, S.K.; Guan, J.L. Breast Cancer: Multiple Subtypes within a Tumor? Trends Cancer 2017, 3, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Eguiara, A.; Elorriaga, K.; Rezola, R.; Martin, A.G. Células madre tumorales: Una diana terapéutica en el cancer de mama. Rev. Senol. Patol. Mamar. 2012, 25, 87–130. [Google Scholar] [CrossRef]
- Deng, G.; Lu, Y.; Zlotnikov, G.; Thor, A.D.; Smith, H.S. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science 1996, 274, 2057–2059. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.R.; Slack, D.N.; Hamoudi, R.A.; Collins, N.; Stratton, M.R.; Sloane, J.P. Detection of allelic imbalance indicates that a proportion of mammary hyperplasia of usual type are clonal, neoplastic proliferations. Lab. Investig. 1996, 74, 129–135. [Google Scholar] [PubMed]
- Tsai, Y.C.; Lu, Y.; Nichols, P.W.; Zlotnikov, G.; Jones, P.A.; Smith, H.S. Contiguous patches of normal human mammary epithelium derived from a single stem cell: Implications for breast carcinogenesis. Cancer Res. 1996, 56, 402–404. [Google Scholar] [PubMed]
- Vermeulen, L.; de Sousa e Melo, F.; Richel, D.J.; Medema, J.P. The developing cancer stem-cell model: Clinical challenges and opportunities. Lancet Oncol. 2012, 13, e83–e89. [Google Scholar] [CrossRef]
- Hernández-Camarero, P.; Jiménez, G.; López-Ruiz, E.; Barungi, S.; Marchal, J.A.; Perán, M. Revisiting the dynamic cancer stem cell model: Importance of tumour edges. Crit. Rev. Oncol. Hematol. 2018, 131, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Yoshioka, Y.; Isohashi, F.; Seo, Y.; Yoshida, K.; Yamazaki, H. Radiotherapy targeting cancer stem cells: Current views and future perspectives. Anticancer Res. 2013, 33, 747–754. [Google Scholar] [PubMed]
- Hicks, D.G.; Yoder, B.J.; Pettay, J.; Swain, E.; Tarr, S.; Hartke, M.; Skacel, M.; Crowe, J.P.; Budd, G.T.; Tubbs, R.R. The incidence of topoisomerase II-alpha genomic alterations in adenocarcinoma of the breast and their relationship to human epidermal growth factor receptor-2 gene amplification: A fluorescence in situ hybridization study. Hum. Pathol. 2005, 36, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Vlashi, E.; Pajonk, F. Cancer stem cells, cancer cell plasticity and radiation therapy. Semin. Cancer Biol. 2015, 31, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.C.; Tsai, C.Y.; Tsai, M.M.; Yeh, C.T.; Lin, K.H. Roles of Long Noncoding RNAs in Recurrence and Metastasis of Radiotherapy-Resistant Cancer Stem Cells. Int. J. Mol. Sci. 2017, 18, 1903. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Ranalli, T.; Musio, D.; Lisi, R.; Rea, F.; Caiazzo, R.; Tombolini, V. Relation between Hypofractionated Radiotherapy, Toxicity and Outcome in Early Breast Cancer. Breast J. 2017, 23, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Valle, L.F.; Agarwal, S.; Bickel, K.E.; Herchek, H.A.; Nalepinski, D.C.; Kapadia, N.S. Hypofractionated whole breast radiotherapy in breast conservation for early-stage breast cancer: A systematic review and meta-analysis of randomized trials. Breast Cancer Res. Treat. 2017, 162, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.A.; Shirvani, S.M.; Lal, L.; Swint, J.M.; Cantor, S.B.; Smith, B.D.; Likhacheva, A. Cost-effectiveness Analysis Comparing Conventional, Hypofractionated, and Intraoperative Radiotherapy for Early-Stage Breast Cancer. J. Natl. Cancer Inst. 2017, 109, djx068. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cabarcas, S.M.; Farrar, W.L. Radioresistance and Cancer Stem Cells: Survival of the Fittest. J. Carcinog Mutagen 2011, 1, 1–12. [Google Scholar] [CrossRef]
