Serum miRNA Are Promising Biomarkers for the Detection of Early Hepatocellular Carcinoma after Treatment with Direct-Acting Antivirals
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Study Population
2.2. Changes in Circulating miRNA after DAA Therapy
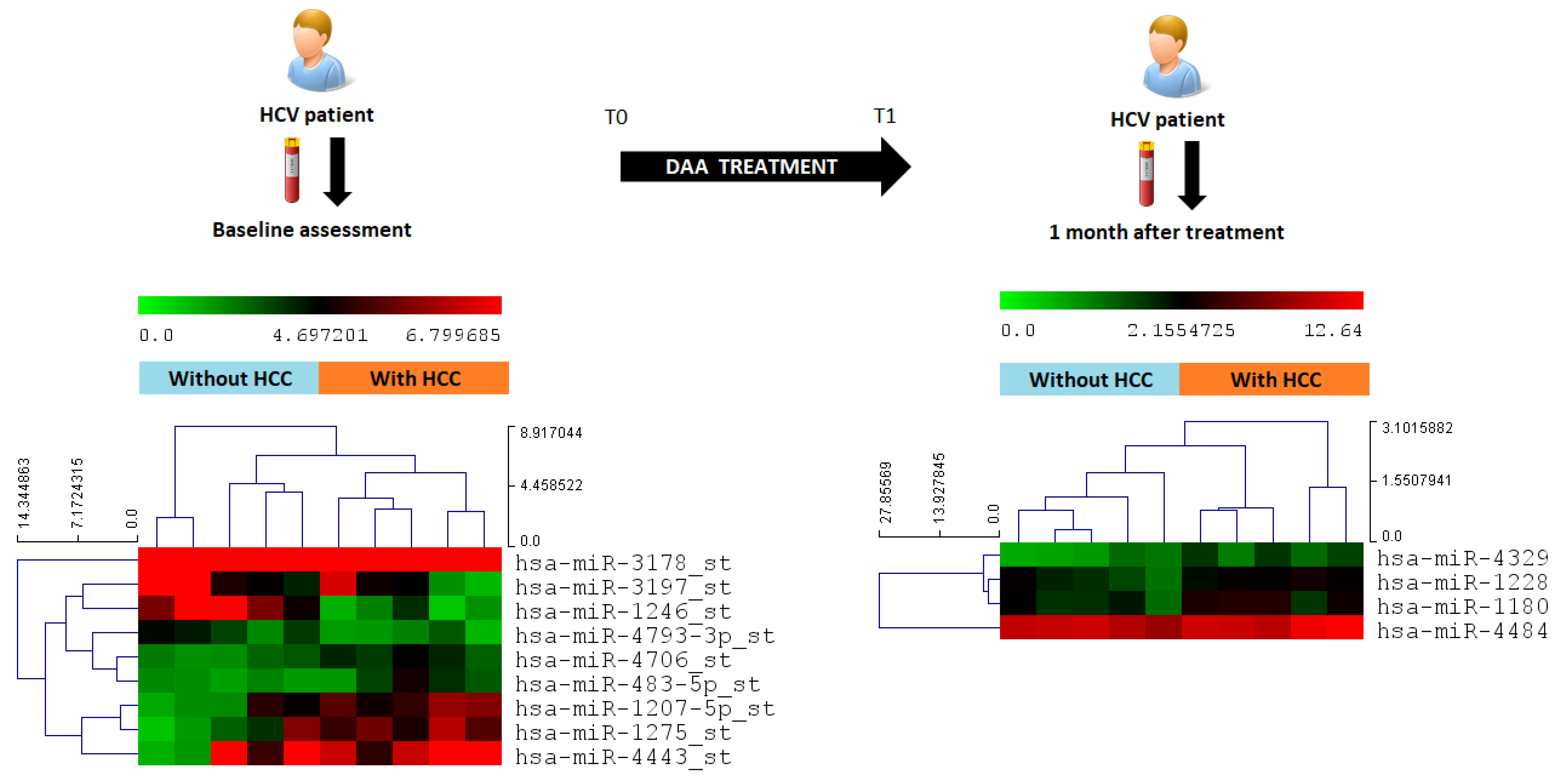
2.3. MicroRNA Screening in the Discovery Cohort, Identification of Predictive miRNA
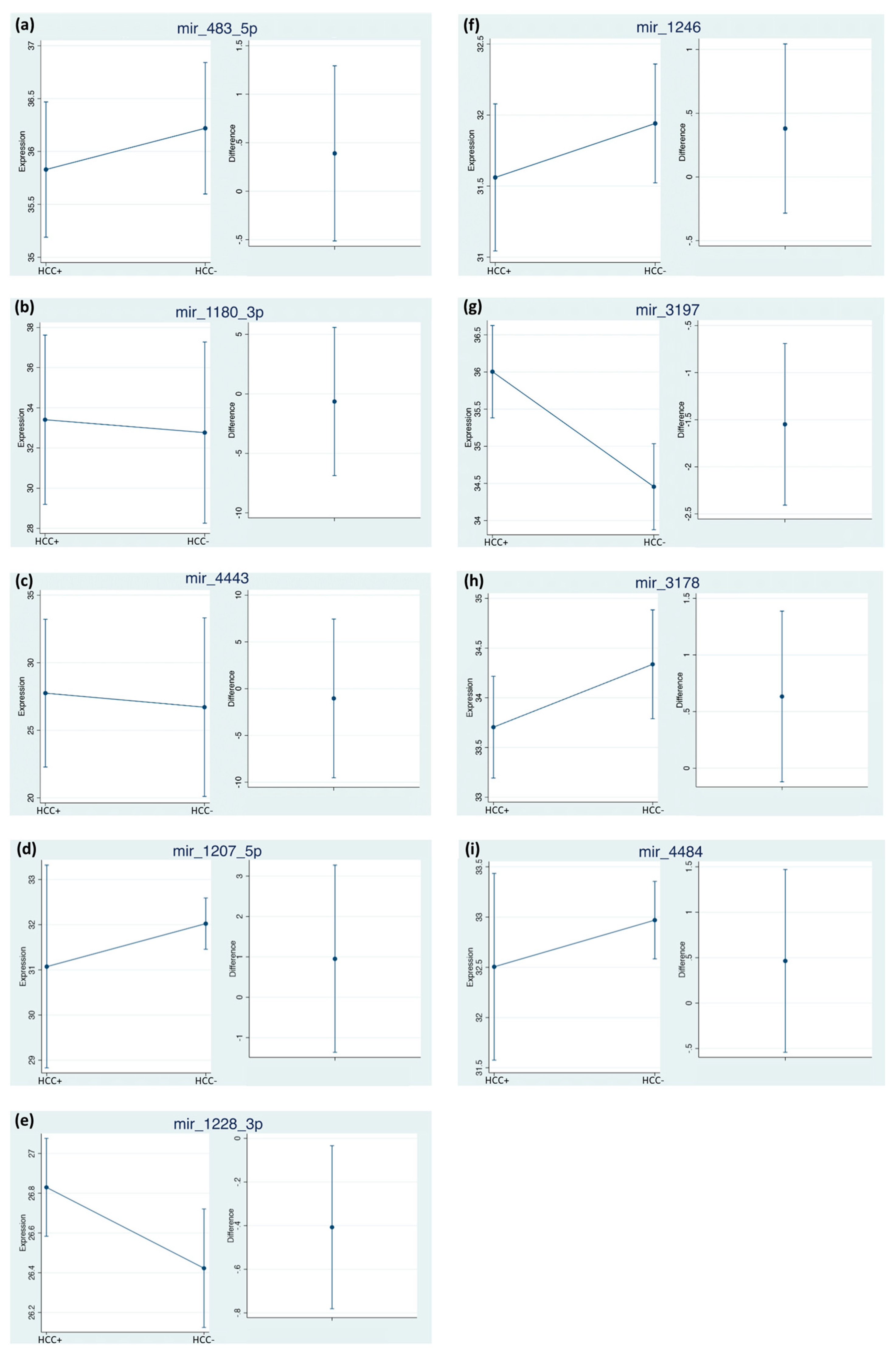
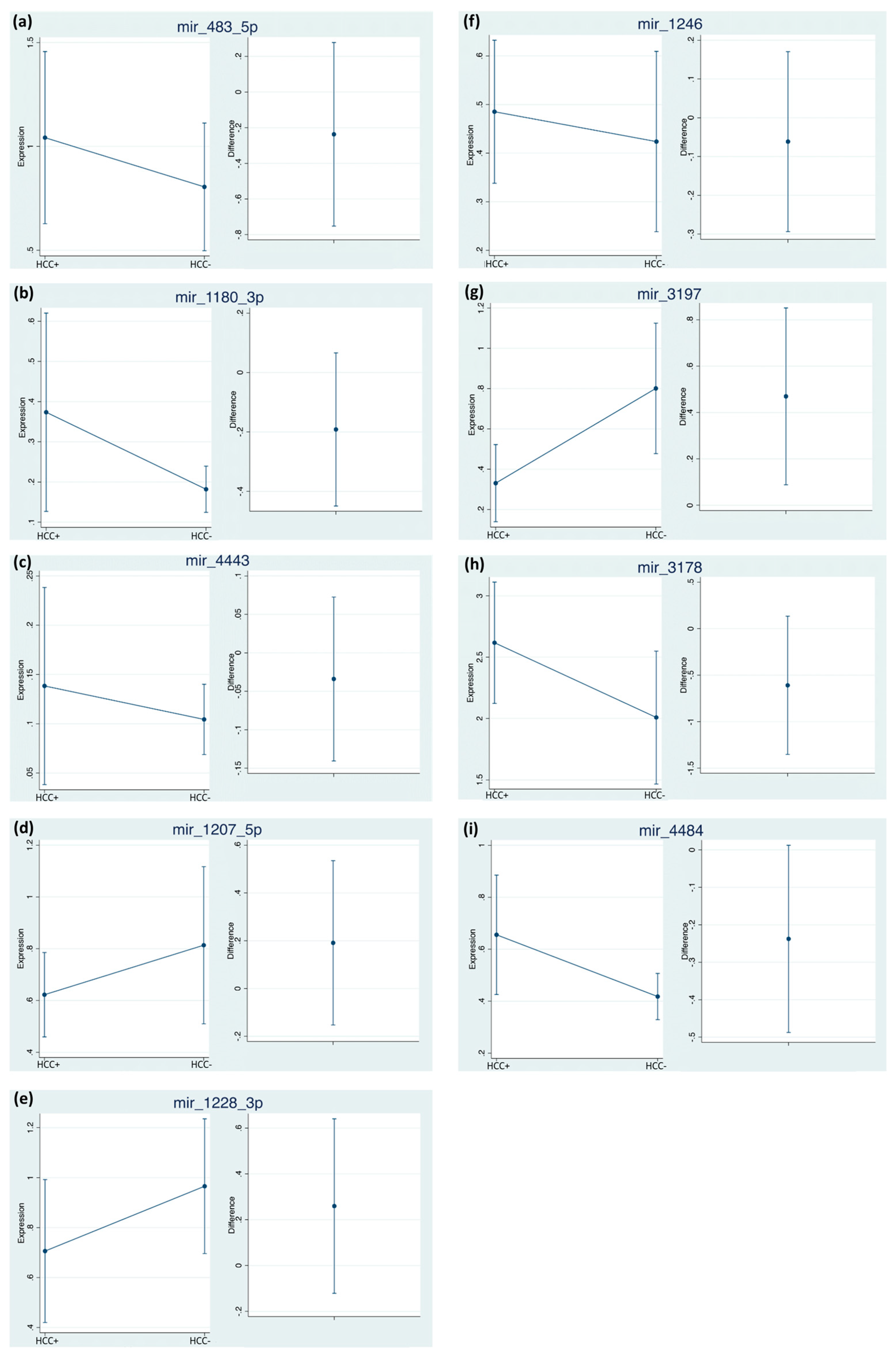
2.4. Differential miRNA Expression Profiles and Identification of miRNA Biomarker Candidates
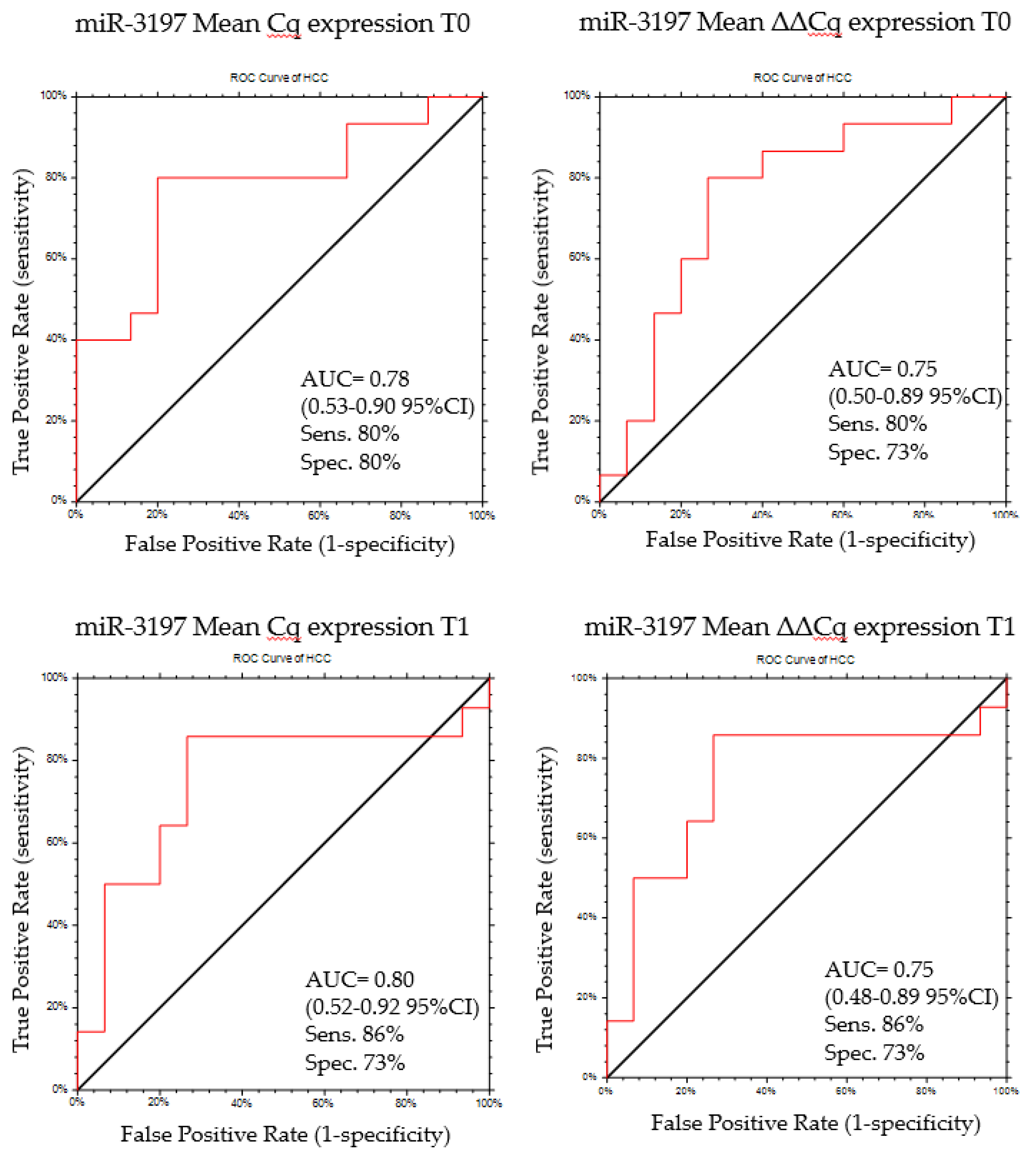
2.5. MiR-3197 as Diagnostic Biomarker
3. Discussion
4. Materials and Methods
4.1. Patient Characteristics
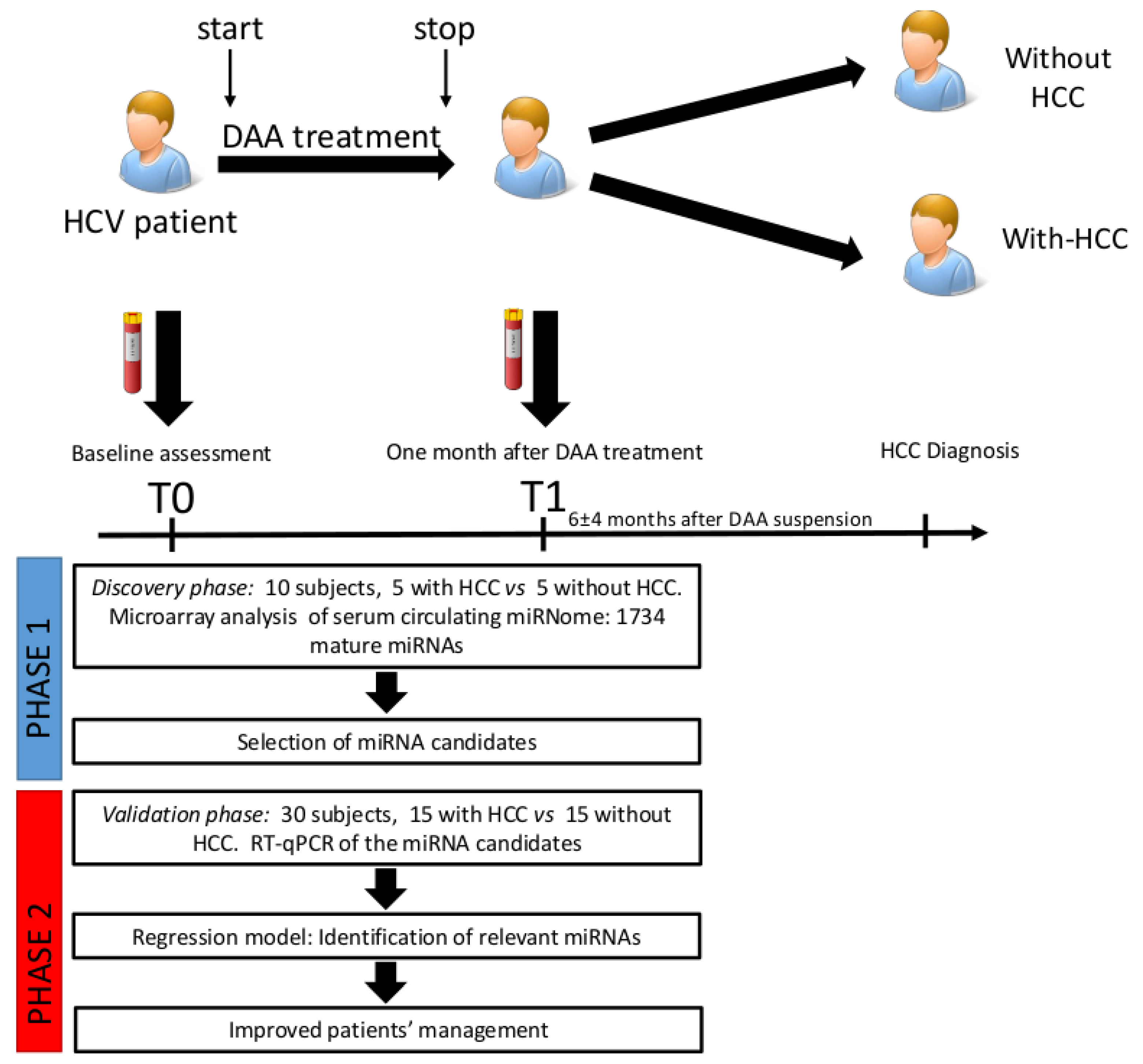
4.2. Study Design
4.3. Serum Collection and RNA Extraction
4.4. Microarray Profiling
4.5. Quantitative Real Time PCR (RT-qPCR)
4.6. Microarray Data Analysis
4.7. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.V.; King, L.Y.; Chung, R.T. Hepatitis C virus-associated cancer. Annu. Rev. Pathol. 2015, 10, 345–370. [Google Scholar] [CrossRef] [PubMed]
- Tampaki, M.; Savvanis, S.; Koskinas, J. Impact of direct-acting antiviral agents on the development of hepatocellular carcinoma: Evidence and pathophysiological issues. Ann. Gastroenterol. 2018, 31, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Falade-Nwulia, O.; Suarez-Cuervo, C.; Nelson, D.R.; Fried, M.W.; Segal, J.B.; Sulkowski, M.S. Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review. Ann. Intern. Med. 2017, 166, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Bourlière, M.; Sulkowski, M.; Omata, M.; Zeuzem, S.; Feld, J.J.; Lawitz, E.; Marcellin, P.; Welzel, T.M.; Hyland, R.; et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: An integrated safety and efficacy analysis. Hepatology 2015, 62, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Saab, S.; Park, S.H.; Mizokami, M.; Omata, M.; Mangia, A.; Eggleton, E.; Zhu, Y.; Knox, S.J.; Pang, P.; Subramanian, M.; et al. Safety and efficacy of ledipasvir/sofosbuvir for the treatment of genotype 1 hepatitis C in subjects aged 65 years or older. Hepatology 2016, 63, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005.e1. