Sarcopenia Is a Negative Prognostic Factor in Patients Undergoing Transarterial Chemoembolization (TACE) for Hepatic Malignancies
Abstract
1. Introduction
2. Patients and Methods
2.1. Design of the Study and Patient Characteristics
2.2. Transarterial Chemoembolization (TACE)
2.3. TACE Response Analysis
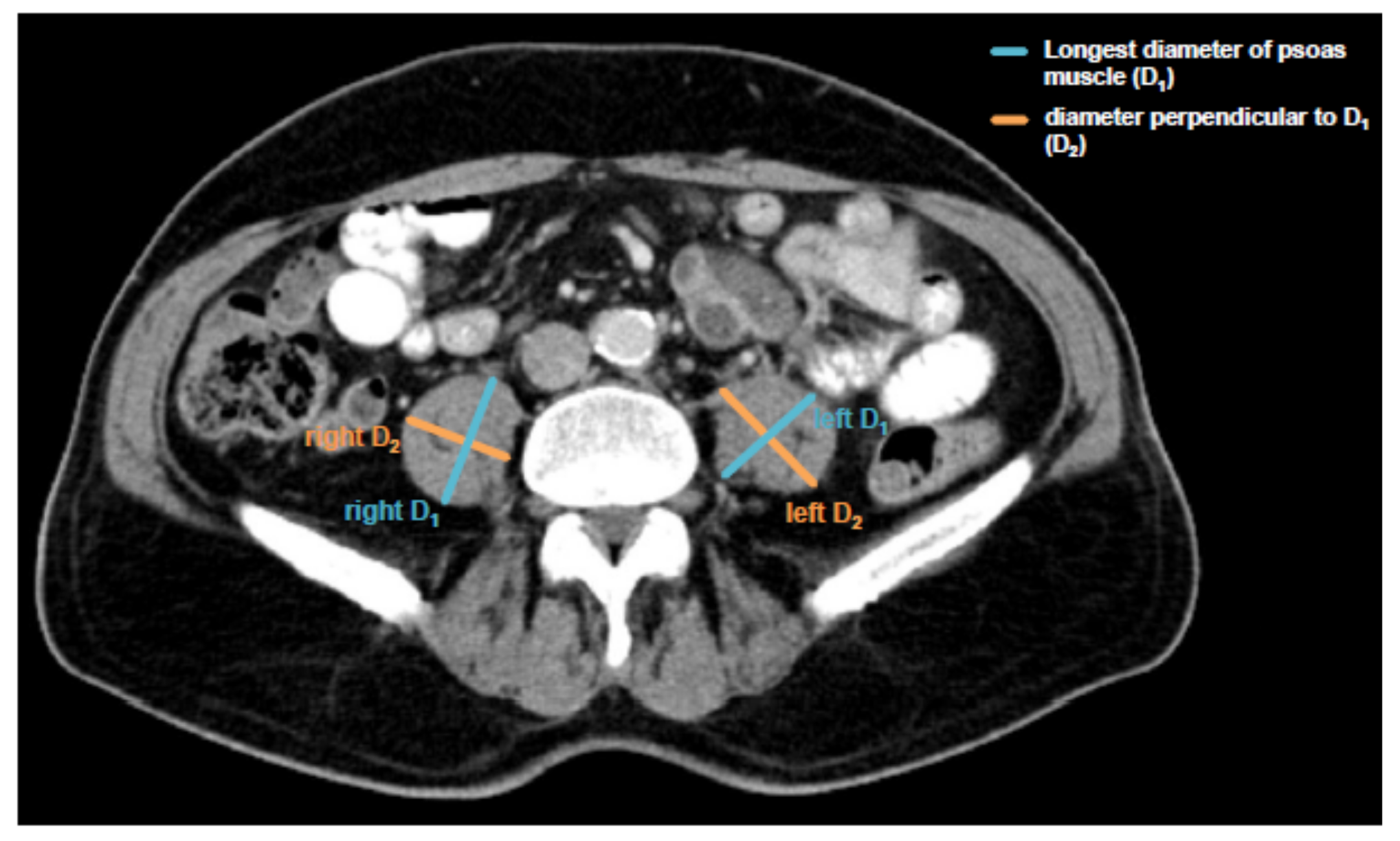
2.4. Assessment of Sarcopenia and Psoas Muscle Index
2.5. Measurement of Cytokine Serum Levels
2.6. Measurement Laboratory Parameters
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
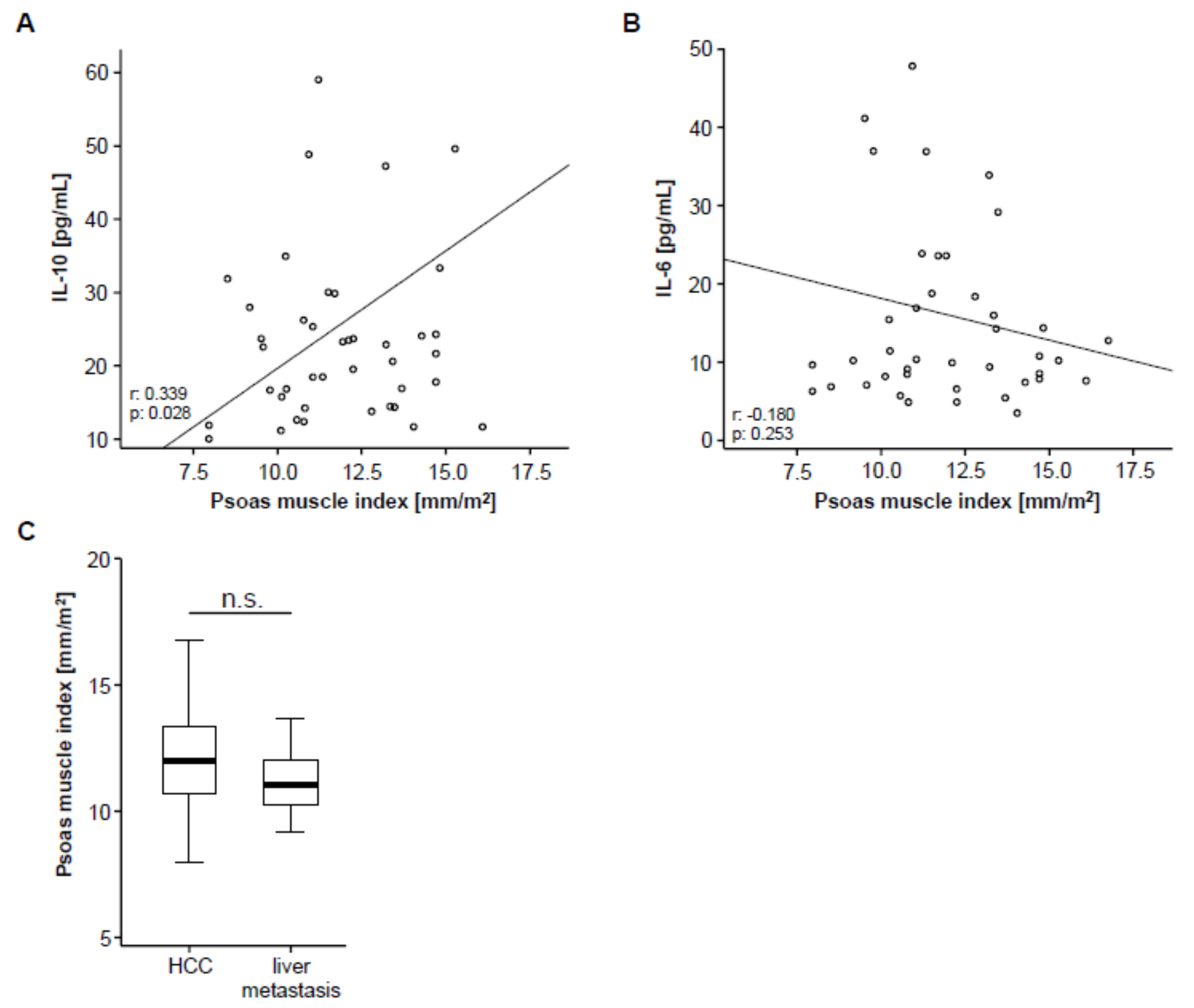
3.2. Psoas Muscle Index (PMI) and Disease Characteristics
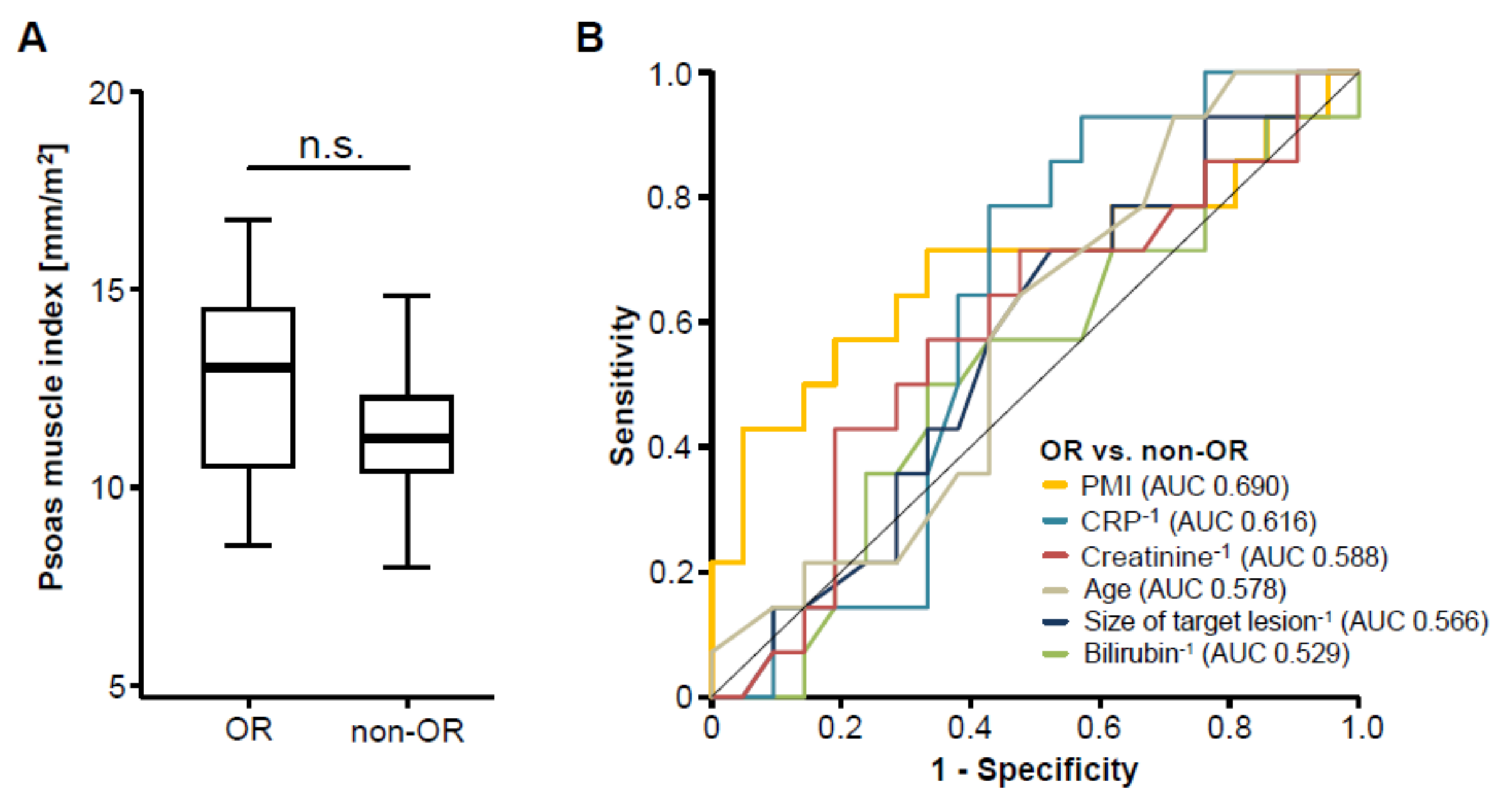
3.3. Pre-Interventional Sarcopenia Does Not Correlate with Treatment Response to TACE Therapy
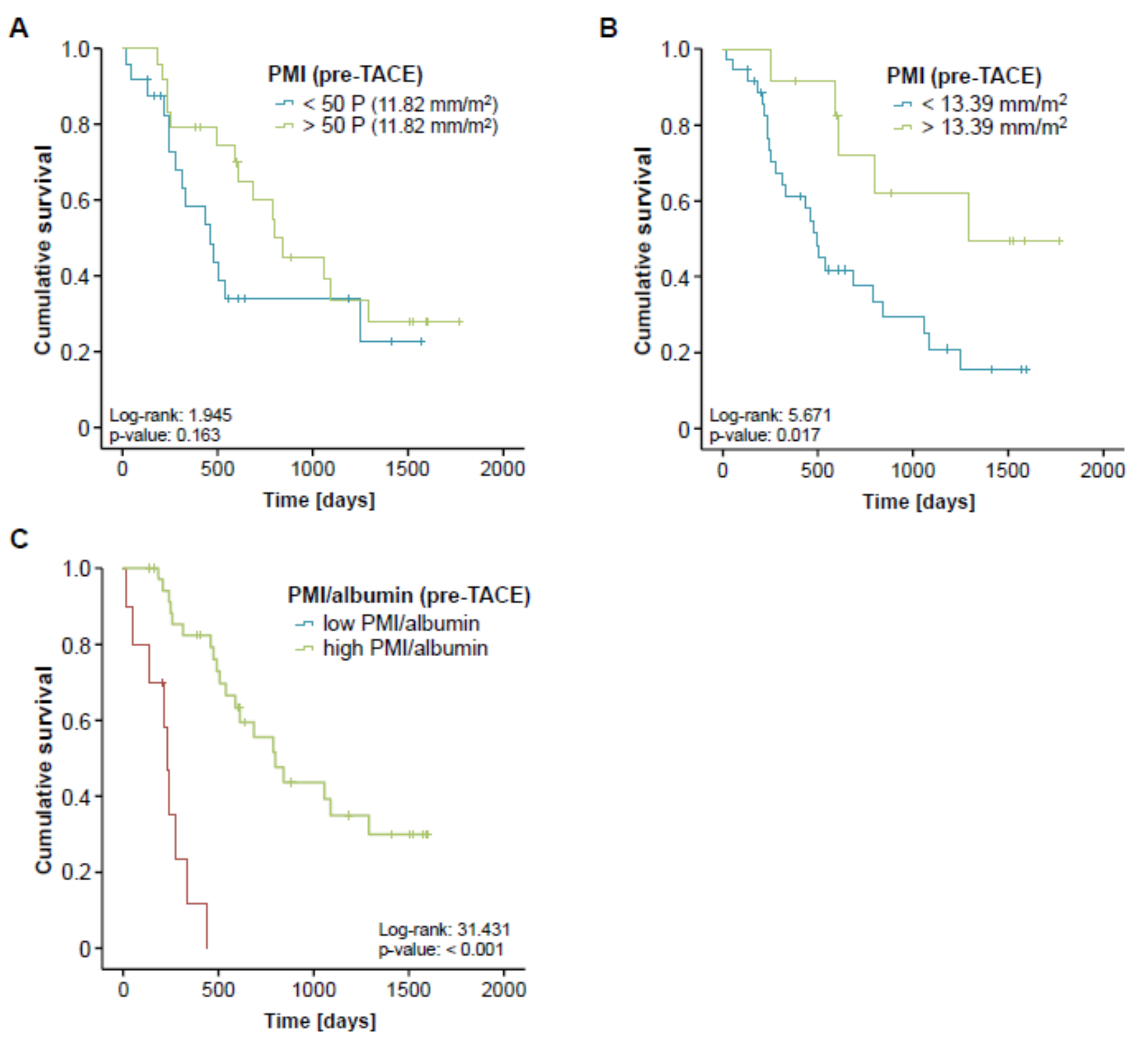
3.4. Pre-Interventional Sarcopenia Is Associated with a Reduced Overall Survival after TACE
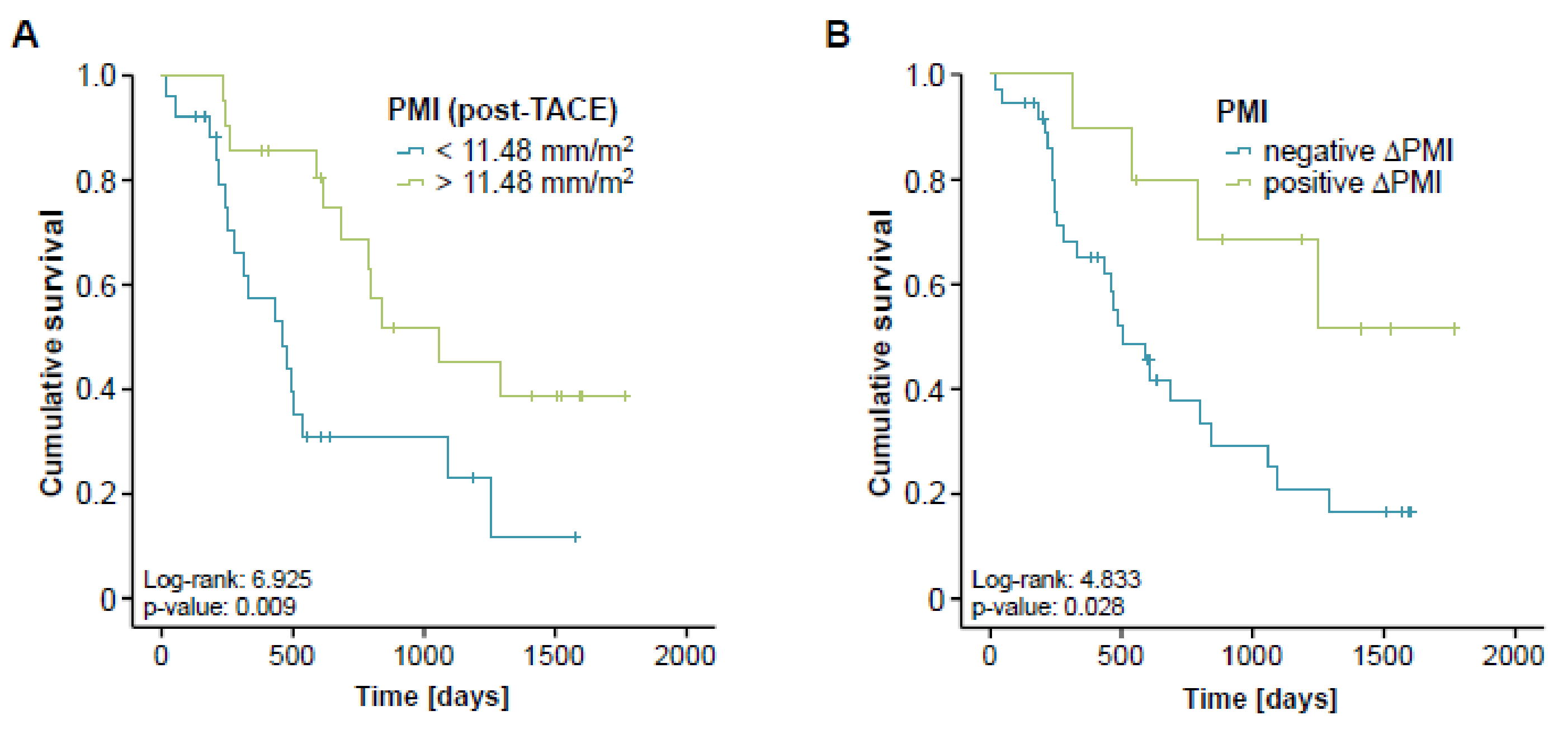
3.5. Post-Interventional Sarcopenia Is Associated with a Poor Prognosis after TACE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TACE | Transarterial chemoembolization |
| HCC | Hepatocellular carcinoma |
| CT | Computed Tomography |
| PMI | Psoas muscle index |
| RWTH | Rheinisch Westfälische Technische Hochschule |
| LVB | Lumbar vertebral body |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| ROC | Receiver Operating Characteristic |
| INR | International Normalized Ratio |
| AST | Aspartate Transaminase |
| ALT | Alanine Transaminase |
