Immune Dysfunctions and Immunotherapy in Colorectal Cancer: The Role of Dendritic Cells
Abstract
1. Introduction
2. Diet, Inflammation and Microbiota in the Pathogenesis of CRC
2.1. Diet and Obesity As Important Factors in the Pathogenesis of CRC
2.2. Relationships Between Diet, Microbiota and Immune Dysfunctions in CRC Pathogenesis
3. The Role of DC in the Regulation of the Inflammatory and Immune Responses in CRC
4. Changes in the Phenotype and Function of DC in CRC Patients
5. DC and Immunotherapy of CRC
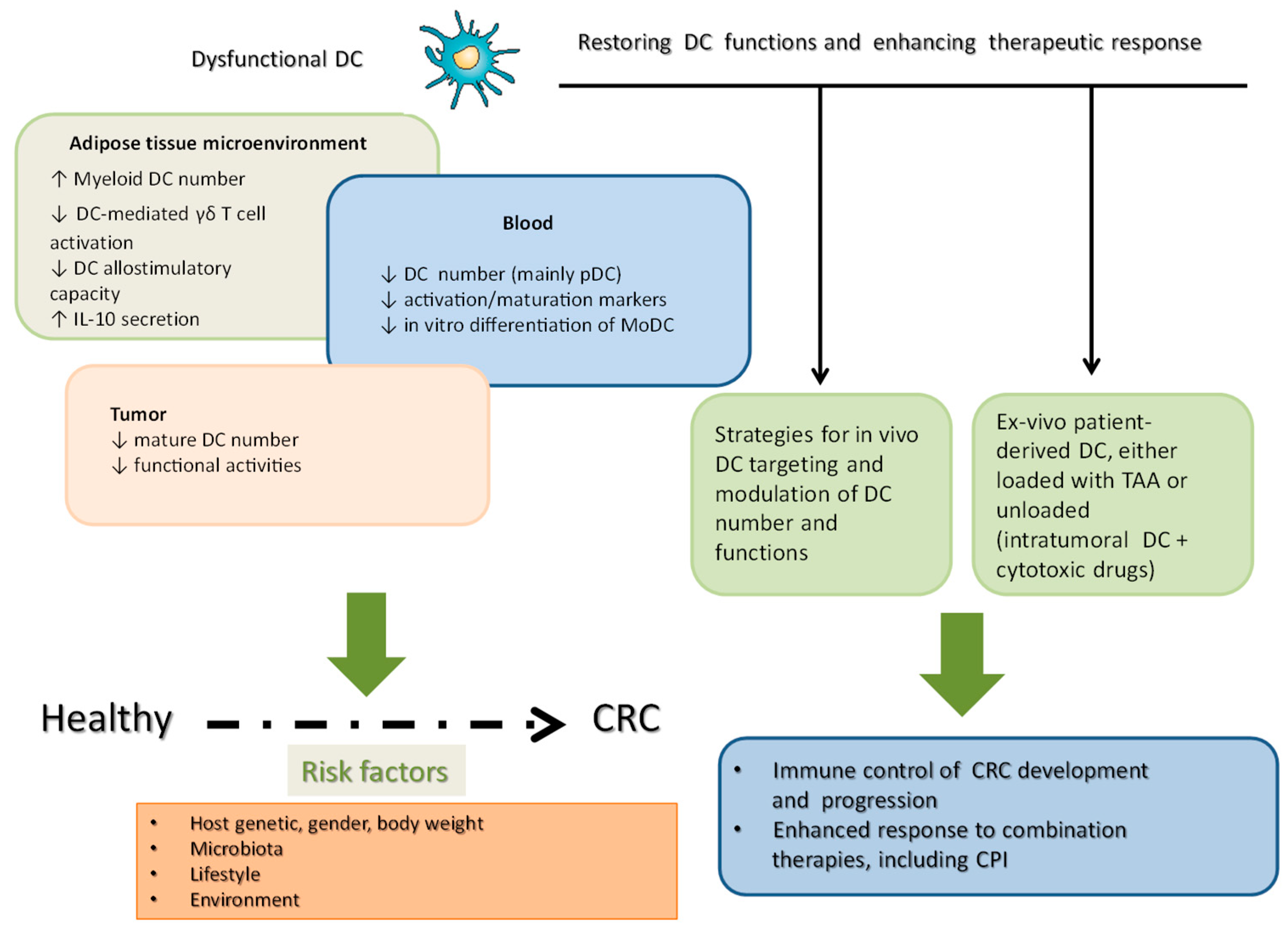
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Van der Geest, L.L.; Lam-Boer, J.; Koopman, M.; Verhoef, C.; Elferink, M.M.; de Wilt, J.J. Nationwide Trends in Incidence, Treatment and Survival of Colorectal Cancer Patients with Synchronous Metastases. Clin. Exp. Metastasis 2015, 32, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.A.; Divya, T.; Kumar, K.; Dineshbabu, V.; Velavan, B.; Sudhandiran, G. Colorectal Carcinogenesis: Insights into the Cell Death and Signal Transduction Pathways: A review. World J. Gastrointest. Oncol. 2018, 10, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.V.; Zhou, S.; Diaz, L.L., Jr.; Kinzler, K.K. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Nowak, J.J.; Hamada, T.; Phipps, A.A.; Peters, U.; Milner, D.D., Jr.; Giovannucci, E.E.; Nishihara, R.; Giannakis, M.; Garrett, W.W.; et al. Integrative Analysis of Exogenous, Endogenous, Tumour and Immune Factors for Precision Medicine. Gut 2018, 67, 1168–1180. [Google Scholar] [CrossRef]
- Kosumi, K.; Mima, K.; Baba, H.; Ogino, S. Dysbiosis of the Gut Microbiota and Colorectal Cancer: The Key Target of Molecular Pathological Epidemiology. J. Lab. Precis. Med. 2018, 3. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Z.; Zheng, W.; Liu, X.; Sun, C.; Laugsand, J.J.; Liu, Z.; Cui, G. Changes of Immunocytic Phenotypes and Functions from Human Colorectal Adenomatous Stage to Cancerous Stage: Update. Immunobiology 2015, 220, 1186–1196. [Google Scholar] [CrossRef]
- McLean, M.M.; Murray, G.G.; Stewart, K.K.; Norrie, G.; Mayer, C.; Hold, G.G.; Thomson, J.; Fyfe, N.; Hope, M.; Mowat, N.N.; et al. The Inflammatory Microenvironment in Colorectal Neoplasia. PLoS ONE 2011, 6, e15366. [Google Scholar] [CrossRef]
- Croci, D.D.; Zacarias, F.M.M.; Rico, M.M.; Matar, P.; Rabinovich, G.G.; Scharovsky, O.O. Dynamic Cross-Talk between Tumor and Immune Cells in Orchestrating the Immunosuppressive Network at the Tumor Microenvironment. Cancer Immunol. Immunother. 2007, 56, 1687–1700. [Google Scholar] [CrossRef]
- Lasry, A.; Zinger, A.; Ben-Neriah, Y. Inflammatory Networks Underlying Colorectal Cancer. Nat. Immunol. 2016, 17, 230–240. [Google Scholar] [CrossRef]
- West, N.N.; McCuaig, S.; Franchini, F.; Powrie, F. Emerging Cytokine Networks in Colorectal Cancer. Nat. Rev. Immunol. 2015, 15, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.M.; Hotamisligil, G.G. Inflammatory Mechanisms in Obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Cai, X.; Zhang, J.; Wang, W.; Sheng, Q.; Hua, H.; Zhou, X. Role of Gut Microbiota in the Development and Treatment of Colorectal Cancer. Digestion 2019, 100, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Pitmon, E.; Wang, K. Microbiome, Inflammation and Colorectal Cancer. Semin. Immunol. 2017, 32, 43–53. [Google Scholar] [CrossRef]
- De Almeida, C.C.; de Camargo, M.M.; Russo, E.; Amedei, A. Role of Diet and Gut Microbiota on Colorectal Cancer Immunomodulation. World J. Gastroenterol. 2019, 25, 151–162. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, A. Dendritic Cells and CC8 T Cell Immunity in Tumor Microenvironment. Front. Immunol. 2018, 9, 3059. [Google Scholar] [CrossRef]
