PATZ1 Is Overexpressed in Pediatric Glial Tumors and Correlates with Worse Event-Free Survival in High-grade Gliomas
Abstract
1. Introduction
2. Results
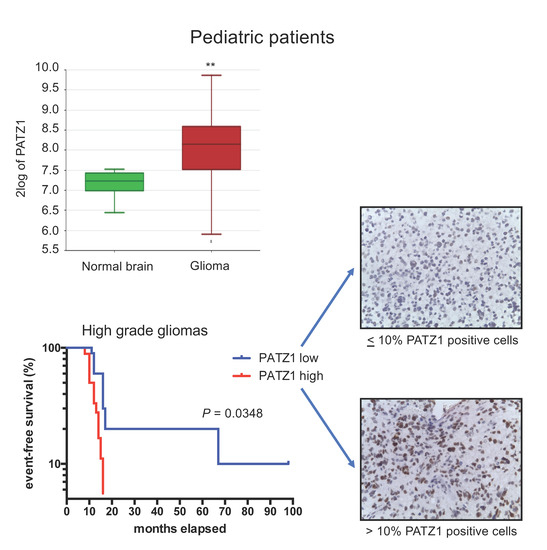
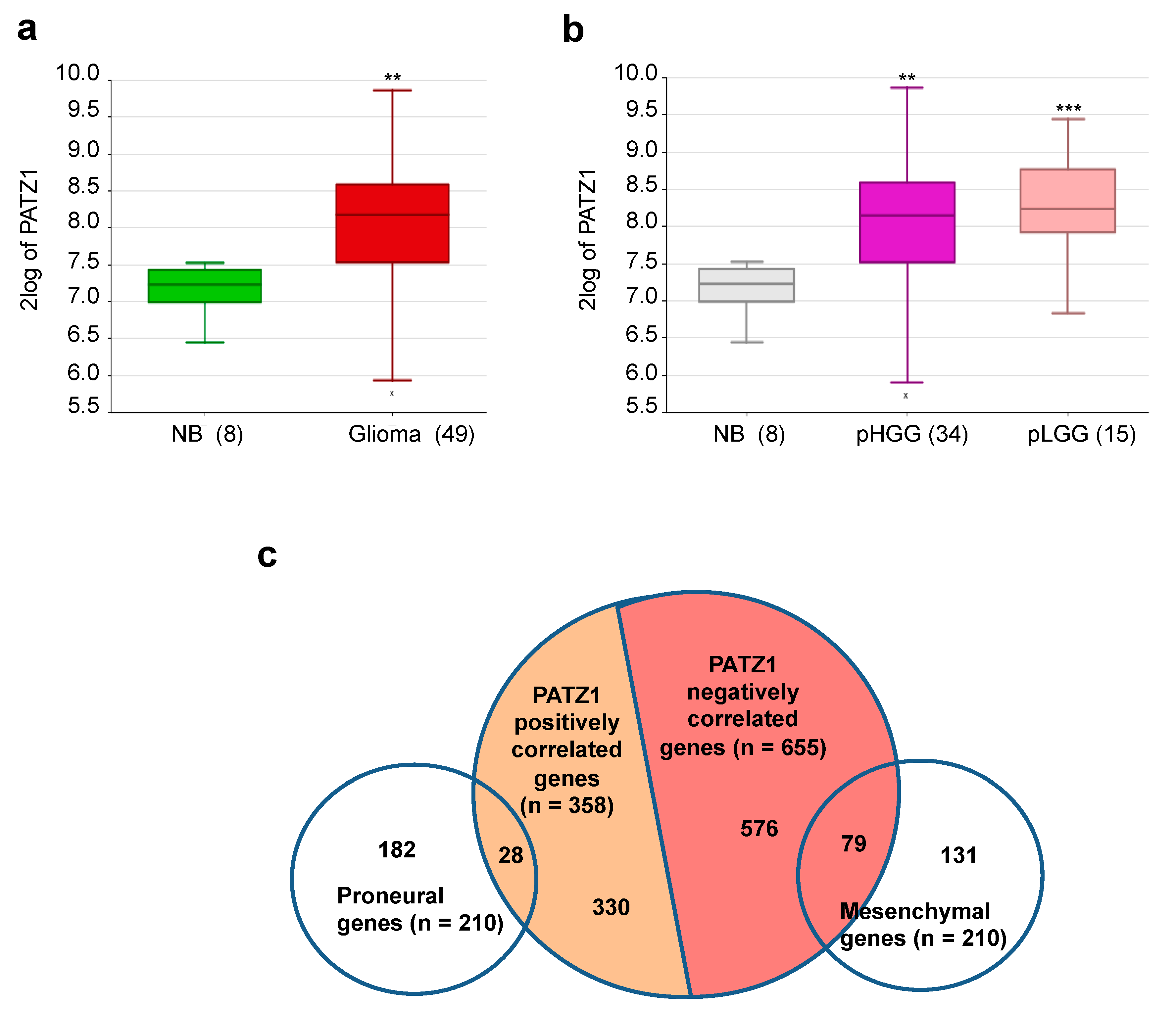
2.1. PATZ1 Gene Expression Is Enriched in Pediatric Glial Tumors and Associated with a Proneural Signature
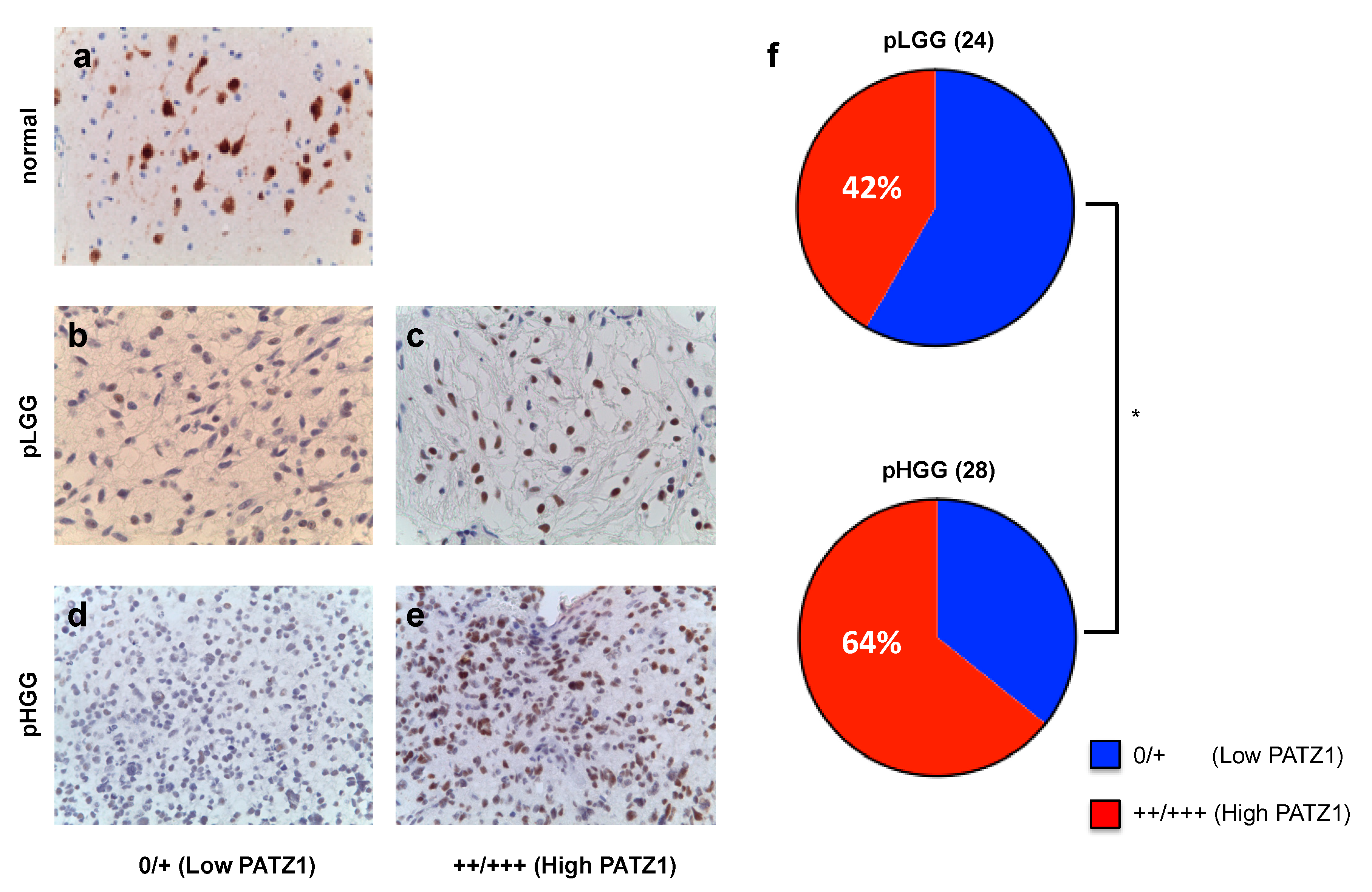
2.2. PATZ1 Protein Expression Is Associated with Tumor State in Pediatric Glioma Tissue and More Frequently Highly Expressed in pHGG than pLGG
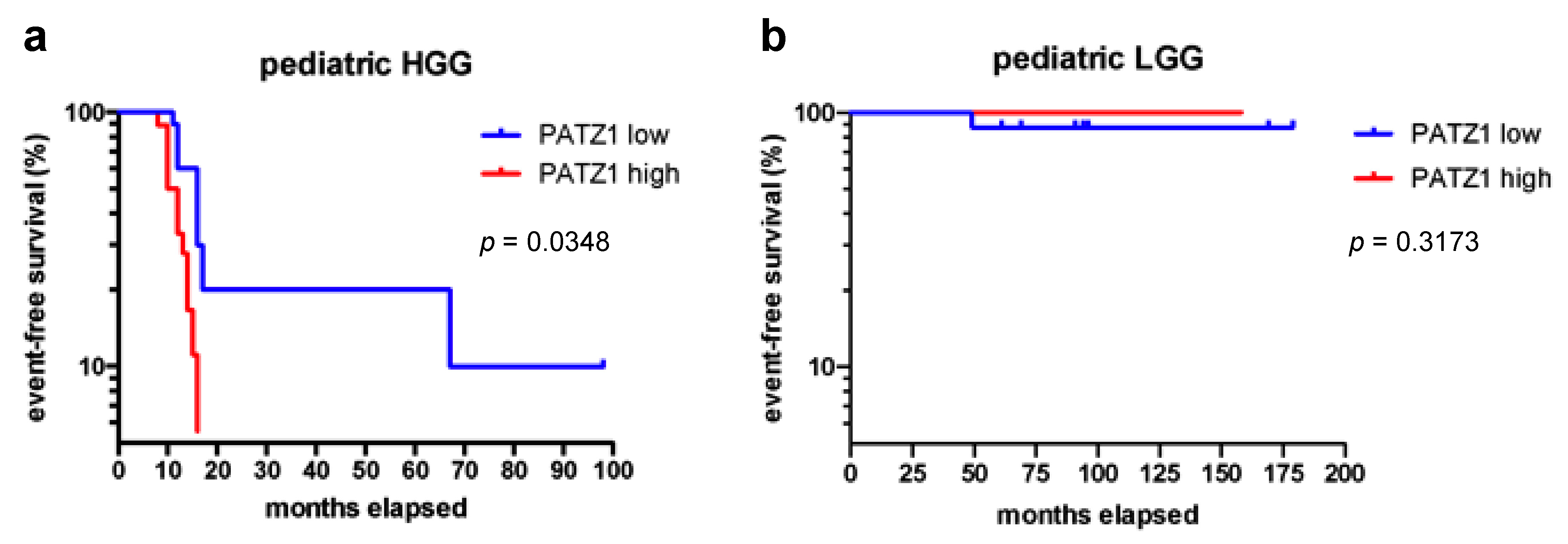
2.3. High PATZ1 Expression Correlates with Worse Event-Free Survival in pHGG
3. Discussion
4. Materials and Methods
4.1. Data Mining
4.2. Patients
4.3. Histopathological Analysis and Immunohistochemistry
4.4. Statistical Analysis and Kaplan-Meier Survival Curves
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Udaka, Y.T.; Packer, R.J. Pediatric Brain Tumors. Neurol. Clin. 2018, 36, 533–556. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.J.; Pollack, I.F.; Zhou, T.; Buxton, A.; Holmes, E.J.; Burger, P.C.; Brat, D.J.; Rosenblum, M.K.; Hamilton, R.L.; Lavey, R.S.; et al. Temozolomide in the treatment of high-grade gliomas in children: A report from the Children’s Oncology Group. Neuro-oncology 2011, 13, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoon, L.A.; Britt, N.; Guevara, A.; Whitt, E.; Severson, E.; Sathyan, P.; Gay, L.; Elvin, J.; Ross, J.S.; Brown, C.; et al. Precision Neuro-oncology: The Role of Genomic Testing in the Management of Adult and Pediatric Gliomas. Curr. Treat. Options Oncol. 2018, 19, 41. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Severson, E.; Gay, L.; Vergilio, J.A.; Elvin, J.; Suh, J.; Daniel, S.; Covert, M.; Frampton, G.M.; Hsu, S.; et al. Comprehensive Genomic Profiling of 282 Pediatric Low-and High-Grade Gliomas Reveals Genomic Drivers, Tumor Mutational Burden, and Hypermutation Signatures. Oncologist 2017, 22, 1478–1490. [Google Scholar] [CrossRef]
- Forst, D.A.; Nahed, B.V.; Loeffler, J.S.; Batchelor, T.T. Low-grade gliomas. Oncologist 2014, 19, 403–413. [Google Scholar] [CrossRef]