| LDH | Lactate dehydrogenase |
| GGT | Gamma-glutamyl Transferase |
| ALP | Alkaline phosphatase |
| CRP | C-reactive protein |
| ORr | Overall response rate |
| AUC | Area under the curve |
| OS | Overall survival |
| HR | Hazard ratio |
| MRI | Magnetic resonance imaging |
References
- Sullivan, K.M.; Kenerson, H.L.; Pillarisetty, V.G.; Riehle, K.J.; Yeung, R.S. Precision oncology in liver cancer. Ann. Transl. Med. 2018, 6, e285. [Google Scholar] [CrossRef] [PubMed]
- Manzini, G.; Henne-Bruns, D.; Porzsolt, F.; Kremer, M. Is there a standard for surgical therapy of hepatocellular carcinoma in healthy and cirrhotic liver? A comparison of eight guidelines. BMJ Open Gastroenterol. 2017, 4, e000129. [Google Scholar] [CrossRef]
- Merle, P.; Camus, P.; Abergel, A.; Pageaux, G.P.; Masliah, C.; Bronowicki, J.P.; Zarski, J.P.; Pelletier, G.; Bouattour, M.; Farloux, L.; et al. Safety and efficacy of intra-arterial hepatic chemotherapy with doxorubicin-loaded nanoparticles in hepatocellular carcinoma. ESMO Open 2017, 2, e000238. [Google Scholar] [CrossRef]
- Yu, S.J. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010-2016. Clin. Mol. Hepatol. 2016, 22, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Matsui, O.; Izumi, N.; Kadoya, M.; Okusaka, T.; Miyayama, S.; Yamakado, K.; Tsuchiya, K.; Ueshima, K.; Hiraoka, A.; et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 2014, 87, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Adhoute, X.; Penaranda, G.; Naude, S.; Raoul, J.L.; Perrier, H.; Bayle, O.; Monnet, O.; Beaurain, P.; Bazin, C.; Pol, B.; et al. Retreatment with TACE: The ABCR SCORE, an aid to the decision-making process. J. Hepatol. 