Clinical Practice Use of Liquid Biopsy to Identify RAS/BRAF Mutations in Patients with Metastatic Colorectal Cancer (mCRC): A Single Institution Experience
Abstract
1. Introduction
2. Results
2.1. Concordance between Tissue and Plasma Samples
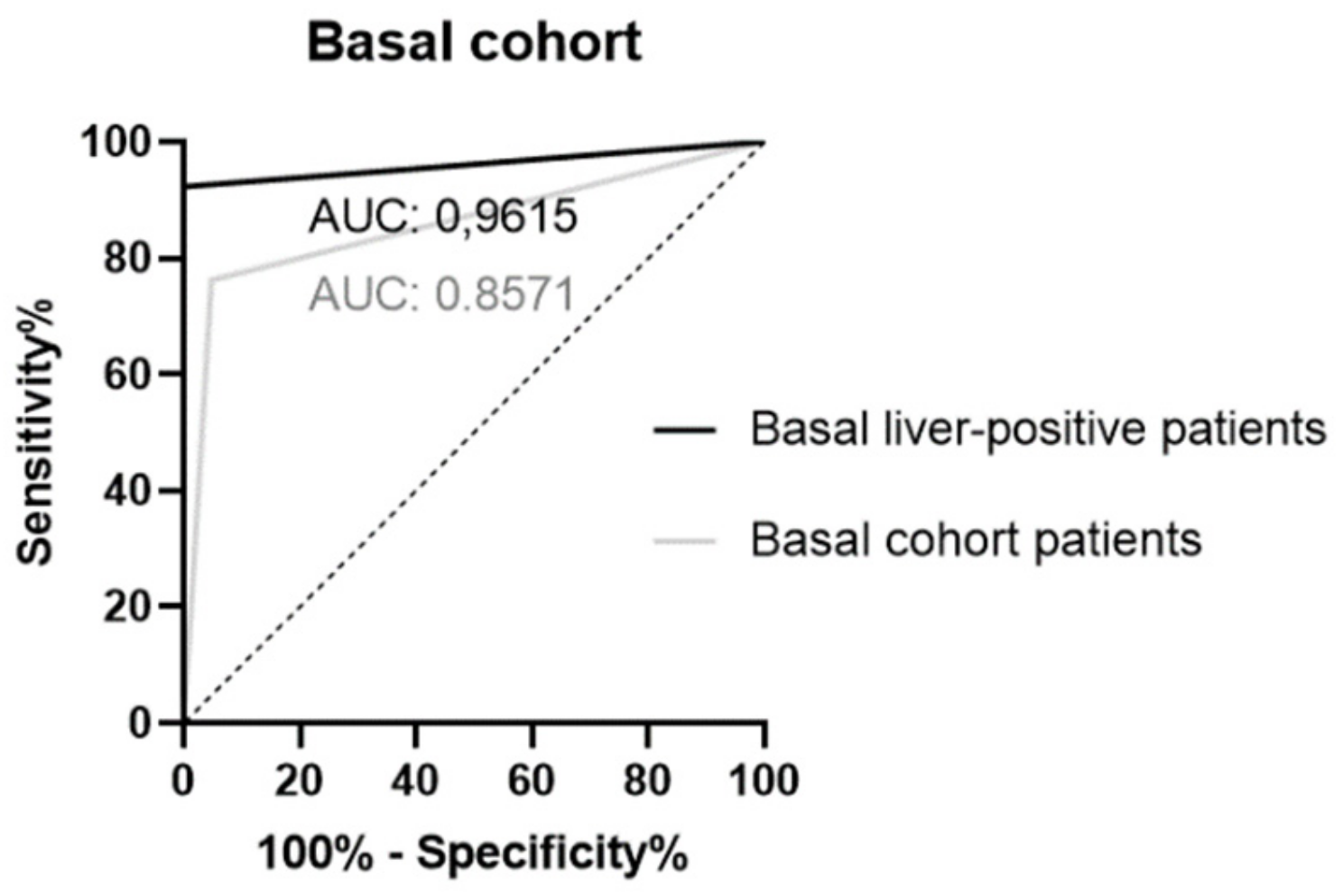
2.2. Sensitivity, Specificity and Positive Predictive Value (PPV)