- Muhammed, A.; Gaber, M.S.; Elbeltagy, M.; El Hemaly, A.; Taha, H.; Refaat, A.; Zaghluol, M.S. Risk stratification of pediatric high-grade glioma: A newly proposed prognostic score. Child’s Nerv. Syst. 2019, 1–8. [Google Scholar] [CrossRef]
- Pollack, I.F.; Agnihotri, S.; Broniscer, A. Childhood brain tumors: Current management, biological insights, and future directions. J. Neurosurg. Pediatric 2019, 23, 261–273. [Google Scholar] [CrossRef]
- Fedele, M.; Pierantoni, G.M.; Pallante, P.; Fusco, A. High mobility group A-interacting proteins in cancer: Focus on chromobox protein homolog 7, homeodomain interacting protein kinase 2 and PATZ. J. Nucl. Acids Inv. 2012, 3, 1. [Google Scholar] [CrossRef]
- Fedele, M.; Crescenzi, E.; Cerchia, L. The POZ/BTB and AT-Hook Containing Zinc Finger 1 (PATZ1) Transcription Regulator: Physiological Functions and Disease Involvement. Int. J. Mol. Sci. 2017, 18, 2524. [Google Scholar] [CrossRef]
- Costoya, J.A. Functional analysis of the role of POK transcriptional repressors. Brief. Funct. Genom. Proteom. 2007, 6, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Valentino, T.; Palmieri, D.; Vitiello, M.; Pierantoni, G.M.; Fusco, A.; Fedele, M. PATZ1 interacts with p53 and regulates expression of p53-target genes enhancing apoptosis or cell survival based on the cellular context. Cell Death Dis. 2013, 4, e963. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Q.; Guo, F.F.; Sun, D.F.; Wang, Y.C.; Yang, L.; Chen, S.L.; Hong, J.; Fang, J.Y. Downregulation of ZNF278 arrests the cell cycle and decreases the proliferation of colorectal cancer cells via inhibition of the ERK/MAPK pathway. Oncol. Rep. 2017, 38, 3685–3692. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.Y.; Liang, C.M.; Liang, S.M. PATZ1 induces PP4R2 to form a negative feedback loop on IKK/NF-κB signaling in lung cancer. Oncotarget 2016, 7, 52255–52269. [Google Scholar] [CrossRef]