2015, 62, 855–862. [Google Scholar] [CrossRef]
- Fatourou, E.M.; Tsochatzis, E.A. ART and science in using transarterial chemoembolization for retreating patients with hepatocellular carcinoma. Hepatobiliary Surg. Nutr. 2014, 3, 415–418. [Google Scholar] [PubMed]
- Kim, B.K.; Shim, J.H.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, K.M.; Lim, Y.-S.; Han, K.-H.; Lee, H.C. Risk prediction for patients with hepatocellular carcinoma undergoing chemoembolization: Development of a prediction model. Liver Int. 2016, 36, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Mislang, A.R.; Di Donato, S.; Hubbard, J.; Krishna, L.; Mottino, G.; Bozzetti, F.; Biganzoli, L. Nutritional management of older adults with gastrointestinal cancers: An international society of geriatric oncology (SIOG) review paper. J. Geriatr. Oncol. 2018, 9, 382–392. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumors: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Li, Y.; Peng, Y.; Lu, D.; Zhang, F.; Cui, X.; Zhang, Q.; Li, Z. Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int. J. Colorectal Dis. 2018, 33, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, R.; Oki, E.; Sasaki, S.; Hirose, K.; Jogo, T.; Edahiro, K.; Korehisa, S.; Taniguchi, D.; Kudo, K.; Kurashige, J.; et al. Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg. Today 2018, 48, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumors: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Edeline, J.; Boucher, E.; Rolland, Y.; Vauléon, E.; Pracht, M.; Perrin, C.; Le Roux, C.; Raoul, J.-L. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer 2012, 118, 147–156. [Google Scholar] [CrossRef]
- Loosen, S.H.; Schulze-Hagen, M.; Leyh, C.; Benz, F.; Vucur, M.; Kuhl, C.; Trautwein, C.; Tacke, F.; Bruners, P.; Roderburg, C.; et al. IL-6 and IL-8 serum levels predict tumor response and overall survival after TACE for primary and secondary hepatic malignancies. Int. J. Mol. Sci. 2018, 19, 1766. [Google Scholar] [CrossRef]
- Loosen, S.H.; Roderburg, C.; Kauertz, K.L.; Pombeiro, I.; Leyh, C.; Benz, F.; Vucur, M.; Longerich, T.; Koch, A.; Braunschweig, T.; et al. Elevated levels of circulating osteopontin are associated with a poor survival after resection of cholangiocarcinoma. J. Hepatol. 2017, 67, 749–757. [Google Scholar] [CrossRef]
- Budczies, J.; Klauschen, F.; Sinn, B.V.; Győrffy, B.; Schmitt, W.D.; Darb-Esfahani, S.; Denkert, C. Cutoff Finder: A comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS ONE 2012, 7, e51862. [Google Scholar] [CrossRef]
- Koch, A.; Voigt, S.; Kruschinski, C.; Sanson, E.; Dückers, H.; Horn, A.; Yagmur, E.; Zimmermann, H.; Trautwein, C.; Tacke, F. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit. Care 2011, 15, e63. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The role of inflammation in age-related sarcopenia. Front. Physiol. 2017, 8, e1045. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, G.; Sarti, D.; Aliberti, C.; Carandina, R.; Mambrini, A.; Guadagni, S. Multidisciplinary approach of colorectal cancer liver metastases. World J. Clin. Oncol. 2017, 8, e190. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Zhu, A.X.; Wigdan, F.; Almasri, J.; Zaiem, F.; Prokop, L.J.; Hassan Murad, M.; Mohammed, K. Therapies for advanced stage hepatocellular carcinoma with macrovascular invasion or metastatic disease: A systematic review and meta-analysis. Hepatology 2017, 67, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; Heimbach, J.K.; Zaiem, F.; Almasri, J.; Prokop, L.J.; Wang, Z.; Murad, M.H.; Mohammed, K. Therapies for patients with hepatocellular carcinoma awaiting for liver transplantation: A systematic review and meta-analysis. Hepatology 2017, 67, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Chen, M.; Colombo, M.; Roberts, L.R.; Schwartz, M.; Chen, P.-J.; Kudo, M.; Johnson, P.; Wagner, S.; Orsini, L.S.; et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study. Liver Int. 2015, 35, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Massmann, A.; Rodt, T.; Marquardt, S.; Seidel, R.; Thomas, K.; Wacker, F.; Richter, G.M.; Kauczor, H.U.; Bücker, A.; Pereira, P.L.; et al. Transarterial chemoembolization (TACE) for colorectal liver metastases--current status and critical review. Langenbeck’s Arch. Surg. 2015, 400, 641–659. [Google Scholar] [CrossRef]