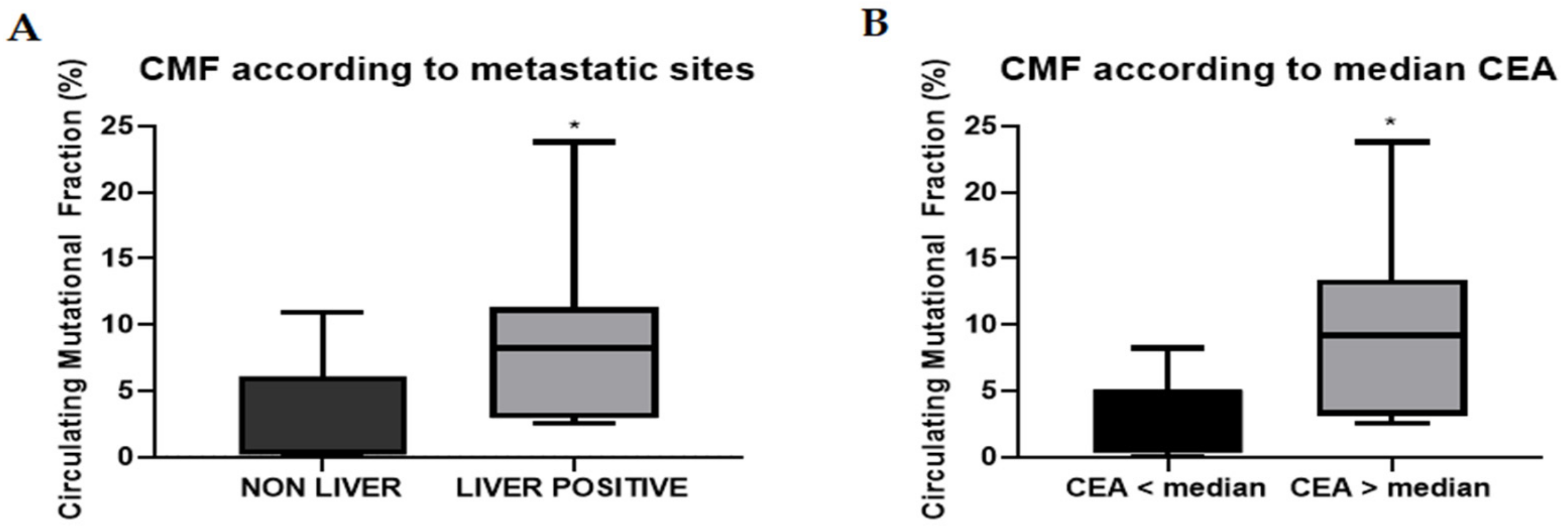
2.3. Circulating Mutational Fraction
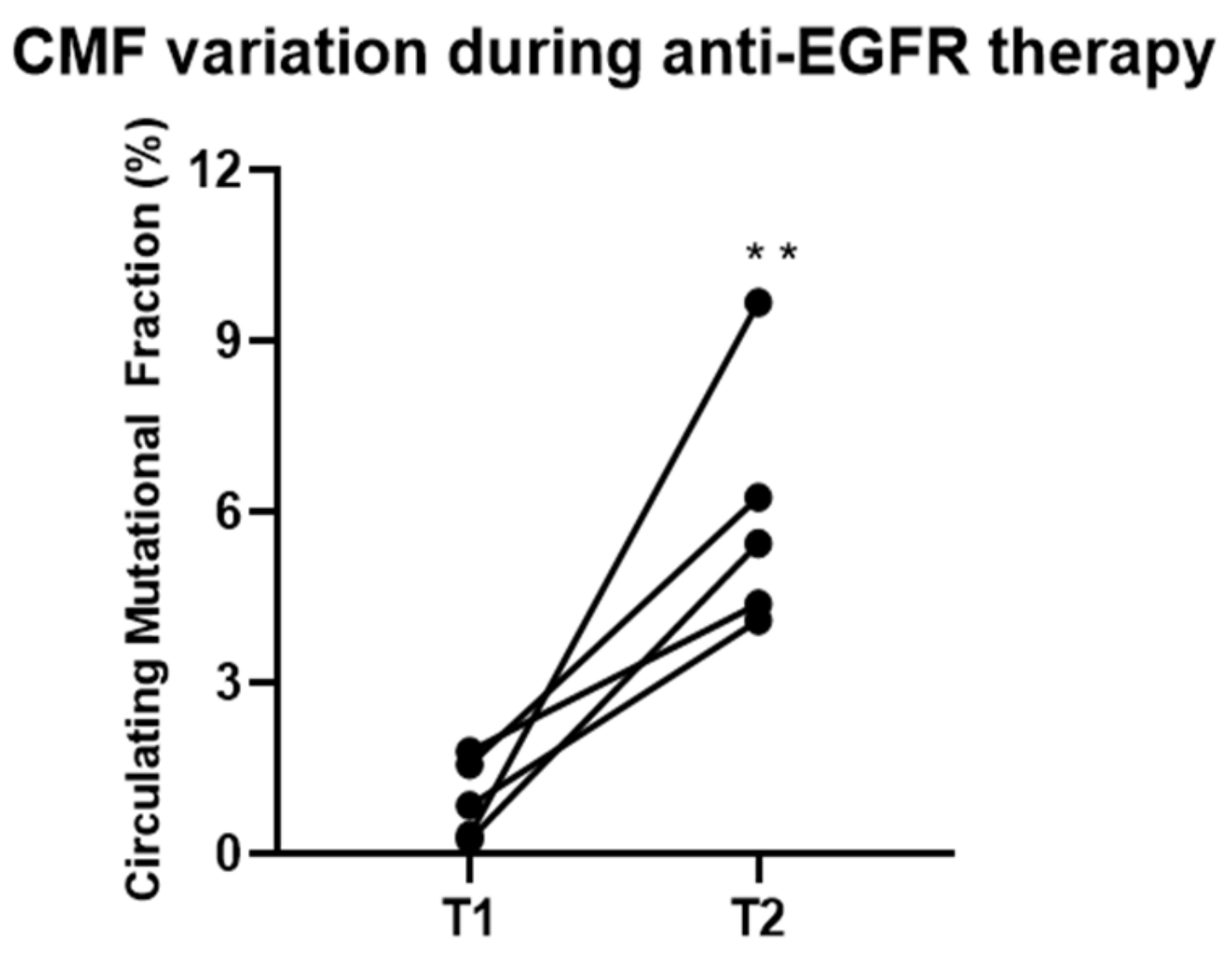
2.4. Post-Anti-EGFR Cohort
3. Discussion
Learning from the Discordant Cases
4. Materials and Methods
4.1. Study Oversight
4.2. Plasma Collection
4.3. Mutational Analyses of Tissue Specimens
4.3.1. Sample Preparation
4.3.2. Library Preparation
4.3.3. Data Analysis
4.4. Mutational Analyses of Plasma
4.5. Concordance
4.6. Sensitivity, Specificity and Positive Predictive Value
4.7. Circulating Mutational Fraction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Latest Global Cancer Data: Cancer Burden Rises to 18.1 Million New Cases and 9.6 Million Cancer Deaths in 2018; WHO: Geneva, Switzerland, 2018; Volume 3.