- Di Cara, G.; Marabeti, M.R.; Musso, R.; Riili, I.; Cancemi, P.; Pucci Minafra, I. New Insights into the Occurrence of Matrix Metalloproteases -2 and -9 in a Cohort of Breast Cancer Patients and Proteomic Correlations. Cells 2018, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Artacho-Cordón, F.; Ríos-Arrabal, S.; Lara, P.C.; Artacho-Cordón, A.; Calvente, I.; Núñez, M.I. Matrix metalloproteinases: Potential therapy to prevent the development of second malignancies after breast radiotherapy. Surg. Oncol. 2012, 21, e143–e151. [Google Scholar] [CrossRef] [PubMed]
- Artacho-Cordón, F.; Ríos-Arrabal, S.; Olivares-Urbano, M.A.; Storch, K.; Dickreuter, E.; Muñoz-Gámez, J.A.; León, J.; Calvente, I.; Torné, P.; Salinas, M.; et al. Valproic acid modulates radiation-enhanced matrix metalloproteinase activity and invasion of breast cancer cells. Int. J. Radiat. Biol. 2015, 91, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Tauro, M.; Lynch, C.C. Cutting to the Chase: How Matrix Metalloproteinase-2 Activity Controls Breast-Cancer-to-Bone Metastasis. Cancers 2018, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Radisky, E.S.; Radisky, D.C. Matrix metalloproteinases as breast cancer drivers and therapeutic targets. Front. Biosci. (Landmark Ed.) 2015, 20, 1144–1163. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, E.; Dasse, E.; Haye, B.; Petitfrere, E. TIMPs as multifacial proteins. Crit. Rev. Oncol. Hematol. 2004, 49, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, I.; Matsubara, H. Recent advances in histone deacetylase targeted cancer therapy. Surg. Today 2010, 40, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Young, D.A.; Lakey, R.L.; Pennington, C.J.; Jones, D.; Kevorkian, L.; Edwards, D.R.; Cawston, T.E.; Clark, I.M. Histone deacetylase inhibitors modulate metalloproteinase gene expression in chondrocytes and block cartilage resorption. Arthritis Res. Ther. 2005, 7, R503–R512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koledova, Z. 3D Cell Culture: An Introduction. In 3D Cell Culture: Methods and Protocols; Koledova, Z., Ed.; Humana Press: New York, NY, USA, 2017; pp. 1–11. [Google Scholar] [CrossRef]
- Li, X.P.; Zhang, X.W.; Zheng, L.Z.; Guo, W.J. Expression of CD44 in pancreatic cancer and its significance. Int. J. Clin. Exp. Pathol. 2015, 8, 6724–6731. [Google Scholar] [PubMed]
- Ohkoshi, E.; Uemura, N. Induced overexpression of CD44 associated with resistance to apoptosis on DNA damage response in human head and neck squamous cell carcinoma cells. Int. J. Oncol. 2017, 50, 387–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.H.; Kim, M.H.; Kim, K.S.; Park, M.J.; Jeong, J.H.; Park, S.W.; Ji, Y.H.; Kim, K.I.; Lee, T.S.; Ryu, P.Y.; et al. In vivo monitoring of CD44+ cancer stem-like cells by γ-irradiation in breast cancer. Int. J. Oncol. 2016, 48, 2277–2286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrose, J.; Livitz, M.; Wessels, D.; Kuhl, S.; Lusche, D.F.; Scherer, A.; Voss, E.; Soll, D.R. Mediated coalescence: A possible mechanism for tumor cellular heterogeneity. Am. J. Cancer Res. 2015, 5, 3485–3504. [Google Scholar] [PubMed]
