Protein Expression Profiling Identifies Key Proteins and Pathways Involved in Growth Inhibitory Effects Exerted by Guggulsterone in Human Colorectal Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Diffeential Inhibition of Cell Proliferation by GS in HCT 116 and SW620 Cell Lines
2.2. Proteomic Profiling of GS Treated HCT 116 Cells
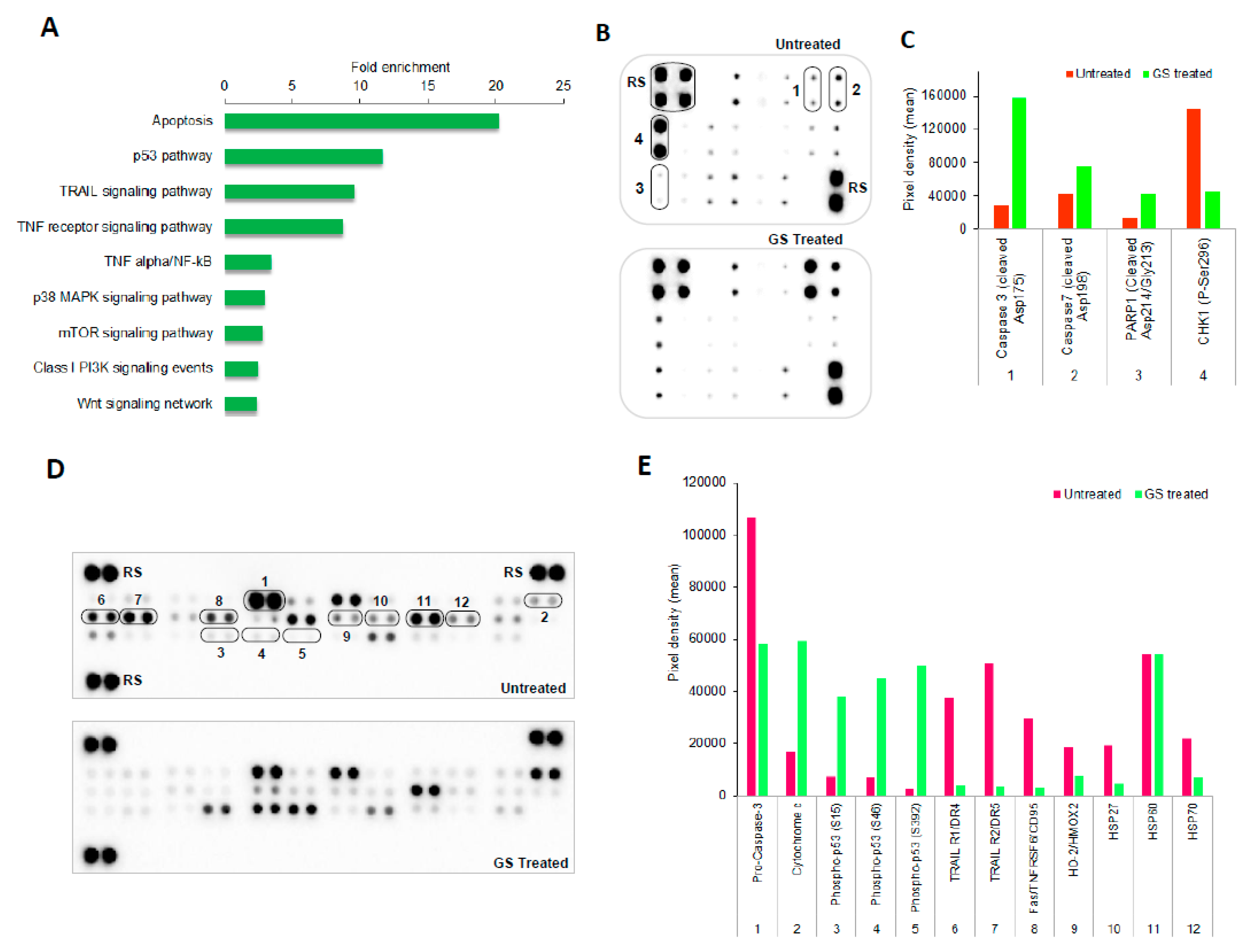
2.3. Functional Annotation of Dysregulated Proteins
2.4. Proteomic Signatures of GS Treated HCT 116 Cells
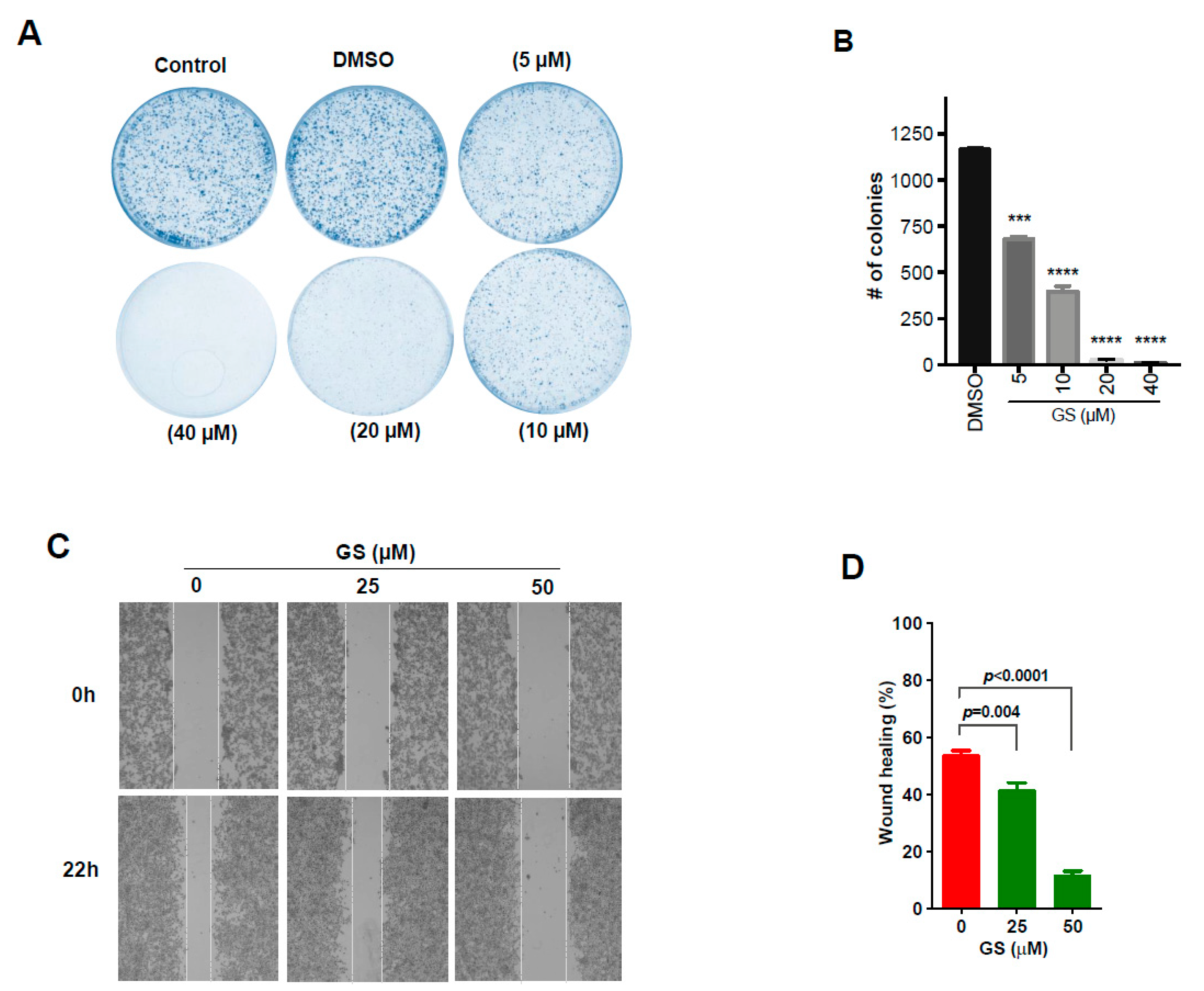
2.5. GS Treatment Reduced Cell Proliferation and Migration in CRC Cells
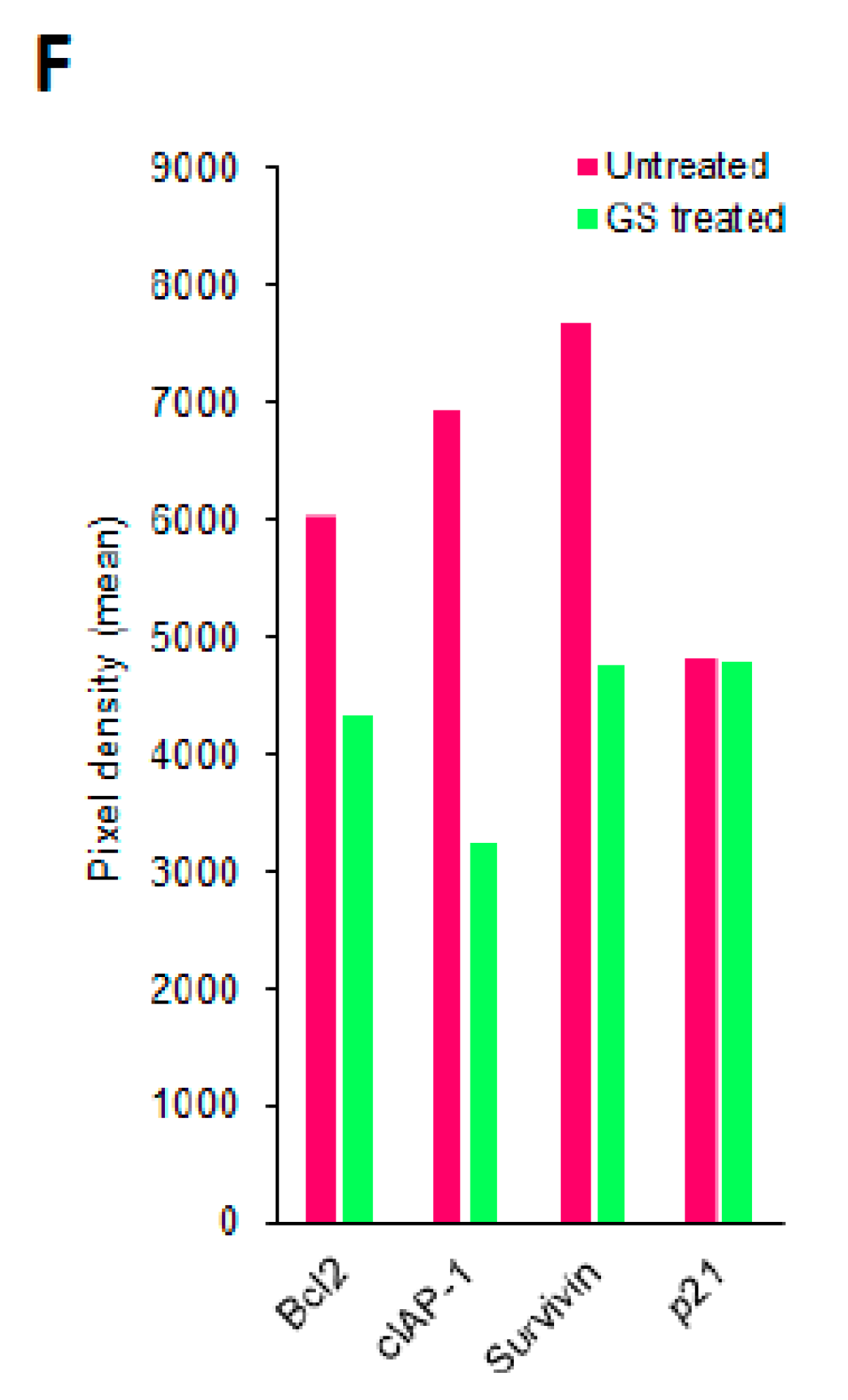
2.6. Pathways Enriched in GS Treated HCT 116 Cells and Their Validation by Protein Arrays
2.7. GS Treatment Induced Apoptosis in HCT 116 Cells
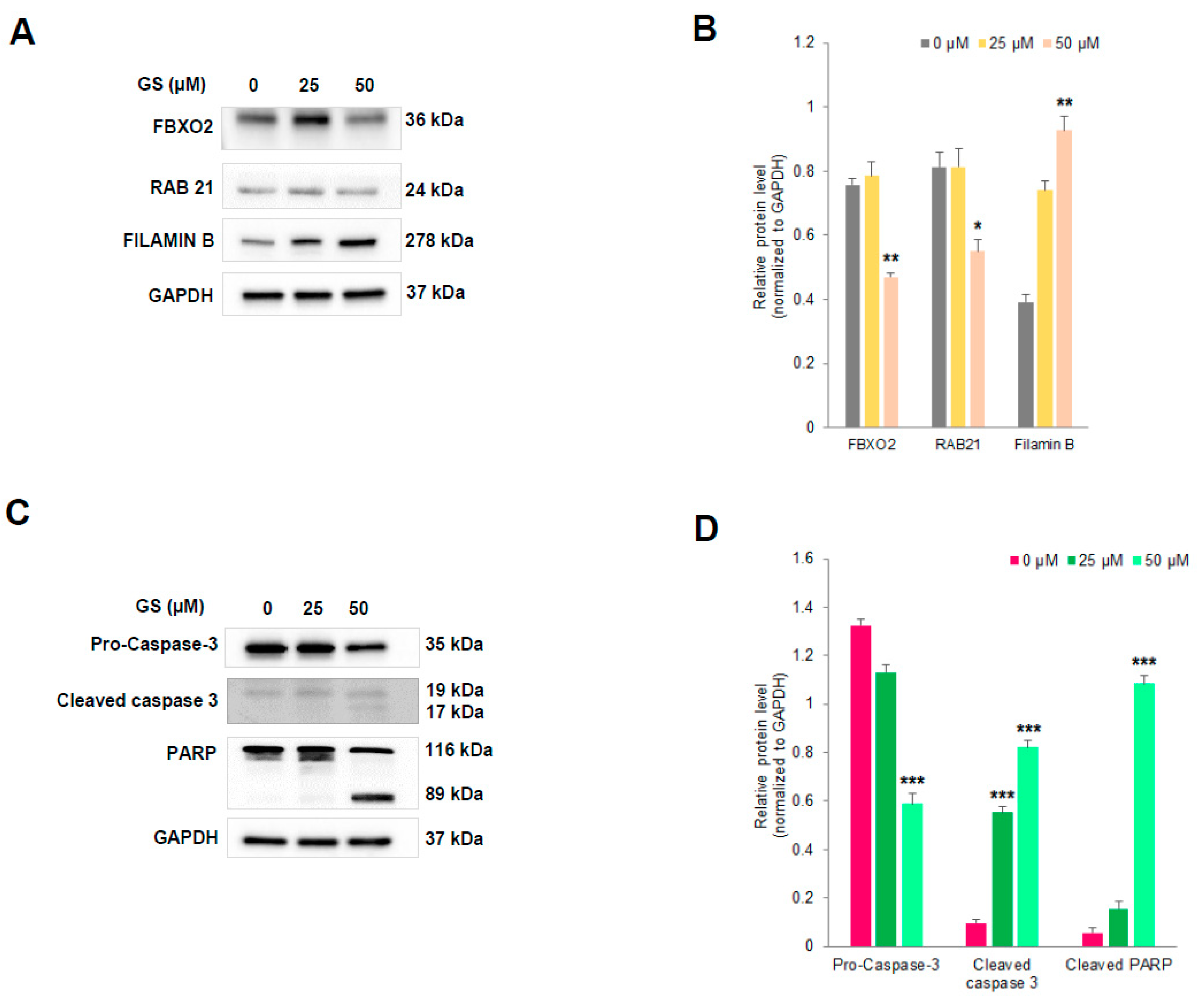
2.8. Validation of Proteomic Signatures and Key Proteins From Antibody Array by Western Blot Anlaysis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Lines and Culture Conditions
4.3. Cell Viability
4.4. Morphological Changes
4.5. Experimental Design
4.6. Sample Preparation for Proteomics
4.7. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analysis
4.8. MS Data Processing and Analysis
4.9. Clonogenic Assay
4.10. Wound Healing Assay
4.11. Protein Array Analysis
4.12. Apoptosis Assay by Annexin V/PI Staining
4.13. Cell Cycle Analysis
4.14. Western Blot Analysis
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Chin, S.-F.; Low, T.Y.; Jamal, R. Probing the colorectal cancer proteome for biomarkers: Current status and perspectives. J. Proteom. 2018, 187, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Favoriti, P.; Carbone, G.; Greco, M.; Pirozzi, F.; Pirozzi, R.E.M.; Corcione, F. Worldwide burden of colorectal cancer: A review. Updates Surg. 2016, 68, 7–11. [Google Scholar] [CrossRef]
- Yamada, T.; Sugimoto, K. Guggulsterone and Its Role in Chronic Diseases. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; Volume 929, pp. 329–361. [Google Scholar]
- Shishodia, S.; Harikumar, K.B.; Dass, S.; Ramawat, K.G.; Aggarwal, B.B. The guggul for chronic diseases: Ancient medicine, modern targets. Anticancer Res. 2008, 28, 3647–3664. [Google Scholar]
