High Expression of MicroRNA-196a is Associated with Progression of Hepatocellular Carcinoma in Younger Patients
Abstract
1. Introduction
2. Results
2.1. Correlation of MiR-196a Expression with Clinical–Pathological Parameters and HCC Progression
2.2. Downregulation of MiR-196a Expression Impaired Sphere Formation and Invasiveness
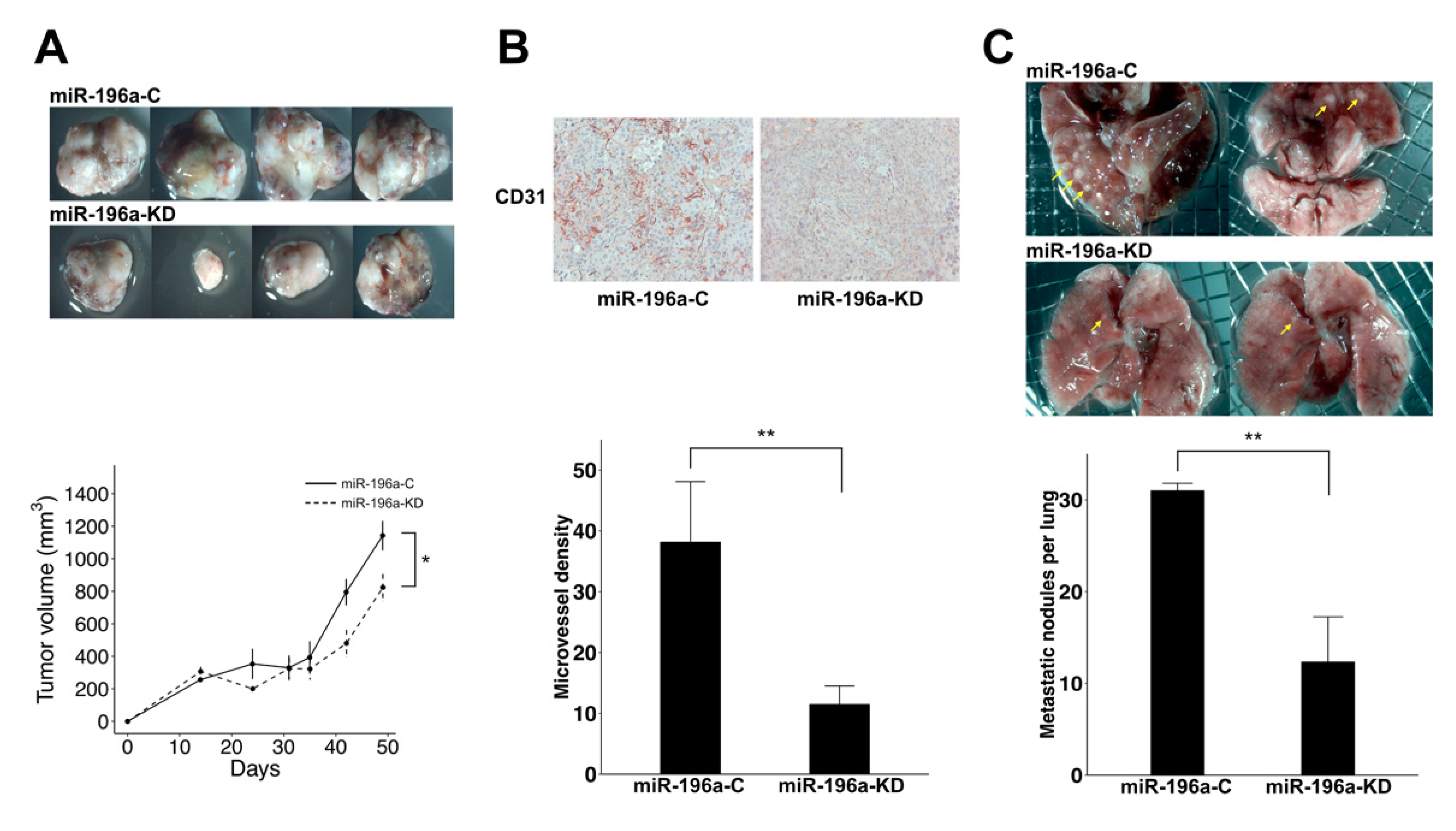
2.3. Knockdown of MiR-196a Decreased Angiogenesis and Lung Metastasis of HCC
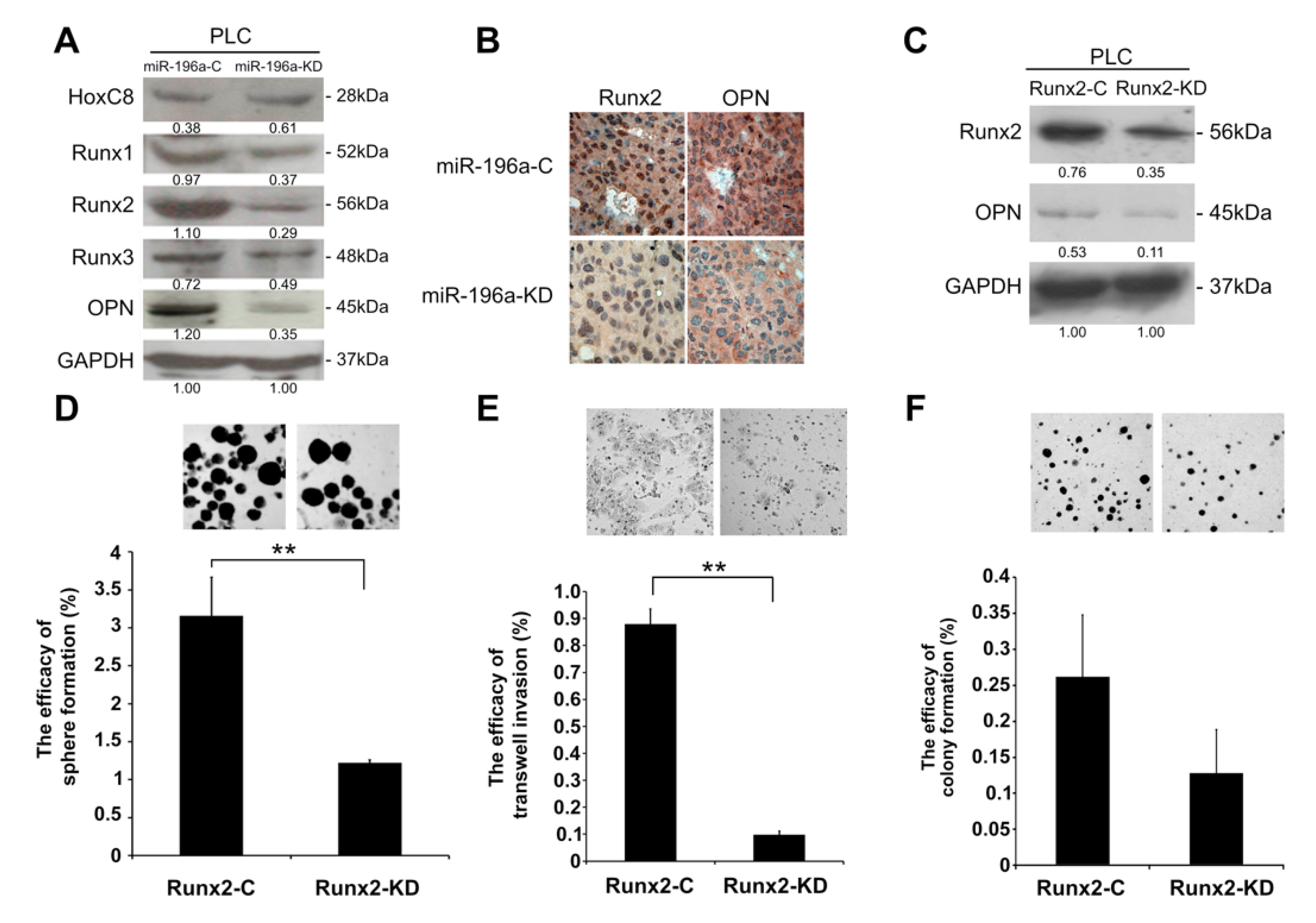
2.4. Knockdown of MiR-196a Modulated EMT Regulators and Stemness Regulators of HCC
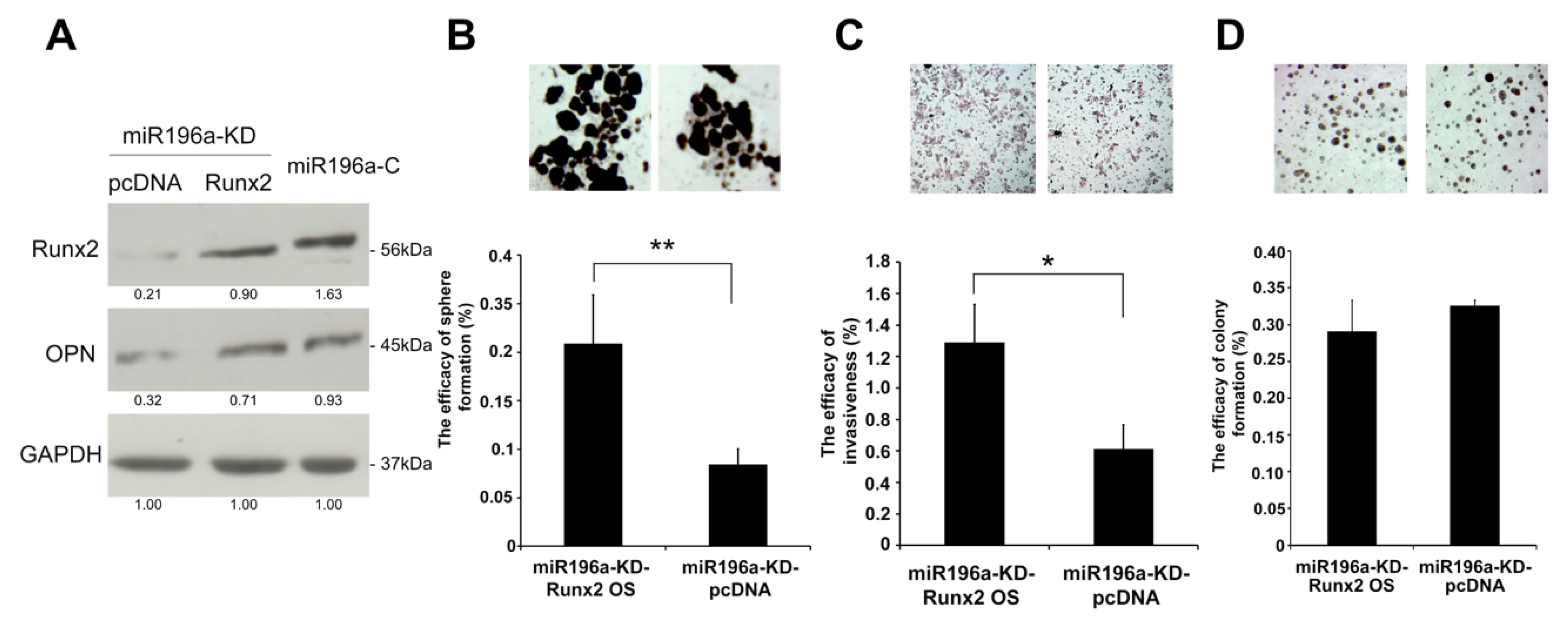
2.5. MiR-196a Promotes HCC Invasiveness by Regulating Runx2 and OPN
3. Discussion
4. Materials and Methods
4.1. Tissue Collection and Total RNA Extraction
4.2. Cell Cultures and Total RNA Extraction of HCC Cell Lines
4.3. Reverse Transcription and Quantitative Real-Time PCR (RT-qPCR)
4.4. Generation of MiR-196a-Knockdown Cell Lines
4.5. Colony Formation Assay
4.6. Invasion Assay
4.7. Sphere Formation Assay
4.8. Transendothelial Invasion Assay
4.9. Protein Extraction and Western Blotting
4.10. Immunohistochemical Staining
4.11. Immunofluorescence Staining
4.12. In Vivo Tumorigenic and Metastatic Study
4.13. Measurement of Intratumoral Microvessel Density
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Schwartz, M.; Mazzaferro, V. Resection and liver transplantation for hepatocellular carcinoma. Semin. Liver Dis. 2005, 25, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.P.; Lau, N.C.; Garrett-Engele, P.; Grimson, A.; Schelter, J.M.; Castle, J.; Bartel, D.P.; Linsley, P.S.; Johnson, J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005, 433, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Yasuda, T.; Saigo, K.; Urashima, T.; Toyoda, H.; Okanoue, T.; Shimotohno, K. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene 2006, 25, 2537–2545. [Google Scholar] [CrossRef] [PubMed]