- Tol, J.; Punt, C.J. Monoclonal antibodies in the treatment of metastatic colorectal cancer: A review. Clin. Ther. 2010, 32, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; van Krieken, J.H.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodokyet, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Mussolin, B.; Buscarino, M.; Corti, G.; Cassingena, A.; Crisafulli, G.; Ponzetti, A.; Cremolini, C.; Amatu, A.; Lauricella, C.; et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat. Med. 2015, 21, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Normanno, N.; Cervantes, A.; Ciardiello, F.; De Luca, A.; Pinto, C. The liquid biopsy in the management of colorectal cancer patients: Current applications and future scenarios. Cancer Treat. Rev. 2018, 70, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumor heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef]
- Diehl, F.; Li, M.; He, Y.; Kinzler, K.W.; Vogelstein, B.; Dressman, D. BEAMing: Single-molecule PCR on microparticles in water-in-oil emulsions. Nat. Methods 2006, 3, 551–559. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Fernández-Landázuri, S.; Rodríguez, C.; Ruth, Z.; María, D.L.; Leyre, Z.; José Luis, P.; Salvador, M.; Alvaro, G. Quantitative cell-free circulating BRAFV600E mutation analysis by use of droplet digital PCR in the follow-up of patients with melanoma being treated with BRAF inhibitors. Clin. Chem. 2015, 61, 297–304. [Google Scholar] [CrossRef]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C.; et al. High-Throughput Droplet Digital PCR System for Absolute Quantitation of DNA Copy Number. Anal. Chem. 2011, 83, 8604–8610. [Google Scholar] [CrossRef]
- Oh, J.E.; Lim, H.S.; An, C.H.; Jeong, E.G.; Han, J.Y.; Lee, S.H.; Yoo, N.J. Detection of Low-Level KRAS Mutations Using PNA-Mediated Asymmetric PCR Clamping and Melting Curve Analysis with Unlabeled Probes. J. Mol. Diagn. 2010, 12, 418–424. [Google Scholar] [CrossRef]
- Watanabe, K.; Fukuhara, T.; Tsukita, Y.; Morita, M.; Suzuki, A.; Tanaka, N.; Terasaki, H.; Nukiwa, T.; Maemondo, M. EGFR Mutation Analysis of Circulating Tumor DNA Using an Improved PNA-LNA PCR Clamp Method. Can. Respir. J. 2016, 2016, 5297329. [Google Scholar] [CrossRef] [PubMed]
- Vivancos, A.; Aranda, E.; Benavides, M.; Élez, E.; Gómez-España, M.A.; Toledano, M.; Alvarez, M.; Parrado, M.R.C.; García-Barberán, V.; Diaz-Rubio, E. Comparison of the Clinical Sensitivity of the Idylla Platform and the OncoBEAM RAS CRC Assay for KRAS Mutation Detection in Liquid Biopsy Samples. Sci. Rep. 2019, 9, 8976. [Google Scholar] [CrossRef] [PubMed]
- Normanno, N.; Esposito Abate, R.; Lambiase, M.; Forgione, L.; Cardone, C.; Iannaccone, A.; Sacco, A.; Rachiglio, A.M.; Martinelli, E.; Rizzi, D.; et al. RAS testing of liquid biopsy correlates with the outcome of metastatic colorectal cancer patients treated with first-line FOLFIRI plus cetuximab in the CAPRI-GOIM trial. Ann. Oncol. 2018, 29, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Laurent-Puig, P.; Pekin, D.; Normand, C.; Steve, K.K.; Philippe, N.; Karla, P.; Rachel, R.; Jeff, O.; Preethi, S.; Delphine, l.; et al. Clinical relevance of KRAS-mutated subclones detected with picodroplet digital PCR in advanced colorectal cancer treated with anti-EGFR therapy. Clin. Cancer Res. 2015, 21, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.; Bellosillo, B.; Vivas, C.S.; García-Alfonso, P.; Carrato, A.; Cano, M.; García-Carbonero, R.; Élez, E.; Losa, F.; Massutí, B.; et al. Ultra-selection of metastatic colorectal cancer patients using next-generation sequencing to improve clinical efficacy of anti-EGFR therapy. Ann. Oncol. 