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Baack, B.; Smith, B.D.; Yartel, A.; Pitasi, M.; Falck-Ytter, Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: A meta-analysis of observational studies. Ann. Intern. Med. 2013, 158, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Buonfiglioli, F.; Scuteri, A.; Crespi, C.; Bolondi, L.; Caraceni, P.; Foschi, F.G.; Lenzi, M.; Mazzella, G.; Verucchi, G.; et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J. Hepatol. 2016, 65, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.C.M.; Walker, A.J.; Hudson, B.E.; Verma, S.; McLauchlan, J.; Mutimer, D.J.; Brown, A.; Gelson, W.T.H.; MacDonald, D.C.; Agarwal, K.; et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J. Hepatol. 2016, 65, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, H.; Vale, A.M.; Rodrigues, S.; Gonçalves, R.; Albuquerque, A.; Pereira, P.; Lopes, S.; Silva, M.; Andrade, P.; Morais, R.; et al. High incidence of hepatocellular carcinoma following successful interferon-free antiviral therapy for hepatitis C associated cirrhosis. J. Hepatol. 2016, 65, 1070–1071. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Mariño, Z.; Perelló, C.; Iñarrairaegui, M.; Ribeiro, A.; Lens, S.; Díaz, A.; Vilana, R.; Darnell, A.; Varela, M.; et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 2016, 65, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Hashimoto, S.; Abiru, S.; Komori, A.; Yamasaki, K.; Nagaoka, S.; Saeki, A.; Bekki, S.; Kugiyama, Y.; Kuroki, T.; et al. Rapidly growing, moderately differentiated HCC: A clinicopathological characteristic of HCC occurrence after IFN-free DAA therapy? J. Hepatol. 2018, 68, 854–855. [Google Scholar] [CrossRef] [PubMed]
- Warzyszyńska, K.; Jonas, M.; Wasiak, D.; Kosieradzki, M.; Małkowski, P. Accelerated hepatocellular carcinoma recurrence rate after postoperative direct-acting antivirals treatment—preliminary report. Clin. Exp. Hepatol. 