- Wrobel, P.; Ahmed, S. Current Status of Immunotherapy in Metastatic Colorectal Cancer. Int. J. Colorectal Dis. 2019, 34, 13–25. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pages, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, Density and Location of Immune Cells within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Diesendruck, Y.; Benhar, I. Novel Immune Check Point Inhibiting Antibodies in Cancer Therapy-Opportunities and Challenges. Drug Resist. Updates 2017, 30, 39–47. [Google Scholar] [CrossRef]
- Gutting, T.; Burgermeister, E.; Hartel, N.; Ebert, M.M. Checkpoints and Beyond—Immunotherapy in Colorectal Cancer. Semin. Cancer. Biol. 2019, 55, 78–89. [Google Scholar] [CrossRef]
- Westdorp, H.; Fennemann, F.F.; Weren, R.R.; Bisseling, T.T.; Ligtenberg, M.M.; Figdor, C.C.; Schreibelt, G.; Hoogerbrugge, N.; Wimmers, F.; de Vries, I.I. Opportunities for Immunotherapy in Microsatellite Instable Colorectal Cancer. Cancer. Immunol. Immunother. 2016, 65, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Zitvogel, L.; Fridman, W.W. Colorectal cancer: The First Neoplasia Found to be under Immunosurveillance and the Last One to Respond to Immunotherapy? Oncoimmunology 2015, 4, e1058597. [Google Scholar] [CrossRef] [PubMed]
- Cozzo, A.A.; Fuller, A.A.; Makowski, L. Contribution of Adipose Tissue to Development of Cancer. Compr. Physiol. 2018, 8, 237–282. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.G.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Guzik, T.T.; Skiba, D.D.; Touyz, R.R.; Harrison, D.D. The Role of Infiltrating Immune Cells in Dysfunctional Adipose Tissue. Cardiovasc. Res. 2017, 113, 1009–1023. [Google Scholar] [CrossRef]
- Del Corno, M.; Conti, L.; Gessani, S. Innate Lymphocytes in Adipose Tissue Homeostasis and Their Alterations in Obesity and Colorectal Cancer. Front. Immunol. 2018, 9, 2556. [Google Scholar] [CrossRef]
- Meiliana, A.; Dewi, N.N.; Wijaya, A. Adipose Tissue Inlammation (Meta-inlammation) and Obesity Management. Indones. Biomed. J. 2015, 7, 129–146. [Google Scholar] [CrossRef]
- Masoodi, M.; Kuda, O.; Rossmeisl, M.; Flachs, P.; Kopecky, J. Lipid Signaling in Adipose Tissue: Connecting Inflammation & Metabolism. Biochim. Biophys. Acta 2015, 1851, 503–518. [Google Scholar] [CrossRef]
- D’Archivio, M.; Scazzocchio, B.; Giammarioli, S.; Fiani, M.M.; Vari, R.; Santangelo, C.; Veneziani, A.; Iacovelli, A.; Giovannini, C.; Gessani, S.; et al. omega3-PUFAs Exert Anti-Inflammatory Activity in Visceral Adipocytes from Colorectal Cancer Patients. PLoS ONE 2013, 8, e77432. [Google Scholar] [CrossRef][Green Version]
- Andersen, C.C.; Murphy, K.K.; Fernandez, M.M. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Bertola, A.; Ciucci, T.; Rousseau, D.; Bourlier, V.; Duffaut, C.; Bonnafous, S.; Blin-Wakkach, C.; Anty, R.; Iannelli, A.; Gugenheim, J.; et al. Identification of Adipose Tissue Dendritic Cells Correlated with Obesity-Associated Insulin-resistance and Inducing, Th17 Responses in Mice and Patients. Diabetes 2012, 61, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Yessoufou, A.; Ategbo, J.J.; Attakpa, E.; Hichami, A.; Moutairou, K.; Dramane, K.K.; Khan, N.N. Peroxisome Proliferator-Activated Receptor-Alpha Modulates Insulin Gene Transcription Factors and Inflammation in Adipose Tissues in Mice. Mol. Cell. Biochem. 2009, 323, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Khan, N.N.; McMurray, D.D.; Prior, I.I.; Wang, N.; Chapkin, R.R. Regulatory Activity of Polyunsaturated Fatty Acids in T.