2019, 30, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, J.; Élez, E.; Caratú, G.; Matito, J.; Santos, C.; Macarulla, T.; Vidal, J.; García, M.; Viéitez, J.M.; Páez, D.; et al. Concordance of blood- and tumor-based detection of RAS mutations to guide anti-EGFR therapy in metastatic colorectal cancer. Ann. Oncol. 2017, 28, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Elez, E.; Chianese, C.; Sanz-García, E.; Martinelli, E.; Noguerido, A.; Mancuso, F.M.; Caratù, G.; Matito, J.; Grasselli, J.; Cardone, C.; et al. Impact of circulating tumor DNA mutant allele fraction on prognosis in RAS -mutant metastatic colorectal cancer. Mol. Oncol. 2019, 13, 1827–1835. [Google Scholar] [CrossRef]
- Bachet, J.B.; Bouché, O.; Taieb, J.; Dubreuil, O.; Garcia, M.L.; Meurisse, A.; Normand, C.; Gornet, J.M.; Artru, P.; Louafi, S.; et al. RAS mutation analysis in circulating tumor DNA from patients with metastatic colorectal cancer: The AGEO RASANC prospective multicenter study. Ann. Oncol. 2018, 29, 1211–1219. [Google Scholar] [CrossRef]
- Taly, V.; Pekin, D.; Benhaïm, L.; Kotsopoulos, S.K.; Le Corre, D.; Li, X.; Atochin, I.; Link, D.R.; Griffiths, A.D.; Pallier, K.; et al. Multiplex Picodroplet Digital PCR to Detect KRAS Mutations in Circulating DNA from the Plasma of Colorectal Cancer Patients. Clin. Chem. 2013, 59, 1722–1731. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- Thierry, A.R.; Mouliere, F.; El Messaoudi, S.; Mollevi, C.; Lopez-Crapez, E.; Rolet, F.; Gillet, B.; Gongora, C.; Déchelotte, P.; Robert, B.; et al. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat. Med. 2014, 20, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Suzuki, K.; Muto, Y.; Ichida, K.; Fukui, T.; Kakizawa, N.; Ishikawa, H.; Watanabe, F.; Hasegawa, F.; Saito, M.; et al. Monitoring circulating tumor DNA revealed dynamic changes in KRAS status in patients with metastatic colorectal cancer. Oncotarget 2018, 9, 24398–24413. [Google Scholar] [CrossRef] [PubMed]
- García-Foncillas, J.; Tabernero, J.; Élez, E.; Aranda, E.; Benavides, M.; Camps, C.; Jantus-Lewintre, E.; López, R.; Muinelo-Romay, L.; Montagut, C.; et al. Prospective multicenter real-world RAS mutation comparison between OncoBEAM-based liquid biopsy and tissue analysis in metastatic colorectal cancer. Br. J. Cancer 2018, 119, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Toledo, R.A.; Cubillo, A.; Vega, E.; Garralda, E.; Alvarez, R.; de la Varga, L.U.; Pascual, J.R.; Sánchez, G.; Sarno, F.; Prieto, S.H.; et al. Clinical validation of prospective liquid biopsy monitoring in patients with wild-type RAS metastatic colorectal cancer treated with FOLFIRI-cetuximab. Oncotarget 2017, 8, 35289–35300. [Google Scholar] [CrossRef]
- Spindler, K.-L.G.; Pallisgaard, N.; Appelt, A.L.; Andersen, R.F.; Schou, J.V.; Nielsen, D.; Pfeiffer, P.; Yilmaz, M.; Johansen, J.S.; Hoegdall, E.V.; et al. Clinical utility of KRAS status in circulating plasma DNA compared to archival tumour tissue from patients with metastatic colorectal cancer treated with anti-epidermal growth factor receptor therapy. Eur. J. Cancer 2015, 51, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Chibaudel, B. Extended RAS Mutational Status Analysis in Circulating Tumor DNA from Patients with Advanced Colorectal Cancer in Daily Clinical Practice. The Franco-British Institute Experience and Recommendations. Biomed. J. Sci. Tech. Res. 2018, 6. [Google Scholar] [CrossRef]