2017, 3, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Green, P.K.; Berry, K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J. Hepatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, A.J.; Feld, J.J.; Hofer, H.; Almasio, P.L.; Calvaruso, V.; Fernández-Rodríguez, C.M.; Aleman, S.; Ganne-Carrié, N.; D’Ambrosio, R.; Pol, S.; et al. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J. Hepatol. 2017, 66, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Feld, J.J. What are the Benefits of a Sustained Virologic Response to Direct-acting Antiviral Therapy for HCV Infection? Gastroenterology 2019, 15, 446–460.e2. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Green, P.K.; Beste, L.A.; Mun, E.J.; Kerr, K.F.; Berry, K. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J. Hepatol. 2018, 69, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Nakagawa, M.; Asahina, Y.; Sato, A.; Asano, Y.; Tsunoda, T.; Miyoshi, M.; Kaneko, S.; Otani, S.; Kawai-Kitahata, F.; et al. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J. Hepatol. 2017, 67, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Goossens, N.; Finn, R.S.; Schmidt, W.N.; Thung, S.N.; Im, G.Y.; Hoshida, Y. Precision Liver Cancer Prevention Consortium Persisting risk of hepatocellular carcinoma after hepatitis C virus cure monitored by a liver transcriptome signature. Hepatology 2017, 66, 1344–1346. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. microRNAs: Tiny Regulators with Great Potential. Cell 2001, 107, 823–826. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Tat Trung, N.; Duong, D.C.; Tong, H.V.; Hien, T.T.T.; Hoan, P.Q.; Bang, M.H.; Binh, M.T.; Ky, T.D.; Tung, N.L.; Thinh, N.T.; et al. Optimisation of quantitative miRNA panels to consolidate the diagnostic surveillance of HBV-related hepatocellular carcinoma. PLoS ONE 2018, 13, e0196081. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Pang, Y.-Y.; He, R.-Q.; Lin, P.; Cen, J.-M.; Yang, H.; Ma, J.; Chen, G. Diagnostic value of strand-specific miRNA-101-3p and miRNA-101-5p for hepatocellular carcinoma and a bioinformatic analysis of their possible mechanism of action. FEBS Open Bio 2018, 8, 64–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, T.; Qiu, Y.; Zhang, T.; Guo, P.; Ma, X.; Wei, Q.; Han, L. Serum microRNA panel for early diagnosis of the onset of hepatocellular carcinoma. Medicine (Baltimore) 2017, 96, e5642. [Google Scholar] [CrossRef] [PubMed]
