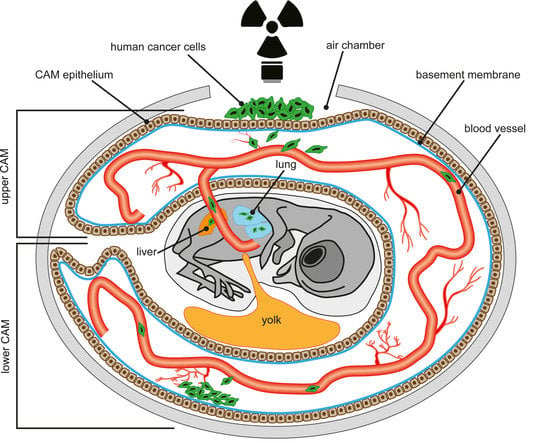
Implementation of the Chick Chorioallantoic Membrane (CAM) Model in Radiation Biology and Experimental Radiation Oncology Research
Abstract
1. Introduction
2. Biologic Optimization of Radiotherapy
3. Current Preclinical Models to Assess Cellular Radiation Responses
4. The Chick Chorioallantoic Membrane (CAM) Assay
5. Advantages and Limitations of the CAM Assay
5.1. Biological Advantages
5.2. Technical Advantages
5.3. Ethical Advantages
6. The Immune System of the CAM
7. Radiation Studies in and Ex Ovo
8. The CAM Model in Invasion Studies Under Hypoxia
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bartelink, H.; Roelofsen, F.; Eschwege, F.; Rougier, P.; Bosset, J.F.; Gonzalez, D.G.; Peiffert, D.; van Glabbeke, M.; Pierart, M. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. JCO 1997, 15, 2040–2049. [Google Scholar] [CrossRef]
- Yu, V.Y.; Nguyen, D.; Pajonk, F.; Kupelian, P.; Kaprealian, T.; Selch, M.; Low, D.A.; Sheng, K. Incorporating cancer stem cells in radiation therapy treatment response modeling and the implication in glioblastoma multiforme treatment resistance. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Begg, A.C.; Stewart, F.A.; Vens, C. Strategies to improve radiotherapy with targeted drugs. Nat. Rev. Cancer 2011, 11, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.M.; Narang, A.K.; Griffith, K.A.; Zalupski, M.M.; Reese, J.B.; Gearhart, S.L.; Azad, N.S.; Chan, J.; Olsen, L.; Efron, J.E.; et al. The quality-of-life effects of neoadjuvant chemoradiation in locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, e15–e19. [Google Scholar] [CrossRef]
- Morgan, M.A.; Parsels, L.A.; Maybaum, J.; Lawrence, T.S. Improving the efficacy of chemoradiation with targeted agents. Cancer Discov. 2014, 4, 280–291. [Google Scholar] [CrossRef]
- Budach, W.; Hehr, T.; Budach, V.; Belka, C.; Dietz, K. A meta-analysis of hyperfractionated and accelerated radiotherapy and combined chemotherapy and radiotherapy regimens in unresected locally advanced squamous cell carcinoma of the head and neck. BMC Cancer 2006, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef]
- Orth, M.; Lauber, K.; Niyazi, M.; Friedl, A.A.; Li, M.; Maihöfer, C.; Schüttrumpf, L.; Ernst, A.; Niemöller, O.M.; Belka, C. Current concepts in clinical radiation oncology. Radiat. Environ. Biophys. 2014, 53, 1–29. [Google Scholar] [CrossRef]
- Held, K.D.; Kawamura, H.; Kaminuma, T.; Paz, A.E.S.; Yoshida, Y.; Liu, Q.; Willers, H.; Takahashi, A. Effects of Charged Particles on Human Tumor Cells. Front. Oncol. 2016, 6, 23. [Google Scholar] [CrossRef]
- Schulz-Ertner, D.; Jäkel, O.; Schlegel, W. Radiation therapy with charged particles. Semin. Radiat. Oncol. 2006, 16, 249–259. [Google Scholar] [CrossRef]
- Paganetti, H.; van Luijk, P. Biological considerations when comparing proton therapy with photon therapy. Semin. Radiat. Oncol. 2013, 23, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Plastaras, J.P.; Berman, A.T.; Freedman, G.M. Special cases for proton beam radiotherapy: Re-irradiation, lymphoma, and breast cancer. Semin. Oncol. 2014, 41, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Suit, H.; DeLaney, T.; Goldberg, S.; Paganetti, H.; Clasie, B.; Gerweck, L.; Niemierko, A.; Hall, E.; Flanz, J.; Hallman, J.; et al. Proton vs carbon ion beams in the definitive radiation treatment of cancer patients. Radiother. Oncol. 2010, 95, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Ertner, D.; Tsujii, H. Particle radiation therapy using proton and heavier ion beams. J. Clin. Oncol. 2007, 25, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Dokic, I.; Mairani, A.; Mein, S.; Brons, S.; Häring, P.; Haberer, T.; Jäkel, O.; Zimmermann, A.; Zenke, F.; et al. Overcoming hypoxia-induced tumor radioresistance in non-small cell lung cancer by targeting DNA-dependent protein kinase in combination with carbon ion irradiation. Radiat. Oncol. 2017, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wei, Q.; Schwager, C.; Hanne, J.; Zhou, C.; Herfarth, K.; Rieken, S.; Lipson, K.E.; Debus, J.; Abdollahi, A. Oncogene addiction and radiation oncology: Effect of radiotherapy with photons and carbon ions in ALK-EML4 translocated NSCLC. Radiat. Oncol. 2018, 13, 1. [Google Scholar] [CrossRef]
- Takahashi, W.; Nakajima, M.; Yamamoto, N.; Yamashita, H.; Nakagawa, K.; Miyamoto, T.; Tsuji, H.; Kamada, T.; Fujisawa, T. A prospective nonrandomized phase I/II study of carbon ion radiotherapy in a favorable subset of locally advanced non-small cell lung cancer (NSCLC). Cancer 2015, 121, 1321–1327. [Google Scholar] [CrossRef]
- Zhou, C.; Moustafa, M.R.; Cao, L.; Kriegsmann, M.; Winter, M.; Schwager, C.; Jones, B.; Wang, S.; Bäuerle, T.; Zhou, P.-K.; et al. Modeling and multiscale characterization of the quantitative imaging based fibrosis index reveals pathophysiological, transcriptome and proteomic correlates of lung fibrosis induced by fractionated irradiation. Int. J. Cancer 2019, 144, 3160–3173. [Google Scholar] [CrossRef]
- Zhou, C.; Jones, B.; Moustafa, M.; Yang, B.; Brons, S.; Cao, L.; Dai, Y.; Schwager, C.; Chen, M.; Jaekel, O.; et al. Determining RBE for development of lung fibrosis induced by fractionated irradiation with carbon ions utilizing fibrosis index and high-LET BED model. Clin. Transl. Radiat. Oncol. 2019, 14, 25–32. [Google Scholar] [CrossRef]
- Graves, P.R.; Siddiqui, F.; Anscher, M.S.; Movsas, B. Radiation pulmonary toxicity: From mechanisms to management. Semin. Radiat. Oncol. 2010, 20, 201–207. [Google Scholar] [CrossRef]
- Nishimura, H.; Miyamoto, T.; Yamamoto, N.; Koto, M.; Sugimura, K.; Tsujii, H. Radiographic pulmonary and pleural changes after carbon ion irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 861–866. [Google Scholar] [CrossRef]
- Hayashi, K.; Yamamoto, N.; Karube, M.; Nakajima, M.; Matsufuji, N.; Tsuji, H.; Ogawa, K.; Kamada, T. Prognostic analysis of radiation pneumonitis: Carbon-ion radiotherapy in patients with locally advanced lung cancer. Radiat. Oncol. 2017, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, X.; Yang, R.; Liu, Y.; Zhao, W.; Si, J.; Ma, X.; Sun, C.; Liu, Y.; Tan, Y.; et al. Effects of carbon ion beam irradiation on lung injury and pulmonary fibrosis in mice. Exp. Ther. Med. 2013, 5, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.S.; Horsman, M.R.; Alsner, J.; Overgaard, J.; Durante, M.; Scholz, M.; Friedrich, T.; Bassler, N. Relative biological effectiveness of carbon ions for tumor control, acute skin damage and late radiation-induced fibrosis in a mouse model. Acta Oncol. 2015, 54, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Krause, M.; Overgaard, J.; Debus, J.; Bentzen, S.M.; Daartz, J.; Richter, C.; Zips, D.; Bortfeld, T. Radiation oncology in the era of precision medicine. Nat. Rev. Cancer 2016, 16, 234–249. [Google Scholar] [CrossRef]
- Bolla, M.; de Reijke, T.M.; van Tienhoven, G.; van den Bergh, A.C.M.; Oddens, J.; Poortmans, P.M.P.; Gez, E.; Kil, P.; Akdas, A.; Soete, G.; et al. Duration of androgen suppression in the treatment of prostate cancer. N. Engl. J. Med. 2009, 360, 2516–2527. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Cohen, R.B.; Jones, C.U.; Sur, R.K.; Raben, D.; Baselga, J.; Spencer, S.A.; Zhu, J.; et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010, 11, 21–28. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Manola, J.; Loffredo, M.; Renshaw, A.A.; DellaCroce, A.; Kantoff, P.W. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. JAMA 2004, 292, 821–827. [Google Scholar] [CrossRef]
