The Targeting of RNA Polymerase I Transcription Using CX-5461 in Combination with Radiation Enhances Tumour Cell Killing Effects in Human Solid Cancers
Abstract
1. Introduction
2. Results
2.1. A Panel of Solid Tumour Cell Lines Showed a Spectrum of Sensitivity Towards CX-5461
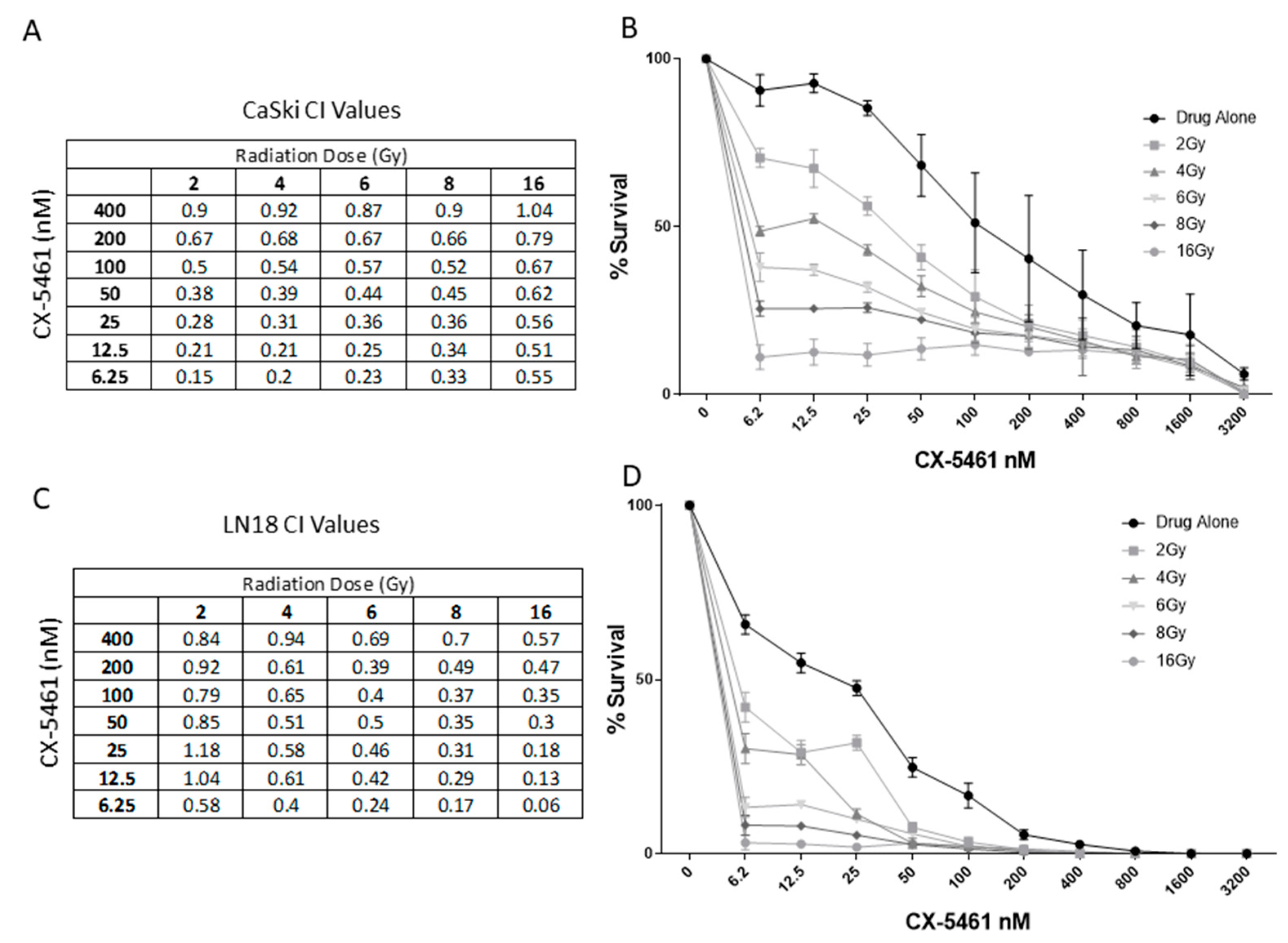
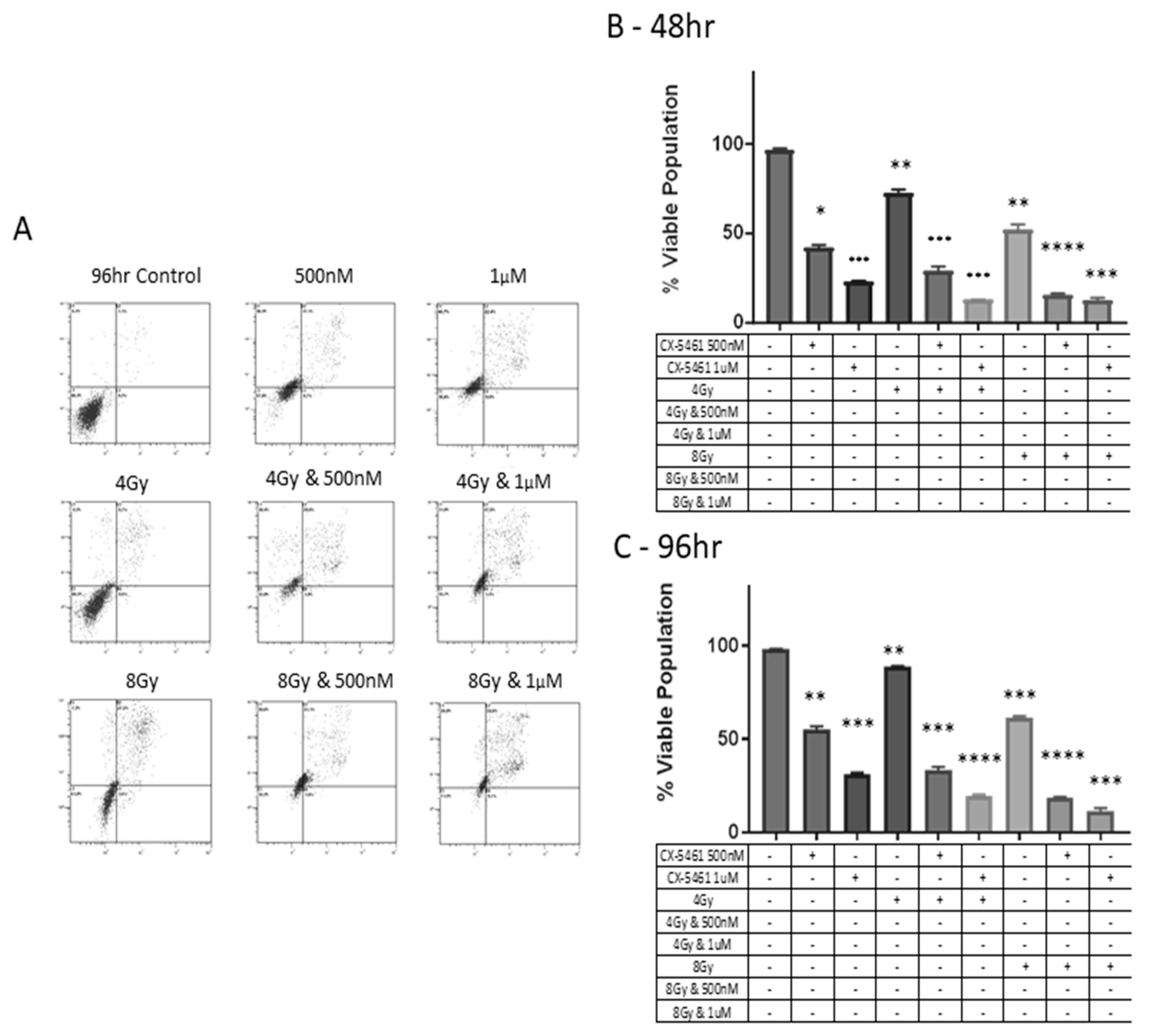
2.2. CX-5461 in Combination with X-rays Show Synergistic Interactions
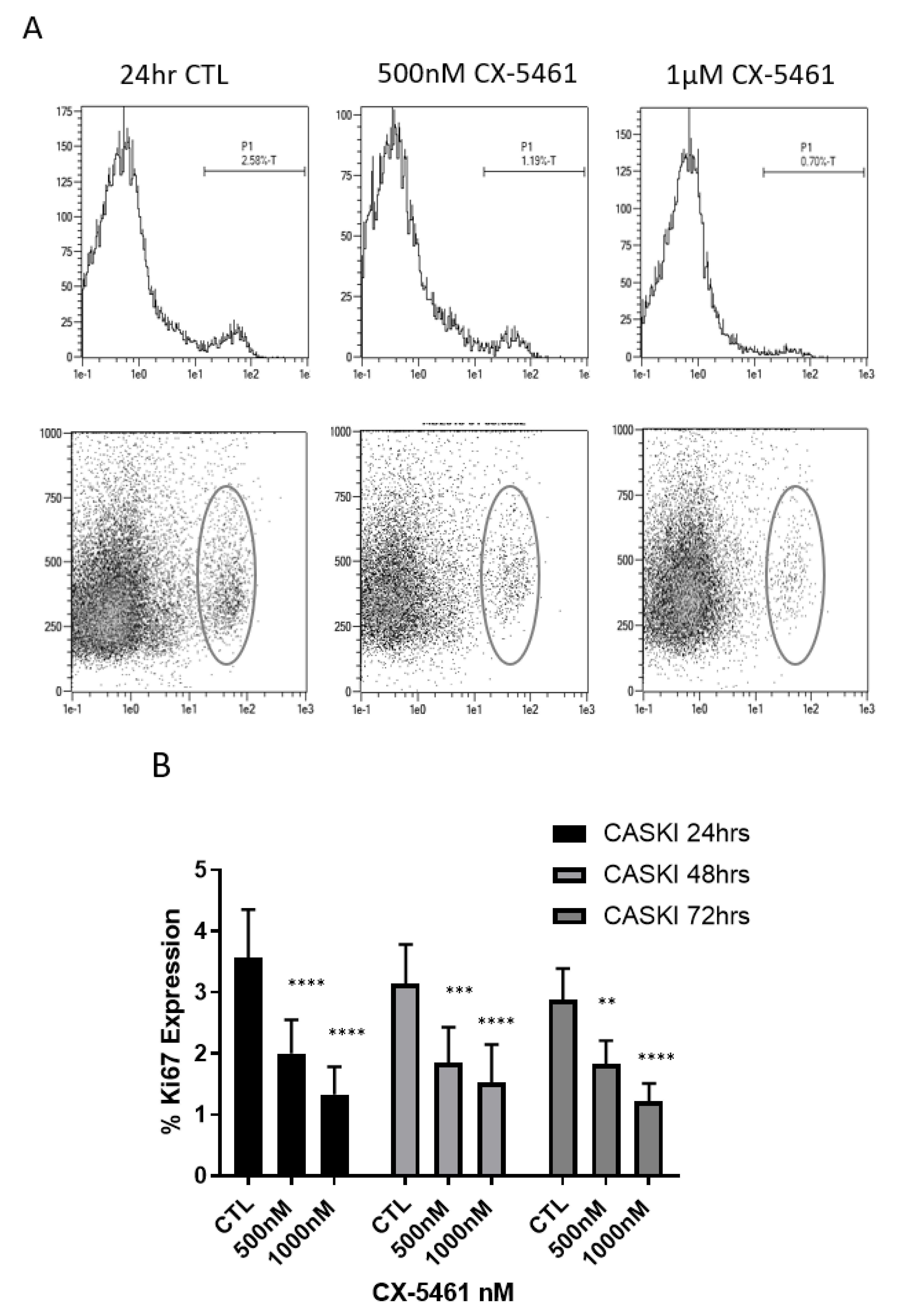
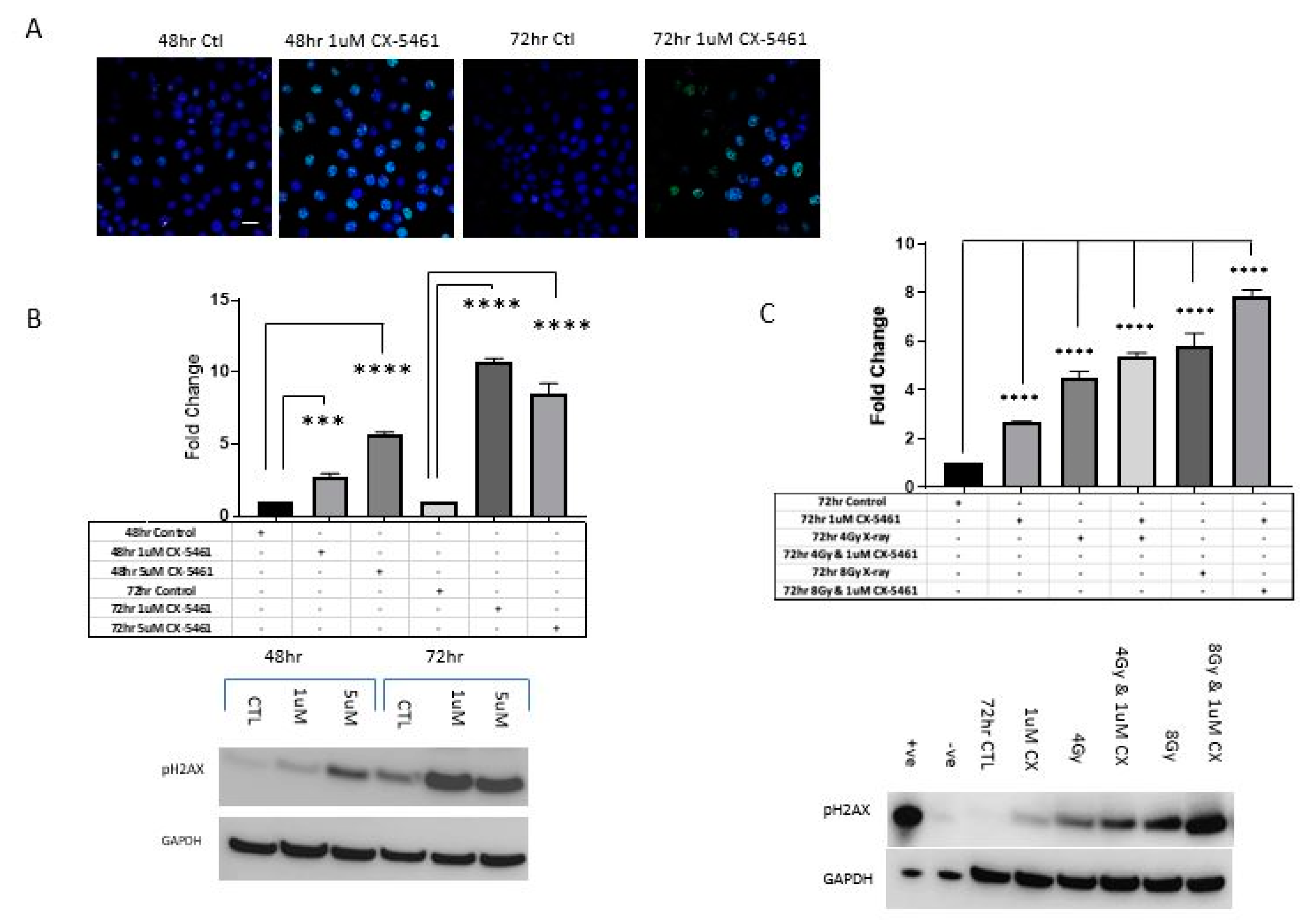
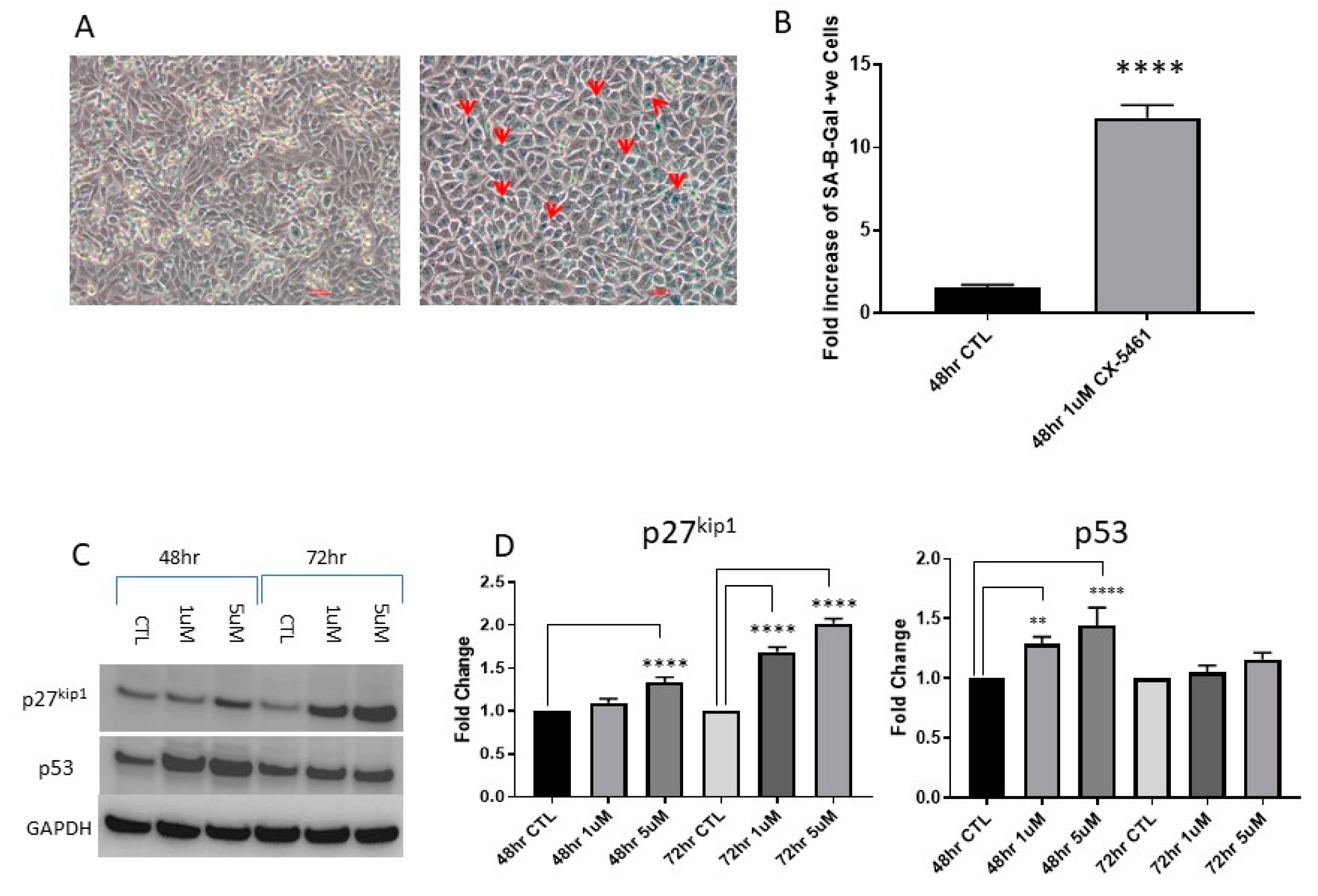
2.3. CX-5461 Induced Reduced Proliferation, Cell Death and DNA Damage
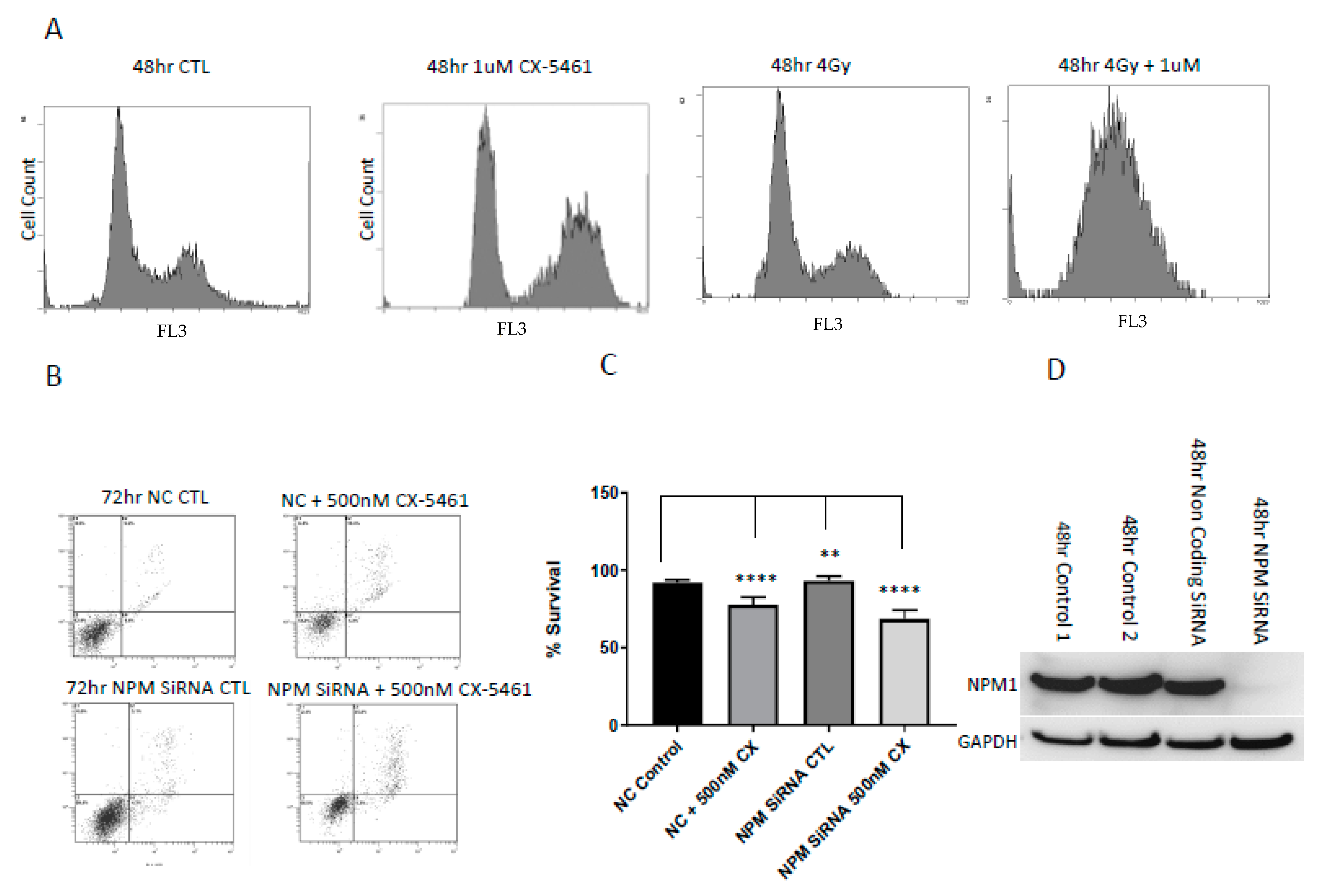
2.4. Cell Cycle Associated Effects Following CX-5461 Exposure
2.5. Low Levels of the Nucleolar Protein Nucleophosmin Enhances the Cell Death Response of Cancer Cells to the Effects of CX-5461
3. Discussion
4. Methods and Materials
4.1. Cell Lines and Reagents
4.2. Cell Viability Assay, Clonogenic Assay
4.3. Flow Cytometry Analysis
4.4. Western Blot Analysis
4.5. Quantitative Polymerase Chain Reaction
4.6. Transient NPM1 RNA Silencing
4.7. Ki-67 Expression Analysis
4.8. Immunofluorescence Staining of LC3 and γH2AX