- Pascut, D.; Krmac, H.; Gilardi, F.; Patti, R.; Calligaris, R.; Crocè, L.S.; Tiribelli, C. A comparative characterization of the circulating miRNome in whole blood and serum of HCC patients. Sci. Rep. 2019, 9, 8265. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, F.; Lin, X.; Huang, C.; Zhang, Y.; Li, Y.; Lin, J.; Chen, W.; Lin, X. MicroRNA-1225-5p inhibits proliferation and metastasis of gastric carcinoma through repressing insulin receptor substrate-1 and activation of β-catenin signaling. Oncotarget 2016, 7, 4647–4663. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhang, D.; Huang, H.; Yu, Y.; Yang, Z.; Niu, Y.; Liu, J. MicroRNA-1225-5p acts as a tumor-suppressor in laryngeal cancer via targeting CDC14B. Biol. Chem. 2019, 400, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Faillaci, F.; Marzi, L.; Critelli, R.; Milosa, F.; Schepis, F.; Turola, E.; Andreani, S.; Vandelli, G.; Bernabucci, V.; Lei, B.; et al. Liver Angiopoietin-2 Is a Key Predictor of De Novo or Recurrent Hepatocellular Cancer After Hepatitis C Virus Direct-Acting Antivirals. Hepatology 2018, 68, 1010–1024. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Matsuoka, S.; Moriyama, M. Early occurrence and recurrence of hepatocellular carcinoma in hepatitis C virus-infected patients after sustained virological response. Hepatol. Int. 2018, 12, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Langhans, B.; Nischalke, H.D.; Krämer, B.; Hausen, A.; Dold, L.; van Heteren, P.; Hüneburg, R.; Nattermann, J.; Strassburg, C.P.; Spengler, U. Increased peripheral CD4+ regulatory T cells persist after successful direct-acting antiviral treatment of chronic hepatitis C. J. Hepatol. 2017, 66, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Debes, J.D.; van Tilborg, M.; Groothuismink, Z.M.A.; Hansen, B.E.; Schulze Zur Wiesch, J.; von Felden, J.; de Knegt, R.J.; Boonstra, A. Levels of Cytokines in Serum Associate With Development of Hepatocellular Carcinoma in Patients With HCV Infection Treated With Direct-Acting Antivirals. Gastroenterology 2018, 154, 515–517.e3. [Google Scholar] [CrossRef] [PubMed]