- Bentzen, J.; Toustrup, K.; Eriksen, J.G.; Primdahl, H.; Andersen, L.J.; Overgaard, J. Locally advanced head and neck cancer treated with accelerated radiotherapy, the hypoxic modifier nimorazole and weekly cisplatin. Results from the DAHANCA 18 phase II study. Acta Oncol. 2015, 54, 1001–1007. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 2009, 10, 718–726. [Google Scholar] [CrossRef]
- Burnette, B.; Fu, Y.-X.; Weichselbaum, R.R. The confluence of radiotherapy and immunotherapy. Front. Oncol. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, A.; Wang, M.; Robins, H.I.; Lautenschlaeger, T.; Curran, W.J.; Brachman, D.G.; Schultz, C.J.; Choucair, A.; Dolled-Filhart, M.; Christiansen, J.; et al. RTOG 0211: A phase 1/2 study of radiation therapy with concurrent gefitinib for newly diagnosed glioblastoma patients. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Crane, C.H.; Winter, K.; Regine, W.F.; Safran, H.; Rich, T.A.; Curran, W.; Wolff, R.A.; Willett, C.G. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. J. Clin. Oncol. 2009, 27, 4096–4102. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Hainsworth, J.D.; Yardley, D.A.; Raefsky, E.; Patton, J.; Peacock, N.; Farley, C.; Burris, H.A.; Greco, F.A. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J. Clin. Oncol. 2010, 28, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Rischin, D.; Peters, L.J.; O’Sullivan, B.; Giralt, J.; Fisher, R.; Yuen, K.; Trotti, A.; Bernier, J.; Bourhis, J.; Ringash, J.; et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): A phase III trial of the Trans-Tasman Radiation Oncology Group. J. Clin. Oncol. 2010, 28, 2989–2995. [Google Scholar] [CrossRef] [PubMed]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death: From specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013, 332, 237–248. [Google Scholar] [CrossRef]
- Kirsch, D.G.; Diehn, M.; Kesarwala, A.H.; Maity, A.; Morgan, M.A.; Schwarz, J.K.; Bristow, R.; Demaria, S.; Eke, I.; Griffin, R.J.; et al. The Future of Radiobiology. J. Natl. Cancer Inst. 2018, 110, 329–340. [Google Scholar] [CrossRef]
- Bristow, R.G.; Berlin, A.; Dal Pra, A. An arranged marriage for precision medicine: Hypoxia and genomic assays in localized prostate cancer radiotherapy. Br. J. Radiol. 2014, 87, 20130753. [Google Scholar] [CrossRef]
- Scott, J.G.; Berglund, A.; Schell, M.J.; Mihaylov, I.; Fulp, W.J.; Yue, B.; Welsh, E.; Caudell, J.J.; Ahmed, K.; Strom, T.S.; et al. A genome-based model for adjusting radiotherapy dose (GARD): A retrospective, cohort-based study. Lancet Oncol. 2017, 18, 202–211. [Google Scholar] [CrossRef]
- Bristow, R.G.; Alexander, B.; Baumann, M.; Bratman, S.V.; Brown, J.M.; Camphausen, K.; Choyke, P.; Citrin, D.; Contessa, J.N.; Dicker, A.; et al. Combining precision radiotherapy with molecular targeting and immunomodulatory agents: A guideline by the American Society for Radiation Oncology. Lancet Oncol. 2018, 19, e240–e251. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Meziani, L.; Yakkala, C.; Vozenin, M.-C. Expanding the therapeutic index of radiation therapy by normal tissue protection. Br. J. Radiol. 2018, 20180008. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Helleday, T. Cancer phenotypic lethality, exemplified by the non-essential MTH1 enzyme being required for cancer survival. Ann. Oncol. 2014, 25, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.A.; Lawrence, T.S. Molecular Pathways: Overcoming Radiation Resistance by Targeting DNA Damage Response Pathways. Clin. Cancer Res. 2015, 21, 2898–2904. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294. [Google Scholar] [CrossRef]
- Fokas, E.; Prevo, R.; Pollard, J.R.; Reaper, P.M.; Charlton, P.A.; Cornelissen, B.; Vallis, K.A.; Hammond, E.M.; Olcina, M.M.; Gillies McKenna, W.; et al. Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell Death Dis. 2012, 3, e441. [Google Scholar] [CrossRef] [PubMed]
- Biddlestone-Thorpe, L.; Sajjad, M.; Rosenberg, E.; Beckta, J.M.; Valerie, N.C.K.; Tokarz, M.; Adams, B.R.; Wagner, A.F.; Khalil, A.; Gilfor, D.; et al. ATM kinase inhibition preferentially sensitizes p53-mutant glioma to ionizing radiation. Clin. Cancer Res. 2013, 19, 3189–3200. [Google Scholar] [CrossRef]
- Parsels, L.A.; Karnak, D.; Parsels, J.D.; Zhang, Q.; Vélez-Padilla, J.; Reichert, Z.R.; Wahl, D.R.; Maybaum, J.; O’Connor, M.J.; Lawrence, T.S.; et al. PARP1 Trapping and DNA Replication Stress Enhance Radiosensitization with Combined WEE1 and PARP Inhibitors. Mol. Cancer Res. 2018, 16, 222–232. [Google Scholar] [CrossRef]
- Koniaras, K.; Cuddihy, A.R.; Christopoulos, H.; Hogg, A.; O’Connell, M.J. Inhibition of Chk1-dependent G2 DNA damage checkpoint radiosensitizes p53 mutant human cells. Oncogene 2001, 20, 7453–7463. [Google Scholar] [CrossRef]
- Gad, H.; Koolmeister, T.; Jemth, A.-S.; Eshtad, S.; Jacques, S.A.; Ström, C.E.; Svensson, L.M.; Schultz, N.; Lundbäck, T.; Einarsdottir, B.O.; et al. MTH1 inhibition eradicates cancer by preventing sanitation of the dNTP pool. Nature 2014, 508, 215–221. [Google Scholar] [CrossRef]
- Van Gent, D.C.; Kanaar, R. Exploiting DNA repair defects for novel cancer therapies. Mol. Biol. Cell 2016, 27, 2145–2148. [Google Scholar] [CrossRef] [PubMed]
- Sanjiv, K.; Hagenkort, A.; Calderón-Montaño, J.M.; Koolmeister, T.; Reaper, P.M.; Mortusewicz, O.; Jacques, S.A.; Kuiper, R.V.; Schultz, N.; Scobie, M.; et al. Cancer-Specific Synthetic Lethality between ATR and CHK1 Kinase Activities. Cell Rep. 2016, 14, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, P.L.; Corso, C.D.; Robinson, N.D.; Scanlon, S.E.; Purshouse, K.R.; Bai, H.; Liu, Y.; Sundaram, R.K.; Hegan, D.C.; Fons, N.R.; et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.; Koritzinsky, M.; Zhao, H.; Bindra, R.; Glazer, P.M.; Powell, S.; Belmaaza, A.; Wouters, B.; Bristow, R.G. Chronic hypoxia decreases synthesis of homologous recombination proteins to offset chemoresistance and radioresistance. Cancer Res. 2008, 68, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Pires, I.M.; Bencokova, Z.; Milani, M.; Folkes, L.K.; Li, J.-L.; Stratford, M.R.; Harris, A.L.; Hammond, E.M. Effects of acute versus chronic hypoxia on DNA damage responses and genomic instability. Cancer Res. 2010, 70, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Stenerlöw, B.; Höglund, E.; Carlsson, J.; Blomquist, E. Rejoining of DNA fragments produced by radiations of different linear energy transfer. Int. J. Radiat. Biol. 2000, 76, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Jakob, B.; Scholz, M.; Taucher-Scholz, G. Biological imaging of heavy charged-particle tracks. Radiat. Res. 2003, 159, 676–684. [Google Scholar] [CrossRef]
- Durante, M.; Loeffler, J.S. Charged particles in radiation oncology. Nat. Rev. Clin. Oncol. 2010, 7, 37–43. [Google Scholar] [CrossRef]
- Sage, E.; Harrison, L. Clustered DNA lesion repair in eukaryotes: Relevance to mutagenesis and cell survival. Mutat. Res. 2011, 711, 123–133. [Google Scholar] [CrossRef]
- Loucas, B.D.; Cornforth, M.N. The LET dependence of unrepaired chromosome damage in human cells: A break too far? Radiat. Res. 2013, 179, 393–405. [Google Scholar] [CrossRef]
