Real-World Impact of Immune Checkpoint Inhibitors in Metastatic Uveal Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Databases
2.2. Treatment and Response
2.3. Statistical Analysis
3. Results
3.1. Patient Population
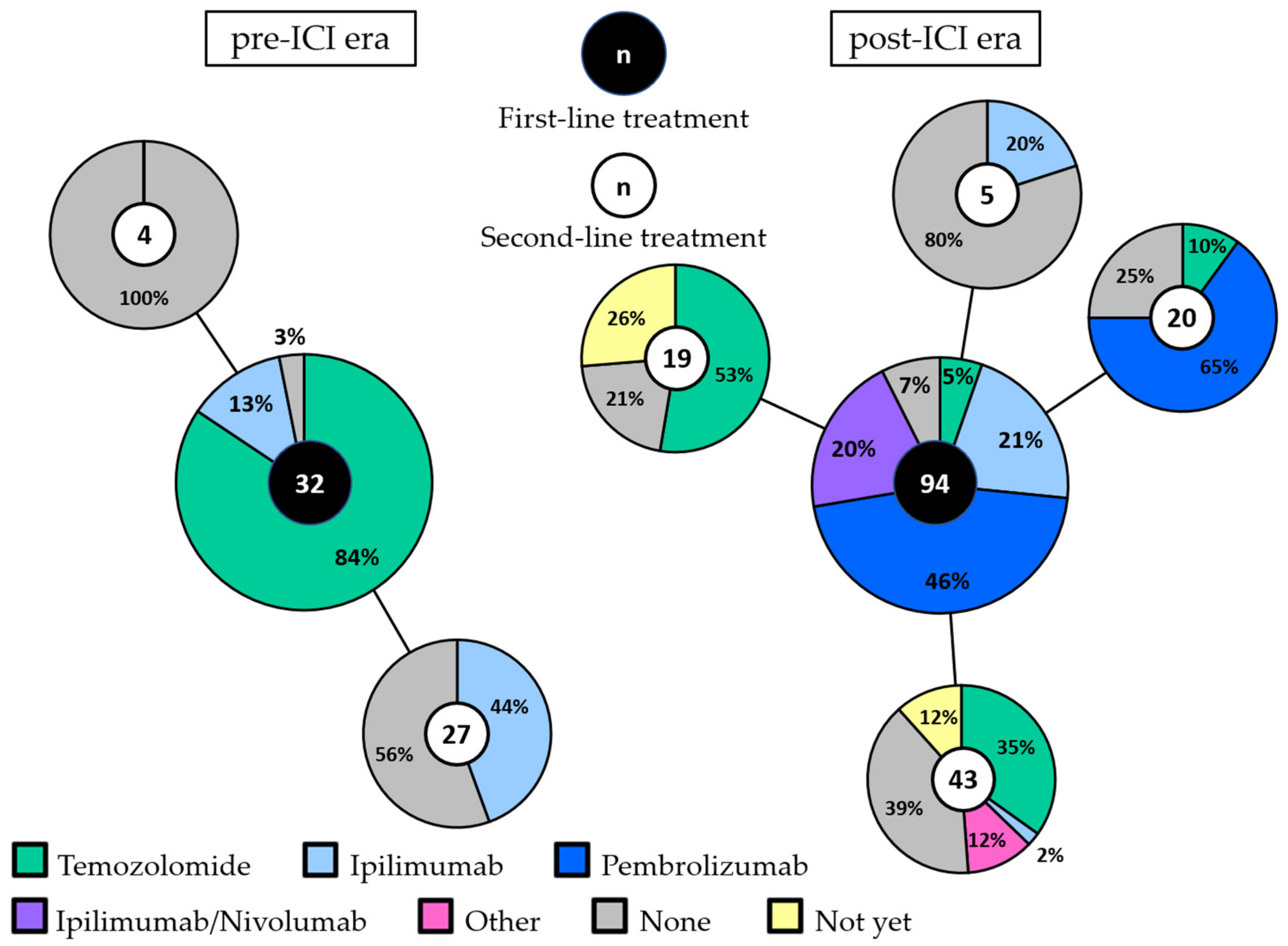
3.2. First-Line Treatment in the pre-ICI and post-ICI Era
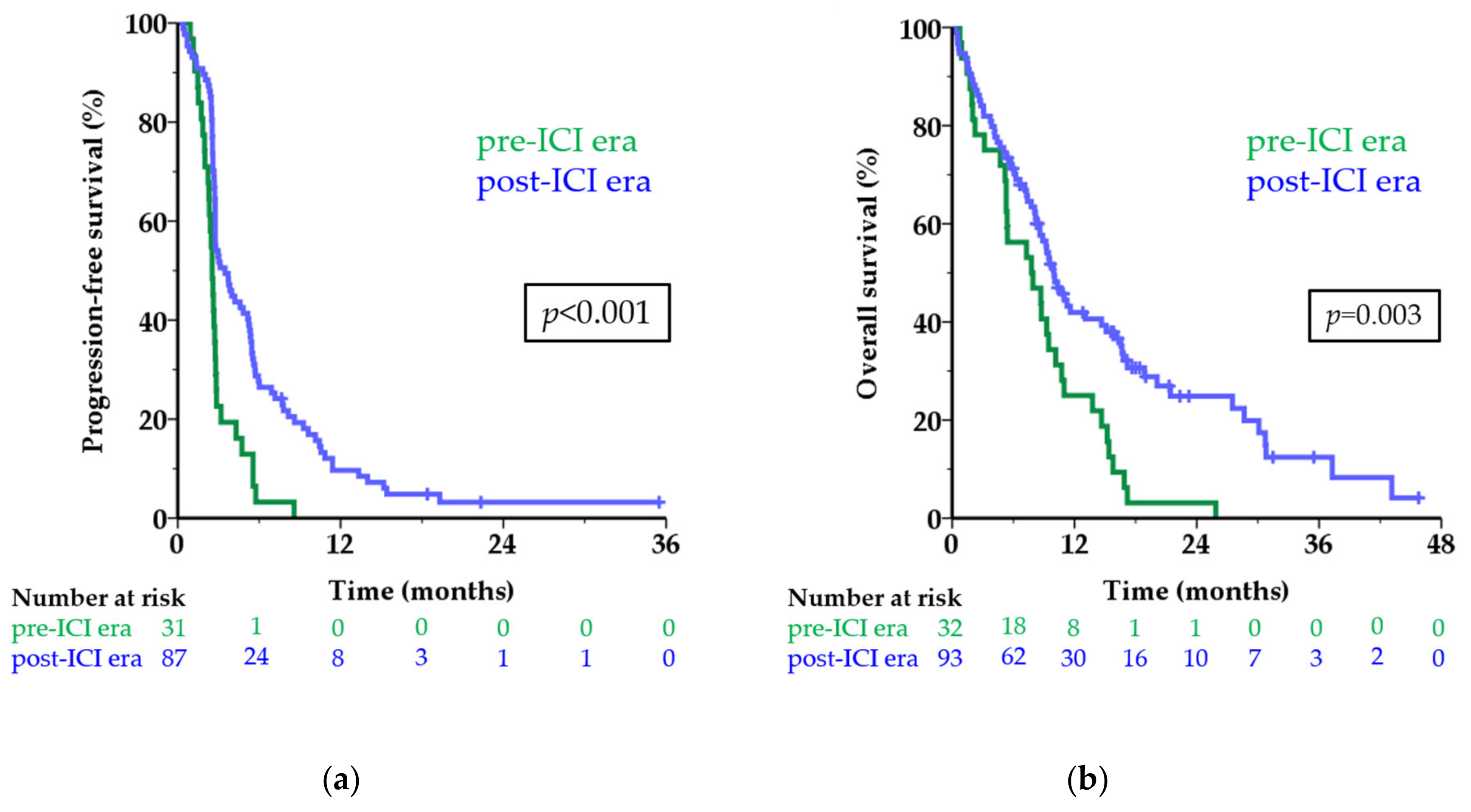
3.3. Survival Per Treatment Era
3.4. Objective Response Rates and Survival per First-Line Treatment
3.5. Retreatment and Second-Line Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AJCC: | American Joint Committee on Cancer |
| CI: | confidence intervals |
| CM: | cutaneous melanoma |
| CR: | complete response |
| ECOG: | Eastern cooperative oncology group |
| HR: | hazard ratio |
| ICI: | immune checkpoint inhibitors |
| LDH: | lactate dehydrogenase |
| OS: | overall survival |
| ORR: | objective response rate |
| PFS: | progression-free survival |
| PR: | partial response |
| SD: | stable disease |
| ULN: | upper limit of normal |
| UM: | uveal melanoma |
References
- Virgili, G.; Gatta, G.; Ciccolallo, L.; Capocaccia, R.; Biggeri, A.; Crocetti, E.; Lutz, J.M.; Paci, E.; EuroCare Working Group. Incidence of uveal melanoma in Europe. Ophthalmology 2007, 114, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Kujala, E.; Makitie, T.; Kivela, T. Very long-term prognosis of patients with malignant uveal melanoma. Invest. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef] [PubMed]
- Rietschel, P.; Panageas, K.S.; Hanlon, C.; Patel, A.; Abramson, D.H.; Chapman, P.B. Variates of survival in metastatic uveal melanoma. J. Clin. Oncol. 2005, 23, 8076–8080. [Google Scholar] [CrossRef]
- Rantala, E.S.; Hernberg, M.; Kivela, T.T. Overall survival after treatment for metastatic uveal melanoma: A systematic review and meta-analysis. Melanoma Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Nathan, P.; Cohen, V.; Coupland, S.; Curtis, K.; Damato, B.; Evans, J.; Fenwick, S.; Kirkpatrick, L.; Li, O.; Marshall, E.; et al. Uveal Melanoma UK National Guidelines. Eur. J. Cancer 2015, 51, 2404–2412. [Google Scholar] [CrossRef]