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Boisvert, F.M.; van Koningsbruggen, S.; Navascués, J.; Lamond, A.I. The multifunctional nucleolus. Nat. Rev. Mol. Cell Biol. 2007, 8, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Drygin, D.; Rice, W.G.; Grummt, I. The RNA Polymerase I transcription machinery—an emerging target for the treatment of cancer. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Villicaña, C.; Cruz, G.; Zurita, M. The basal transcription machinery as a target for cancer therapy. Cancer Cell Int. 2014, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.; Lu, Y.J.; Fang, C.; Pritchard-Jones, K.; Shipley, J. Nascent pre-rRNA overexpression correlates with an adverse prognosis in alveolar rhabdomyosarcoma. Genes Chromosomes Cancer 2006, 45, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Derenzini, M.; Trere, D.; Pession, A.; Govoni, M.; Sirri, V.; Chieco, P. Nucleolar size indicates the rapidity of cell proliferation in cancer tissues. J. Pathol. 2000, 191, 181–186. [Google Scholar] [CrossRef]
- Burger, K.; Muhl, B.; Harasim, T.; Rohrmoser, M.; Malamoussi, A.; Orban, M.; Kellner, M.; Gruber-Eber, A.; Kremmer, E.; Hölzel, M.; et al. Chemotheraputic drugs inhibit ribosome biogenesis at various levels. J. Biol. Chem. 2010, 285, 12416–12425. [Google Scholar] [CrossRef] [PubMed]