- Singh, S.V.; Zeng, Y.; Xiao, D.; Vogel, V.G.; Nelson, J.B.; Dhir, R.; Tripathi, Y.B. Caspase-dependent apoptosis induction by guggulsterone, a constituent of Ayurvedic medicinal plant Commiphora mukul, in PC-3 human prostate cancer cells is mediated by Bax and Bak. Mol. Cancer Ther. 2005, 4, 1747–1754. [Google Scholar] [CrossRef]
- Shishodia, S.; Sethi, G.; Ahn, K.S.; Aggarwal, B.B. Guggulsterone inhibits tumor cell proliferation, induces S-phase arrest, and promotes apoptosis through activation of c-Jun N-terminal kinase, suppression of Akt pathway, and downregulation of antiapoptotic gene products. Biochem. Pharmacol. 2007, 74, 118–130. [Google Scholar] [CrossRef]
- Cui, J.; Huang, L.; Zhao, A.; Lew, J.L.; Yu, J.; Sahoo, S.; Meinke, P.T.; Royo, I.; Peláez, F.; Wright, S.D. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J. Biol. Chem. 2003, 278, 10214–10220. [Google Scholar] [CrossRef]
- Mazzanti, R.; Solazzo, M.; Fantappié, O.; Elfering, S.; Pantaleo, P.; Bechi, P.; Cianchi, F.; Ettl, A.; Giulivi, C. Differential expression proteomics of human colon cancer. Am. J. Physiol. Liver Physiol. 2006, 290, G1329–G1338. [Google Scholar] [CrossRef]
- Gygi, S.P.; Rochon, Y.; Franza, B.R.; Aebersold, R. Correlation between Protein and mRNA Abundance in Yeast. Mol. Cell. Biol. 1999, 19, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Seilhamer, J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis 1997, 18, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Sanchez, J.-C.; Gooley, A.A.; Appel, R.D.; Humphery-Smith, I.; Hochstrasser, D.F.; Williams, K.L. Progress with Proteome Projects: Why all Proteins Expressed by a Genome Should be Identified and How To Do It. Biotechnol. Genet. Eng. Rev. 1996, 13, 19–50. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Aguilar, J.; Chik, J.; Nicholson, J.; Semaan, C.; McKay, M.J.; Molloy, M.P. Quantitative mass spectrometry for colorectal cancer proteomics. Proteom.-Clin. Appl. 2013, 7, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Suárez, E.; Whetton, A.D. The application of quantification techniques in proteomics for biomedical research. Mass Spectrom. Rev. 2013, 32, 1–26. [Google Scholar] [CrossRef] [PubMed]