- Moore, S.; Stanley, F.K.T.; Goodarzi, A.A. The repair of environmentally relevant DNA double strand breaks caused by high linear energy transfer irradiation--no simple task. DNA Repair (Amst) 2014, 17, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Lorat, Y.; Brunner, C.U.; Schanz, S.; Jakob, B.; Taucher-Scholz, G.; Rübe, C.E. Nanoscale analysis of clustered DNA damage after high-LET irradiation by quantitative electron microscopy—The heavy burden to repair. DNA Repair (Amst) 2015, 28, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Grosse, N.; Fontana, A.O.; Hug, E.B.; Lomax, A.; Coray, A.; Augsburger, M.; Paganetti, H.; Sartori, A.A.; Pruschy, M. Deficiency in homologous recombination renders Mammalian cells more sensitive to proton versus photon irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Oeck, S.; Szymonowicz, K.; Wiel, G.; Krysztofiak, A.; Lambert, J.; Koska, B.; Iliakis, G.; Timmermann, B.; Jendrossek, V. Relating Linear Energy Transfer to the Formation and Resolution of DNA Repair Foci After Irradiation with Equal Doses of X-ray Photons, Plateau, or Bragg-Peak Protons. Int. J. Mol. Sci. 2018, 19, 3779. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Unger, K.; Orth, M.; Schötz, U.; Schüttrumpf, L.; Zangen, V.; Gimenez-Aznar, I.; Michna, A.; Schneider, L.; Stamp, R.; et al. Genomic amplification of Fanconi anemia complementation group A (FancA) in head and neck squamous cell carcinoma (HNSCC): Cellular mechanisms of radioresistance and clinical relevance. Cancer Lett. 2017, 386, 87–99. [Google Scholar] [CrossRef]
- Michna, A.; Schötz, U.; Selmansberger, M.; Zitzelsberger, H.; Lauber, K.; Unger, K.; Hess, J. Transcriptomic analyses of the radiation response in head and neck squamous cell carcinoma subclones with different radiation sensitivity: Time-course gene expression profiles and gene association networks. Radiat. Oncol. 2016, 11, 94. [Google Scholar] [CrossRef]
- Michna, A.; Braselmann, H.; Selmansberger, M.; Dietz, A.; Hess, J.; Gomolka, M.; Hornhardt, S.; Blüthgen, N.; Zitzelsberger, H.; Unger, K. Natural Cubic Spline Regression Modeling Followed by Dynamic Network Reconstruction for the Identification of Radiation-Sensitivity Gene Association Networks from Time-Course Transcriptome Data. PLoS ONE 2016, 11, e0160791. [Google Scholar] [CrossRef]
- Jehmlich, N.; Stegmaier, P.; Golatowski, C.; Salazar, M.G.; Rischke, C.; Henke, M.; Völker, U. Differences in the whole saliva baseline proteome profile associated with development of oral mucositis in head and neck cancer patients undergoing radiotherapy. J. Proteom. 2015, 125, 98–103. [Google Scholar] [CrossRef]
- Cheng, D.; Cao, N.; Chen, J.; Yu, X.; Shuai, X. Multifunctional nanocarrier mediated co-delivery of doxorubicin and siRNA for synergistic enhancement of glioma apoptosis in rat. Biomaterials 2012, 33, 1170–1179. [Google Scholar] [CrossRef]
- Park, C.C.; Zhang, H.J.; Yao, E.S.; Park, C.J.; Bissell, M.J. Beta1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res. 2008, 68, 4398–4405. [Google Scholar] [CrossRef]
- Ahmed, K.M.; Zhang, H.; Park, C.C. NF-κB regulates radioresistance mediated by β1-integrin in three-dimensional culture of breast cancer cells. Cancer Res. 2013, 73, 3737–3748. [Google Scholar] [CrossRef] [PubMed]
- Eke, I.; Zscheppang, K.; Dickreuter, E.; Hickmann, L.; Mazzeo, E.; Unger, K.; Krause, M.; Cordes, N. Simultaneous β1 integrin-EGFR targeting and radiosensitization of human head and neck cancer. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Barros, M.; Paris, F.; Cordon-Cardo, C.; Lyden, D.; Rafii, S.; Haimovitz-Friedman, A.; Fuks, Z.; Kolesnick, R. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003, 300, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Potiron, V.A.; Abderrahmani, R.; Clément-Colmou, K.; Marionneau-Lambot, S.; Oullier, T.; Paris, F.; Supiot, S. Improved functionality of the vasculature during conventionally fractionated radiation therapy of prostate cancer. PLoS ONE 2013, 8, e84076. [Google Scholar] [CrossRef] [PubMed]
- Klein, D. The Tumor Vascular Endothelium as Decision Maker in Cancer Therapy. Front. Oncol. 2018, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D. A Century of Radiation Therapy and Adaptive Immunity. Front. Immunol. 2017, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Brix, N.; Tiefenthaller, A.; Anders, H.; Belka, C.; Lauber, K. Abscopal, immunological effects of radiotherapy: Narrowing the gap between clinical and preclinical experiences. Immunol. Rev. 2017, 280, 249–279. [Google Scholar] [CrossRef]
- Ganss, R.; Ryschich, E.; Klar, E.; Arnold, B.; Hämmerling, G.J. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002, 62, 1462–1470. [Google Scholar] [PubMed]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef]
- Lee, Y.; Auh, S.L.; Wang, Y.; Burnette, B.; Wang, Y.; Meng, Y.; Beckett, M.; Sharma, R.; Chin, R.; Tu, T.; et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood 2009, 114, 589–595. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.-D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Rech, A.J.; Dada, H.; Kotzin, J.J.; Henao-Mejia, J.; Minn, A.J.; Twyman-Saint Victor, C.; Vonderheide, R.H. Radiotherapy and CD40 Activation Separately Augment Immunity to Checkpoint Blockade in Cancer. Cancer Res. 2018, 78, 4282–4291. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.M.; Hanoteau, A.; Liu, H.-C.; Gaspero, A.; Parikh, F.; Gartrell-Corrado, R.D.; Hart, T.D.; Laoui, D.; van Ginderachter, J.A.; Dharmaraj, N.; et al. Immune microenvironment modulation unmasks therapeutic benefit of radiotherapy and checkpoint inhibition. J. Immunother. Cancer 2019, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Herrera, F.G.; Bourhis, J.; Coukos, G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J. Clin. 2017, 67, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Dar, T.B.; Henson, R.M.; Shiao, S.L. Targeting Innate Immunity to Enhance the Efficacy of Radiation Therapy. Front. Immunol. 2018, 9, 3077. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.B.; Evers, G.; Kerkhoff, A.; Mohr, M.; Schliemann, C.; Berdel, W.E.; Schmidt, L.H. Future Options of Molecular-Targeted Therapy in Small Cell Lung Cancer. Cancers (Basel) 2019, 11, 690. [Google Scholar] [CrossRef]
- Sevenich, L. Turning “Cold” Into “Hot” Tumors-Opportunities and Challenges for Radio-Immunotherapy Against Primary and Metastatic Brain Cancers. Front. Oncol. 2019, 9, 163. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef]
- Burnette, B.; Weichselbaum, R.R. Radiation as an immune modulator. Semin. Radiat. Oncol. 2013, 23, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Rudqvist, N.-P.; Golden, E.; Cooper, B.; Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Friedman, K.; Ferrari de Andrade, L.; Wucherpfennig, K.W.; et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018, 24, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Vacchelli, E.; Bloy, N.; Aranda, F.; Buqué, A.; Cremer, I.; Demaria, S.; Eggermont, A.; Formenti, S.C.; Fridman, W.H.; Fucikova, J.; et al. Trial Watch: Immunotherapy plus radiation therapy for oncological indications. Oncoimmunology 2016, 5, e1214790. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.B.; Krishnan, S.; Hodge, J.W.; Chang, J.Y. Immunotherapy and stereotactic ablative radiotherapy (ISABR): A curative approach? Nat. Rev. Clin. Oncol. 2016, 13, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Shaverdian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Weichselbaum, R.R.; Liang, H.; Deng, L.; Fu, Y.-X. Radiotherapy and immunotherapy: A beneficial liaison? Nat. Rev. Clin. Oncol. 2017, 14, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Benci, J.L.; Xu, B.; Qiu, Y.; Wu, T.J.; Dada, H.; Twyman-Saint Victor, C.; Cucolo, L.; Lee, D.S.M.; Pauken, K.E.; Huang, A.C.; et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell 2016, 167, 1540–1554.e.12. [Google Scholar] [CrossRef] [PubMed]
- Wirsdörfer, F.; Jendrossek, V. Modeling DNA damage-induced pneumopathy in mice: Insight from danger signaling cascades. Radiat. Oncol. 2017, 12, 142. [Google Scholar] [CrossRef]
- Arscott, W.T.; Zhu, S.; Plastaras, J.P.; Maity, A.; Alonso-Basanta, M.; Jones, J. Acute neurologic toxicity of palliative radiotherapy for brain metastases in patients receiving immune checkpoint blockade. Neurooncol. Pract. 2019, 6, 297–304. [Google Scholar] [CrossRef]