-cell Signaling. Prog. Lipid Res. 2010, 49, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Del Corno, M.; D’Archivio, M.; Conti, L.; Scazzocchio, B.; Vari, R.; Donninelli, G.; Varano, B.; Giammarioli, S.; De Meo, S.; Silecchia, G.; et al. Visceral Fat Adipocytes From Obese and Colorectal Cancer Subjects Exhibit Distinct Secretory and Omega6 Polyunsaturated Fatty Acid Profiles and Deliver Immunosuppressive Signals to Innate Immunity Cells. Oncotarget 2016, 7, 63093–63105. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, B.; Vari, R.; Silenzi, A.; Giammarioli, S.; Masotti, A.; Baldassarre, A.; Santangelo, C.; D’Archivio, M.; Giovannini, C.; Del Corno, M.; et al. Dietary Habits Affect Fatty Acid Composition of Visceral Adipose Tissue in Subjects with Colorectal Cancer or Obesity. Eur. J. Nutr. 2019. [CrossRef] [PubMed]
- Kong, L.L.; Holmes, B.B.; Cotillard, A.; Habi-Rachedi, F.; Brazeilles, R.; Gougis, S.; Gausseres, N.; Cani, P.P.; Fellahi, S.; Bastard, J.J.; et al. Dietary Patterns Differently Associate with Inflammation and Gut Microbiota in Overweight and Obese Subjects. PLoS ONE 2014, 9, e109434. [Google Scholar] [CrossRef]
- Mokkala, K.; Houttu, N.; Cansev, T.; Laitinen, K. Interactions of Dietary Fat with the Gut Microbiota: Evaluation of Mechanisms and Metabolic Consequences. Clin. Nutr. 2019. [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.C.M.; Alou, M.M.; Daillere, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.M.; et al. Gut Microbiome Influences Efficacy of PP-1-based Immunotherapy Against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, T.; Tu, X.; Huang, Y.; Zhang, H.; Tan, D.; Jiang, W.; Cai, S.; Zhao, P.; Song, R.; et al. Gut Microbiome Affects the Response To Anti-PD-1 Immunotherapy in Patients with Hepatocellular Carcinoma. J. Immunother. Cancer 2019, 7, 193. [Google Scholar] [CrossRef]
- Debedat, J.; Clement, K.; Aron-Wisnewsky, J. Gut Microbiota Dysbiosis in Human Obesity: Impact of Bariatric Surgery. Curr. Obes. Rep. 2019, 8, 229–242. [Google Scholar] [CrossRef]
- Ahn, J.; Sinha, R.; Pei, Z.; Dominianni, C.; Wu, J.; Shi, J.; Goedert, J.J.; Hayes, R.R.; Yang, L. Human Gut Microbiome and Risk for Colorectal Cancer. J. Natl. Cancer Inst. 2013, 105, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Huipeng, W.; Lifeng, G.; Chuang, G.; Jiaying, Z.; Yuankun, C. The Differences in Colonic Mucosal Microbiota between Normal Individual and Colon Cancer Patients by Polymerase Chain Reaction-Denaturing Gradient Gel Electrophoresis. J. Clin. Gastroenterol. 2014, 48, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Solano-Galvez, S.S.; Tovar-Torres, S.S.; Tron-Gomez, M.M.; Weiser-Smeke, A.A.; Alvarez-Hernandez, D.D.; Franyuti-Kelly, G.G.; Tapia-Moreno, M.; Ibarra, A.; Gutierrez-Kobeh, L.; Vazquez-Lopez, R. Human Dendritic Cells: Ontogeny and Their Subsets in Health and Disease. Med. Sci. 2018, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Palucka, K.; Banchereau, J. Cancer Immunotherapy via Dendritic Cells. Nat. Rev. Cancer 2012, 12, 265–277. [Google Scholar] [CrossRef]
- Gabrilovich, D. Mechanisms and Functional Significance of Tumour-Induced Dendritic-Cell Defects. Nat. Rev. Immunol. 2004, 4, 941–952. [Google Scholar] [CrossRef]
- Legitimo, A.; Consolini, R.; Failli, A.; Orsini, G.; Spisni, R. Dendritic Cell Defects in the Colorectal Cancer. Hum. Vaccines Immunother. 2014, 10, 3224–3235. [Google Scholar] [CrossRef]
- Alwarawrah, Y.; Kiernan, K.; MacIver, N.N. Changes in Nutritional Status Impact Immune Cell Metabolism and Function. Front. Immunol. 2018, 9, 1055. [Google Scholar] [CrossRef]
- Donninelli, G.; Del Corno, M.; Pierdominici, M.; Scazzocchio, B.; Vari, R.; Varano, B.; Pacella, I.; Piconese, S.; Barnaba, V.; D’Archivio, M.; et al. Distinct Blood and Visceral Adipose Tissue Regulatory T Cell and Innate Lymphocyte Profiles Characterize Obesity and Colorectal Cancer. Front. Immunol. 2017, 8, 643. [Google Scholar] [CrossRef]