- An, M.J.; Cheon, J.H.; Kim, S.W.; Kim, E.S.; Kim, T.I.; Kim, W.H. Guggulsterone induces apoptosis in colon cancer cells and inhibits tumor growth in murine colorectal cancer xenografts. Cancer Lett. 2009, 279, 93–100. [Google Scholar] [CrossRef]
- Nishio, K.; Yoshida, Y.; Tanaka, K.; Mizushima, T. Structural analysis of a function-associated loop mutant of the substrate-recognition domain of Fbs1 ubiquitin ligase. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2016, 72, 619–626. [Google Scholar] [CrossRef]
- Wei, X.; Bu, J.; Mo, X.; Lv, B.; Wang, X.; Hou, B. The prognostic significance of FBXO2 expression in colorectal cancer. Int. J. Clin. Exp. Pathol. 2018, 11, 5054–5062. [Google Scholar]
- Zhang, Z.; Chang, Y.; Zhang, J.; Lu, Y.; Zheng, L.; Hu, Y.; Zhang, F.; Li, X.; Zhang, W.; Li, X. HMGB3 promotes growth and migration in colorectal cancer by regulating WNT/β-catenin pathway. PLoS ONE 2017, 12, e0179741. [Google Scholar] [CrossRef]
- Pellinen, T.; Arjonen, A.; Vuoriluoto, K.; Kallio, K.; Fransen, J.A.M.; Ivaska, J. Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of β1-integrins. J. Cell Biol. 2006, 173, 767–780. [Google Scholar] [CrossRef]
- Lisanti, M.P.; Scherer, P.E.; Tang, Z.; Sargiacomo, M. Caveolae, caveolin and caveolin-rich membrane domains: A signalling hypothesis. Trends Cell Biol. 1994, 4, 231–235. [Google Scholar] [CrossRef]
- Fu, P.; Chen, F.-C.; Pan, Q.; Zhao, X.; Zhao, C.; Cho, W.; Chen, H. The different functions and clinical significances of caveolin-1 in human adenocarcinoma and squamous cell carcinoma. Onco Targets Ther. 2017, 10, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Goetz, J.G.; Lajoie, P.; Wiseman, S.M.; Nabi, I.R. Caveolin-1 in tumor progression: The good, the bad and the ugly. Cancer Metastasis Rev. 2008, 27, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Fine, S.W.; Lisanti, M.P.; Galbiati, F.; Li, M. Elevated Expression of Caveolin-1 in Adenocarcinoma of the Colon. Am. J. Clin. Pathol. 2001, 115, 719–724. [Google Scholar] [CrossRef]