- Bandiera, S.; Pfeffer, S.; Baumert, T.F.; Zeisel, M.B. miR-122—A key factor and therapeutic target in liver disease. J. Hepatol. 2015, 62, 448–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waring, J.F.; Dumas, E.O.; Abel, S.; Coakley, E.; Cohen, D.E.; Davis, J.W.; Podsadecki, T.; Dutta, S. Serum miR-122 may serve as a biomarker for response to direct acting antivirals: Effect of paritaprevir/R with dasabuvir or ombitasvir on miR-122 in HCV-infected subjects. J. Viral. Hepat. 2016, 23, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Dong, L.; Shi, H.; Li, H.; Lu, X.; Guo, X.; Wang, J. MicroRNA-1207-5p inhibits hepatocellular carcinoma cell growth and invasion through the fatty acid synthase-mediated Akt/mTOR signalling pathway. Oncol. Rep. 2016, 36, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Guo, D.; Chen, X.; Xiong, W.; Jie, S.; Li, H. Abnormal miRNAs Targeting Chromosome Open Reading Frame Genes were Enriched in Microvesicles Derived from the Circulation of HCC. Biochem. Genet. 2016, 54, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhao, J. miR-1228 prevents cellular apoptosis through targeting of MOAP1 protein. Apoptosis 2012, 17, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Liu, D.; Liang, H.; Xue, L.; Su, C.; Liu, M. MiR-1228 promotes breast cancer cell growth and metastasis through targeting SCAI protein. Int. J. Clin. Exp. Pathol. 2015, 8, 6646–6655. [Google Scholar] [PubMed]
- Liese, J.; Peveling-Oberhag, J.; Doering, C.; Schnitzbauer, A.A.; Herrmann, E.; Zangos, S.; Hansmann, M.L.; Moench, C.; Welker, M.W.; Zeuzem, S.; et al. A possible role of microRNAs as predictive markers for the recurrence of hepatocellular carcinoma after liver transplantation. Transpl. Int. 2016, 29, 369–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.U.; Seo, Y.-S.; Lee, Y.-H.; Park, J.; Hwang, I.; Kang, K.J.; Nam, J.; Kim, S.-W.; Kim, J.Y. Altered microRNA expression profile in hepatitis B virus-related hepatocellular carcinoma. Gene 2015, 573, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhu, H.