- Barker, C.A.; Salama, A.K. New NCCN Guidelines for Uveal Melanoma and Treatment of Recurrent or Progressive Distant Metastatic Melanoma. J. Natl. Compr. Cancer Netw. 2018, 16, 646–650. [Google Scholar] [CrossRef]
- Rimoldi, D.; Salvi, S.; Lienard, D.; Lejeune, F.J.; Speiser, D.; Zografos, L.; Cerottini, J.C. Lack of BRAF mutations in uveal melanoma. Cancer Res. 2003, 63, 5712–5715. [Google Scholar]
- Cruz, F., 3rd; Rubin, B.P.; Wilson, D.; Town, A.; Schroeder, A.; Haley, A.; Bainbridge, T.; Heinrich, M.C.; Corless, C.L. Absence of BRAF and NRAS mutations in uveal melanoma. Cancer Res. 2003, 63, 5761–5766. [Google Scholar]
- Carvajal, R.D.; Piperno-Neumann, S.; Kapiteijn, E.; Chapman, P.B.; Frank, S.; Joshua, A.M.; Piulats, J.M.; Wolter, P.; Cocquyt, V.; Chmielowski, B.; et al. Selumetinib in Combination With Dacarbazine in Patients With Metastatic Uveal Melanoma: A Phase III, Multicenter, Randomized Trial (SUMIT). J. Clin. Oncol. 2018, 36, 1232–1239. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.-F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.M.P.; de Olza, M.O.; Codes, M.; Lopez-Martin, J.A.; Berrocal, A.; García, M.; Gurpide, A.; Homet, B.; Martin-Algarra, S. Phase II study evaluating ipilimumab as a single agent in the first-line treatment of adult patients (Pts) with metastatic uveal melanoma (MUM): The GEM-1 trial. J. Clin. Oncol. 2014, 32, 9033. [Google Scholar] [CrossRef]
- Zimmer, L.; Vaubel, J.; Mohr, P.; Hauschild, A.; Utikal, J.; Simon, J.; Garbe, C.; Herbst, R.; Enk, A.; Kampgen, E.; et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naive patients with metastatic uveal melanoma. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Joshua, A.M.; Monzon, J.G.; Mihalcioiu, C.; Hogg, D.; Smylie, M.; Cheng, T. A phase 2 study of tremelimumab in patients with advanced uveal melanoma. Melanoma Res. 2015, 25, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Danielli, R.; Ridolfi, R.; Chiarion-Sileni, V.; Queirolo, P.; Testori, A.; Plummer, R.; Boitano, M.; Calabro, L.; Rossi, C.D.; Giacomo, A.M.; et al. Ipilimumab in pretreated patients with metastatic uveal melanoma: Safety and clinical efficacy. Cancer Immunol. Immunother. 2012, 61, 41–48. [Google Scholar] [CrossRef]