- Patlolla, J.; Swamy, M.; Raju, J.; Rao, C. Overexpression of caveolin-1 in experimental colon adenocarcinomas and human colon cancer cell lines. Oncol. Rep. 2004, 11, 957–963. [Google Scholar] [CrossRef]
- Kau, T.R.; Way, J.C.; Silver, P.A. Nuclear transport and cancer: From mechanism to intervention. Nat. Rev. Cancer 2004, 4, 106–117. [Google Scholar] [CrossRef]
- Christiansen, A.; Dyrskjøt, L. The functional role of the novel biomarker karyopherin α 2 (KPNA2) in cancer. Cancer Lett. 2013, 331, 18–23. [Google Scholar] [CrossRef]
- Yu, L.; Wang, G.; Zhang, Q.; Gao, L.; Huang, R.; Chen, Y.; Tang, Q.; Liu, J.; Liu, C.; Wang, H.; et al. Karyopherin alpha 2 expression is a novel diagnostic and prognostic factor for colorectal cancer. Oncol. Lett. 2017, 13, 1194–1200. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Yu, F.; Lu, S.; Sun, H.; Tang, H.; Peng, Z. Karyopherin alpha 2 is a novel prognostic marker and a potential therapeutic target for colon cancer. J. Exp. Clin. Cancer Res. 2015, 34, 145. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, H.; Ban, S.; Oh, S.; Ji, S.; Kim, D.; Ahn, T.S.; Kim, H.J.; Bae, S.B.; Kwon, H.Y.; et al. Karyopherin α-2 is a reliable marker for identification of patients with high-risk stage II colorectal cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 2493–2503. [Google Scholar] [CrossRef]
- Zhao, Q.; Rank, G.; Tan, Y.T.; Li, H.; Moritz, R.L.; Simpson, R.J.; Cerruti, L.; Curtis, D.J.; Patel, D.J.; Allis, C.D.; et al. PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat. Struct. Mol. Biol. 2009, 16, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Dong, S.; Zhu, R.; Hu, C.; Hou, J.; Li, Y.; Zhao, Q.; Shao, X.; Bu, Q.; Li, H.; et al. Targeting protein arginine methyltransferase 5 inhibits colorectal cancer growth by decreasing arginine methylation of eIF4E and FGFR3. Oncotarget 2015, 6, 22799–22811. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, L.; Wei, H.; Chen, L.; Demir, Ö.; Sandusky, G.; Sun, E.; Wang, J.; Mo, J.; Zeng, L.; Fishel, M.; et al. Adapting AlphaLISA high throughput screen to discover a novel small-molecule inhibitor targeting protein arginine methyltransferase 5 in pancreatic and colorectal cancers. Oncotarget 2017, 8, 39963–39977. [Google Scholar] [CrossRef] [PubMed]