- Swartz, M.A.; Iida, N.; Roberts, E.W.; Sangaletti, S.; Wong, M.H.; Yull, F.E.; Coussens, L.M.; DeClerck, Y.A. Tumor microenvironment complexity: Emerging roles in cancer therapy. Cancer Res. 2012, 72, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Upreti, M.; Jyoti, A.; Sethi, P. Tumor microenvironment and nanotherapeutics. Transl. Cancer Res. 2013, 2, 309–319. [Google Scholar] [PubMed]
- Faurobert, E.; Bouin, A.P.; Albiges-Rizo, C. Microenvironment, tumor cell plasticity, and cancer. Curr. Opin. Oncol. 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ailles, L.E.; Weissman, I.L. Cancer stem cells in solid tumors. Curr. Opin. Biotechnol. 2007, 18, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.H.; Murph, M.M.; Chang, J.Y. Cancer stem cell radioresistance and enrichment: Where frontline radiation therapy may fail in lung and esophageal cancers. Cancers 2011, 3, 1232–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Casal, R.; Bhattacharya, C.; Ganesh, N.; Bailey, L.; Basse, P.; Gibson, M.; Epperly, M.; Levina, V. Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelial-mesenchymal transition phenotypes. Mol. Cancer 2013, 12, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, M.; Krause, M.; Hill, R. Exploring the role of cancer stem cells in radioresistance. Nat. Rev. Cancer 2008, 8, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Köhrmann, A.; Kammerer, U.; Kapp, M.; Dietl, J.; Anacker, J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literature. BMC Cancer 2009, 9, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Tada, M.; Hida, Y.; Asano, T.; Kuramae, T.; Takemoto, N.; Hamada, J.; Miyamoto, M.; Hirano, S.; Kondo, S.; et al. High MMP-1 mRNA expression is a risk factor for disease-free and overall survivals in patients with invasive breast carcinoma. J. Surg. Res. 2008, 146, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.J.; Kuo, Y.L.; Chen, C.C.; Chen, M.J.; Cheng, Y.M. MMP1 expression is activated by Slug and enhances multi-drug resistance (MDR) in breast cancer. PLoS ONE 2017, 12, e0174487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.P.; Nair, S.; Shyamasundar, S.; Chua, P.J.; Muniasamy, U.; Matsumoto, K.; Gunaratne, J.; Bay, B.H. Silencing Y-box binding protein-1 inhibits triple-negative breast cancer cell invasiveness via regulation of MMP1 and beta-catenin expression. Cancer Lett. 2019, 452, 119–131. [Google Scholar] [CrossRef] [PubMed]
- He, E.; Pan, F.; Li, G.; Li, J. Fractionated ionizing radiation promotes epithelial-mesenchymal transition in human esophageal cancer cells through PTEN deficiency-mediated akt activation. PLoS ONE 2015, 10, e0126149. [Google Scholar] [CrossRef]
- Kim, E.; Youn, H.; Kwon, T.; Son, B.; Kang, J.; Yang, H.J.; Seong, K.M.; Kim, W.; Youn, B. PAK1 tyrosine phosphorylation is required to induce epithelial-mesenchymal transition and radioresistance in lung cancer cells. Cancer Res. 2014, 74, 5520–5531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.S.; Jiang, J.; Liang, X.H.; Tang, Y.L. Links between cancer stem cells and epithelial-mesenchymal transition. Onco Targets Ther. 2015, 8, 2973–2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Huang, X.; Zheng, X.; Wang, X.; Li, S.; Zhang, L.; Yang, Z.; Xia, Z. Enrichment of prostate cancer stem-like cells from human prostate cancer cell lines by culture in serum-free medium and chemoradiotherapy. Int. J. Biol. Sci. 2013, 9, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Lagadec, C.; Vlashi, E.; Della Donna, L.; Dekmezian, C.; Pajonk, F. Radiation-induced reprogramming of breast cancer cells. Stem Cells 2012, 30, 833–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghisolfi, L.; Keates, A.C.; Hu, X.; Lee, D.K.; Li, C.J. Ionizing radiation induces stemness in cancer cells. PLoS ONE 2012, 7, e43628. [Google Scholar] [CrossRef] [PubMed]