- Kelderman, S.; van der Kooij, M.K.; van den Eertwegh, A.J.; Soetekouw, P.M.; Jansen, R.L.; van den Brom, R.R.; Hospers, G.A.; Haanen, J.B.; Kapiteijn, E.; Blank, C.U. Ipilimumab in pretreated metastastic uveal melanoma patients. Results of the Dutch Working group on Immunotherapy of Oncology (WIN-O). Acta Oncol. 2013, 52, 1786–1788. [Google Scholar] [CrossRef] [PubMed]
- Maio, M.; Danielli, R.; Chiarion-Sileni, V.; Pigozzo, J.; Parmiani, G.; Ridolfi, R.; De Rosa, F.; Del Vecchio, M.; Di Guardo, L.; Queirolo, P.; et al. Efficacy and safety of ipilimumab in patients with pre-treated, uveal melanoma. Ann. Oncol. 2013, 24, 2911–2915. [Google Scholar] [CrossRef]
- Luke, J.J.; Callahan, M.K.; Postow, M.A.; Romano, E.; Ramaiya, N.; Bluth, M.; Giobbie-Hurder, A.; Lawrence, D.P.; Ibrahim, N.; Ott, P.A.; et al. Clinical activity of ipilimumab for metastatic uveal melanoma: A retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer 2013, 119, 3687–3695. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Qian, W.; Ellis, S.; Mason, E.; Khattak, M.A.; Gupta, A.; Shaw, H.; Quinton, A.; Kovarikova, J.; Thillai, K.; et al. Ipilimumab in the real world: The UK expanded access programme experience in previously treated advanced melanoma patients. Melanoma Res. 2015, 25, 432–442. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Ascierto, P.A.; Haanen, J.B.A.G.; Espinosa, E.; Demidov, L.V.; Garbe, C.; Lorigan, P.; Gogas, H.; Hoeller, C.; Guren, T.K.; et al. Efficacy and safety of nivolumab (NIVO) in patients with advanced melanoma (MEL) and poor prognostic factors who progressed on or after ipilimumab (IPI): Results from a phase II study (CheckMate 172). J. Clin. Oncol. 2017, 35, 9524. [Google Scholar] [CrossRef]
- Algazi, A.P.; Tsai, K.K.; Shoushtari, A.N.; Munhoz, R.R.; Eroglu, Z.; Piulats, J.M.; Ott, P.A.; Johnson, D.B.; Hwang, J.; Daud, A.I.; et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016, 122, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- Karydis, I.; Chan, P.Y.; Wheater, M.; Arriola, E.; Szlosarek, P.W.; Ottensmeier, C.H. Clinical activity and safety of Pembrolizumab in Ipilimumab pre-treated patients with uveal melanoma. Oncoimmunology 2016, 5. [Google Scholar] [CrossRef]