- Gulubova, M.M.; Ananiev, J.J.; Vlaykova, T.T.; Yovchev, Y.; Tsoneva, V.; Manolova, I.I. Role of Dendritic Cells in Progression and Clinical Outcome of Colon Cancer. Int. J. Colorectal Dis. 2012, 27, 159–169. [Google Scholar] [CrossRef]
- Nagorsen, D.; Voigt, S.; Berg, E.; Stein, H.; Thiel, E.; Loddenkemper, C. Tumor-Infiltrating Macrophages and Dendritic Cells in Human Colorectal Cancer: Relation to Local Regulatory T Cells, Systemic T-Cell Response Against Tumor-Associated Antigens and Survival. J. Transl. Med. 2007, 5, 62. [Google Scholar] [CrossRef]
- Dadabayev, A.A.; Sandel, M.M.; Menon, A.A.; Morreau, H.; Melief, C.C.; Offringa, R.; van der Burg, S.S.; Janssen-van Rhijn, C.; Ensink, N.N.; Tollenaar, R.R.; et al. Dendritic Cells in Colorectal Cancer Correlate with Other Tumor-Infiltrating Immune Cells. Cancer Immunol. Immunother. 2004, 53, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Schwaab, T.; Weiss, J.J.; Schned, A.A.; Barth, R.R., Jr. Dendritic Cell Infiltration in Colon Cancer. J. Immunother. 2001, 24, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, A.A.; Hogan, A.A.; Marry, J.; Tosetto, M.; Cox, F.; Hyland, J.J.; Sheahan, K.K.; O’Donoghue, D.D.; Mulcahy, H.H.; Ryan, E.E.; et al. Tumour Tissue Microenvironment can Inhibit Dendritic Cell Maturation in Colorectal Cancer. PLoS ONE 2011, 6, e27944. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, A.A.; Noonan, S.; Martin, P.; Tosetto, M.; Marry, J.; Biniecka, M.; Maguire, A.A.; Hyland, J.J.; Sheahan, K.K.; O’Donoghue, D.D.; et al. Inhibition of Dendritic Cell Maturation by the Tumor Microenvironment Correlates with the Survival of Colorectal Cancer Patients Following Bevacizumab Treatment. Mol. Cancer Ther. 2012, 11, 1829–1837. [Google Scholar] [CrossRef]
- Bauer, K.; Michel, S.; Reuschenbach, M.; Nelius, N.; von Knebel Doeberitz, M.; Kloor, M. Dendritic Cell and Macrophage Infiltration in Microsatellite-Unstable and Microsatellite-Stable Colorectal Cancer. Fam. Cancer 2011, 10, 557–565. [Google Scholar] [CrossRef]
- Bellik, L.; Gerlini, G.; Parenti, A.; Ledda, F.; Pimpinelli, N.; Neri, B.; Pantalone, D. Role of Conventional Treatments on Circulating and Monocyte-Derived Dendritic Cells in Colorectal Cancer. Clin. Immunol. 2006, 121, 74–80. [Google Scholar] [CrossRef]
- Orsini, G.; Legitimo, A.; Failli, A.; Ferrari, P.; Nicolini, A.; Spisni, R.; Miccoli, P.; Consolini, R. Defective generation and maturation of dendritic cells from monocytes in Colorectal Cancer Patients During the Course of Disease. Int. J. Mol. Sci. 2013, 14, 22022–22041. [Google Scholar] [CrossRef]
- Huang, A.; Gilmour, J.J.; Imami, N.; Amjadi, P.; Henderson, D.D.; Allen-Mersh, T.T. Increased Serum Transforming Growth Factor-Beta1 in Human Colorectal Cancer Correlates with Reduced Circulating Dendritic Cells and Increased Colonic, Langerhans Cell Infiltration. Clin. Exp. Immunol. 2003, 134, 270–278. [Google Scholar] [CrossRef]
- Porta, M.D.; Danova, M.; Rigolin, G.G.; Brugnatelli, S.; Rovati, B.; Tronconi, C.; Fraulini, C.; Rossi, A.R.; Riccardi, A.; Castoldi, G. Dendritic Cells and Vascular Endothelial Growth Factor in Colorectal Cancer: Correlations with Clinicobiological Findings. Oncology 2005, 68, 276–284. [Google Scholar] [CrossRef]
- Orsini, G.; Legitimo, A.; Failli, A.; Ferrari, P.; Nicolini, A.; Spisni, R.; Miccoli, P.; Consolini, R. Quantification of Blood Dendritic Cells in Colorectal Cancer Patients During the Course of Disease. Pathol. Oncol. Res. Por. 2014, 20, 267–276. [Google Scholar] [CrossRef]