-Q.; Ma, C.-Q.; Li, H.-G.; Liu, F.-F.; Chang, H.; Lu, J. MiR-1180 promoted the proliferation of hepatocellular carcinoma cells by repressing TNIP2 expression. Biomed. Pharmacother. 2016, 79, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.T.-P.; Lo, C.M.; Wong, N.; Li, C.X.; Qi, X.; Liu, X.B.; Geng, W.; Yeung, O.W.-H.; Ma, Y.Y.; Chan, S.C.; et al. Early-phase circulating miRNAs predict tumor recurrence and survival of hepatocellular carcinoma patients after liver transplantation. Oncotarget 2016, 7, 19824–19839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Wang, A.; Wang, Q.; Gurvich, I.; Siegel, A.B.; Remotti, H.; Santella, R.M. Exploration of genome-wide circulating microRNA in hepatocellular carcinoma: MiR-483-5p as a potential biomarker. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2364–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gui, J.; Tian, Y.; Wen, X.; Zhang, W.; Zhang, P.; Gao, J.; Run, W.; Tian, L.; Jia, X.; Gao, Y. Serum microRNA characterization identifies miR-885-5p as a potential marker for detecting liver pathologies. Clin. Sci. (Lond.) 2011, 120, 183–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Quetglas, I.; Pinyol, R.; Dauch, D.; Torrecilla, S.; Tovar, V.; Moeini, A.; Alsinet, C.; Portela, A.; Rodriguez-Carunchio, L.; Solé, M.; et al. IGF2 Is Up-regulated by Epigenetic Mechanisms in Hepatocellular Carcinomas and Is an Actionable Oncogene Product in Experimental Models. Gastroenterology 2016, 151, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.O.; Nabil, M.M.; Abdelmaksoud, A.H.; Shousha, H.I.; Hashem, M.B.; Hassan, E.M.; Salah, A.; Omran, D.A.; Elbaz, T.M. Tumor behavior of hepatocellular carcinoma after hepatitis C treatment by direct-acting antivirals: Comparative analysis with non-direct-acting antivirals-treated patients. Eur. J. Gastroenterol. Hepatol. 