- Chiappetta, G.; Valentino, T.; Vitiello, T.; Pasquinelli, R.; Monaco, M.; Palma, G.; Sepe, R.; Luciano, A.; Pallante, P.; Palmieri, D.; et al. PATZ1 acts as a tumor suppressor in thyroid cancer via targeting p53-dependent genes involved in EMT and cell migration. Oncotarget 2015, 6, 5310–5323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yan, M.; Li, C.; Feng, Z. POZ/BTB and AT-Hook-Containing Zinc Finger Protein 1 (PATZ1) Suppresses Progression of Ovarian Cancer and Serves as an Independent Prognosis Factor. Med. Sci. Monit. 2018, 24, 4262–4270. [Google Scholar] [CrossRef]
- Franco, R.; Scognamiglio, G.; Valentino, E.; Vitiello, M.; Luciano, A.; Palma, G.; Arra, C.; La Mantia, E.; Panico, L.; Tenneriello, V.; et al. PATZ1 expression correlates positively with BAX and negatively with BCL6 and survival in human diffuse large B cell lymphomas. Oncotarget 2016, 7, 59158–59172. [Google Scholar] [CrossRef]
- Yao, T.; Wang, Q.; Zhang, W.; Bian, A.; Zhang, J. Identification of genes associated with renal cell carcinoma using gene expression profiling analysis. Oncol. Lett. 2016, 12, 73–78. [Google Scholar] [CrossRef]
- Guadagno, E.; Vitiello, M.; Francesca, P.; Calì, G.; Caponnetto, F.; Cesselli, D.; Camorani, S.; Borrelli, G.; Califano, M.; Cappabianca, P.; et al. PATZ1 is a new prognostic marker of glioblastoma associated with the stem-like phenotype and enriched in the proneural subtype. Oncotarget 2017, 8, 59282–59300. [Google Scholar] [CrossRef]
- Alvarez-breckenridge, C.; Miller, J.J.; Nayyar, N.; Gill, C.M.; Kaneb, A.; D’Andrea, M.; Le, L.P.; Lee, J.; Cheng, J.; Zheng, Z.; et al. Clinical and radiographic response following targeting of BCAN-NTRK1 fusion in glioneuronal tumor. NPJ Precis. Oncol. 2017, 1, 5. [Google Scholar] [CrossRef]
- Siegfried, A.; Rousseau, A.; Maurage, C.A.; Pericart, S.; Nicaise, Y.; Escudie, F.; Grand, D.; Delrieu, A.; Gomez-Brouchet, A.; Le Guellec, S.; et al. EWSR1-PATZ1 gene fusion may define a new glioneuronal tumor entity. Brain Pathol. 2019, 29, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, A.M.; Birks, D.K.; Donson, A.M.; Amani, V.; Hoffman, L.M.; Waziri, A.; Wang, M.; Handler, M.H.; Foreman, N.K. Characterization of distinct immunophenotypes across pediatric brain tumor types. J. Immunol. 2013, 191, 4880–4888. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.K.; Raborn, J.; Kelly, V.M.; Cassady, K.A.; Markert, J.M.; Gillespie, G.Y. Pediatric glioma stem cells: Biologic strategies for oncolytic HSV virotherapy. Front. Oncol. 2013, 3, 28. [Google Scholar] [CrossRef]