- Shibaki, R.; Akamatsu, H.; Fujimoto, M.; Koh, Y.; Yamamoto, N. Nivolumab induced radiation recall pneumonitis after two years of radiotherapy. Ann. Oncol. 2017, 28, 1404–1405. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Luo, Y.; Tian, X.; Ma, S.; Sun, Y.; You, C.; Gong, Y.; Xie, C. Impact of Radiotherapy Concurrent with Anti-PD-1 Therapy on the Lung Tissue of Tumor-Bearing Mice. Radiat. Res. 2019, 191, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Pilones, K.A.; García-Martínez, E.; Rudqvist, N.-P.; Formenti, S.C.; Demaria, S. Barriers to Radiation-Induced In Situ Tumor Vaccination. Front. Immunol. 2017, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Wirsdörfer, F.; de Leve, S.; Jendrossek, V. Combining Radiotherapy and Immunotherapy in Lung Cancer: Can We Expect Limitations Due to Altered Normal Tissue Toxicity? Int. J. Mol. Sci. 2018, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P. Metabolic microenvironment of tumor cells: A key factor in malignant progression. Exp. Oncol. 2010, 32, 125–127. [Google Scholar] [PubMed]
- Harris, A.L. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Cao, Y.; Moeller, B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat. Rev. Cancer 2008, 8, 425–437. [Google Scholar] [CrossRef]
- Horsman, M.R.; Mortensen, L.S.; Petersen, J.B.; Busk, M.; Overgaard, J. Imaging hypoxia to improve radiotherapy outcome. Nat. Rev. Clin. Oncol. 2012, 9, 674–687. [Google Scholar] [CrossRef]
- Horsman, M.R.; Vaupel, P. Pathophysiological Basis for the Formation of the Tumor Microenvironment. Front. Oncol. 2016, 6, 66. [Google Scholar] [CrossRef]
- Gray, L.H.; Conger, A.D.; Ebert, M.; Hornsey, S.; Scott, O.C. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 1953, 26, 638–648. [Google Scholar] [CrossRef]
- Nordsmark, M.; Bentzen, S.M.; Rudat, V.; Brizel, D.; Lartigau, E.; Stadler, P.; Becker, A.; Adam, M.; Molls, M.; Dunst, J.; et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother. Oncol. 2005, 77, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, L.S.; Johansen, J.; Kallehauge, J.; Primdahl, H.; Busk, M.; Lassen, P.; Alsner, J.; Sørensen, B.S.; Toustrup, K.; Jakobsen, S.; et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: Results from the DAHANCA 24 trial. Radiother. Oncol. 2012, 105, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.R.; Overgaard, J. The impact of hypoxia and its modification of the outcome of radiotherapy. J. Radiat. Res. 2016, 57 (Suppl. 1), i90–i98. [Google Scholar] [CrossRef]
- Bristow, R.G.; Hill, R.P. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Liauw, S.L.; Connell, P.P.; Weichselbaum, R.R. New paradigms and future challenges in radiation oncology: An update of biological targets and technology. Sci. Transl. Med. 2013, 5, 173sr2. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Harrison, L. Tumor hypoxia: Causative factors, compensatory mechanisms, and cellular response. Oncologist 2004, 9 (Suppl. 5), 4–9. [Google Scholar] [CrossRef]
- Overgaard, J. Sensitization of hypoxic tumour cells—Clinical experience. Int. J. Radiat. Biol. 1989, 56, 801–811. [Google Scholar] [CrossRef]
- Secomb, T.W.; Hsu, R.; Ong, E.T.; Gross, J.F.; Dewhirst, M.W. Analysis of the effects of oxygen supply and demand on hypoxic fraction in tumors. Acta Oncol. 1995, 34, 313–316. [Google Scholar] [CrossRef]
- Secomb, T.W.; Hsu, R.; Braun, R.D.; Ross, J.R.; Gross, J.F.; Dewhirst, M.W. Theoretical simulation of oxygen transport to tumors by three-dimensional networks of microvessels. Adv. Exp. Med. Biol. 1998, 454, 629–634. [Google Scholar] [CrossRef]
- Secomb, T.W.; Hsu, R.; Dewhirst, M.W. Synergistic effects of hyperoxic gas breathing and reduced oxygen consumption on tumor oxygenation: A theoretical model. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Ashton, T.M.; Fokas, E.; Kunz-Schughart, L.A.; Folkes, L.K.; Anbalagan, S.; Huether, M.; Kelly, C.J.; Pirovano, G.; Buffa, F.M.; Hammond, E.M.; et al. The anti-malarial atovaquone increases radiosensitivity by alleviating tumour hypoxia. Nat. Commun. 2016, 7, 12308. [Google Scholar] [CrossRef] [PubMed]
- De Mey, S.; Jiang, H.; Corbet, C.; Wang, H.; Dufait, I.; Law, K.; Bastien, E.; Verovski, V.; Gevaert, T.; Feron, O.; et al. Antidiabetic Biguanides Radiosensitize Hypoxic Colorectal Cancer Cells Through a Decrease in Oxygen Consumption. Front. Pharmacol. 2018, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Benej, M.; Hong, X.; Vibhute, S.; Scott, S.; Wu, J.; Graves, E.; Le, Q.-T.; Koong, A.C.; Giaccia, A.J.; Yu, B.; et al. Papaverine and its derivatives radiosensitize solid tumors by inhibiting mitochondrial metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 10756–10761. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, M.W. A potential solution for eliminating hypoxia as a cause for radioresistance. Proc. Natl. Acad. Sci. USA 2018, 115, 10548–10550. [Google Scholar] [CrossRef] [PubMed]
- Bacon, A.L.; Harris, A.L. Hypoxia-inducible factors and hypoxic cell death in tumour physiology. Ann. Med. 2004, 36, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Comerford, K.M.; Wallace, T.J.; Karhausen, J.; Louis, N.A.; Montalto, M.C.; Colgan, S.P. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002, 62, 3387–3394. [Google Scholar] [PubMed]
- Liu, C.; Lin, Q.; Yun, Z. Cellular and molecular mechanisms underlying oxygen-dependent radiosensitivity. Radiat. Res. 2015, 183, 487–496. [Google Scholar] [CrossRef]
- Wouters, B.G.; Koritzinsky, M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat. Rev. Cancer 2008, 8, 851–864. [Google Scholar] [CrossRef]
- Span, P.N.; Bussink, J. Biology of hypoxia. Semin. Nucl. Med. 2015, 45, 101–109. [Google Scholar] [CrossRef]
- Lee, C.-T.; Boss, M.-K.; Dewhirst, M.W. Imaging tumor hypoxia to advance radiation oncology. Antioxid. Redox Signal. 2014, 21, 313–337. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Radiat. Oncol. 2004, 14, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ljungkvist, A.S.E.; Bussink, J.; Kaanders, J.H.A.M.; van der Kogel, A.J. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiat. Res. 2007, 167, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Yasui, H.; Mitchell, J.B.; Krishna, M.C. Imaging cycling tumor hypoxia. Cancer Res. 2010, 70, 10019–10023. [Google Scholar] [CrossRef] [PubMed]
- Tellier, C.; Desmet, D.; Petit, L.; Finet, L.; Graux, C.; Raes, M.; Feron, O.; Michiels, C. Cycling hypoxia induces a specific amplified inflammatory phenotype in endothelial cells and enhances tumor-promoting inflammation in vivo. Neoplasia 2015, 17, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Rankin, E.B.; Giaccia, A.J. Hypoxic control of metastasis. Science 2016, 352, 175–180. [Google Scholar] [CrossRef]
- Dewhirst, M.W. Intermittent hypoxia furthers the rationale for hypoxia-inducible factor-1 targeting. Cancer Res. 2007, 67, 854–855. [Google Scholar] [CrossRef]
- Hlouschek, J.; Ritter, V.; Wirsdörfer, F.; Klein, D.; Jendrossek, V.; Matschke, J. Targeting SLC25A10 alleviates improved antioxidant capacity and associated radioresistance of cancer cells induced by chronic-cycling hypoxia. Cancer Lett. 2018, 439, 24–38. [Google Scholar] [CrossRef]
- Matschke, J.; Riffkin, H.; Klein, D.; Handrick, R.; Lüdemann, L.; Metzen, E.; Shlomi, T.; Stuschke, M.; Jendrossek, V. Targeted Inhibition of Glutamine-Dependent Glutathione Metabolism Overcomes Death Resistance Induced by Chronic Cycling Hypoxia. Antioxid. Redox Signal. 2016, 25, 89–107. [Google Scholar] [CrossRef]