2019, 31, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Herrell, F. Regression Modeling Strategies; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Mettke, F.; Schlevogt, B.; Deterding, K.; Wranke, A.; Smith, A.; Port, K.; Manns, M.P.; Vogel, A.; Cornberg, M.; Wedemeyer, H. Interferon-free therapy of chronic hepatitis C with direct-acting antivirals does not change the short-term risk for de novo hepatocellular carcinoma in patients with liver cirrhosis. Aliment. Pharmacol. Ther. 2018, 47, 516–525. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Study Group | § p-Level | ||
|---|---|---|---|---|
| Overall 40 Patients | Discovery 10 Patients | Validation 30 Patients | ||
| Age, (years ± SD) | 56.3 ± 10.3 | 59.4 ± 6.3 | 55.0 ± 11.1 | 0.07 |
| Gender male/female, (% males) | 26/14 (67.5) | 6/4 (70) | 20/10 (66.6) | 0.84 |
| BMI, (kg/m2 ± SD) | 25.8 ± 3.1 | 27.3 ± 2.8 | 25.3 ± 3.1 | 0.08 |
| Genotype HCV-1/non-1, (% HCV-1) | 27/13 (67.5) | 6/4 (60) | 21/9 (70) | 0.84 |
| Child-Pugh score A5/A6-B7, (% A5) | 30/10 (75) | 8/2 (80) | 22/8 (73.3) | 1.0 |
| Naive/Experienced, (% naive) | 13/27 (32.5) | 1/9 (10) | 12/18 (40) | 0.17 |
| Sofosbuvir-based schedule, (%) | 35/5 (87.5) | 8/2 (80) | 27/3 (90) | 0.91 |
| Ribavirin combination use, (%) | 38/2 (95) | 10/0 (100) | 28/2 (93.3) | 1.0 |
| Treatment duration, (weeks ± SD) | 18.3 ± 5.6 | 20.4 ± 5.1 | 18.6 ± 5.6 | 0.16 |
| SVR/Relapse, (% SVR) | 32/8 (80) | 7/3 (70) | 25/5 (83.3) | 0.64 |
| ALT (U/L ± SD) | 114.9 ± 79.1 | 103.7 ± 87.6 | 110.2 ± 76.5 | 0.56 |
| Albumin (mg/dL ± SD) | 40.9 ± 3.4 | 41.1 ± 3.5 | 39.2 ±3.4 | 0.15 |
| Bilirubin (μmol/L ± SD) | 15.7 ± 7.1 | 14.5 ± 6.2 | 16.2 ± 8.3 | 0.36 |
| PT (INR ± SD) | 1.1 ± 0.08 | 1.0 ± 0.05 | 1.1 ± 0.09 | 0.76 |
| PLTS (×109/L ± SD) | 164 ± 110 | 120 ± 70 | 179 ± 120 | 0.61 |
| Alfa-fetoprotein (μg/L ± SD) | 19 ± 32 | 17 ± 11 | 21 ± 36 | 0.14 |
| HCC development, (%) | 20 (50) | 5 (50) | 15 (50) | 0.71 |
| Time to HCC diagnosis from begin of DAAs (months ± SD) | 8.9 ± 5.6 | 9.2 ± 5.1 | 8.8 ± 5.6 | 0.8 |
| miRNA | With HCC Mean Cq (95% CI) | With HCC Mean ΔΔCq (95% CI) | Without HCC Mean Cq (95% CI) | Without HCC Mean ΔΔCq (95% CI) |
|---|---|---|---|---|
| miR-483-5p | 35.83 (35.19–36.47) | 1.04 (0.62–1.47) | 36.22 (35.60–36.83) | 0.80 (0.51–1.10) |
| miR-1246 | 31.56 (31.06–32.06) | 0.48 (0.34–0.63) | 31.94 (31.50–32.38) | 0.42 (0.24–0.61) |