- Paugh, B.; Qu, C.; Jones, C.; Liu, Z.; Adamowicz-Brice, M.; Zhang, J.; Bax, D.A.; Coyle, B.; Barrow, J.; Hargrave, D.; et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J. Clin. Oncol. 2010, 28, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Fedele, M.; Cerchia, L.; Pegoraro, S.; Sgarra, R.; Manfioletti, G. Proneural-Mesenchymal Transition: Phenotypic plasticity to acquire multitherapy resistance in glioblastoma. Int. J. Mol. Sci. 2019, 20, 2746. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Karajannis, M.A.; Jones, D.T.W.; Kieran, M.W.; Monje, M.; Baker, S.J.; Becher, O.J.; Cho, Y.J.; Gupta, N.; Hawkins, C.; et al. Pediatric high-grade glioma: Biologically and clinically in need of new thinking. Neuro-oncology 2017, 19, 153–161. [Google Scholar] [CrossRef]
- Tritz, R.; Mueller, B.M.; Hickey, M.J.; Lin, A.H.; Gomez, G.G.; Hadwiger, P.; Sah, D.W.; Muldoon, L.; Neuwelt, E.A.; Kruse, C.A. siRNA Down-regulation of the PATZ1 Gene in Human Glioma Cells Increases Their Sensitivity to Apoptotic Stimuli. Cancer Ther. 2008, 6, 865–876. [Google Scholar]
- Osuka, S.; Van Meir, E.G. Overcoming therapeutic resistance in glioblastoma: The way forward. J. Clin. Investig. 2017, 127, 415–426. [Google Scholar] [CrossRef]
- Ma, H.; Ow, J.R.; Tan, B.C.; Goh, Z.; Feng, B.; Loh, Y.H.; Fedele, M.; Li, H.; Wu, Q. The dosage of Patz1 modulates reprogramming process. Sci. Rep. 2014, 4, 7519. [Google Scholar] [CrossRef]
- Meel, M.H.; Schaper, S.A.; Kaspers, G.J.L.; Hulleman, E. Signaling pathways and mesenchymal transition in pediatric high-grade glioma. Cell Mol. Life Sci. 2018, 75, 871–887. [Google Scholar] [CrossRef] [PubMed]