- Onishi, H.; Morisaki, T.; Baba, E.; Kuga, H.; Kuroki, H.; Matsumoto, K.; Tanaka, M.; Katano, M. Dysfunctional and Short-Lived Subsets in Monocyte-Derived Dendritic Cells from Patients with Advanced Cancer. Clin. Immunol. 2002, 105, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, H.; Nagayama, H.; Sato, K.; Enomoto, M.; Takeda, Y.; Takahashi, T.T.; Hasumi, K.; Eriguchi, M. Dysfunctional Regulation of the Development of Monocyte-Derived Dendritic Cells in Cancer Patients. Biomed. Pharmacother. 2000, 54, 291–298. [Google Scholar] [CrossRef]
- Kvistborg, P.; Bechmann, C.C.; Pedersen, A.A.; Toh, H.H.; Claesson, M.M.; Zocca, M.M. Comparison of Monocyte-Derived Dendritic Cells from Colorectal Cancer Patients, Non-Small-Cell-Lung-Cancer patients and Healthy Donors. Vaccine 2009, 28, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, N.; Soltani, A.; Ghazvini, K.; Hassanian, S.S.; Hashemy, S.S. PD-1/PD-L1 Blockade As a Novel Treatment for Colorectal Cancer. Biomed. Pharmacother. 2019, 110, 312–318. [Google Scholar] [CrossRef]
- Garg, A.A.; Vara, P.M.; Schaaf, M.; Agostinis, P.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial Watch: Dendritic Cell-Based Anticancer Immunotherapy. Oncoimmunology 2017, 6, e1328341. [Google Scholar] [CrossRef]
- Garg, A.A.; Coulie, P.P.; Van den Eynde, B.B.; Agostinis, P. Integrating Next-Generation Dendritic Cell Vaccines into the Current Cancer Immunotherapy Landscape. Trends Immunol. 2017, 38, 577–593. [Google Scholar] [CrossRef]
- Karaki, S.; Anson, M.; Tran, T.; Giusti, D.; Blanc, C.; Oudard, S.; Tartour, E. Is There Still Room for Cancer Vaccines at the Era of Checkpoint Inhibitors. Vaccines 2016, 4, 37. [Google Scholar] [CrossRef]
- Garris, C.C.; Arlauckas, S.S.; Kohler, R.R.; Trefny, M.M.; Garren, S.; Piot, C.; Engblom, C.; Pfirschke, C.; Siwicki, M.; Gungabeesoon, J.; et al. Successful, Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IIN-gamma and II-12. Immunity 2018, 49, 1148–1161 e1147. [Google Scholar] [CrossRef]
- Santini, S.S.; Lapenta, C.; Logozzi, M.; Parlato, S.; Spada, M.; Di Pucchio, T.; Belardelli, F. Type I Interferon As a Powerful Adjuvant for Monocyte-Derived Dendritic Cell Development and Activity in vitro and in Hu-PBL-SCID Mice. J. Exp. Med. 2000, 191, 1777–1788. [Google Scholar] [CrossRef]
- Lapenta, C.; Donati, S.; Spadaro, F.; Castaldo, P.; Belardelli, F.; Cox, M.M.; Santini, S.S. NK Cell Activation in the Antitumor Response Induced by IIN-alpha Dendritic Cells Loaded with Apoptotic Cells from Follicular Lymphoma Patients. J. Immunol. 2016, 197, 795–806. [Google Scholar] [CrossRef]
- Rozera, C.; Cappellini, G.G.; D’Agostino, G.; Santodonato, L.; Castiello, L.; Urbani, F.; Macchia, I.; Arico, E.; Casorelli, I.; Sestili, P.; et al. Intratumoral Injection of IIN-Alpha Dendritic Cells after Dacarbazine Activates Anti-Tumor Immunity: Results from a Phase I Trial in Advanced Melanoma. J. Transl. Med. 2015, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.M.; Castiello, L.; Mattei, M.; Santodonato, L.; D’Agostino, G.; Muraro, E.; Martorelli, D.; Lapenta, C.; Di Napoli, A.; Di Landro, F.; et al. Clinical and Antitumor Immune Responses in Relapsed/Refractory Follicular Lymphoma Patients after Intranodal Injections of IINalpha-Dendritic Cells and Rituximab. Clin. Cancer Res. 2019. [CrossRef]