- Song, C.; Hong, B.-J.; Bok, S.; Lee, C.-J.; Kim, Y.-E.; Jeon, S.-R.; Wu, H.-G.; Lee, Y.-S.; Cheon, G.J.; Paeng, J.C.; et al. Real-time Tumor Oxygenation Changes After Single High-dose Radiation Therapy in Orthotopic and Subcutaneous Lung Cancer in Mice: Clinical Implication for Stereotactic Ablative Radiation Therapy Schedule Optimization. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1022–1031. [Google Scholar] [CrossRef]
- Leung, E.; Cairns, R.A.; Chaudary, N.; Vellanki, R.N.; Kalliomaki, T.; Moriyama, E.H.; Mujcic, H.; Wilson, B.C.; Wouters, B.G.; Hill, R.; et al. Metabolic targeting of HIF-dependent glycolysis reduces lactate, increases oxygen consumption and enhances response to high-dose single-fraction radiotherapy in hypoxic solid tumors. BMC Cancer 2017, 17, 418. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.X.; Jalali, F.; Cuddihy, A.; Chan, N.; Bindra, R.S.; Glazer, P.M.; Bristow, R.G. Hypoxia down-regulates DNA double strand break repair gene expression in prostate cancer cells. Radiother. Oncol. 2005, 76, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Glazer, P.M.; Hegan, D.C.; Lu, Y.; Czochor, J.; Scanlon, S.E. Hypoxia and DNA repair. Yale J. Biol. Med. 2013, 86, 443–451. [Google Scholar] [PubMed]
- Rey, S.; Schito, L.; Koritzinsky, M.; Wouters, B.G. Molecular targeting of hypoxia in radiotherapy. Adv. Drug Deliv. Rev. 2017, 109, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.; Ali, M.; McCallum, G.P.; Kumareswaran, R.; Koritzinsky, M.; Wouters, B.G.; Wells, P.G.; Gallinger, S.; Bristow, R.G. Hypoxia provokes base excision repair changes and a repair-deficient, mutator phenotype in colorectal cancer cells. Mol. Cancer Res. 2014, 12, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Sitkovsky, M.V.; Kjaergaard, J.; Lukashev, D.; Ohta, A. Hypoxia-adenosinergic immunosuppression: Tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin. Cancer Res. 2008, 14, 5947–5952. [Google Scholar] [CrossRef]
- Ohta, A. A Metabolic Immune Checkpoint: Adenosine in Tumor Microenvironment. Front. Immunol. 2016, 7, 109. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Hypoxia-/HIF-1α-Driven Factors of the Tumor Microenvironment Impeding Antitumor Immune Responses and Promoting Malignant Progression. Adv. Exp. Med. Biol. 2018, 1072, 171–175. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Mowery, Y.M.; Mitchell, J.B.; Cherukuri, M.K.; Secomb, T.W. Rationale for hypoxia assessment and amelioration for precision therapy and immunotherapy studies. J. Clin. Investing. 2019, 129, 489–491. [Google Scholar] [CrossRef]
- Chan, N.; Pires, I.M.; Bencokova, Z.; Coackley, C.; Luoto, K.R.; Bhogal, N.; Lakshman, M.; Gottipati, P.; Oliver, F.J.; Helleday, T.; et al. Contextual synthetic lethality of cancer cell kill based on the tumor microenvironment. Cancer Res. 2010, 70, 8045–8054. [Google Scholar] [CrossRef]
- Olcina, M.; Lecane, P.S.; Hammond, E.M. Targeting hypoxic cells through the DNA damage response. Clin. Cancer Res. 2010, 16, 5624–5629. [Google Scholar] [CrossRef] [PubMed]
- Sitkovsky, M.; Ohta, A. Targeting the hypoxia-adenosinergic signaling pathway to improve the adoptive immunotherapy of cancer. J. Mol. Med. 2013, 91, 147–155. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.C.; Chafe, S.C.; Dedhar, S. Overcoming Hypoxia-Mediated Tumor Progression: Combinatorial Approaches Targeting pH Regulation, Angiogenesis and Immune Dysfunction. Front. Cell Dev. Biol. 2016, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, S.; Veszeleiova, K.; Steingold, J.; Sethuraman, J.; Sitkovsky, M. Mechanistic Justifications of Systemic Therapeutic Oxygenation of Tumors to Weaken the Hypoxia Inducible Factor 1α-Mediated Immunosuppression. Adv. Exp. Med. Biol. 2019, 1136, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Helleday, T. Putting poly (ADP-ribose) polymerase and other DNA repair inhibitors into clinical practice. Curr. Opin. Oncol. 2013, 25, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Smith, G.C.M.; Curtin, N.J. Poly(ADP-Ribose) polymerase-1 and DNA-dependent protein kinase have equivalent roles in double strand break repair following ionizing radiation. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Giaccia, A.J. Molecular Radiobiology: The State of the Art. JCO 2014, 32, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- Eke, I.; Koch, U.; Hehlgans, S.; Sandfort, V.; Stanchi, F.; Zips, D.; Baumann, M.; Shevchenko, A.; Pilarsky, C.; Haase, M.; et al. PINCH1 regulates Akt1 activation and enhances radioresistance by inhibiting PP1alpha. J. Clin. Investing. 2010, 120, 2516–2527. [Google Scholar] [CrossRef]
- Nagle, P.W.; Hosper, N.A.; Barazzuol, L.; Jellema, A.L.; Baanstra, M.; van Goethem, M.-J.; Brandenburg, S.; Giesen, U.; Langendijk, J.A.; van Luijk, P.; et al. Lack of DNA Damage Response at Low Radiation Doses in Adult Stem Cells Contributes to Organ Dysfunction. Clin. Cancer Res. 2018, 24, 6583–6593. [Google Scholar] [CrossRef]
- Bläuer, M.; Tammela, T.L.; Ylikomi, T. A novel tissue-slice culture model for non-malignant human prostate. Cell Tissue Res. 2008, 332, 489–498. [Google Scholar] [CrossRef]
- Coppes, R.P.; Stokman, M.A. Stem cells and the repair of radiation-induced salivary gland damage. Oral Dis. 2011, 17, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, K.K.; Cattaneo, C.M.; Weeber, F.; Chalabi, M.; van de Haar, J.; Fanchi, L.F.; Slagter, M.; van der Velden, D.L.; Kaing, S.; Kelderman, S.; et al. Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell 2018, 174, 1586–1598.e12. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Clevers, H. Studying cellular heterogeneity and drug sensitivity in colorectal cancer using organoid technology. Curr. Opin. Genet. Dev. 2018, 52, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Barkauskas, C.E.; Chung, M.-I.; Fioret, B.; Gao, X.; Katsura, H.; Hogan, B.L.M. Lung organoids: Current uses and future promise. Development 2017, 144, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Tuveson, D.; Clevers, H. Cancer modeling meets human organoid technology. Science 2019, 364, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Kocher, S.; Beyer, B.; Lange, T.; Nordquist, L.; Volquardsen, J.; Burdak-Rothkamm, S.; Schlomm, T.; Petersen, C.; Rothkamm, K.; Mansour, W.Y. A functional ex vivo assay to detect PARP1-EJ repair and radiosensitization by PARP-inhibitor in prostate cancer. Int. J. Cancer 2019, 144, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Naipal, K.A.T.; Verkaik, N.S.; Sánchez, H.; van Deurzen, C.H.M.; den Bakker, M.A.; Hoeijmakers, J.H.J.; Kanaar, R.; Vreeswijk, M.P.G.; Jager, A.; van Gent, D.C. Tumor slice culture system to assess drug response of primary breast cancer. BMC Cancer 2016, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Meijer, T.G.; Naipal, K.A.; Jager, A.; van Gent, D.C. Ex vivo tumor culture systems for functional drug testing and therapy response prediction. Future Sci. OA 2017, 3, FSO190. [Google Scholar] [CrossRef]
- Naipal, K.A.T.; Verkaik, N.S.; Ameziane, N.; van Deurzen, C.H.M.; Ter Brugge, P.; Meijers, M.; Sieuwerts, A.M.; Martens, J.W.; O’Connor, M.J.; Vrieling, H.; et al. Functional ex vivo assay to select homologous recombination-deficient breast tumors for PARP inhibitor treatment. Clin. Cancer Res. 2014, 20, 4816–4826. [Google Scholar] [CrossRef]
- Nagle, P.W.; Plukker, J.T.M.; Muijs, C.T.; van Luijk, P.; Coppes, R.P. Patient-derived tumor organoids for prediction of cancer treatment response. Semin. Cancer Biol. 2018, 53, 258–264. [Google Scholar] [CrossRef]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Huch, M. Disease modelling in human organoids. Dis. Model. Mech. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Marini, P.; Budach, W.; Niyazi, M.; Junginger, D.; Stickl, S.; Jendrossek, V.; Belka, C. Combination of the pro-apoptotic TRAIL-receptor antibody mapatumumab with ionizing radiation strongly increases long-term tumor control under ambient and hypoxic conditions. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Budach, W.; Budach, V.; Stuschke, M.; Dinges, S.; Sack, H. The TCD50 and regrowth delay assay in human tumor xenografts: Differences and implications. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 259–268. [Google Scholar] [CrossRef]