- Gnekow, A.K.; Walker, D.A.; Kandels, D.; Picton, S.; Perilongo, G.; Grill, J.; Stokland, T.; Sandstrom, P.E.; Warmuth-Metz, M.; Pietsch, T.; et al. A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (≤16 years) low-grade glioma—A final report. Eur. J. Cancer 2017, 81, 206–225. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.C.; Haley, K.; Patel, N.; Dhall, G.; Gardner, S.; Allen, J.; Torkildson, J.; Cornelius, A.; Rassekh, R.; Bedros, A.; et al. Outcome of young children with high-grade glioma treated with irradiation-avoiding intensive chemotherapy regimens: Final report of the Head Start II and III trials. Pediatric Blood Cancer 2016, 63, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Massimino, M.; Gandola, L.; Biassoni, V.; Spreafico, F.; Schiavello, E.; Poggi, G.; Pecori, E.; Vajna De Pava, M.; Modena, P.; Antonelli, M.; et al. Evolving of Therapeutic Strategies for CNS-PNET. Pediatric Blood Cancer 2013, 60, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
| Proneural Signature | Mesenchymal Signature | ||||
|---|---|---|---|---|---|
| Gene | r 2 | p | Gene | r | p |
| PAFAH1B3 | 0.785 | 4.5 × 10−5 | ITGAM | −0.832 | 6.1 × 10−6 |
| LOC81691 | 0.745 | 1.4 × 10−4 | PLS3 | −0.817 | 1.3 × 10−5 |
| CHD7 | 0.713 | 3.7 × 10−4 | MAN1A1 | −0.780 | 5.5 × 10−5 |
| MAP2 | 0.673 | 1.0 × 10−3 | CASP4 | −0.776 | 5.9 × 10−5 |
| HDAC2 | 0.663 | 1.2 × 10−3 | S100A13 | −0.773 | 6.4 × 10−5 |
| MTSS1 | 0.639 | 2.2 × 10−3 | PTPRC | −0.768 | 7.7 × 10−5 |
| DLL3 | 0.633 | 2.5 × 10−3 | AMPD3 | −0.767 | 7.3 × 10−5 |
| SOX4 | 0.630 | 2.6 × 10−3 | ALOX5 | −0.755 | 1.0 × 10−4 |
| MARCKSL1 | 0.630 | 2.6 × 10−3 | VDR | −0.751 | 1.2 × 10−4 |
| HN1 | 0.624 | 3.0 × 10−3 | TNFRSF11A | −0.742 | 1.6 × 10−4 |
| PKMYT1 | 0.624 | 3.0 × 10−3 | CASP8 | −0.741 | 1.6 × 10−4 |
| PODXL2 | 0.620 | 3.2 × 10−3 | PLA2G5 | −0.741 | 1.6 × 10−4 |
| CASK | 0.614 | 3.6 × 10−3 | MFSD1 | −0.737 | 1.9 × 10−4 |
| DBN1 | 0.609 | 4.1 × 10−3 | RUNX2 | −0.732 | 2.1 × 10−4 |
| GNG4 | 0.609 | 4.1 × 10−3 | RAC2 | −0.726 | 2.7 × 10−4 |
| SEZ6L | 0.608 | 4.1 × 10−3 | CASP1 | −0.726 | 2.7 × 10−4 |
| SLCO5A1 | 0.605 | 4.4 × 10−3 | LY75 | −0.724 | 2.8 × 10−4 |
| MCM10 | 0.602 | 4.6 × 10−3 | CAST | −0.719 | 3.3 × 10−4 |
| CSNK1E | 0.598 | 5.0 × 10−3 | IL15 | −0.719 | 3.4 × 10−4 |
| RAB33A | 0.591 | 5.8 × 10−3 | LY96 | −0.717 | 3.4 × 10−4 |