- Babatz, J.; Rollig, C.; Lobel, B.; Folprecht, G.; Haack, M.; Gunther, H.; Kohne, C.C.; Ehninger, G.; Schmitz, M.; Bornhauser, M. Induction of Cellular Immune Responses Against Carcinoembryonic Antigen in Patients with Metastatic Tumors after Vaccination with Altered Peptide Ligand-Loaded Dendritic Cells. Cancer Immunol. Immunother. 2006, 55, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.M.; Clay, T.T.; Hobeika, A.A.; Osada, T.; Khan, S.; Chui, S.; Niedzwiecki, D.; Panicali, D.; Schlom, J.; Lyerly, H.H. Phase I Study of Immunization with Dendritic Cells Modified with Fowlpox Encoding Carcinoembryonic Antigen and Costimulatory Molecules. Clin. Cancer Res. 2005, 11, 3017–3024. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, B.; Ko, A.; Venook, A.; Margolin, K.; Zeh, H.; Lotze, M.; Schillinger, B.; Liu, W.; Lu, Y.; Mitsky, P.; et al. Vaccination of Metastatic Colorectal Cancer Patients with Matured Dendritic Cells Loaded with Multiple Major Histocompatibility Complex Class I Peptides. J Immunother 2007, 30, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.R., Jr.; Fisher, D.D.; Wallace, P.P.; Channon, J.J.; Noelle, R.R.; Gui, J.; Ernstoff, M.M. A Randomized Trial of ex vivo CC40L Activation of a Dendritic Cell Vaccine in Colorectal Cancer Patients: Tumor-Specific Immune Responses are Associated with Improved Survival. Clin. Cancer Res. 2010, 16, 5548–5556. [Google Scholar] [CrossRef]
- Sakakibara, M.; Kanto, T.; Hayakawa, M.; Kuroda, S.; Miyatake, H.; Itose, I.; Miyazaki, M.; Kakita, N.; Higashitani, K.; Matsubara, T.; et al. Comprehensive Immunological Analyses of Colorectal Cancer Patients in the Phase I/II Study of Quickly Matured Dendritic Cell Vaccine Pulsed with Carcinoembryonic Antigen Peptide. Cancer Immunol. Immunother. 2011, 60, 1565–1575. [Google Scholar] [CrossRef]
- Morse, M.M.; Niedzwiecki, D.; Marshall, J.J.; Garrett, C.; Chang, D.D.; Aklilu, M.; Crocenzi, T.T.; Cole, D.D.; Dessureault, S.; Hobeika, A.A.; et al. A Randomized Phase II Study of Immunization with Dendritic Cells Modified with Poxvectors Encoding CCA and MMC1 Compared with the Same Poxvectors Plus GG-CSF for Resected Metastatic Colorectal Cancer. Ann. Surg. 2013, 258, 879–886. [Google Scholar] [CrossRef]
- Hunyadi, J.; Andras, C.; Szabo, I.; Szanto, J.; Szluha, K.; Sipka, S.; Kovacs, P.; Kiss, A.; Szegedi, G.; Altorjay, I.; et al. Autologous dendritic cell based adoptive immunotherapy of patients with colorectal cancer-A phase I-II study. Pathol. Oncol. Res. Por. 2014, 20, 357–365. [Google Scholar] [CrossRef]
- Gao, D.; Li, C.; Xie, X.; Zhao, P.; Wei, X.; Sun, W.; Liu, H.H.; Alexandrou, A.A.; Jones, J.; Zhao, R.; et al. Autologous Tumor Lysate-Pulsed Dendritic Cell Immunotherapy with Cytokine-Induced Killer Cells Improves Survival in Gastric and Colorectal Cancer Patients. PLoS ONE 2014, 9, e93886. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, X.; Li, J.; Ren, Y.; Zhang, T.; Zhang, C.; Zhang, J.; Pang, Y. Immune Response Safety and Survival and Quality of Life Outcomes for Advanced Colorectal Cancer Patients Treated with Dendritic Cell Vaccine and Cytokine-Induced Killer Cell Therapy. Biomed. Res. Int. 2014, 2014, 603871. [Google Scholar] [CrossRef]
- Liu, K.K.; Chao, T.T.; Chang, J.J.; Cheng, A.A.; Chang, H.H.; Kao, W.W.; Wu, Y.Y.; Yu, W.W.; Chung, T.T.; Whang-Peng, J. A Phase I Clinical Study of Immunotherapy for Advanced Colorectal Cancers Using Carcinoembryonic Antigen-Pulsed Dendritic Cells Mixed with Tetanus Toxoid and Subsequent II-2 Treatment. J. Biomed. Sci. 2016, 23, 64. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Banos, M.; Benitez-Ribas, D.; Tabera, J.; Varea, S.; Vilana, R.; Bianchi, L.; Ayuso, J.J.; Pages, M.; Carrera, G.; Cuatrecasas, M.; et al. Phase II Randomised Trial of Autologous Tumour Lysate Dendritic Cell Plus Best Supportive Care Compared with Best Supportive Care in Pre-Treated Advanced Colorectal Cancer Patients. Eur. J. Cancer 2016, 64, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Murthy, R.; Hong, D.D.; Prins, R.R.; Hosing, C.; Hendricks, K.; Kolli, D.; Noffsinger, L.; Brown, R.; McGuire, M.; et al. Cytokines Produced by Dendritic Cells Administered Intratumorally Correlate with Clinical Outcome in Patients with Diverse Cancers. Clin. Cancer Res. 2018, 24, 3845–3856. [Google Scholar] [CrossRef] [PubMed]