- Landgraf, M.; McGovern, J.A.; Friedl, P.; Hutmacher, D.W. Rational Design of Mouse Models for Cancer Research. Trends Biotechnol. 2018, 36, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.J.; Bird, G.; Refaeli, Y.; Jimeno, A. Humanized Mouse Xenograft Models: Narrowing the Tumor-Microenvironment Gap. Cancer Res. 2016, 76, 6153–6158. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Goodwin, N.; Ishikawa, F.; Hosur, V.; Lyons, B.L.; Greiner, D.L. Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb. Protoc. 2014, 2014, 694–708. [Google Scholar] [CrossRef]
- Byrne, A.T.; Alférez, D.G.; Amant, F.; Annibali, D.; Arribas, J.; Biankin, A.V.; Bruna, A.; Budinská, E.; Caldas, C.; Chang, D.K.; et al. Interrogating open issues in cancer medicine with patient-derived xenografts. Nat. Rev. Cancer 2017, 17, 632. [Google Scholar] [CrossRef]
- Lai, Y.; Wei, X.; Lin, S.; Qin, L.; Cheng, L.; Li, P. Current status and perspectives of patient-derived xenograft models in cancer research. J. Hematol. Oncol. 2017, 10, 106. [Google Scholar] [CrossRef]
- Willey, C.D.; Gilbert, A.N.; Anderson, J.C.; Gillespie, G.Y. Patient-Derived Xenografts as a Model System for Radiation Research. Semin. Radiat. Oncol. 2015, 25, 273–280. [Google Scholar] [CrossRef]
- Khan, M.; Walters, L.L.; Li, Q.; Thomas, D.G.; Miller, J.M.L.; Zhang, Q.; Sciallis, A.P.; Liu, Y.; Dlouhy, B.J.; Fort, P.E.; et al. Characterization and pharmacologic targeting of EZH2, a fetal retinal protein and epigenetic regulator, in human retinoblastoma. Lab. Investing. 2015, 95, 1278–1290. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.; Tofilon, P.J.; Camphausen, K. Preclinical models in radiation oncology. Radiat. Oncol. 2012, 7, 223. [Google Scholar] [CrossRef] [PubMed]
- Koontz, B.F.; Verhaegen, F.; de Ruysscher, D. Tumour and normal tissue radiobiology in mouse models: How close are mice to mini-humans? Br. J. Radiol. 2017, 90, 20160441. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Vaeteewoottacharn, K.; Kariya, R. Application of Highly Immunocompromised Mice for the Establishment of Patient-Derived Xenograft (PDX) Models. Cells 2019, 8, 889. [Google Scholar] [CrossRef] [PubMed]
- Unkel, S.; Belka, C.; Lauber, K. On the analysis of clonogenic survival data: Statistical alternatives to the linear-quadratic model. Radiat. Oncol. 2016, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Barcellos-Hoff, M.H.; Lyden, D.; Wang, T.C. The evolution of the cancer niche during multistage carcinogenesis. Nat. Rev. Cancer 2013, 13, 511–518. [Google Scholar] [CrossRef]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of ionizing radiation on biological molecules—Mechanisms of damage and emerging methods of detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef]
- Russell, J.S.; Brown, J.M. The irradiated tumor microenvironment: Role of tumor-associated macrophages in vascular recovery. Front. Physiol. 2013, 4, 157. [Google Scholar] [CrossRef]
- Lauber, K.; Ernst, A.; Orth, M.; Herrmann, M.; Belka, C. Dying cell clearance and its impact on the outcome of tumor radiotherapy. Front. Oncol. 2012, 2, 116. [Google Scholar] [CrossRef]
- Wirsdörfer, F.; Jendrossek, V. The Role of Lymphocytes in Radiotherapy-Induced Adverse Late Effects in the Lung. Front. Immunol. 2016, 7, 591. [Google Scholar] [CrossRef]
- Schaue, D.; Micewicz, E.D.; Ratikan, J.A.; Xie, M.W.; Cheng, G.; McBride, W.H. Radiation and inflammation. Semin. Radiat. Oncol. 2015, 25, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Moding, E.J.; Castle, K.D.; Perez, B.A.; Oh, P.; Min, H.D.; Norris, H.; Ma, Y.; Cardona, D.M.; Lee, C.-L.; Kirsch, D.G. Tumor cells, but not endothelial cells, mediate eradication of primary sarcomas by stereotactic body radiation therapy. Sci. Transl. Med. 2015, 7, 278ra34. [Google Scholar] [CrossRef] [PubMed]
- Avraham, T.; Yan, A.; Zampell, J.C.; Daluvoy, S.V.; Haimovitz-Friedman, A.; Cordeiro, A.P.; Mehrara, B.J. Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-beta1-mediated tissue fibrosis. Am. J. Physiol. Cell Physiol. 2010, 299, C589–C605. [Google Scholar] [CrossRef] [PubMed]
- Paris, F.; Fuks, Z.; Kang, A.; Capodieci, P.; Juan, G.; Ehleiter, D.; Haimovitz-Friedman, A.; Cordon-Cardo, C.; Kolesnick, R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 2001, 293, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Yang, M.; Fan, Z.; Li, S.; Gao, T.; Fang, Z. Associations of chemo- and radio-resistant phenotypes with the gap junction, adhesion and extracellular matrix in a three-dimensional culture model of soft sarcoma. J. Exp. Clin. Cancer Res. 2015, 34, 58. [Google Scholar] [CrossRef] [PubMed]
- Dickreuter, E.; Eke, I.; Krause, M.; Borgmann, K.; van Vugt, M.A.; Cordes, N. Targeting of β1 integrins impairs DNA repair for radiosensitization of head and neck cancer cells. Oncogene 2016, 35, 1353–1362. [Google Scholar] [CrossRef]
- Liang, H.; Deng, L.; Chmura, S.; Burnette, B.; Liadis, N.; Darga, T.; Beckett, M.A.; Lingen, M.W.; Witt, M.; Weichselbaum, R.R.; et al. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J. Immunol. 2013, 190, 5874–5881. [Google Scholar] [CrossRef]
- Balermpas, P.; Rödel, F.; Liberz, R.; Oppermann, J.; Wagenblast, J.; Ghanaati, S.; Harter, P.N.; Mittelbronn, M.; Weiss, C.; Rödel, C.; et al. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br. J. Cancer 2014, 111, 1509–1518. [Google Scholar] [CrossRef]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane as an In Vivo Assay to Study Antiangiogenesis. Pharmaceuticals (Basel) 2010, 3, 482–513. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane as a model for tumor biology. Exp. Cell Res. 2014, 328, 314–324. [Google Scholar] [CrossRef]
- Liu, M.; Xie, S.; Zhou, J. Use of animal models for the imaging and quantification of angiogenesis. Exp. Anim. 2018, 67, 1–6. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef]
- Cimpean, A.M.; Ribatti, D.; Raica, M. The chick embryo chorioallantoic membrane as a model to study tumor metastasis. Angiogenesis 2008, 11, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Quigley, J.P. Chapter 2. Chick Embryo Chorioallantoic Membrane Models to Quantify Angiogenesis Induced by Inflammatory and Tumor Cells or Purified Effector Molecules. Methods Enzymol. 2008, 444, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Soulet, F.; Kilarski, W.W.; Roux-Dalvai, F.; Herbert, M.J.; Sacewicz, I.; Mouton-Barbosa, E.; Bicknell, R.; Lalor, P.; Monsarrat, B.; Bikfalvi, A. Mapping the extracellular and membrane proteome associated with the vasculature and the stroma in the embryo. Mol. Cell. Proteom. 2013, 12, 2293–2312. [Google Scholar] [CrossRef] [PubMed]
- Mangir, N.; Raza, A.; Haycock, J.W.; Chapple, C.; Macneil, S. An Improved In Vivo Methodology to Visualise Tumour Induced Changes in Vasculature Using the Chick Chorionic Allantoic Membrane Assay. In Vivo 2018, 32, 461–472. [Google Scholar] [CrossRef]
- Zijlstra, A.; Mellor, R.; Panzarella, G.; Aimes, R.T.; Hooper, J.D.; Marchenko, N.D.; Quigley, J.P. A quantitative analysis of rate-limiting steps in the metastatic cascade using human-specific real-time polymerase chain reaction. Cancer Res. 2002, 62, 7083–7092. [Google Scholar]
- Palmer, T.D.; Lewis, J.; Zijlstra, A. Quantitative analysis of cancer metastasis using an avian embryo model. J. Vis. Exp. 2011. [Google Scholar] [CrossRef]