| ASCL1 | 0.591 | 5.7 × 10−3 | TRIM38 | −0.709 | 4.2 × 10−4 |
| HMGB3 | 0.590 | 5.8 × 10−3 | ARSJ | −0.706 | 4.5 × 10−4 |
| SOX11 | 0.587 | 6.1 × 10−3 | SQRDL | −0.697 | 5.6 × 10−4 |
| AMOTL2 | 0.582 | 6.6 × 10−3 | TLR2 | −0.691 | 6.4 × 10−4 |
| OLIG2 | 0.576 | 7.3 × 10−3 | SYPL1 | −0.688 | 7.0 × 10−4 |
| DPF1 | 0.569 | 8.4 × 10−3 | PTRF | −0.684 | 7.8 × 10−4 |
| TOPBP1 | 0.567 | 8.6 × 10−3 | VAMP5 | −0.680 | 8.6 × 10−4 |
| CRMP1 | 0.566 | 8.8 × 10−3 | ITGB2 | −0.675 | 9.6 × 10−4 |
| SCPEP1 | −0.674 | 9.8 × 10−4 | |||
| PTGER4 | −0.672 | 1.0 × 10−3 | |||
| RAB27A | −0.667 | 1.2 × 10−3 | |||
| TNFRSF1A | −0.667 | 1.1 × 10−3 | |||
| CD14 | −0.667 | 1.2 × 10−3 | |||
| CHI3L1 | −0.666 | 1.2 × 10−3 | |||
| TRADD | −0.664 | 1.2 × 10−3 | |||
| IQGAP1 | −0.664 | 1.2 × 10−3 | |||
| LCP2 | −0.660 | 1.3 × 10−3 | |||
| MSR1 | −0.654 | 1.6 × 10−3 | |||
| ARPC1B | −0.653 | 1.6 × 10−3 | |||
| TGFBR2 | −0.649 | 1.8 × 10−3 | |||
| LAPTM5 | −0.647 | 1.8 × 10−3 | |||
| PHF11 | −0.646 | 1.9 × 10−3 | |||
| NPC2 | −0.643 | 2.0 × 10−3 | |||
| LAIR1 | −0.642 | 2.0 × 10−3 | |||
| CTSB | −0.641 | 2.1 × 10−3 | |||
| MAFB | −0.641 | 2.1 × 10−3 | |||
| NCF2 | −0.640 | 2.1 × 10−3 | |||
| ASL | −0.637 | 2.3 × 10−3 | |||
| ANXA1 | −0.637 | 2.3 × 10−3 | |||
| S100A11 | −0.629 | 2.6 × 10−3 | |||
| WWTR1 | −0.629 | 2.6 × 10−3 | |||
| COL8A2 | −0.626 | 2.9 × 10−3 | |||
| CSTA | −0.625 | 2.9 × 10−3 | |||
| IL4R | −0.619 | 3.3 × 10−3 | |||
| STAB1 | −0.617 | 3.4 × 10−3 | |||
| S100A4 | −0.613 | 3.7 × 10−3 | |||
| MGST2 | −0.609 | 4.1 × 10−3 | |||
| P4HA2 | −0.608 | 4.1 × 10−3 | |||
| MVP | −0.607 | 4.2 × 10−3 | |||
| MFSD1 | −0.601 | 4.7 × 10−3 | |||
| LCP1 | −0.599 | 5.0 × 10−3 | |||
| STAT6 | −0.593 | 5.5 × 10−3 | |||
| HFE | −0.592 | 5.7 × 10−3 | |||
| PLAUR | −0.592 | 5.7 × 10−3 | |||
| FCGR2B | −0.591 | 5.7 × 10−3 | |||
| ANXA4 | −0.589 | 5.9 × 10−3 | |||
| SP100 | −0.588 | 5.9 × 10−3 | |||
| COPZ2 | −0.584 | 6.4 × 10−3 | |||
| THBD | −0.584 | 6.4 × 10−3 | |||
| FCGR2A | −0.582 | 6.6 × 10−3 | |||
| CYBRD1 | −0.579 | 7.0 × 10−3 | |||
| LILRB3 | −0.579 | 7.0 × 10−3 | |||
| IL15RA | −0.576 | 7.4 × 10−3 | |||