- Furihata, T.; Kishida, S.; Sugiura, H.; Kamiichi, A.; Iikura, M.; Chiba, K. Functional Analysis of Purine Nucleoside Phosphorylase as a Key Enzyme in Ribavirin Metabolism. Drug Metab. Pharmacokinet. 2014, 29, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Bantia, S.; Ananth, S.L.; Parker, C.D.; Horn, L.L.; Upshaw, R. Mechanism of inhibition of T-acute lymphoblastic leukemia cells by PNP inhibitor—BCX-1777. Int. Immunopharmacol. 2003, 3, 879–887. [Google Scholar] [CrossRef]
- Ravandi, F.; Gandhi, V. Novel purine nucleoside analogues for T-cell-lineage acute lymphoblastic leukaemia and lymphoma. Expert Opin. Investig. Drugs 2006, 15, 1601–1613. [Google Scholar] [CrossRef]
- Kojima, S.; Chiyomaru, T.; Kawakami, K.; Yoshino, H.; Enokida, H.; Nohata, N.; Fuse, M.; Ichikawa, T.; Naya, Y.; Nakagawa, M.; et al. Tumour suppressors miR-1 and miR-133a target the oncogenic function of purine nucleoside phosphorylase (PNP) in prostate cancer. Br. J. Cancer 2012, 106, 405–413. [Google Scholar] [CrossRef]
- Chen, H.; Xia, M.; Lin, M.; Yang, H.; Kuhlenkamp, J.; Li, T.; Sodir, N.M.; Chen, Y.; Josef–Lenz, H.; Laird, P.W.; et al. Role of Methionine Adenosyltransferase 2A and S-adenosylmethionine in Mitogen-Induced Growth of Human Colon Cancer Cells. Gastroenterology 2007, 133, 207–218. [Google Scholar] [CrossRef]
- Dou, N.; Yang, D.; Yu, S.; Wu, B.; Gao, Y.; Li, Y. SNRPA enhances tumour cell growth in gastric cancer through modulating NGF expression. Cell Prolif. 2018, 51, e12484. [Google Scholar] [CrossRef]
- Zhuang, L.; Yang, Z.; Meng, Z. Upregulation of BUB1B, CCNB1, CDC7, CDC20, and MCM3 in Tumor Tissues Predicted Worse Overall Survival and Disease-Free Survival in Hepatocellular Carcinoma Patients. BioMed Res. Int. 2018, 2018, 7897346. [Google Scholar] [CrossRef]