| miR-1180-3p | 33.41 (29.39–37.42) | 0.37 (0.13–0.62) | 32.77 (28.18–37.35) | 0.18 (0.12–0.24) |
| miR-3197 | 36.00 (35.38–36.63) | 0.33 (0.14–0.52) | 34.46 (33.89–35.01) | 0.80 (0.48–1.12) |
| miR-4443 | 27.75 (22.35–33.15) | 0.14 (0.04–0.24) | 26.71 (20.24–33.18) | 0.10 (0.07–0.14) |
| miR-3178 | 33.70 (33.20–34.21) | 2.61 (2.12–3.12 | 34.34 (33.82–34.85) | 2.01 (1.48–2.53) |
| miR-1207-5p | 31.07 (28.91–33.23) | 0.62 (0.46–0.79) | 32.02 (31.48–32.57) | 0.81 (0.51–1.12) |
| miR-1228-3p | 26.83 (26.59–27.07) | 0.71 (0.42–0.99) | 26.42 (26.14–26.71) | 0.97 (0.71–1.2) |
| miR-4484 | 32.5 (31.58–33.43) | 0.66 (0.43–0.88) | 32.97 (32.56–33.38) | 0.42 (0.33–0.51) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascut, D.; Cavalletto, L.; Pratama, M.Y.; Bresolin, S.; Trentin, L.; Basso, G.; Bedogni, G.; Tiribelli, C.; Chemello, L. Serum miRNA Are Promising Biomarkers for the Detection of Early Hepatocellular Carcinoma after Treatment with Direct-Acting Antivirals. Cancers 2019, 11, 1773. https://doi.org/10.3390/cancers11111773
Pascut D, Cavalletto L, Pratama MY, Bresolin S, Trentin L, Basso G, Bedogni G, Tiribelli C, Chemello L. Serum miRNA Are Promising Biomarkers for the Detection of Early Hepatocellular Carcinoma after Treatment with Direct-Acting Antivirals. Cancers. 2019; 11(11):1773. https://doi.org/10.3390/cancers11111773
Chicago/Turabian StylePascut, Devis, Luisa Cavalletto, Muhammad Yogi Pratama, Silvia Bresolin, Luca Trentin, Giuseppe Basso, Giorgio Bedogni, Claudio Tiribelli, and Liliana Chemello. 2019. "Serum miRNA Are Promising Biomarkers for the Detection of Early Hepatocellular Carcinoma after Treatment with Direct-Acting Antivirals" Cancers 11, no. 11: 1773. https://doi.org/10.3390/cancers11111773
APA StylePascut, D., Cavalletto, L., Pratama, M. Y., Bresolin, S., Trentin, L., Basso, G., Bedogni, G., Tiribelli, C., & Chemello, L. (2019). Serum miRNA Are Promising Biomarkers for the Detection of Early Hepatocellular Carcinoma after Treatment with Direct-Acting Antivirals. Cancers, 11(11), 1773. https://doi.org/10.3390/cancers11111773