- Sacco, R.; Tapete, G.; Simonetti, N.; Sellitri, R.; Natali, V.; Melissari, S.; Cabibbo, G.; Biscaglia, L.; Bresci, G.; Giacomelli, L. Transarterial chemoembolization for the treatment of hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2017, 4, 105–110. [Google Scholar] [CrossRef]
- Kadalayil, L.; Benini, R.; Pallan, L.; O’Beirne, J.; Marelli, L.; Yu, D.; Hackshaw, A.; Fox, R.; Johnson, P.; Burroughs, A.K.; et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann. Oncol. 2013, 24, 2565–2570. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Montesano, A.; Senesi, P.; Luzi, L.; Benedini, S.; Terruzzi, I. Potential therapeutic role of L-carnitine in skeletal muscle oxidative stress and atrophy conditions. Oxid. Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, Y.; Wang, X.; Li, H.; Zhang, H.; Gong, J.; Shen, S.; Yin, W.; Hu, H. Efficacy and safety of oral branched-chain amino acid supplementation in patients undergoing interventions for hepatocellular carcinoma: A meta-analysis. Nutr. J. 2015, 14, e67. [Google Scholar] [CrossRef] [PubMed]
- Koya, S.; Kawaguchi, T.; Hashida, R.; Goto, E.; Matsuse, H.; Saito, H.; Hirota, K.; Taira, R.; Matsushita, Y.; Imanaga, M.; et al. Effects of in-hospital exercise on liver function, physical ability, and muscle mass during treatment of hepatoma in patients with chronic liver disease. Hepatol. Res. 2017, 47, 22–34. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Study Cohort |