- Piperno-Neumann, S.; Servois, V.; Mariani, P.; Cassoux, N.; Barnhill, R.; Rodrigues, M.J. Activity of anti-PD1 drugs in uveal melanoma patients. J. Clin. Oncol. 2016, 34, 9588. [Google Scholar] [CrossRef]
- Bender, C.; Enk, A.; Gutzmer, R.; Hassel, J.C. Anti-PD-1 antibodies in metastatic uveal melanoma: A treatment option? Cancer Med. 2017, 6, 1581–1586. [Google Scholar] [CrossRef]
- Van der Kooij, M.K.; Joosse, A.; Speetjens, F.M.; Hospers, G.A.; Bisschop, C.; de Groot, J.W.; Koornstra, R.; Blank, C.U.; Kapiteijn, E. Anti-PD1 treatment in metastatic uveal melanoma in the Netherlands. Acta Oncol. 2017, 56, 101–103. [Google Scholar] [CrossRef]
- Heppt, M.V.; Heinzerling, L.; Kahler, K.C.; Forschner, A.; Kirchberger, M.C.; Loquai, C.; Meissner, M.; Meier, F.; Terheyden, P.; Schell, B.; et al. Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur. J. Cancer 2017, 82, 56–65. [Google Scholar] [CrossRef]
- Rossi, E.; Pagliara, M.M.; Orteschi, D.; Dosa, T.; Sammarco, M.G.; Caputo, C.G.; Petrone, G.; Rindi, G.; Zollino, M.; Blasi, M.A.; et al. Pembrolizumab as first-line treatment for metastatic uveal melanoma. Cancer Immunol. Immunother. 2019, 68, 1179–1185. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Shoushtari, A.N.; Navid-Azarbaijani, P.; Friedman, C.F.; Panageas, K.; Postow, M.A.; Callahan, M.K.; Momtaz, P.; Campbell, S.C.; Shames, Y.; Prempeh-Keteku, N.A.; et al. Efficacy of nivolumab and ipilimumab (Nivo + Ipi) combination in melanoma patients (pts) treated at a single institution on an expanded-access program (EAP). J. Clin. Oncol. 2016, 34, 9554. [Google Scholar] [CrossRef]
- Rodriguez, J.M.P.; Merino, L.d.l.C.; Espinosa, E.; Carrión, L.A.; Algarra, S.M.; López-Castro, R.; García, M.T.C.; Abreu, D.R.; Iriarte, A.J.R.; Jaime, A.B. Phase II multicenter, single arm, open label study of Nivolumab in combination with Ipilimumab in untreated patients with metastatic uveal melanoma. Ann. Oncol. 2018, 29, viii442–viii466. [Google Scholar] [CrossRef]