- Pavlopoulou, A.; Oktay, Y.; Vougas, K.; Louka, M.; Vorgias, C.E.; Georgakilas, A.G. Determinants of resistance to chemotherapy and ionizing radiation in breast cancer stem cells. Cancer Lett. 2016, 380, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.L.; Reinke, L.M.; Damerow, M.S.; Perez, D.; Chodosh, L.A.; Yang, J.; Cheng, C. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial- mesenchymal transition and breast cancer progression. J. Clin. Investig. 2011, 121, 1064–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011, 19, 387–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yae, T.; Tsuchihashi, K.; Ishimoto, T.; Motohara, T.; Yoshikawa, M.; Yoshida, G.J.; Wada, T.; Masuko, T.; Mogushi, K.; Tanaka, H.; et al. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat. Commun. 2012, 3, 883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, M.; Tsuchihashi, K.; Ishimoto, T.; Yae, T.; Motohara, T.; Sugihara, E.; Onishi, N.; Masuko, T.; Yoshizawa, K.; Kawashiri, S.; et al. xCT inhibition depletes CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy in head and neck squamous cell carcinoma. Cancer Res. 2013, 73, 1855–1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosulski, A.; Horn, H.; Zhang, L.; Coletti, C.; Vathipadiekal, V.; Castro, C.M.; Birrer, M.J.; Nagano, O.; Saya, H.; Lage, K.; et al. CD44 splice variant v8-10 as a marker of serious ovarian cancer prognosis. PLoS ONE 2016, 11, e0156595. [Google Scholar] [CrossRef] [PubMed]
- Tsubouchi, K.; Minami, K.; Hayashi, N.; Yokoyama, Y.; Mori, S.; Yamamoto, H.; Koizumi, M. The CD44 standard isoform contributes to radioresistance of pancreatic cancer cells. J. Radiat. Res. 2017, 58, 816–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.S.; Bhatt, M.L.B.; Kushwaha, V.S.; Singh, A.; Kumar, R.; Gupta, R.; Parmar, D. Role of matrix metalloproteinase 13 gene expression in the evaluation of radiation response in oral squamous cell carcinoma. J. Carcinog. 2017, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Takahashi, H.; Harada, H.; Kohno, S.; Ohue, S.; Kobayashi, K.; Yano, H.; Tanaka, J.; Ohnishi, T. Cancer stem-like cells of glioblastoma characteristically express MMP-13 and display highly invasive activity. Int. J. Oncol. 2010, 37, 1121–1131. [Google Scholar] [PubMed] [Green Version]
- Wagner, J.M.; Hackanson, B.; Lübbert, M.; Jung, M. Histone deacetylase (HDAC) inhibitors in recent clinical trials for cancer therapy. Clin. Epigenetics 2010, 1, 117–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Seto, E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasajima, A.; Lehmann, A.; Prinzler, J.; Budczies, J.; Winzer, K.J.; Dietel, M.; Weichert, W.; Denkert, C. Differential expression of histone deacetylases HDAC1, 2 and 3 in human breast cance--overexpressión of HDAC2 and HDAC3 is associated with clinicopathological indicators of disease progression. BMC Cancer 2013, 13, 215. [Google Scholar] [CrossRef] [Green Version]