|---|---|
| TACE patients | 56 |
| Sex (%): | |
| male-female | 78.6–21.4 |
| Age (years, median and range) | 65 (30–89) |
| Hepatic malignancy (%): | |
| HCC | 82.1 |
| Liver metastasis (CRC) | 10.7 |
| Liver metastasis (gastric cancer) | 1.8 |
| Liver metastasis (pancreatic cancer) | 3.6 |
| Liver metastasis (CCA) | 1.8 |
| Size of target lesion (mm, median and range) | 27 (10–129) |
| HCC tumor stage (%): | |
| BCLC A | 34.8 |
| BCLC B | 58.7 |
| BCLC C | 6.5 |
| Child-Pugh Score (HCC only) (%): | |
| No liver cirrhosis | 2.2 |
| CHILD A: | 80.4 |
| 5 points | 56.5 |
| 6 points | 23.9 |
| CHILD B: | 17.4 |
| 7 points | 13 |
| 8 points | 4.4 |
| OR to TACE therapy (%): | |
| Yes–No | 41.5–58.5 |
| Deceased during follow-up (%): | |
| Yes–No | 60.0–40.0 |
| Median overall survival (days/month) | 611/20.4 |
| Characteristic | Univariate Cox Regression | Multivariate Cox Regression | ||
|---|---|---|---|---|
| Parameter | p-Value | Hazard-Ratio (95% CI) | p-Value | Hazard-Ratio (95% CI) |
| PMI < 13.39 mm/m2 | 0.023 | 3.075 (1.165–8.117) | 0.041 | 2.876 (1.044–7.922) |
| Size of target lesion | 0.319 | 1.006 (0.995–1.016) | ||
| Age | 0.162 | 1.021 (0.992–1.052) | 0.435 | 1.017 (0.975–1.060) |
| Sex | 0.878 | 0.973 (0.405–2.165) | ||
| Creatinine | 0.164 | 1.491 (0.850–2.615) | 0.223 | 1.436 (0.802–2.570) |
| ALT | 0.614 | 0.998 (0.992–1.005) | ||
| LDH | 0.934 | 1.000 (0.997–1.002) | ||
| Albumin | 0.063 | 0.495 (0.236–1.039) | 0.035 | 0.314 (0.107–0.924) |