- Drygin, D.; Lin, A.; Bliesath, J.; Ho, C.B.; O’Brien, S.E.; Proffitt, C.; Omori, M.; Haddach, M.; Schwaebe, M.K.; Siddiqui-Jain, A.; et al. Targeting RNA polymerase I with an oral small molecule CX-5461 inhibits ribosomal RNA synthesis and solid tumor growth. Cancer Res. 2011, 71, 1418–1430. [Google Scholar] [CrossRef] [PubMed]
- Bywater, M.J.; Poortinga, G.; Sanij, E.; Hein, N.; Peck, A.; Cullinane, C.; Wall, M.; Cluse, L.; Drygin, D.; Anderes, K.; et al. Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer specific activation of p53. Cancer Cell 2012, 22, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Zhao, J.; Fan, S.; Wang, L.; Li, X. CX-5461 induces autophagy and inhibits tumor growth via mammalian target of rapamycin-related signaling pathways in osteosarcoma. Onco. Targets Ther. 2016, 9, 5985–5997. [Google Scholar] [CrossRef] [PubMed]
- Hein, N.; Bywater, M.J.; Stanley, K.; Verbrugge, I.; Cullinane, C.; Baker, A.; Zuber, H.; Rappaport, A.; Drygin, D.; Huser, N.; et al. Inhibition of RNA Polymerase I Transcription by CX-5461 As a Therapeutic Strategy for the Cancer Specific Activation of p53 in MLL-Reaaranged Acute Myeloid Leukemias. Blood 2011, 118, 1548. [Google Scholar]
- Pradier, O.; Rave-Fränk, M.; Lehmann, J.; Lücke, E.; Boghun, O.; Hess, C.F.; Schmidberger, H. Effects of docetaxel in combination with radiation on human head and neck cancer cells (ZMK-1) and cervical squamous cell carcinoma cells (CaSki). Int. J. Cancer 2001, 91, 840–845. [Google Scholar] [CrossRef]