- Kao, G.D.; McKenna, W.G.; Guenther, M.G.; Muschel, R.J.; Lazar, M.A.; Yen, T.J. Histone deacetylase 4 interacts with 53BP1 to mediate the DNA damage response. J. Cell Biol. 2003, 160, 1017–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marampon, F.; Megiorni, F.; Camero, S.; Crescioli, C.; McDowell, H.P.; Sferra, R.; Vetuschi, A.; Pompili, S.; Ventura, L.; De Felice, F.; et al. HDAC4 and HDAC6 sustain DNA double strand break repair and stem-like phenotype by promoting radioresistance in glioblastoma cells. Cancer Lett. 2017, 397, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bayat, S.; Mansoori Derakhshan, S.; Mansoori Derakhshan, N.; Shekari Khaniani, M.; Alivand, M.R. Downregulation of HDAC2 and HDAC3 via oleuropein as a potent prevention and therapeutic agent in MCF-7 breast cancer cells. J. Cell Biochem. 2019, 120, 9172–9180. [Google Scholar] [CrossRef] [PubMed]
- La Noce, M.; Paino, F.; Mele, L.; Papaccio, G.; Regad, T.; Lombardi, A.; Papaccio, F.; Desiderio, V.; Tirino, V. HDAC2 depletion promotes osteosarcoma’s stemness both in vitro and in vivo: A study on a putative new target for CSCs directed therapy. J. Exp. Clin. Cancer Res. 2018, 37, 296. [Google Scholar] [CrossRef] [PubMed]
- Cid, S.; Eiro, N.; Fernández, B.; Sánchez, R.; Andicoechea, A.; Fernández-Muñiz, P.I.; González, L.O.; Vizoso, F.J. Prognostic Influence of tumor stroma on breast cancer subtypes. Clin. Breast Cancer 2018, 18, e123–e133. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.T.; Hsu, H.T.; Lee, C.C.; Lee, H.C.; Kuo, Y.J.; Hua, K.; Hsia, C.Y.; Chi, C.W. Krüppel-like factor 4 modulates the migration and invasion of hepatoma cells by suppressing TIMP-1 and TIMP-2. Oncol. Rep. 2015, 34, 439–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, A.; Reid, H.M.; Baker, A.H.; McGlynn, H. Modifications of the radiosensitivity of a renal cancer cell line as a consequence of stable TIMP-1 overexpression. Int. J. Radiat. Biol. 2007, 83, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Varela, I.; Menendez, P.; Sanjuan-Pla, A. Intratumoral heterogeneity and clonal evolution in blood malignancies and solid tumors. Oncotarget 2017, 8, 66742–66746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, G.; Hackenberg, M.; Catalina, P.; Boulaiz, H.; Griñán-Lisón, C.; García, M.A.; Perán, M.; López-Ruiz, E.; Ramírez, A.; Morata-Tarifa, C.; et al. Mesenchymal stem cell’s secretome promotes selective enrichment of cancer stem-like cells with specific cytogenetic profile. Cancer Lett. 2018, 429, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Brueckmann, I.; Scheel, C.; Kaestli, A.J.; Wiggins, P.A.; Rodrigues, L.O.; Brooks, M.; Reinhardt, F.; Su, Y.; Polyak, K.; et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc. Natl. Acad. Sci. USA 2011, 108, 7950–7955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, G.J. The heterogeneity of cancer stem-like cells at the invasive front. Cancer Cell Int. 2017, 17, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Lin, X.; Liu, Y.; Gong, W.; Ma, X.; Yu, Y.; Xie, Y.; Sun, X.; Feng, Y.; Janzen, V.; et al. Transformation of epithelial ovarian cancer stemlike cells into mesenchymal lineage via EMT results in cellular heterogeneity and supports tumor engraftment. Mol. Med. 2012, 18, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.D.C.; Lopes, C. Implications of different cancer stem cell phenotypes in breast cancer. Anticancer Res. 2017, 37, 2173–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinel Lamas, D.J.; Cortina, J.E.; Ventura, C.; Sterle, H.A.; Valli, E.; Balestrasse, K.B.; Blanco, H.; Graciela, A.; Cremaschi, G.A.; Elena, S.; et al. Enhancement of ionizing radiation response by histamine in vitro and in vivo in human breast cancer. Cancer Biol. Ther. 2015, 16, 137–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcellos-Hoff, M.H.; Park, C.; Wright, E.G. Radiation and the microenvironment-tumorigenesis and therapy. Nat. Rev. Cancer 2005, 5, 867–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodward, W.A.; Chen, M.S.; Behbod, F.; Alfaro, M.P.; Buchholz, T.A.; Rosen, J.M. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc. Natl. Acad. Sci. USA 2007, 104, 618–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghotra, V.P.; Puigvert, J.C.; Danen, E.H. The cancer stem cell microenvironment and anti-cancer therapy. Int. J. Radiat. Biol. 2009, 85, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, S.L.; Finniss, S.; Xiang, C.; Decarvalho, A.; Umansky, F.; Kalkanis, S.N.; Mikkelsen, T.; Brodie, C. The induction of autophagy by gamma-radiation contributes to the radioresistance of glioma stem cells. Int. J. Cancer 2009, 125, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Vyas, P.; Enver, T. Molecular targeting of cancer stem cells. Cell Stem Cell 2009, 5, 125–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rassi, H. Stem cell therapy for hereditary breast cancer. Tsitol. Genet. 2009, 43, 80–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubor, P.; Gondova, A.; Polivka, J., Jr.; Kasajova, P.; Konieczka, K.; Danko, J.; Golubnitschaja, O. Breast cancer and Flammer syndrome: Any symptoms in common for prediction, prevention and personalised medicalapproach? EPMA J. 2017, 8, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golubnitschaja, O.; Filep, N.; Yeghiazaryan, K.; Blom, H.J.; Hofmann-Apitius, M.; Kuhn, W. Multi-omic approach decodes paradoxes of the triple-negative breast cancer: Lessons for Predictive, Preventive and Personalised Medicine. Amino Acids 2018, 50, 383–395. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivares-Urbano, M.A.; Griñán-Lisón, C.; Ríos-Arrabal, S.; Artacho-Cordón, F.; Torralbo, A.I.; López-Ruiz, E.; Marchal, J.A.; Núñez, M.I. Radiation and Stemness Phenotype May Influence Individual Breast Cancer Outcomes: The Crucial Role of MMPs and Microenvironment. Cancers 2019, 11, 1781. https://doi.org/10.3390/cancers11111781
Olivares-Urbano MA, Griñán-Lisón C, Ríos-Arrabal S, Artacho-Cordón F, Torralbo AI, López-Ruiz E, Marchal JA, Núñez MI. Radiation and Stemness Phenotype May Influence Individual Breast Cancer Outcomes: The Crucial Role of MMPs and Microenvironment. Cancers. 2019; 11(11):1781. https://doi.org/10.3390/cancers11111781
Chicago/Turabian StyleOlivares-Urbano, María Auxiliadora, Carmen Griñán-Lisón, Sandra Ríos-Arrabal, Francisco Artacho-Cordón, Ana Isabel Torralbo, Elena López-Ruiz, Juan Antonio Marchal, and María Isabel Núñez. 2019. "Radiation and Stemness Phenotype May Influence Individual Breast Cancer Outcomes: The Crucial Role of MMPs and Microenvironment" Cancers 11, no. 11: 1781. https://doi.org/10.3390/cancers11111781
APA StyleOlivares-Urbano, M. A., Griñán-Lisón, C., Ríos-Arrabal, S., Artacho-Cordón, F., Torralbo, A. I., López-Ruiz, E., Marchal, J. A., & Núñez, M. I. (2019). Radiation and Stemness Phenotype May Influence Individual Breast Cancer Outcomes: The Crucial Role of MMPs and Microenvironment. Cancers, 11(11), 1781. https://doi.org/10.3390/cancers11111781