- Harris, J.J. The human tumor grown in the egg. Ann. N. Y. Acad. Sci. 1958, 76, 764–769. [Google Scholar] [CrossRef]
- Kaufman, N.; Kinney, T.D.; Mason, E.J.; Prieto, L.C. Maintenance of human neoplasm on the chick chorioallantoic membrane. Am. J. Pathol. 1956, 32, 271–285. [Google Scholar] [PubMed]
- Dagg, C.P.; Karnofsky, D.A.; Roddy, J. Growth of transplantable human tumors in the chick embryo and hatched chick. Cancer Res. 1956, 16, 589–594. [Google Scholar] [PubMed]
- Dohle, D.S.; Pasa, S.D.; Gustmann, S.; Laub, M.; Wissler, J.H.; Jennissen, H.P.; Dünker, N. Chick ex ovo culture and ex ovo CAM assay: How it really works. J. Vis. Exp. 2009. [Google Scholar] [CrossRef] [PubMed]
- Ausprunk, D.H.; Knighton, D.R.; Folkman, J. Vascularization of normal and neoplastic tissues grafted to the chick chorioallantois. Role of host and preexisting graft blood vessels. Am. J. Pathol. 1975, 79, 597–618. [Google Scholar] [PubMed]
- Ausprunk, D.H.; Folkman, J. Vascular injury in transplanted tissues. Fine structural changes in tumor, adult, and embryonic blood vessels. Virchows Arch. B Cell Pathol. 1976, 21, 31–44. [Google Scholar]
- Ossowski, L.; Reich, E. Experimental model for quantitative study of metastasis. Cancer Res. 1980, 40, 2300–2309. [Google Scholar] [PubMed]
- Chambers, A.F.; Shafir, R.; Ling, V. A model system for studying metastasis using the embryonic chick. Cancer Res. 1982, 42, 4018–4025. [Google Scholar] [PubMed]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef] [PubMed]
- Swadi, R.; Mather, G.; Pizer, B.L.; Losty, P.D.; See, V.; Moss, D. Optimising the chick chorioallantoic membrane xenograft model of neuroblastoma for drug delivery. BMC Cancer 2018, 18. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef] [PubMed]
- DeBord, L.C.; Pathak, R.R.; Villaneuva, M.; Liu, H.-C.; Harrington, D.A.; Yu, W.; Lewis, M.T.; Sikora, A.G. The chick chorioallantoic membrane (CAM) as a versatile patient-derived xenograft (PDX) platform for precision medicine and preclinical research. Am. J. Cancer Res. 2018, 8, 1642–1660. [Google Scholar] [PubMed]
- Palmeira-de-Oliveira, R.; Monteiro Machado, R.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Testing vaginal irritation with the Hen’s Egg Test-Chorioallantoic Membrane assay. ALTEX 2018, 35, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Philippeit, C.; Weise, A.; Dünker, N. Re-characterization of established human retinoblastoma cell lines. Histochem. Cell Biol. 2015, 143, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Große-Kreul, J.; Wirtz, J.J.; Beier, M.; Stephan, H.; Royer-Pokora, B.; Metz, K.; Dünker, N. Reduction of the tumorigenic potential of human retinoblastoma cell lines by TFF1 overexpression involves p53/caspase signaling and miR-18a regulation. Int. J. Cancer 2017, 141, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Papior, D.; Stephan, H.; Dünker, N. Characterization of etoposide- and cisplatin-chemoresistant retinoblastoma cell lines. Oncol. Rep. 2018, 39, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yu, W.; Kovalski, K.; Ossowski, L. Requirement for specific proteases in cancer cell intravasation as revealed by a novel semiquantitative PCR-based assay. Cell 1998, 94, 353–362. [Google Scholar] [CrossRef]
- Große-Kreul, J.; Busch, M.; Winter, C.; Pikos, S.; Stephan, H.; Dünker, N. Forced Trefoil Factor Family Peptide 3 (TFF3) Expression Reduces Growth, Viability, and Tumorigenicity of Human Retinoblastoma Cell Lines. PLoS ONE 2016, 11, e0163025. [Google Scholar] [CrossRef][Green Version]
- Auerbach, R.; Kubai, L.; Knighton, D.; Folkman, J. A simple procedure for the long-term cultivation of chicken embryos. Dev. Biol. 1974, 41, 391–394. [Google Scholar] [CrossRef]
- Kauffmann, P.; Troeltzsch, M.; Cordesmeyer, R.; Heidekrueger, P.I.; Schliephake, H.; Canis, M.; Wolff, H.A.; Rave-Fraenk, M.; Stroebel, P.; Kehrer, A.; et al. Presentation of a variation of the chorioallantoic membrane set up as a potential model for individual therapy for squamous cell carcinoma of the oropharynx. Clin. Hemorheol. Microcirc. 2017, 67, 453–457. [Google Scholar] [CrossRef]
- Farzaneh, M.; Attari, F.; Khoshnam, S.E.; Mozdziak, P.E. The method of chicken whole embryo culture using the eggshell windowing, surrogate eggshell and ex ovo culture system. Br. Poult. Sci. 2018, 59, 240–244. [Google Scholar] [CrossRef]
- Willetts, L.; Bond, D.; Stoletov, K.; Lewis, J.D. Quantitative Analysis of Human Cancer Cell Extravasation Using Intravital Imaging. Methods Mol. Biol. 2016, 1458, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.S.; Chambers, A.F.; Lewis, J.D. Assessing cancer cell migration and metastatic growth in vivo in the chick embryo using fluorescence intravital imaging. Methods Mol. Biol. 2012, 872, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dupertuis, Y.M.; Delie, F.; Cohen, M.; Pichard, C. In ovo method for evaluating the effect of nutritional therapies on tumor development, growth and vascularization. Clin. Nutr. Exp. 2015, 2, 9–17. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane in the study of tumor angiogenesis. Rom. J. Morphol. Embryol. 2008, 49, 131–135. [Google Scholar] [PubMed]
- Ribatti, D. Chapter 5. Chick Embryo Chorioallantoic Membrane as a Useful Tool to Study Angiogenesis. Int. Rev. Cell. Mol. Biol. 2008, 270, 181–224. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM) assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef]
- Bobek, V.; Plachy, J.; Pinterova, D.; Kolostova, K.; Boubelik, M.; Jiang, P.; Yang, M.; Hoffman, R.M. Development of a green fluorescent protein metastatic-cancer chick-embryo drug-screen model. Clin. Exp. Metastasis 2004, 21, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.B.; Rous, P. The Behavior of Chicken Sarcoma Implanted in the Developing Embryo. J. Exp. Med. 1912, 15, 119–132. [Google Scholar] [CrossRef]
- Korngold, L.; Lipari, R. Tissue antigens of human tumors grown in rats, hamsters, and eggs. Cancer Res. 1955, 15, 159–161. [Google Scholar]
- Abe, C.; Uto, Y.; Nakae, T.; Shinmoto, Y.; Sano, K.; Nakata, H.; Teraoka, M.; Endo, Y.; Maezawa, H.; Masunaga, S.-I.; et al. Evaluation of the in vivo Radiosensitizing Activity of Etanidazole Using Tumor-bearing Chick Embryo. JRR 2011, 52, 208–214. [Google Scholar] [CrossRef]
- Joyce, J.A.; Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, I.; Hulsart-Billstrom, G.; Lanham, S.A.; Janeczek, A.A.; Kontouli, N.; Kanczler, J.M.; Evans, N.D.; Oreffo, R.O. The chorioallantoic membrane (CAM) assay for the study of human bone regeneration: A refinement animal model for tissue engineering. Sci. Rep. 2016, 6, 32168. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, M.; Becker, J.; Eberth, S.; Kube, D.; Wilting, J. The chick chorioallantoic membrane as an in vivo xenograft model for Burkitt lymphoma. BMC Cancer 2014, 14, 339. [Google Scholar] [CrossRef] [PubMed]
- Comşa, Ş.; Popescu, R.; Avram, Ş.; Ceaușu, R.A.; Cîmpean, A.M.; Raica, M. Bevacizumab Modulation of the Interaction Between the MCF-7 Cell Line and the Chick Embryo Chorioallantoic Membrane. In Vivo 2017, 31, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Kleibeuker, E.A.; ten Hooven, M.A.; Castricum, K.C.; Honeywell, R.; Griffioen, A.W.; Verheul, H.M.; Slotman, B.J.; Thijssen, V.L. Optimal treatment scheduling of ionizing radiation and sunitinib improves the antitumor activity and allows dose reduction. Cancer Med. 2015, 4, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Boyineni, J.; Tanpure, S.; Gnanamony, M.; Antony, R.; Fernández, K.S.; Lin, J.; Pinson, D.; Gondi, C.S. SPARC overexpression combined with radiation retards angiogenesis by suppressing VEGF-A via miR-410 in human neuroblastoma cells. Int. J. Oncol. 2016, 49, 1394–1406. [Google Scholar] [CrossRef]