- Wang, J.; Yang, T.; Chen, H.; Li, H.; Zheng, S. Oncogene RPA1 promotes proliferation of hepatocellular carcinoma via CDK4/Cyclin-D pathway. Biochem. Biophys. Res. Commun. 2018, 498, 424–430. [Google Scholar] [CrossRef]
- Kuijpers, G.A.J.; Lee, G.; Pollard, H.B. Immunolocalization of synexin (annexin VII) in adrenal chromaffin granules and chromaffin cells: Evidence for a dynamic role in the secretory process. Cell Tissue Res. 1992, 269, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Naidu, D.G.; Raha, A.; Chen, X.-L.; Spitzer, A.R.; Chander, A. Partial truncation of the NH2-terminus affects physical characteristics and membrane binding, aggregation, and fusion properties of annexin A7. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2005, 1734, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M. Prognostic Impact of ANX7-GTPase in Metastatic and HER2-Negative Breast Cancer Patients. Clin. Cancer Res. 2004, 10, 2344–2350. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duncan, R.; Carpenter, B.; Main, L.C.; Telfer, C.; Murray, G.I. Characterisation and protein expression profiling of annexins in colorectal cancer. Br. J. Cancer 2008, 98, 426–433. [Google Scholar] [CrossRef]
- Srivastava, M.; Bubendorf, L.; Srikantan, V.; Fossom, L.; Nolan, L.; Glasman, M.; Leighton, X.; Fehrle, W.; Pittaluga, S.; Raffeld, M.; et al. ANX7, a candidate tumor suppressor gene for prostate cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 4575–4580. [Google Scholar] [CrossRef]
- Ye, W.; Li, Y.; Fan, L.; Zhao, Q.; Yuan, H.; Tan, B.; Zhang, Z. Annexin A7 expression is downregulated in late-stage gastric cancer and is negatively correlated with the differentiation grade and apoptosis rate. Oncol. Lett. 2018, 15, 9836–9844. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Nair, P.; Lu, M.; Petersen, S.; Ashkenazi, A. Apoptosis Initiation Through the Cell-Extrinsic Pathway. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 544, pp. 99–128. [Google Scholar]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial Membrane Permeabilization in Cell Death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Strasser, A.; Harris, A.W.; Huang, D.C.; Krammer, P.H.; Cory, S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995, 14, 6136–6147. [Google Scholar] [CrossRef]