- Maurel, J.; Alonso, V.; Escudero, P.; Fernández-Martos, C.; Salud, A.; Méndez, M.V.; Gallego, V.; Rodriguez, J.R.; Martín-Richard, M.; Fernández-Plana, J.; et al. Clinical Impact of Circulating Tumor RAS and BRAF Mutation Dynamics in Patients with Metastatic Colorectal Cancer Treated with First-Line Chemotherapy Plus Anti–Epidermal Growth Factor Receptor Therapy. JCO Precis. Oncol. 2019, 1–16. [Google Scholar] [CrossRef]
- Normanno, N.; Barone, C.; Maiello, E.; Di Costanzo, F.; Cassata, A.; Tamburini, E.; Tonini, G.; Bordonaro, R.; Rosati, G.; Zaniboni, A.; et al. Analysis of liquid biopsies from metastatic colorectal carcinoma (mCRC) patients (pts) enrolled in the ERMES clinical trial. J. Clin. Oncol. 2018, 36, e15507. [Google Scholar] [CrossRef]
- Raimondi, C.; Nicolazzo, C.; Belardinilli, F.; Loreni, F.; Gradilone, A.; Mahdavian, Y.; Gelibter, A.; Giannini, G.; Cortesi, E.; Gazzaniga, P. Transient Disappearance of RAS Mutant Clones in Plasma: A Counterintuitive Clinical Use of EGFR Inhibitors in RAS Mutant Metastatic Colorectal Cancer. Cancers 2019, 11, 42. [Google Scholar] [CrossRef]
- Berger, A.W.; Schwerdel, D.; Welz, H.; Marienfeld, R.; Schmidt, S.A.; Kleger, A.; Ettrich, T.J.; Seufferlein, T. Treatment monitoring in metastatic colorectal cancer patients by quantification and KRAS genotyping of circulating cell-free DNA. PLoS ONE 2017, 12, e0174308. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.R.; El Messaoudi, S.; Mollevi, C.; Raoul, J.L.; Guimbaud, R.; Pezet, D.; Artru, P.; Assenat, E.; Borg, C.; Mathonnet, M.; et al. Clinical utility of circulating DNA analysis for rapid detection of actionable mutations to select metastatic colorectal patients for anti-EGFR treatment. Ann. Oncol. 2017, 28, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- ctNRAS-BRAF cartridge Technical Sheet. Available online: https://media.biocartis.com/biocartis/documents/Tech_Sheet-ctNRAS-BRAF-IVD-A4_WEB.pdf (accessed on 29 June 2019).
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Bellosillo, B.; Pages, J.; Collin, C.; Pasmans, R.; Montagut, C. The Cost of Molecular Diagnostic Testing in Oncology—A Workflow Analysis. Value Heal. 2015, 18, A336–A337. [Google Scholar] [CrossRef][Green Version]
| A | N° | TP | TN | FP | FN | Concordance % | Sensitivity % | Specificity % | PPV % |
| Global cohort | 72 | 17 | 42 | 8 | 5 | 81.94 | 77.27 | 84 | 68 |
| Non-liver | 23 | 4 | 13 | 2 | 4 | 73.91 | 50 | 86.67 | 66.67 |
| Liver positive | 49 | 12 | 30 | 6 | 1 | 85.71 | 92.31 | 83.33 | 66.67 |
| Liver only | 13 | 2 | 8 | 2 | 1 | 76.92 | 66.67 | 80 | 50 |
| Liver and other | 36 | 10 | 22 | 4 | 0 | 88.89 | 100 | 84.62 | 71.43 |
| B | N° | TP | TN | FP | FN | Concordance % | Sensitivity % | Specificity % | PPV % |
| Basal cohort | 42 | 16 | 20 | 1 | 5 | 85.71 | 76.19 | 95.24 | 94.12 |
| Non-liver | 16 | 4 | 7 | 1 | 4 | 68.75 | 50 | 87.5 | 80 |
| Liver positive | 26 | 12 | 13 | 0 | 1 | 96.15 | 92.31 | 100 | 100 |
| Basal liver only | 8 | 3 | 4 | 0 | 1 | 87.5 | 75 | 100 | 100 |