- Budhu, A.; Jia, H.L.; Forgues, M.; Liu, C.G.; Goldstein, D.; Lam, A.; Zanetti, K.A.; Ye, Q.H.; Qin, L.X.; Croce, C.M.; et al. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology 2008, 47, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, X.H.; Li, J.H.; Yang, J.S.; Zhang, E.B.; Yin, D.D.; Liu, Z.L.; Zhou, J.; Ding, Y.; Li, S.Q.; et al. MiR-196a is upregulated in gastric cancer and promotes cell proliferation by downregulating p27(kip1). Mol. Cancer Ther. 2012, 11, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Luthra, R.; Singh, R.R.; Luthra, M.G.; Li, Y.X.; Hannah, C.; Romans, A.M.; Barkoh, B.A.; Chen, S.S.; Ensor, J.; Maru, D.M.; et al. MicroRNA-196a targets annexin A1: A microRNA-mediated mechanism of annexin A1 downregulation in cancers. Oncogene 2008, 27, 6667–6678. [Google Scholar] [CrossRef]
- Kim, Y.J.; Bae, S.W.; Yu, S.S.; Bae, Y.C.; Jung, J.S. miR-196a regulates proliferation and osteogenic differentiation in mesenchymal stem cells derived from human adipose tissue. J. Bone Miner. Res. 2009, 24, 816–825. [Google Scholar] [CrossRef]
- Wai, P.Y.; Kuo, P.C. The role of Osteopontin in tumor metastasis. J. Surg. Res. 2004, 121, 228–241. [Google Scholar] [CrossRef]
- Abu El Makarem, M.A.; Abdel-Aleem, A.; Ali, A.; Saber, R.; Shatat, M.; Rahem, D.A.; Sayed, D. Diagnostic significance of plasma osteopontin in hepatitis C virus-related hepatocellular carcinoma. Ann. Hepatol. 2011, 10, 296–305. [Google Scholar] [CrossRef]
- Xie, H.; Song, J.; Du, R.; Liu, K.; Wang, J.; Tang, H.; Bai, F.; Liang, J.; Lin, T.; Liu, J.; et al. Prognostic significance of osteopontin in hepatitis B virus-related hepatocellular carcinoma. Dig. Liver Dis. 2007, 39, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Casadei Gardini, A.; Marisi, G.; Scarpi, E.; Scartozzi, M.; Faloppi, L.; Silvestris, N.; Masi, G.; Vivaldi, C.; Brunetti, O.; Tamberi, S.; et al. Effects of metformin on clinical outcome in diabetic patients with advanced HCC receiving sorafenib. Expert Opin. Pharmacother. 2015, 16, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Chen, C.L.; Chau, G.Y.; Chiou, S.H.; Su, C.W.; Chou, T.Y.; Peng, W.L.; Wu, J.C. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology 2009, 50, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Tay, Y.; Zhang, J.; Thomson, A.M.; Lim, B.; Rigoutsos, I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature 2008, 455, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Forgues, M.; Wang, W.; Kim, J.W.; Ye, Q.; Jia, H.; Budhu, A.; Zanetti, K.A.; Chen, Y.; Qin, L.X.; et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008, 68, 1451–1461. [Google Scholar] [CrossRef]
- Wang, H.W.; Hsieh, T.H.; Huang, S.Y.; Chau, G.Y.; Tung, C.Y.; Su, C.W.; Wu, J.C. Forfeited hepatogenesis program and increased embryonic stem cell traits in young hepatocellular carcinoma (HCC) comparing to elderly HCC. BMC Genomics 2013, 14, 736. [Google Scholar] [CrossRef]
- Chen, C.H.; Chang, T.T.; Cheng, K.S.; Su, W.W.; Yang, S.S.; Lin, H.H.; Wu, S.S.; Lee, C.M.; Changchien, C.S.; Chen, C.J.; et al. Do young hepatocellular carcinoma patients have worse prognosis? The paradox of age as a prognostic factor in the survival of hepatocellular carcinoma patients. Liver Int. 2006, 26, 766–773. [Google Scholar] [CrossRef]
- Chang, P.E.; Ong, W.C.; Lui, H.F.; Tan, C.K. Is the prognosis of young patients with hepatocellular carcinoma poorer than the prognosis of older patients? A comparative analysis of clinical characteristics, prognostic features, and survival outcome. J. Gastroenterol. 2008, 43, 881–888. [Google Scholar] [CrossRef]
- Su, C.W.; Lei, H.J.; Chau, G.Y.; Hung, H.H.; Wu, J.C.; Hsia, C.Y.; Lui, W.Y.; Su, Y.H.; Wu, C.W.; Lee, S.D. The effect of age on the long-term prognosis of patients with hepatocellular carcinoma after resection surgery: A propensity score matching analysis. Arch. Surg. 2012, 147, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Tsai, Y.S.; Huang, Y.H.; Liang, Y.J.; Sun, Y.Y.; Su, C.W.; Chau, G.Y.; Yeh, Y.C.; Chang, Y.S.; Hu, J.T.; et al. Lymphoid Enhancer Factor 1 Contributes to Hepatocellular Carcinoma Progression Through Transcriptional Regulation of Epithelial-Mesenchymal Transition Regulators and Stemness Genes. Hepatol. Commun. 2018, 2, 1392–1407. [Google Scholar] [CrossRef] [PubMed]