- Green, D.R. Apoptotic Pathways: Ten Minutes to Dead. Cell 2005, 121, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y. The Role of p21 in Apoptosis, Proliferation, Cell Cycle Arrest, and Antioxidant Activity in UVB-Irradiated Human HaCaT Keratinocytes. Med. Sci. Monit. Basic Res. 2015, 21, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Rappsilber, J.; Mann, M.; Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007, 2, 1896–1906. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A Peptide Search Engine Integrated into the MaxQuant Environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate Proteome-wide Label-free Quantification by Delayed Normalization and Maximal Peptide Ratio Extraction, Termed MaxLFQ. Mol. Cell. Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Pathan, M.; Keerthikumar, S.; Ang, C.-S.; Gangoda, L.; Quek, C.Y.J.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Rafehi, H.; Orlowski, C.; Georgiadis, G.T.; Ververis, K.; El-Osta, A.; Karagiannis, T.C. Clonogenic Assay: Adherent Cells. J. Vis. Exp. 2011, 49, e2573. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, G. Dot Blot Analyzer: Software development using the macro language of ImageJ. In Proceedings of the ImageJ User and Developer Conference; Centre de Recherche Public Henri Tudor: Esch-sur-Alzette, Luxembourg, 2008; Volume 189, pp. 3–5. ISBN 2-919941-06-2. [Google Scholar]
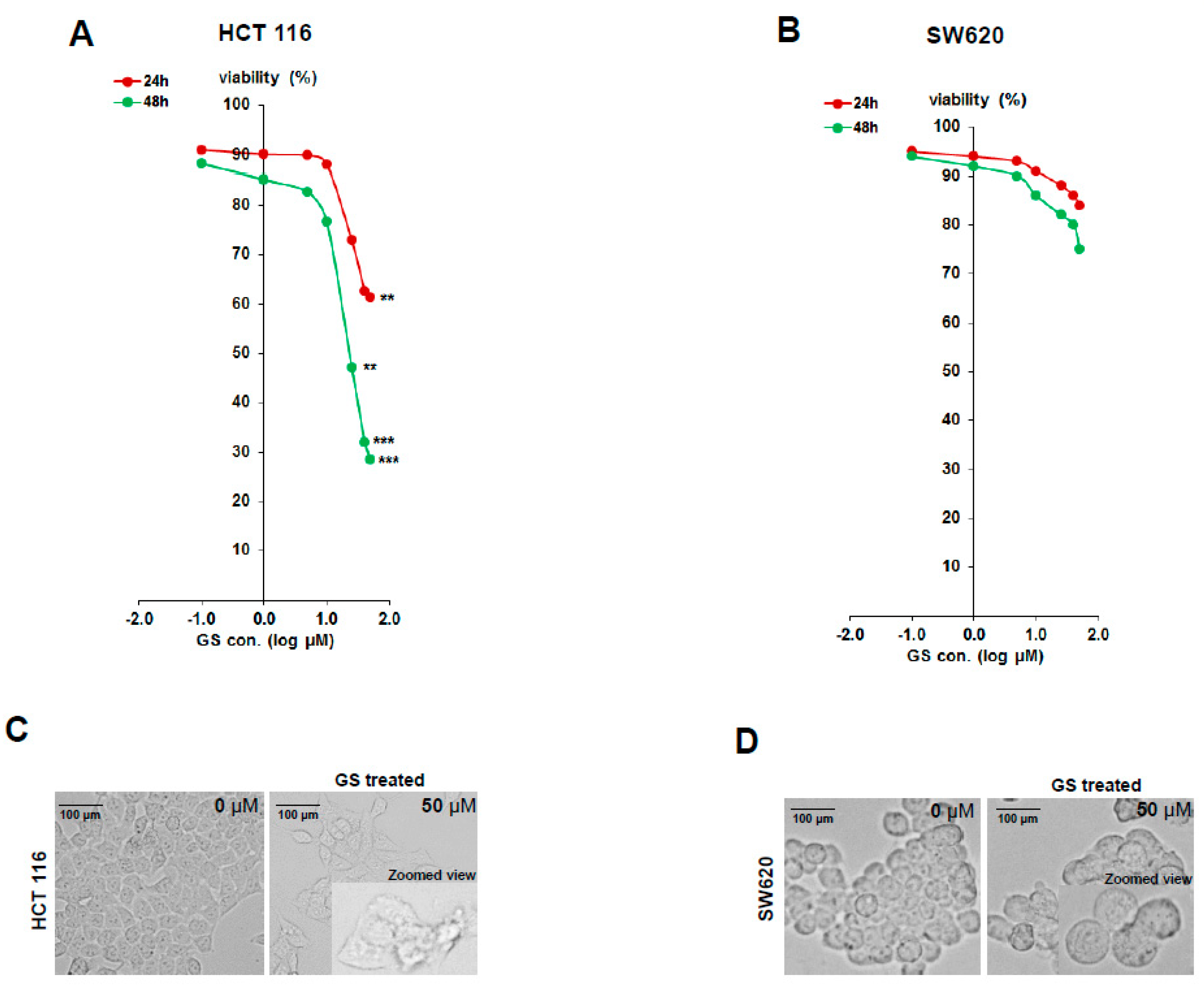
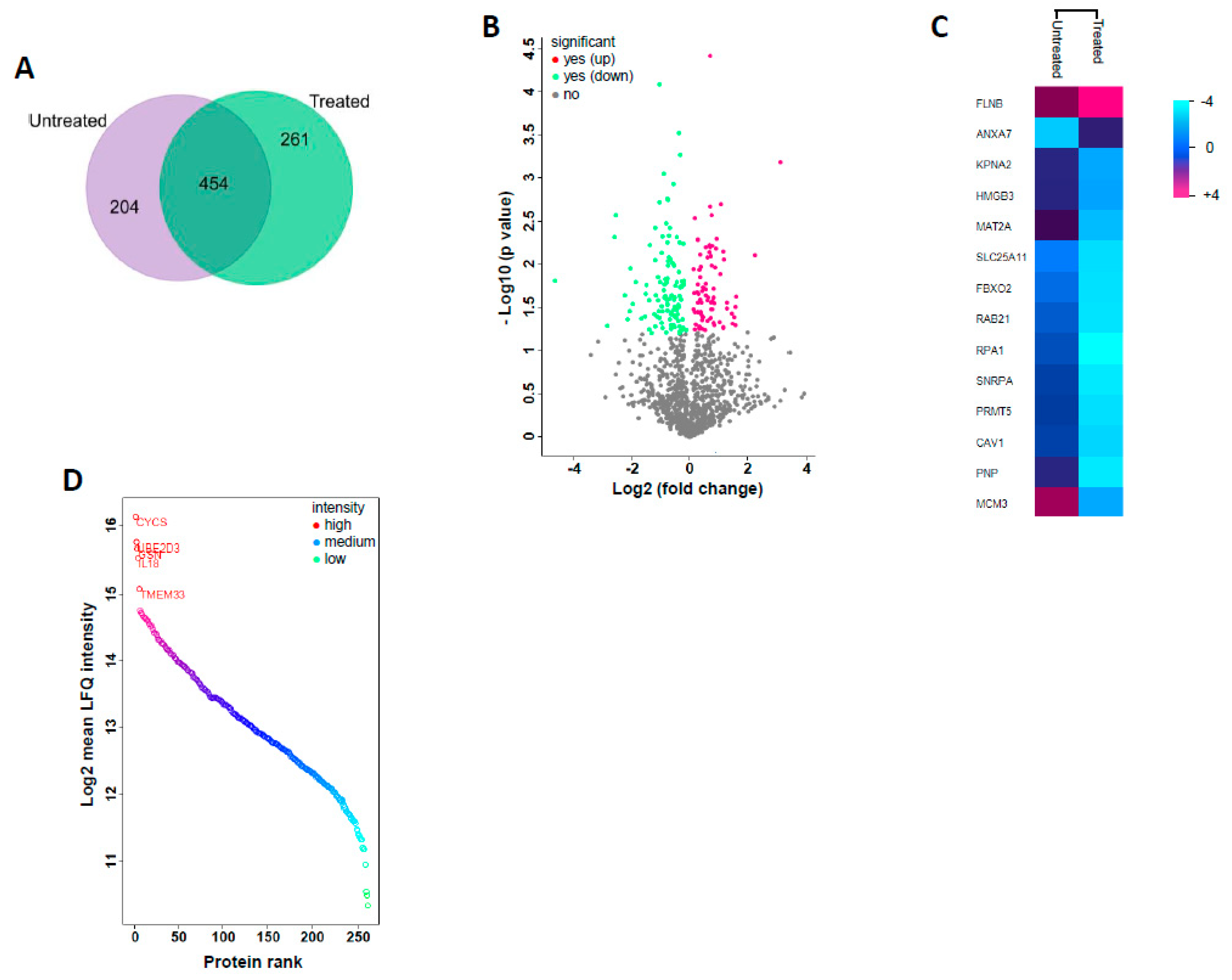
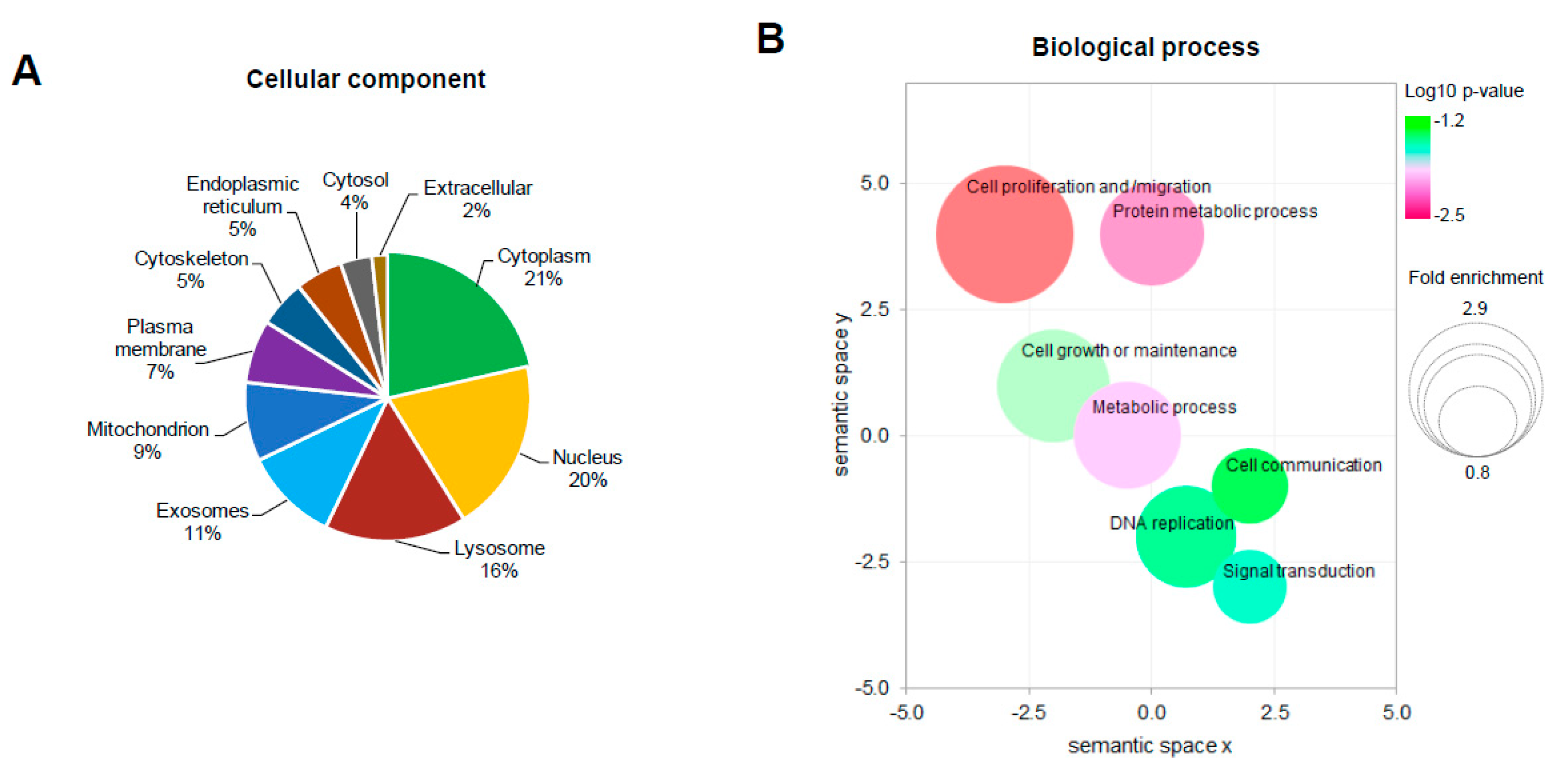
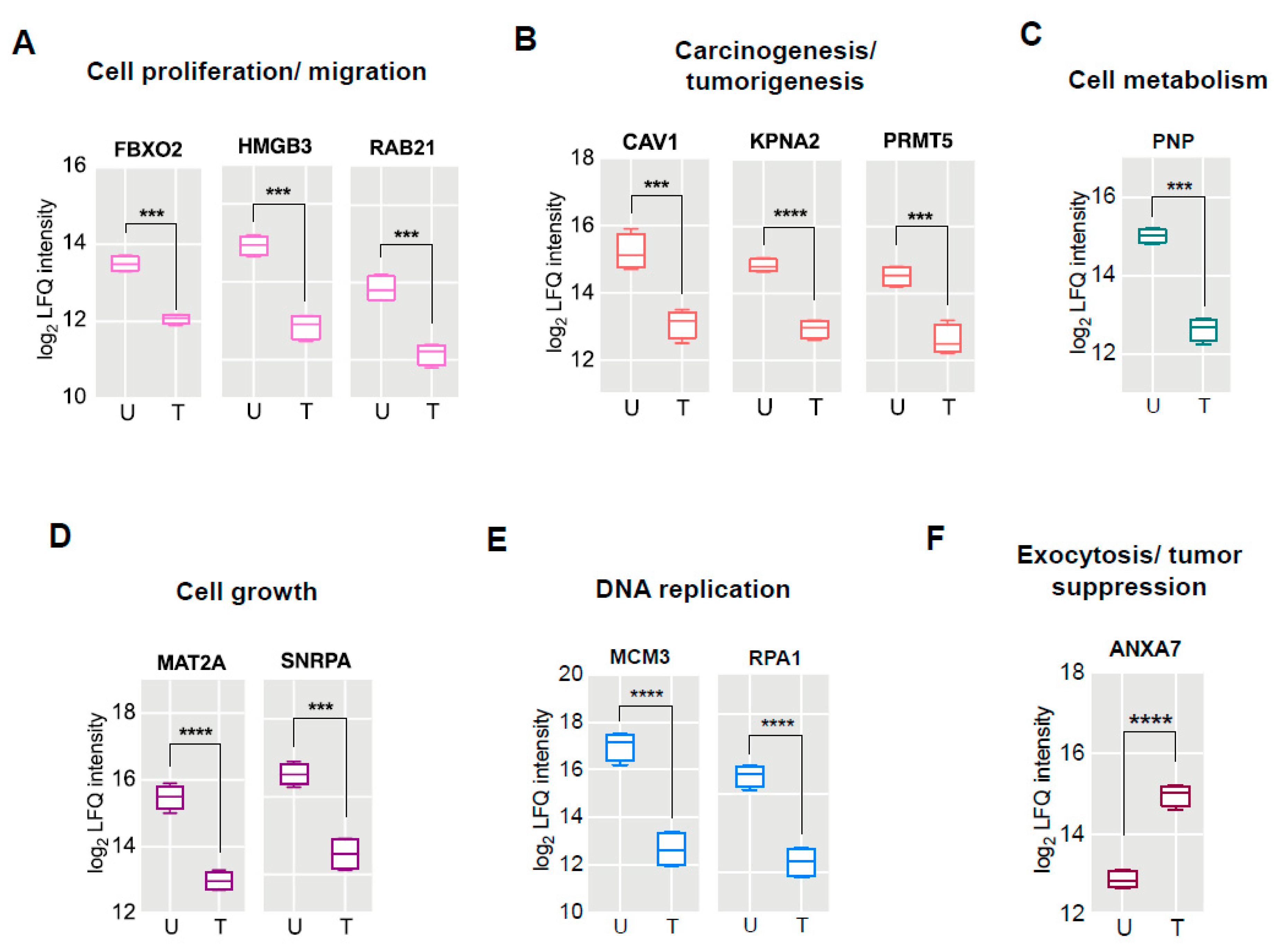

| Uniprot Accession | Gene Symbol | Protein Name | Fold Change | p-Value (−log10) |
|---|---|---|---|---|
| R4GNH2 | FBXO2 | F-box only protein 2 | −1.5 | 1.6580 |
| I3L1P8 | SLC25A11 | Mitochondrial 2-oxoglutarate/malate carrier protein | −1.5 | 1.3996 |
| Q9UL25 | RAB21 | Ras-related protein Rab-21 | −1.7 | 1.3732 |
| P52292 | KPNA2 | Importin subunit alpha-1 | −1.9 | 1.8005 |
| M0R0G9 | SNRPA | U1 small nuclear ribonucleoprotein A | −2.0 | 1.5435 |