- Koivusalo, R.; Krausz, E.; Ruotsalainen, P.; Helenius, H.; Hietanen, S. Chemoradiation of Cervical Cancer Cells-Targeting Human Papillomavirus E6 and p53 Leads to Either Augmented or Attenuated Apoptosis Depending on the Platinum Carrier Ligand. Cancer Res. 2002, 62, 7364–7371. [Google Scholar] [PubMed]
- Lee, C.M.; Fuhrman, C.B.; Planelles, V.; Peltier, M.R.; Gaffney, D.K.; Soisson, A.P.; Dodson, M.K.; Tolley, H.D.; Green, C.L.; Zempolich, K.A. Phosphatidylinositol 3-kinase inhibition by LY294002 radiosensitizes human cervical cancer cell lines. Clin. Cancer Res. 2006, 12, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Erkin, O.C.; Kwon, M.J.; Kim, S.H.; Jung, J.I.; Oh, Y.K.; Her, S.W.; Ju, W.; Choi, Y.L.; Song, S.Y.; et al. The synergistic therapeutic effect of cisplatin with Human papillomavirus E6/E7 short interfering RNA on cervical cancer cell lines in vitro and in vivo. Int. J. Cancer 2012, 130, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Cornelison, R.; Dobbin, Z.C.; Katre, A.A.; Jeong, D.H.; Zhang, Y.; Chen, D.; Petrova, Y.; Llaneza, D.C.; Steg, A.D.; Parsons, L.; et al. Targeting RNA-Polymerase I in Both Chemosensitive and Chemoresistant Populations in Epithelial Ovarian Cancer. Clin. Cancer Res. 2017, 23, 6529–6540. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shen, J.; Cao, J.; Zhou, G.; Lei, T.; Sun, Y.; Gao, H.; Ding, Y.; Xu, W.; Zhan, Z.; et al. Alternative splicing of human telomerase reverse transcriptase in gliomas and its modulation mediated by CX-5461. J. Exp. Clin. Cancer Res. 2018, 37, 78. [Google Scholar] [CrossRef] [PubMed]
- Hein, N.; Cameron, D.P.; Hannan, K.M.; Nguyen, N.N.; Fong, C.Y.; Sornkom, J.; Wall, M.; Pavy, M.; Cullinane, C.; Diesch, J.; et al. Inhibition of Pol I transcription treats murine and human AML by targeting the leukemia-initiating cell population. Blood 2017, 129, 2882–2895. [Google Scholar] [CrossRef] [PubMed]
- Rothkamm, K.; Barnard, S.; Moquet, J.; Ellender, M.; Rana, Z.; Burdak-Rothkamm, S. DNA damage foci, Meaning and significance. Environ. Mol. Mutagen. 2015, 56, 491–504. [Google Scholar] [CrossRef]
- Quin, J.; Chan, K.T.; Devlin, J.R.; Cameron, D.P.; Diesch, J.; Cullinane, C.; Ahern, J.; Khot, A.; Hein, N.; George, A.J.; et al. Inhibition of RNA polymerase I transcription initiation by CX-5461 activates non-canonical ATM/ATR signalling. Oncotarget 2016, 7, 49800–49818. [Google Scholar] [CrossRef]
- Dhillon, K.K.; Swisher, E.M.; Taniguchi, T. Secondary mutations of BRCA1/2 and drug resistance. Cancer Sci. 2011, 102, 663–669. [Google Scholar] [CrossRef]