- Mager, L.L.; Wasmer, M.M.; Rau, T.T.; Krebs, P. Cytokine-Induced Modulation of Colorectal Cancer. Front. Oncol. 2016, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Katlinski, K.K.; Gui, J.; Katlinskaya, Y.Y.; Ortiz, A.; Chakraborty, R.; Bhattacharya, S.; Carbone, C.C.; Beiting, D.D.; Girondo, M.M.; Peck, A.A.; et al. Inactivation of Interferon Receptor Promotes the Establishment of Immune Privileged Tumor Microenvironment. Cancer Cell 2017, 31, 194–207. [Google Scholar] [CrossRef]
- Barry, K.K.; Hsu, J.; Broz, M.M.; Cueto, F.F.; Binnewies, M.; Combes, A.A.; Nelson, A.A.; Loo, K.; Kumar, R.; Rosenblum, M.M.; et al. A Natural Killer-Dendritic Cell Axis Defines Checkpoint Therapy-Responsive Tumor Microenvironments. Nat. Med. 2018, 24, 1178–1191. [Google Scholar] [CrossRef]
- Binnewies, M.; Mujal, A.A.; Pollack, J.J.; Combes, A.A.; Hardison, E.E.; Barry, K.K.; Tsui, J.; Ruhland, M.M.; Kersten, K.; Abushawish, M.M.; et al. Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CC4(+) T Cell Immunity. Cell 2019, 177, 556–571 e516. [Google Scholar] [CrossRef]
- Lapenta, C.; Santini, S.S.; Spada, M.; Donati, S.; Urbani, F.; Accapezzato, D.; Franceschini, D.; Andreotti, M.; Barnaba, V.; Belardelli, F. IFN-Alpha-Conditioned Dendritic Cells are Highly Efficient in Inducing Cross-Priming CC8(+) T Cells Against Exogenous Viral Antigens. Eur. J. Immunol. 2006, 36, 2046–2060. [Google Scholar] [CrossRef]
- Maccalli, C.; Di Cristanziano, V.; Fodale, V.; Corsi, D.; D’Agostino, G.; Petrangeli, V.; Laurenti, L.; Guida, S.; Mazzocchi, A.; Arienti, F.; et al. Induction of Both CC8+ and CC4+ T-Cell-Mediated Responses in Colorectal Cancer Patients by Colon Antigen-1. Clin. Cancer Res. 2008, 14, 7292–7303. [Google Scholar] [CrossRef]
- Kim, S.S.; Paik, H.H.; Yoon, H.; Lee, J.J.; Kim, N.; Sung, M.M. Sex- and Gender-Specific Disparities in Colorectal Cancer Risk. World J. Gastroenterol. 2015, 21, 5167–5175. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Unno, T.; Kim, B.B.; Park, M.M. Sex Differences in Gut Microbiota. World J. Men’s Health 2019. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Epidemiology of Gender Differences in Diabetes and Obesity. Adv. Exp. Med. Biol. 2017, 1043, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Vari, R.; Scazzocchio, B.; D’Amore, A.; Giovannini, C.; Gessani, S.; Masella, R. Gender-Related Differences in Lifestyle May Affect Health Status. Ann. Dell’istituto Super. Di Sanita 2016, 52, 158–166. [Google Scholar] [CrossRef]
- Capone, I.; Marchetti, P.; Ascierto, P.P.; Malorni, W.; Gabriele, L. Sexual Dimorphism of Immune Responses: A New Perspective in Cancer Immunotherapy. Front. Immunol. 2018, 9, 552. [Google Scholar] [CrossRef] [PubMed]
- Soldati, L.; Di Renzo, L.; Jirillo, E.; Ascierto, P.P.; Marincola, F.F.; De Lorenzo, A. The Influence of Diet on Anti-Cancer Immune Responsiveness. J. Transl. Med. 2018, 16. [Google Scholar] [CrossRef] [PubMed]
| Patients | DC generation | Ag loading | DC administration | N | Major findings | Ref. |
|---|---|---|---|---|---|---|
| Metastatic, CEA+, HLA-*0201; Phase I | GM-CSF/IL-4, + TNFα, PGE2, IL-1β | CEA altered peptide | 1–5 × 107, i.v.; 4 times, every 2nd week | 7 | In vivo expansion of peptide-specific CD8+ T cells | [73] |
| Metastatic, CEA+; Phase I | GM-CSF/IL-4 | Fowl-pox vector encoding rCEA and costimulatory molecules | 5 × 105; s.c./i.d; 1 or 2 cycles of 4 weekly injections | 11 | Induction of CEA-specific T cells; trend of correlation with clinical response | [74] |
| Metastatic, HLA-A2+, Phase I | IL-13/GM-CSF, maturation factors | 6 CEA peptides | 35 × 106, i.d., 4 injections every 3 weeks | 11 | Progressive disease in spite of T cell response to tumor associated antigens | [75] |