- Abba, M.L.; Patil, N.; Leupold, J.H.; Allgayer, H. MicroRNA Regulation of Epithelial to Mesenchymal Transition. J. Clin. Med. 2016, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Liu, Y.N.; Yin, J.J.; Abou-Kheir, W.; Hynes, P.G.; Casey, O.M.; Fang, L.; Yi, M.; Stephens, R.M.; Seng, V.; Sheppard-Tillman, H.; et al. MiR-1 and miR-200 inhibit EMT via Slug-dependent and tumorigenesis via Slug-independent mechanisms. Oncogene 2013, 32, 296–306. [Google Scholar] [CrossRef]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Zhang, H.; Tu, F.; Qiang, Y.; Nie, C. Decreased expression of ZO-1 is associated with tumor metastases in liver cancer. Oncol. Lett. 2019, 17, 1859–1864. [Google Scholar] [CrossRef]
- Yu, M.C.; Lee, Y.S.; Lin, S.E.; Wu, H.Y.; Chen, T.C.; Lee, W.C.; Chen, M.F.; Tsai, C.N. Recurrence and poor prognosis following resection of small hepatitis B-related hepatocellular carcinoma lesions are associated with aberrant tumor expression profiles of glypican 3 and osteopontin. Ann. Surg. Oncol. 2012, 19 (Suppl. 3), S455–S463. [Google Scholar] [CrossRef]
- Pan, H.W.; Ou, Y.H.; Peng, S.Y.; Liu, S.H.; Lai, P.L.; Lee, P.H.; Sheu, J.C.; Chen, C.L.; Hsu, H.C. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer 2003, 98, 119–127. [Google Scholar] [CrossRef]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.H.; Inada, M.; et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef]
- Inman, C.K.; Shore, P. The osteoblast transcription factor Runx2 is expressed in mammary epithelial cells and mediates osteopontin expression. J. Biol. Chem. 2003, 278, 48684–48689. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Q.; Tare, R.; Lee, S.H.; Mandeville, M.; Weiner, B.; Montecino, M.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. HOXA10 controls osteoblastogenesis by directly activating bone regulatory and phenotypic genes. Mol. Cell. Biol. 2007, 27, 3337–3352. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Zhang, L.; Weakley, S.M.; Yao, Q. MicroRNA-196: Critical roles and clinical applications in development and cancer. J. Cell. Mol. Med. 2011, 15, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yang, X.; Chen, D.; Chang, Z.; Cao, X. Smad1 interacts with homeobox DNA-binding proteins in bone morphogenetic protein signaling. J. Biol. Chem. 1999, 274, 13711–13717. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhang, X.; Ni, W.; Fan, H.; Xu, J.; Chen, Y.; Zhu, J.; Gu, X.; Yang, L.; Ni, R.; et al. Upregulated HOXC8 Expression Is Associated with Poor Prognosis and Oxaliplatin Resistance in Hepatocellular Carcinoma. Dig. Dis. Sci. 2015, 60, 3351–3363. [Google Scholar] [CrossRef]
- Chou, C.H.; Shrestha, S.; Yang, C.D.; Chang, N.W.; Lin, Y.L.; Liao, K.W.; Huang, W.C.; Sun, T.H.; Tu, S.J.; Lee, W.H.; et al. miRTarBase update 2018: A resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018, 46, D296–D302. [Google Scholar] [CrossRef]
- Lian, J.B.; Stein, G.S.; van Wijnen, A.J.; Stein, J.L.; Hassan, M.Q.; Gaur, T.; Zhang, Y. MicroRNA control of bone formation and homeostasis. Nat. Rev. Endocrinol. 2012, 8, 212–227. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, S.; Wang, B.; Huang, J.; Lu, W.W.; Chen, D. Runx2 and microRNA regulation in bone and cartilage diseases. Ann. N. Y. Acad. Sci. 2016, 1383, 80–87. [Google Scholar] [CrossRef]
- Shen, S.; Pan, J.; Lu, X.; Chi, P. Role of miR-196 and its target gene HoxB8 in the development and proliferation of human colorectal cancer and the impact of neoadjuvant chemotherapy with FOLFOX4 on their expression. Oncol. Lett. 2016, 12, 4041–4047. [Google Scholar] [CrossRef]
- Lin, M.W.; Tseng, Y.W.; Shen, C.C.; Hsu, M.N.; Hwu, J.R.; Chang, C.W.; Yeh, C.J.; Chou, M.Y.; Wu, J.C.; Hu, Y.C. Synthetic switch-based baculovirus for transgene expression control and selective killing of hepatocellular carcinoma cells. Nucleic Acids Res. 2018, 46, e93. [Google Scholar] [CrossRef] [PubMed]
- Oefinger, P.E.; Bronson, D.L.; Dreesman, G.R. Induction of hepatitis B surface antigen in human hepatoma-derived cell lines. J. Gen. Virol. 1981, 53, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res. Treat. 1995, 36, 169–180. [Google Scholar] [CrossRef] [PubMed]