- Bagger, M.; Smidt-Nielsen, I.; Andersen, M.K.; Jensen, P.K.; Heegaard, S.; Andersen, K.K.; Friis, S.; Kiilgaard, J.F. Long-Term Metastatic Risk after Biopsy of Posterior Uveal Melanoma. Ophthalmology 2018, 125, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Mailankody, S.; Prasad, V. Overall Survival in Cancer Drug Trials as a New Surrogate End Point for Overall Survival in the Real World. JAMA Oncol. 2017, 3, 889–890. [Google Scholar] [CrossRef] [PubMed]
- Mignard, C.; Deschamps Huvier, A.; Gillibert, A.; Duval Modeste, A.B.; Dutriaux, C.; Khammari, A.; Avril, M.F.; Kramkimel, N.; Mortier, L.; Marcant, P.; et al. Efficacy of Immunotherapy in Patients with Metastatic Mucosal or Uveal Melanoma. J. Oncol. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Mobuchon, L.; Houy, A.; Fievet, A.; Gardrat, S.; Barnhill, R.L.; Popova, T.; Servois, V.; Rampanou, A.; Mouton, A.; et al. Outlier response to anti-PD1 in uveal melanoma reveals germline MBD4 mutations in hypermutated tumors. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
| Characteristic | Pre-ICI era n = 32 (%) | Post-ICI era n = 94 (%) | |
|---|---|---|---|
| Age | median (range) | 62 (34–89) | 65 (22–87) |
| Sex | male female | 14 (43.8%) 18 (56.3%) | 47 (50.0%) 47 (50.0%) |
| AJCC stage at diagnosis | stage I stage IIA stage IIB stage IIIA stage IIIB stage IIIC stage IV unknown | 1 (3.1%) 11 (34.4%) 11 (34.4%) 6 (18.8%) 2 (6.3%) 0 (0%) 1 (3.1%) 0 (0%) | 7 (7.4%) 25 (26.6%) 24 (25.5%) 20 (21.3%) 10 (10.6%) 3 (3.2%) 4 (4.3%) 1 (1.1%) |
| ECOG performance status | 0 1 2 3 unknown | 11 (57.9%) 5 (26.3%) 2 (10.5%) 1 (5.3%) 13 | 53 (60.2%) 25 (28.4%) 9 (10.2%) 1 (1.1%) 6 |
| Metastatic sites | liver only extrahepatic only liver + extrahepatic | 15 (46.9%) 2 (6.3%) 15 (46.9%) | 39 (41.5%) 8 (8.5%) 47 (50.0%) |
| LDH | LDH ≤ ULN LDH 1-2× ULN LDH > 2× ULN unknown | 4 (25.0%) 6 (37.5%) 6 (37.5%) 16 | 34 (38.2%) 33 (37.1%) 22 (24.7%) 5 |