| CRP | 0.002 | 1.026 (1.009–1.042) | 0.058 | 1.019 (0.999–1.039) |
| Bilirubin | 0.696 | 0.857 (0.395–1.859) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loosen, S.H.; Schulze-Hagen, M.; Bruners, P.; Tacke, F.; Trautwein, C.; Kuhl, C.; Luedde, T.; Roderburg, C. Sarcopenia Is a Negative Prognostic Factor in Patients Undergoing Transarterial Chemoembolization (TACE) for Hepatic Malignancies. Cancers 2019, 11, 1503. https://doi.org/10.3390/cancers11101503
Loosen SH, Schulze-Hagen M, Bruners P, Tacke F, Trautwein C, Kuhl C, Luedde T, Roderburg C. Sarcopenia Is a Negative Prognostic Factor in Patients Undergoing Transarterial Chemoembolization (TACE) for Hepatic Malignancies. Cancers. 2019; 11(10):1503. https://doi.org/10.3390/cancers11101503
Chicago/Turabian StyleLoosen, Sven H., Maximilian Schulze-Hagen, Philipp Bruners, Frank Tacke, Christian Trautwein, Christiane Kuhl, Tom Luedde, and Christoph Roderburg. 2019. "Sarcopenia Is a Negative Prognostic Factor in Patients Undergoing Transarterial Chemoembolization (TACE) for Hepatic Malignancies" Cancers 11, no. 10: 1503. https://doi.org/10.3390/cancers11101503
APA StyleLoosen, S. H., Schulze-Hagen, M., Bruners, P., Tacke, F., Trautwein, C., Kuhl, C., Luedde, T., & Roderburg, C. (2019). Sarcopenia Is a Negative Prognostic Factor in Patients Undergoing Transarterial Chemoembolization (TACE) for Hepatic Malignancies. Cancers, 11(10), 1503. https://doi.org/10.3390/cancers11101503