- Xu, H.; Di Antonio, M.; McKinney, S.; Mathew, V.; Ho, B.; O’Neil, N.J.; Santos, N.D.; Silvester, J.; Wei, V.; Garcia, J.; et al. CX-5461 is a DNA G quadruplex stabilizer with selective lethality in BRCA1/2 deficient tumours. Nat. Commun. 2017, 8, 14432. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.S.; Lyon, C.E.; Fox, A.H.; Leung, A.K.; Lam, Y.W.; Steen, H.; Mann, M.; Lamond, A.I. Directed proteomic analysis of the human nucleolus. Curr. Biol. 2002, 12, 1–11. [Google Scholar] [CrossRef]
- Grisendi, S.; Mecucci, C.; Falini, B.; Pandol, P.P. Nucleophosmin and cancer. Nat. Rev. Cancer 2006, 6, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, M.S. NPM1/B23, A Multifunctional Chaperone in Ribosome Biogenesis and Chromatin Remodeling. Biochem. Res. Int. 2011, 2011, 195209. [Google Scholar] [CrossRef]
- Katagiri, N.; Kuroda, T.; Kishimoto, H.; Hayashi, Y.; Kumazawa, T.; Kimura, K. The nucleolar protein nucleophosmin is essential for autophagy induced by inhibiting Pol I transcription. Sci. Rep. 2015, 5, 8903. [Google Scholar] [CrossRef]
- Cruz, I.N.; Coley, H.M.; Kramer, H.B.; Madhuri, T.K.; Safuwan, N.A.; Angelino, A.R.; Yang, M. Proteomics Analysis of Ovarian Cancer Cell Lines and Tissues Reveals Drug Resistance-associated Proteins. Cancer Genom. Proteom. 2017, 14, 35–51. [Google Scholar] [CrossRef]