| Metastatic, after resection of metastases; Phase I-II | GM-CSF/IL-4 | Autologous tumor lysate, KLH | 5 × 106 into 2 inguinal lymph nodes under ultrasound guidance; week 1, 3 and 6 | 26 | Tumor specific T cell response (63%); correlation with recurrence-free survival; no difference if DC were further treated or not with CD40L | [76] |
| Metastatic, CEA+, HLA-A*2402; Phase I-II | IL-4/GM-CSF/IFNα, streptococcus pyrogenes | CEA peptide | 11–115 × 106, s.c., 2-8 injections | 8 | Trend of correlation between CEA-specific cytotoxic T cells and clinical efficacy | [77] |
| Metastatic, after metastasis resection; Phase II | IL-4/GM-CSF | Poxvectors encoding CEA, MUC-1 and costimulatory molecules | 107, s.c./i.d. 3 times per month/3 months; comparison with patients injected with poxvectors + GM-CSF | 37 | Both DC-poxCEA and poxCEA +GM-CSF treatments showed similar response; longer survival time compared to contemporary unvaccinated group | [78] |
| Stage Dukes B2 and Dukes C; Phase I-II | IL-4/GM-CSF | TCL, rCEA protein | 5 × 106–2 × 107, s.c.; days 1, 14, 28, 56 | 12 ^ | Suggestion of clinical effect with TCL-DC, but no effect with CEA-DC | [79] |
| Metastatic, after resection of metastases; pretreatment with low dose chemotherapy; Phase I-II | IL-4/GM-CSF | TCL | Average DC dosage: 188 × 106, s.c.; 3–5 injections in 2 weeks; patients also received i.v. injections of CIK cells | 13 | Reduction of post-operative disease risk; increase of overall survival | [80] |
| Metastatic, unresectable; Phase II | IL-4/GM-CSF/TNFα | TCL | 107, i.v., for the first 3 weeks; i.d. for the last 3 weeks; i.v. CIK cell infusions for 4 days | 100 | DC/CIK therapy can induce anti-CRC immune response (DTH) with a potential impact on survival and quality life with respect to control group | [81] |
| Metastatic, resistant to standard therapies; Phase I-II | IL-4/GM-CSF, + maturation factors | rCEA protein | 106, s.c., mixed with tetanus toxoid; 3 other s.c. injections of the same DC number | 12 | T cell reactive against CEA in 2 patients; 2 patients with stable disease; 10 patients showed progression; need to enhance antitumor T cell response | [82] |
| Metastatic, phase II; DC vaccine + best supportive care versus best supportive care | IL-4/GM-CSF + maturation factors | Autologous TCL | 5 × 106 (1, 10, 20, 40, 120 days), s.c. | 28 | Induction of tumor specific T cell response; no increase of overall survival with respect to the “best supportive care” group | [83] |
| Metastatic, resistant to standard therapies; Phase I-II | GM-CSF + killed BCG mycobacteria + IFNα | No in vitro antigen loading | 2–15 × 106; 2–6 injections, i.t. using image guidance | 7 | Cytokines produced by DC (IL-8 and IL-12p40) correlate with clinical outcome | [84] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gessani, S.; Belardelli, F. Immune Dysfunctions and Immunotherapy in Colorectal Cancer: The Role of Dendritic Cells. Cancers 2019, 11, 1491. https://doi.org/10.3390/cancers11101491
Gessani S, Belardelli F. Immune Dysfunctions and Immunotherapy in Colorectal Cancer: The Role of Dendritic Cells. Cancers. 2019; 11(10):1491. https://doi.org/10.3390/cancers11101491
Chicago/Turabian StyleGessani, Sandra, and Filippo Belardelli. 2019. "Immune Dysfunctions and Immunotherapy in Colorectal Cancer: The Role of Dendritic Cells" Cancers 11, no. 10: 1491. https://doi.org/10.3390/cancers11101491
APA StyleGessani, S., & Belardelli, F. (2019). Immune Dysfunctions and Immunotherapy in Colorectal Cancer: The Role of Dendritic Cells. Cancers, 11(10), 1491. https://doi.org/10.3390/cancers11101491