| Basal liver and other | 18 | 9 | 9 | 0 | 0 | 100 | 100 | 100 | 100 |
| Case | Results of Tissue | Results of Liquid Biopsy | Clinical Characteristics | Possible Explanations |
|---|---|---|---|---|
| A | KRAS MUT (G12C) | KRAS WT, NRAS WT, BRAF WT | Pelvic-infiltrating, inoperable rectal cancer. No distant metastasis. | The absence of distant metastases is associated with low abundance of circulating tumor DNA (ctDNA). |
| B | KRAS MUT (G13D) | KRAS WT, NRAS WT, BRAF WT | Left colon cancer with loco-regional disease. | The absence of distant metastases is associated with low abundance of circulating tumor DNA (ctDNA). |
| C | KRAS MUT (G12V) | KRAS WT, NRAS WT, BRAF WT | Left colon cancer with subcentimetric nodal disease. | The absence of distant metastases is associated with low abundance of circulating tumor DNA (ctDNA). |
| D | NRAS MUT (Q61H) | KRAS WT, NRAS WT, BRAF WT | Left colon cancer with multiple subcentimetric liver metastases. | Low MAF for this mutation on ctDNA (predicted sensitivity is optimal for MAF > 5%). The liquid biopsy performed at disease progression (tumor burden increased) confirmed NRAS mutation. |
| E | BRAF MUT (V600E) | KRAS WT, NRAS WT, BRAF WT | Right colon cancer with multiple centimetric lung metastases. | Patient with non-liver metastatic disease and a low burden of disease. Low MAF for this mutation on ctDNA (predicted sensitivity is optimal for MAF > 5%). |
| F | KRAS WT, NRAS WT, BRAF WT | NRAS MUT (G13D) | Nodal-limited recurrence of left colon cancer. | The relapsed cancer is enriched with NRAS mutant cells, that were missed on tissue analysis. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitiello, P.P.; De Falco, V.; Giunta, E.F.; Ciardiello, D.; Cardone, C.; Vitale, P.; Zanaletti, N.; Borrelli, C.; Poliero, L.; Terminiello, M.; et al. Clinical Practice Use of Liquid Biopsy to Identify RAS/BRAF Mutations in Patients with Metastatic Colorectal Cancer (mCRC): A Single Institution Experience. Cancers 2019, 11, 1504. https://doi.org/10.3390/cancers11101504
Vitiello PP, De Falco V, Giunta EF, Ciardiello D, Cardone C, Vitale P, Zanaletti N, Borrelli C, Poliero L, Terminiello M, et al. Clinical Practice Use of Liquid Biopsy to Identify RAS/BRAF Mutations in Patients with Metastatic Colorectal Cancer (mCRC): A Single Institution Experience. Cancers. 2019; 11(10):1504. https://doi.org/10.3390/cancers11101504
Chicago/Turabian StyleVitiello, Pietro Paolo, Vincenzo De Falco, Emilio Francesco Giunta, Davide Ciardiello, Claudia Cardone, Pasquale Vitale, Nicoletta Zanaletti, Carola Borrelli, Luca Poliero, Marinella Terminiello, and et al. 2019. "Clinical Practice Use of Liquid Biopsy to Identify RAS/BRAF Mutations in Patients with Metastatic Colorectal Cancer (mCRC): A Single Institution Experience" Cancers 11, no. 10: 1504. https://doi.org/10.3390/cancers11101504
APA StyleVitiello, P. P., De Falco, V., Giunta, E. F., Ciardiello, D., Cardone, C., Vitale, P., Zanaletti, N., Borrelli, C., Poliero, L., Terminiello, M., Arrichiello, G., Caputo, V., Famiglietti, V., Mattera Iacono, V., Marrone, F., Di Liello, A., Martini, G., Napolitano, S., Caraglia, M., ... Martinelli, E. (2019). Clinical Practice Use of Liquid Biopsy to Identify RAS/BRAF Mutations in Patients with Metastatic Colorectal Cancer (mCRC): A Single Institution Experience. Cancers, 11(10), 1504. https://doi.org/10.3390/cancers11101504