- Londero, A.P.; Orsaria, M.; Tell, G.; Marzinotto, S.; Capodicasa, V.; Poletto, M.; Vascotto, C.; Sacco, C.; Mariuzzi, L. Expression and prognostic significance of APE1/Ref-1 and NPM1 proteins in high-grade ovarian serous cancer. Am. J. Clin. Pathol. 2014, 141, 404–414. [Google Scholar] [CrossRef]
- Holmberg Olausson, K.; Elsir, T.; Moazemi Goudarzi, K.; Nistér, M.; Lindström, M.S. NPM1 histone chaperone is upregulated in Glioblastoma to promote cell survival and maintain nucleolar shape. Sci. Rep. 2015, 5, 16495. [Google Scholar] [CrossRef]
- Kalra, R.S.; Bapat, S.A. Enhanced levels of double-strand DNA break repair proteins protect ovarian cancer cells against genotoxic stress-induced apoptosis. J. Ovarian Res. 2013, 6, 66. [Google Scholar] [CrossRef]
- Chen, S.; He, H.; Wang, Y.; Liu, L.; Liu, Y.; You, H.; Dong, Y.; Lyu, J. Poor prognosis of nucleophosmin overexpression in solid tumors, a meta-analysis. BMC Cancer 2018, 18, 838. [Google Scholar] [CrossRef] [PubMed]
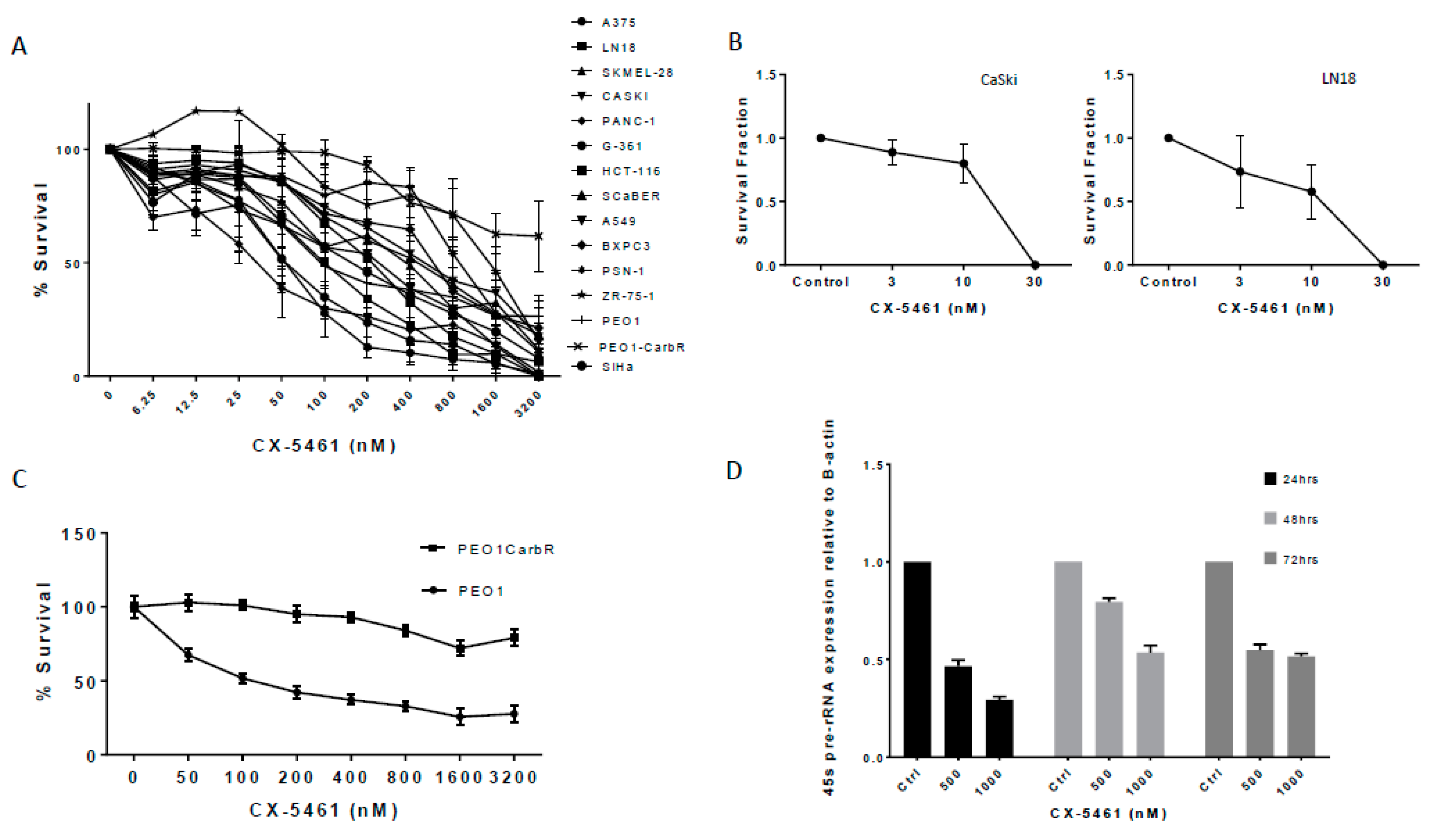

| Cell Line | IC50 nM and SD | p53 Status |
|---|---|---|
| PANC-1 | 35 (−/+ 2.20) | Mut |
| A375 | 53 (−/+ 2.28) | WT |
| G-361 | 55 (−/+ 1.58) | WT |
| PEO1 | 98 (−/+ 4.32) | Mut |
| LN18 | 104 (−/+ 3.01) | Mut |
| A549 | 169 (−/+ 3.74) | WT |
| HCT-116 | 178 (−/+ 2.54) | WT |
| SKMEL-28 | 188 (−/+ 4.70) | WT |
| SCaber | 215 (−/+ 2.1) | Mut |
| SiHa | 229 (−/+ 5.68) | WT |
| BXPC3 | 257 (−/+ 2.65) | Mut |
| CaSKI | 408 (−/+ 4.08) | WT |