| O14744 | PRMT5 | Protein arginine N-methyltransferase 5 | −2.0 | 1.9611 |
| E9PES6 | HMGB3 | High mobility group protein B3 | −2.1 | 1.4630 |
| Q03135 | CAV1 | Caveolin-1 | −2.1 | 1.3701 |
| P27694 | RPA1 | Replication protein A 70 kDa DNA-binding subunit | −2.3 | 1.6471 |
| P31153 | MAT2A | S-adenosylmethionine synthase isoform type-2 | −2.5 | 2.5716 |
| P00491 | PNP | Purine nucleoside phosphorylase | −2.6 | 2.3222 |
| P25205 | MCM3 | DNA replication licensing factor MCM3 | −4.6 | 1.8156 |
| O75369 | FLNB | Filamin-B | 3.1 | 3.1921 |
| P20073 | ANXA7 | Annexin A7 | 2.3 | 2.1067 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leo, R.; Therachiyil, L.; Siveen, S.K.; Uddin, S.; Kulinski, M.; Buddenkotte, J.; Steinhoff, M.; Krishnankutty, R. Protein Expression Profiling Identifies Key Proteins and Pathways Involved in Growth Inhibitory Effects Exerted by Guggulsterone in Human Colorectal Cancer Cells. Cancers 2019, 11, 1478. https://doi.org/10.3390/cancers11101478
Leo R, Therachiyil L, Siveen SK, Uddin S, Kulinski M, Buddenkotte J, Steinhoff M, Krishnankutty R. Protein Expression Profiling Identifies Key Proteins and Pathways Involved in Growth Inhibitory Effects Exerted by Guggulsterone in Human Colorectal Cancer Cells. Cancers. 2019; 11(10):1478. https://doi.org/10.3390/cancers11101478
Chicago/Turabian StyleLeo, Rari, Lubna Therachiyil, Sivaraman K. Siveen, Shahab Uddin, Michal Kulinski, Joerg Buddenkotte, Martin Steinhoff, and Roopesh Krishnankutty. 2019. "Protein Expression Profiling Identifies Key Proteins and Pathways Involved in Growth Inhibitory Effects Exerted by Guggulsterone in Human Colorectal Cancer Cells" Cancers 11, no. 10: 1478. https://doi.org/10.3390/cancers11101478
APA StyleLeo, R., Therachiyil, L., Siveen, S. K., Uddin, S., Kulinski, M., Buddenkotte, J., Steinhoff, M., & Krishnankutty, R. (2019). Protein Expression Profiling Identifies Key Proteins and Pathways Involved in Growth Inhibitory Effects Exerted by Guggulsterone in Human Colorectal Cancer Cells. Cancers, 11(10), 1478. https://doi.org/10.3390/cancers11101478