- Eder, S.; Arndt, A.; Lamkowski, A.; Daskalaki, W.; Rump, A.; Priller, M.; Genze, F.; Wardelmann, E.; Port, M.; Steinestel, K. Baseline MAPK signaling activity confers intrinsic radioresistance to KRAS-mutant colorectal carcinoma cells by rapid upregulation of heterogeneous nuclear ribonucleoprotein K (hnRNP K). Cancer Lett. 2017, 385, 160–167. [Google Scholar] [CrossRef]
- Hammer-Wilson, M.J.; Akian, L.; Espinoza, J.; Kimel, S.; Berns, M.W. Photodynamic parameters in the chick chorioallantoic membrane (CAM) bioassay for topically applied photosensitizers. J. Photochem. Photobiol. B Biol. 1999, 53, 44–52. [Google Scholar] [CrossRef][Green Version]
- Saw, C.L.L.; Olivo, M.; Chin, W.W.L.; Soo, K.C.; Heng, P.W.S. Transport of hypericin across chick chorioallantoic membrane and photodynamic therapy vasculature assessment. Biol. Pharm. Bull. 2005, 28, 1054–1060. [Google Scholar] [CrossRef][Green Version]
- Gottfried, V.; Lindenbaum, E.S.; Kimel, S. The chick chorioallantoic membrane (CAM) as an in vivo model for photodynamic therapy. J. Photochem. Photobiol. B Biol. 1992, 12, 204–207. [Google Scholar] [CrossRef]
- Kimel, S.; Svaasand, L.O.; Hammer-Wilson, M.; Gottfried, V.; Cheng, S.; Svaasand, E.; Berns, M.W. Demonstration of synergistic effects of hyperthermia and photodynamic therapy using the chick chorioallantoic membrane model. Lasers Surg. Med. 1992, 12, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Honda, N.; Kariyama, Y.; Hazama, H.; Ishii, T.; Kitajima, Y.; Inoue, K.; Ishizuka, M.; Tanaka, T.; Awazu, K. Optical properties of tumor tissues grown on the chorioallantoic membrane of chicken eggs: Tumor model to assay of tumor response to photodynamic therapy. J. Biomed. Opt. 2015, 20, 125001. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Covelo-Fernandez, A.; von Bonin, F.; Kube, D.; Wilting, J. Specific tumor-stroma interactions of EBV-positive Burkitt’s lymphoma cells in the chick chorioallantoic membrane. Vasc. Cell 2012, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Fergelot, P.; Bernhard, J.-C.; Soulet, F.; Kilarski, W.W.; Léon, C.; Courtois, N.; Deminière, C.; Herbert, M.J.; Antczak, P.; Falciani, F.; et al. The experimental renal cell carcinoma model in the chick embryo. Angiogenesis 2013, 16, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.T.; Pinto, M.L.; Velho, S.; Pinto, M.T.; Cardoso, A.P.; Figueira, R.; Monteiro, A.; Marques, M.; Seruca, R.; Barbosa, M.A.; et al. Intricate Macrophage-Colorectal Cancer Cell Communication in Response to Radiation. PLoS ONE 2016, 11, e0160891. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, M.; Bottaro, D.P.; Liguri, G.; Gulisano, M.; Peruzzi, B.; Pacini, S. 0.2 T magnetic field inhibits angiogenesis in chick embryo chorioallantoic membrane. Bioelectromagnetics 2004, 25, 390–396. [Google Scholar] [CrossRef]
- Auerbach, R.; Arensman, R.; Kubai, L.; Folkman, J. Tumor-induced angiogenesis: Lack of inhibition by irradiation. Int. J. Cancer 1975, 15, 241–245. [Google Scholar] [CrossRef]
- Brooks, P.C.; Roth, J.M.; Lymberis, S.C.; DeWyngaert, K.; Broek, D.; Formenti, S.C. Ionizing radiation modulates the exposure of the HUIV26 cryptic epitope within collagen type IV during angiogenesis. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1194–1201. [Google Scholar] [CrossRef]
- Hatjikondi, O.; Ravazoula, P.; Kardamakis, D.; Dimopoulos, J.; Papaioannou, S. In vivo experimental evidence that the nitric oxide pathway is involved in the X-ray-induced antiangiogenicity. Br. J. Cancer 1996, 74, 1916–1923. [Google Scholar] [CrossRef][Green Version]
- Karnabatidis, D.; Dimopoulos, J.C.; Siablis, D.; Papazafiropoulos, D.; Kalogeropoulou, C.P.; Nikiforidis, G. Quantification of the ionising radiation effect over angiogenesis in the chick embryo and its chorioallantoic membrane by computerised analysis of angiographic images. Acta Radiol. 2001, 42, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Kardamakis, D.; Hadjimichael, C.; Ginopoulos, P.; Papaioannou, S. Effects of paclitaxel in combination with ionizing radiation on angiogenesis in the chick embryo chorioallantoic membrane. A radiobiological study. Strahlenther. Onkol. 2004, 180, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Demir, R.; Naschberger, L.; Demir, I.; Melling, N.; Dimmler, A.; Papadopoulus, T.; Sturzl, M.; Klein, P.; Hohenberger, W. Hypoxia generates a more invasive phenotype of tumour cells: An in vivo experimental setup based on the chorioallantoic membrane. Pathol. Oncol. Res. 2009, 15, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lin, P.; Qin, Z.; Liu, Y.; Deng, L.-L.; Lu, C. Hypoxia promotes HO-8910PM ovarian cancer cell invasion via Snail-mediated MT1-MMP upregulation. Exp. Biol. Med. (Maywood) 2015, 240, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Chai, H.; Yu, Z.; Ge, W.; Kang, N.; Xia, W.; Che, Y. HIF-1α effects on angiogenic potential in human small cell lung carcinoma. J. Exp. Clin. Cancer Res. 2011, 30, 77. [Google Scholar] [CrossRef] [PubMed]
- Sihn, G.; Walter, T.; Klein, J.-C.; Queguiner, I.; Iwao, H.; Nicolau, C.; Lehn, J.-M.; Corvol, P.; Gasc, J.-M. Anti-angiogenic properties of myo-inositol trispyrophosphate in ovo and growth reduction of implanted glioma. FEBS Lett. 2007, 581, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Fluegen, G.; Avivar-Valderas, A.; Wang, Y.; Padgen, M.R.; Williams, J.K.; Nobre, A.R.; Calvo, V.; Cheung, J.F.; Bravo-Cordero, J.J.; Entenberg, D.; et al. Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nat. Cell Biol. 2017, 19, 120–132. [Google Scholar] [CrossRef] [PubMed]
| Issue | Studies in Mice | CAM Assay | Advantages CAM | Limitations CAM |
|---|---|---|---|---|
| Duration | 4–9 (-12) weeks | 3–5, max. 7 days | High throughput | Limited time frame for tumor growth and effects |
| Experimental burden | Middle to high burden due to invasive treatment and tumor growth | No to low burden due to mainly extraembryonic tumor development | Meeting the 3R principle | |
| Costs | High expenses for breeding, keeping, feeding | Low expenses for eggs and transport | Cost-saving (approx. 60% saving of expenses) | |
| Space requirements | High; specific condition required | Low | Space-saving | |
| Permission requirements | Protocol approval by animal welfare and ethics committee | No approval by welfare or ethics committee required * | No administrative burden; quicker study start | |
| Functional analyses | Availability of antibodies, cytokines, primers | Limited number of avian- compatible antibodies, cytokines, primers |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dünker, N.; Jendrossek, V. Implementation of the Chick Chorioallantoic Membrane (CAM) Model in Radiation Biology and Experimental Radiation Oncology Research. Cancers 2019, 11, 1499. https://doi.org/10.3390/cancers11101499
Dünker N, Jendrossek V. Implementation of the Chick Chorioallantoic Membrane (CAM) Model in Radiation Biology and Experimental Radiation Oncology Research. Cancers. 2019; 11(10):1499. https://doi.org/10.3390/cancers11101499
Chicago/Turabian StyleDünker, Nicole, and Verena Jendrossek. 2019. "Implementation of the Chick Chorioallantoic Membrane (CAM) Model in Radiation Biology and Experimental Radiation Oncology Research" Cancers 11, no. 10: 1499. https://doi.org/10.3390/cancers11101499
APA StyleDünker, N., & Jendrossek, V. (2019). Implementation of the Chick Chorioallantoic Membrane (CAM) Model in Radiation Biology and Experimental Radiation Oncology Research. Cancers, 11(10), 1499. https://doi.org/10.3390/cancers11101499