- R Core Team (2018). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: http://www.R-project.org/ (accessed on 12 October 2019).



| Variable | Total | miR-196a Expression | p Value | |
|---|---|---|---|---|
| (n = 83) | High (n = 42) | Low (n = 41) | ||
| Patient demographics | ||||
| Age 1 | 51 (44–63.5) | 47.5 (43–58.3) | 56 (45–64) | 0.0369 |
| Male, No (%) | 70 (84.3) | 35 (83.3) | 35 (85.4) | 1.0 |
| Cirrhosis, No (%) | 38 (45.8) | 20 (47.6) | 18 (43.9) | 0.9049 |
| Diabetes mellitus, No (%) | 10 (12.0) | 5 (11.9) | 5 (12.2) | 1.0 |
| Serum biochemistries1 | ||||
| Albumin (g/dL) | 4.0 (3.6–4.1) | 3.9 (3.4–4.1) | 4.0 (3.8–4.3) | 0.0386 |
| ALT (IU/L) | 43 (34–56.8) | 44 (34–65) | 40 (34–55) | 0.4064 |
| AST (IU/L) | 39.5 (30–58.5) | 42 (31.5–60.3) | 37 (29.8–56.5) | 0.4132 |
| Total bilirubin (mg/dL) | 0.8 (0.6–1.0) | 0.8 (0.6–1.0) | 0.8 (0.6–1.0) | 0.8798 |
| ALP (IU/L) | 84 (62.5–107) | 84 (61–103) | 83 (65–107) | 0.8695 |
| Viral factors | ||||
| HBeAg (Positive/Negative) | 10/46 | 8/22 | 2/24 | 0.1338 |
| HBV genotype (B/C) | 49/27 | 25/14 | 24/13 | 1.0 |
| HBV DNA (copies/mL) 1 | 1.96 × 105 (1.41 × 104–1.81 × 106) | 2.02 × 105 (1.81 × 104–1.46 × 106) | 1.16 × 105 (1.09 × 104–2.06 × 106) | 0.9242 |
| Tumor factors | ||||
| Tumor size (cm) 1 | 4 (2.5–7.3) | 4.5 (2.5–8.3) | 3.5 (2.5–6.5) | 0.2981 |
| AFP (ng/mL), < 20 vs ≥ 20 | 37/44 | 12/28 | 25/16 | 0.0100 |
| Differentiation (Well/Moderate/Poor) | 2/67/14 | 1/33/8 | 1/34/6 | 0.8656 |
| HCC pattern (Solitary/Multiple) | 64/19 | 31/11 | 33/8 | 0.6435 |
| Microvascular invasion (Yes/No) | 32/51 | 19/23 | 13/28 | 0.298 |
| Macrovascular invasion (Yes/No) | 9/74 | 8/34 | 1/40 | 0.0375 |
| Tumor stage (I vs II + III + IV) | 42/41 | 16/26 | 26/15 | 0.0369 |
| Follow-up (months) 1 | 42 (26.5–51.5) | 40.5 (26.5–47.8) | 43 (27–53) | 0.3942 |
| Variable | Univariate Analyses | Multivariate Analyses | ||||
|---|---|---|---|---|---|---|
| Crude HR | 95% CI | p Value | Adjusted HR | 95% CI | p Value | |
| Host factors | ||||||
| Age ≥ 50 | 0.991 | 0.542–1.811 | 0.9756 | |||