| ZR-75-1 | 506 (−/+ 1.69) | WT |
| PSN-1 | 656 (−/+ 13.49) | Mut |
| PEO1CarbR | >3200 (−/+ 5.77) | Mut |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismael, M.; Webb, R.; Ajaz, M.; Kirkby, K.J.; Coley, H.M. The Targeting of RNA Polymerase I Transcription Using CX-5461 in Combination with Radiation Enhances Tumour Cell Killing Effects in Human Solid Cancers. Cancers 2019, 11, 1429. https://doi.org/10.3390/cancers11101429
Ismael M, Webb R, Ajaz M, Kirkby KJ, Coley HM. The Targeting of RNA Polymerase I Transcription Using CX-5461 in Combination with Radiation Enhances Tumour Cell Killing Effects in Human Solid Cancers. Cancers. 2019; 11(10):1429. https://doi.org/10.3390/cancers11101429
Chicago/Turabian StyleIsmael, Mohammed, Roger Webb, Mazhar Ajaz, Karen J. Kirkby, and Helen M. Coley. 2019. "The Targeting of RNA Polymerase I Transcription Using CX-5461 in Combination with Radiation Enhances Tumour Cell Killing Effects in Human Solid Cancers" Cancers 11, no. 10: 1429. https://doi.org/10.3390/cancers11101429
APA StyleIsmael, M., Webb, R., Ajaz, M., Kirkby, K. J., & Coley, H. M. (2019). The Targeting of RNA Polymerase I Transcription Using CX-5461 in Combination with Radiation Enhances Tumour Cell Killing Effects in Human Solid Cancers. Cancers, 11(10), 1429. https://doi.org/10.3390/cancers11101429