| GNA15 | −0.574 | 7.6 × 10−3 | |||
| PTPN6 | −0.572 | 7.9 × 10−3 | |||
| SLC11A1 | −0.571 | 8.1 × 10−3 | |||
| CLCF1 | −0.570 | 8.2 × 10−3 | |||
| SYNGR2 | −0.566 | 8.9 × 10−3 | |||
| DSC2 | −0.560 | 9.9 × 10−3 | |||
| Patient | Gender 2 | Age (months) | Site 3 | Subtype | Metastases | PATZ1 |
|---|---|---|---|---|---|---|
| 1 | M | 49 | ch | LGG | NO | 50% |
| 2 | F | 38 | ch | LGG | NO | 5% |
| 3 | F | 6 | ch | LGG | NO | 10% |
| 4 | F | 102 | ch | LGG | NO | 5% |
| 5 | F | 45 | ch | LGG | NO | 5% |
| 6 | F | 12 | ch | LGG | NO | 20% |
| 7 | F | 93 | ch | LGG | NO | 20% |
| 8 | F | 33 | ch | LGG | NO | 20% |
| 9 | F | 14 | ch | LGG | NO | 40% |
| 10 | F | 88 | pv | LGG | NO | 60% |
| 11 | F | 14 | ch | LGG | NO | 0 |
| 12 | F | 144 | pv | LGG | NO | 0 |
| 13 | F | 23 | ch | LGG | NO | 0 |
| 14 | F | 28 | ch | LGG | NO | 0 |
| 15 | M | 57 | ch | LGG | NO | 10 |
| 16 | M | 57 | ch | LGG | NO | 10 |
| 17 | M | 77 | ch | LGG | NO | 5 |
| 18 | M | 33 | ch | LGG | NO | 25 |
| 19 | M | 180 | pv | LGG | NO | 30 |
| 20 | M | 12 | ch | LGG | NO | 40 |
| 21 | M | 7 | ch | LGG | NO | 55 |
| 22 | M | 44 | pv | LGG | NO | 0 |
| 23 | F | 65 | co | LGG | NO | 0 |
| 24 | F | 9 | pv | LGG | NO | 0 |
| 25 | F | 171 | th | HGG | YES | 20 |
| 26 | F | 87 | ch | HGG | YES | 50 |
| 27 | F | 140 | tr | HGG | YES | 30 |
| 28 | F | 152 | co | HGG | YES | 0 |
| 29 | M | 119 | tr | HGG | YES | 50 |
| 30 | M | 52 | th | HGG | YES | 20 |
| 31 | F | 109 | tr | HGG | NO | 30 |
| 32 | F | 121 | th | HGG | NO | 40 |
| 33 | F | 149 | co | HGG | NO | 20 |
| 34 | F | 125 | co | HGG | NO | 40 |
| 35 | F | 143 | tr | HGG | NO | 80 |
| 36 | F | 164 | th | HGG | NO | 0 |
| 37 | M | 192 | th | HGG | NO | 5 |
| 38 | M | 153 | tr | HGG | NO | 5 |
| 39 | M | 101 | co | HGG | NO | 5 |
| 40 | M | 76 | pv | HGG | NO | 5 |
| 41 | M | 89 | th | HGG | NO | 5 |
| 42 | M | 56 | th | HGG | NO | 40 |
| 43 | M | 108 | tr | HGG | NO | 30 |
| 44 | M | 96 | co | HGG | NO | 40 |
| 45 | M | 139 | co | HGG | NO | 30 |
| 46 | M | 68 | tr | HGG | NO | 90 |
| 47 | M | 65 | tr | HGG | NO | 70 |
| 48 | M | 40 | co | HGG | NO | 55 |