| Male | 1.074 | 0.474–2.434 | 0.8634 | |||
| Cirrhosis | 0.990 | 0.537–1.825 | 0.973 | |||
| Diabetes mellitus | 1.238 | 0.521–2.942 | 0.629 | |||
| Viral factors | ||||||
| Positive HBeAg | 0.809 | 0.306–2.141 | 0.6694 | |||
| HBV DNA ≥ 104 copies/mL | 1.978 | 0.776–5.040 | 0.1529 | |||
| HBV genotype C vs. B | 1.585 | 0.849–2.957 | 0.1481 | |||
| Tumor factors | ||||||
| Tumor size ≥ 5 cm | 1.422 | 0.773–2.613 | 0.2573 | |||
| AFP ≥ 20 ng/mL | 1.327 | 0.711–2.474 | 0.3741 | |||
| Well differentiation | 0.893 | 0.123–6.504 | 0.9111 | |||
| Multinodularity | 1.799 | 0.900–3.595 | 0.0964 | |||
| Microvascular invasion | 3.429 | 1.831–6.419 | <0.001 | 4.582 | 2.215–9.479 | <0.001 |
| Macrovascular invasion | 2.196 | 0.853–5.656 | 0.1031 | |||
| High miR-196a expression | 2.124 | 1.148–3.929 | 0.016 | 2.395 | 1.207–4.752 | 0.0125 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-Y.; Chen, C.-L.; Hu, Y.-C.; Chi, Y.; Huang, Y.-H.; Su, C.-W.; Jeng, W.-J.; Liang, Y.-J.; Wu, J.-C. High Expression of MicroRNA-196a is Associated with Progression of Hepatocellular Carcinoma in Younger Patients. Cancers 2019, 11, 1549. https://doi.org/10.3390/cancers11101549
Wang S-Y, Chen C-L, Hu Y-C, Chi Y, Huang Y-H, Su C-W, Jeng W-J, Liang Y-J, Wu J-C. High Expression of MicroRNA-196a is Associated with Progression of Hepatocellular Carcinoma in Younger Patients. Cancers. 2019; 11(10):1549. https://doi.org/10.3390/cancers11101549
Chicago/Turabian StyleWang, Shen-Yung, Chih-Li Chen, Yu-Chen Hu, Yi Chi, Yen-Hua Huang, Chien-Wei Su, Wen-Juei Jeng, Yuh-Jin Liang, and Jaw-Ching Wu. 2019. "High Expression of MicroRNA-196a is Associated with Progression of Hepatocellular Carcinoma in Younger Patients" Cancers 11, no. 10: 1549. https://doi.org/10.3390/cancers11101549
APA StyleWang, S.-Y., Chen, C.-L., Hu, Y.-C., Chi, Y., Huang, Y.-H., Su, C.-W., Jeng, W.-J., Liang, Y.-J., & Wu, J.-C. (2019). High Expression of MicroRNA-196a is Associated with Progression of Hepatocellular Carcinoma in Younger Patients. Cancers, 11(10), 1549. https://doi.org/10.3390/cancers11101549