| Time from primary diagnosis to metastatic disease | <1 year 1–3 years >3 years | 3 (9.4%) 10 (31.3%) 19 (59.4%) | 22 (23.4%) 27 (28.7%) 45 (47.9%) |
| Time to start systemic treatment * | <6 months 6–12 months >12 months | 30 (96.7%) 1 (3.2%) 0 (0%) | 75 (86.2%) 7 (8.0%) 5 (5.7%) |
| Temozolomide n = 32 (%) | Ipilimumab n = 24 (%) | Pembrolizumab n = 43 (%) | Ipilimumab/Nivolumab n = 19 (%) | |
|---|---|---|---|---|
| CR | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| PR | 0 (0%) | 0 (0%) | 3 (7.0%) | 4 (21.1%) |
| SD ≥ 24 weeks | 2 (6.3%) | 6 (25.0%) | 12 (27.9%) | 2 (10.5%) |
| PD | 30 (93.4%) | 18 (75.0%) | 28 (65.1%) | 13 (68.4%) |
| PFS, median | 2.5 | 3.0 | 4.8 | 3.7 |
| 6-month PFS rate | 3.1% | 16.7% | 32.6% | 31.6% |
| OS, median | 5.7 | 9.9 | 10.3 | 18.9 |
| 1-year OS rate | 18.8% | 50.0% | 38.7% | 57.6% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bol, K.F.; Ellebaek, E.; Hoejberg, L.; Bagger, M.M.; Larsen, M.S.; Klausen, T.W.; Køhler, U.H.; Schmidt, H.; Bastholt, L.; Kiilgaard, J.F.; et al. Real-World Impact of Immune Checkpoint Inhibitors in Metastatic Uveal Melanoma. Cancers 2019, 11, 1489. https://doi.org/10.3390/cancers11101489
Bol KF, Ellebaek E, Hoejberg L, Bagger MM, Larsen MS, Klausen TW, Køhler UH, Schmidt H, Bastholt L, Kiilgaard JF, et al. Real-World Impact of Immune Checkpoint Inhibitors in Metastatic Uveal Melanoma. Cancers. 2019; 11(10):1489. https://doi.org/10.3390/cancers11101489
Chicago/Turabian StyleBol, Kalijn Fredrike, Eva Ellebaek, Lise Hoejberg, Mette Marie Bagger, Mathilde Skaarup Larsen, Tobias Wirenfeldt Klausen, Ulrich Heide Køhler, Henrik Schmidt, Lars Bastholt, Jens Folke Kiilgaard, and et al. 2019. "Real-World Impact of Immune Checkpoint Inhibitors in Metastatic Uveal Melanoma" Cancers 11, no. 10: 1489. https://doi.org/10.3390/cancers11101489
APA StyleBol, K. F., Ellebaek, E., Hoejberg, L., Bagger, M. M., Larsen, M. S., Klausen, T. W., Køhler, U. H., Schmidt, H., Bastholt, L., Kiilgaard, J. F., Donia, M., & Svane, I. M. (2019). Real-World Impact of Immune Checkpoint Inhibitors in Metastatic Uveal Melanoma. Cancers, 11(10), 1489. https://doi.org/10.3390/cancers11101489