| 49 | M | 126 | co | HGG | NO | 0 |
| 50 | M | 91 | th | HGG | NO | 0 |
| 51 | M | 82 | tr | HGG | NO | 0 |
| 52 | M | 148 | co | HGG | NO | 0 |
| Variables | Number | PATZ1 Expression | p value 1 | |
|---|---|---|---|---|
| High | Low | |||
| Gender | ||||
| Male | 28 | 16 | 12 | 0.407 |
| Female | 24 | 12 | 12 | |
| Age (years) | ||||
| ≤3 | 11 | 5 | 7 | 0.674 |
| >3 ≤10 | 27 | 16 | 10 | |
| >10 ≤ 16 | 14 | 7 | 7 | |
| Grade | ||||
| LGG | 24 | 10 | 14 | 0.088 |
| HGG | 28 | 18 | 10 | |
| Location | ||||
| Chiasma/Thalamus | 27 | 14 | 13 | 0.461 |
| Trunk | 9 | 7 | 2 | |
| Cortex | 10 | 5 | 5 | |
| posterior ventricle | 6 | 2 | 4 | |
| Metastasis | ||||
| Yes | 7 | 6 | 1 | 0.076 |
| No | 45 | 22 | 23 | |
| Relapse | ||||
| Yes | 20 | 10 | 10 | 0.438 |
| No | 32 | 18 | 14 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passariello, A.; Errico, M.E.; Donofrio, V.; Maestrini, M.; Zerbato, A.; Cerchia, L.; Capasso, M.; Capasso, M.; Fedele, M. PATZ1 Is Overexpressed in Pediatric Glial Tumors and Correlates with Worse Event-Free Survival in High-grade Gliomas. Cancers 2019, 11, 1537. https://doi.org/10.3390/cancers11101537
Passariello A, Errico ME, Donofrio V, Maestrini M, Zerbato A, Cerchia L, Capasso M, Capasso M, Fedele M. PATZ1 Is Overexpressed in Pediatric Glial Tumors and Correlates with Worse Event-Free Survival in High-grade Gliomas. Cancers. 2019; 11(10):1537. https://doi.org/10.3390/cancers11101537
Chicago/Turabian StylePassariello, Annalisa, Maria Elena Errico, Vittoria Donofrio, Manuela Maestrini, Alia Zerbato, Laura Cerchia, Maria Capasso, Mario Capasso, and Monica Fedele. 2019. "PATZ1 Is Overexpressed in Pediatric Glial Tumors and Correlates with Worse Event-Free Survival in High-grade Gliomas" Cancers 11, no. 10: 1537. https://doi.org/10.3390/cancers11101537
APA StylePassariello, A., Errico, M. E., Donofrio, V., Maestrini, M., Zerbato, A., Cerchia, L., Capasso, M., Capasso, M., & Fedele, M. (2019). PATZ1 Is Overexpressed in Pediatric Glial Tumors and Correlates with Worse Event-Free Survival in High-grade Gliomas. Cancers, 11(10), 1537. https://doi.org/10.3390/cancers11101537