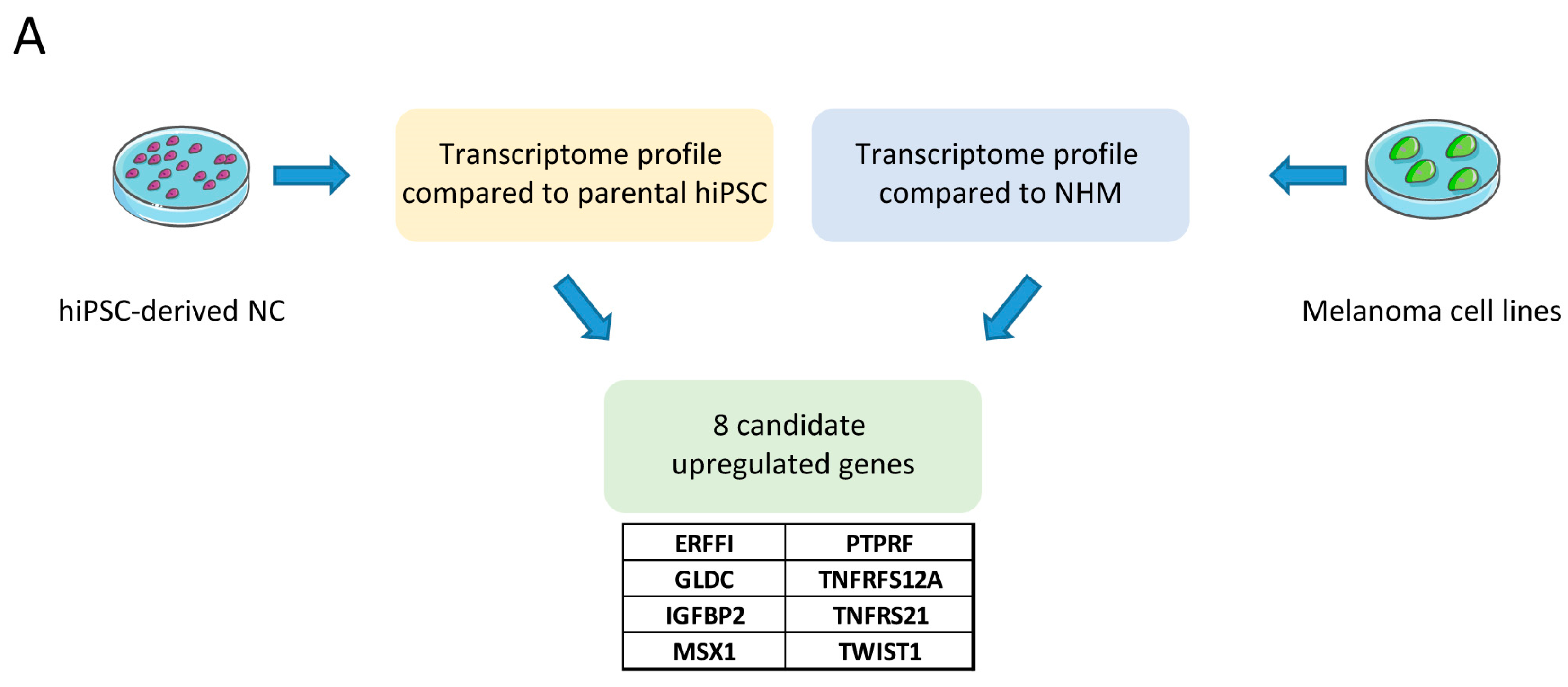
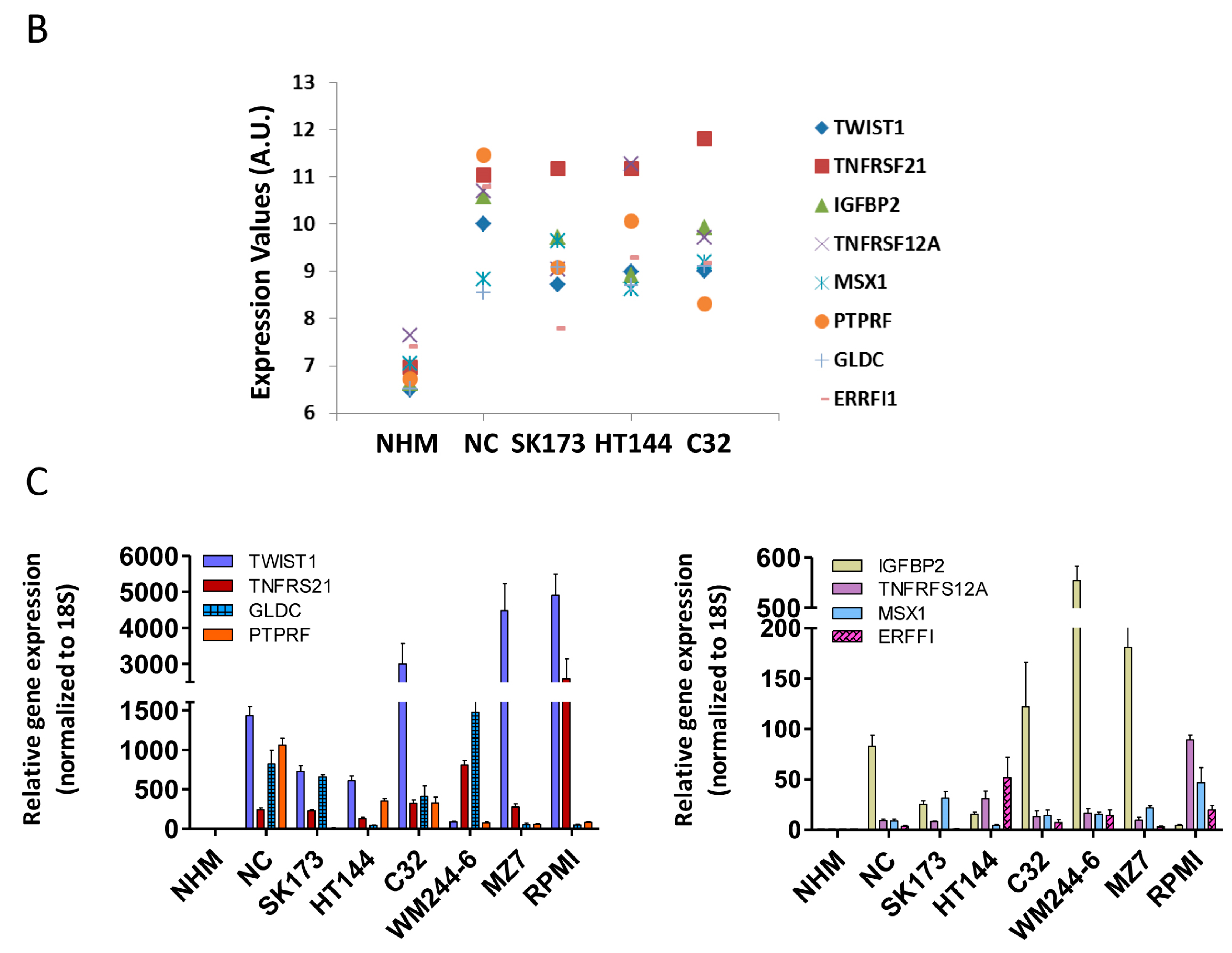
3. Discussion
Next to tumor thickness and ulceration of primary melanoma, the extent of regional and distant metastasis only lactate dehydrogenase (LDH) is used as a prognostic marker in the TMN classification system of the American Joint Committee on Cancer (AJCC) 2017. However, these markers are used for staging and often do not predict accurately the patient’s outcome. LDH is only used as a prognostic marker in clinic when distant metastasis already occurred [
12]. Additionally, LDH is not specific for malignancy as it is also elevated in cases of cell damage, inflammatory processes, and hemolysis [
13]. In our study, we show that genes that are highly expressed not only in neural crest cells but also in malignant melanoma cells are associated with prognosis. Upregulation of CD271, ERRFI1, and GLDC is in line with a bad prognosis. Additionally, upregulation of TWIST and GLDC is associated with early metastasis and therefore can be used as prognostic markers before metastasis occurred.
The role of CD271 in tumor transformation and progression has been widely investigated. It has also been suggested as a marker of cancer stem cell-like population in human melanoma tissues [
3]. The results of these studies are, however, not always on the same line. CD271 has been described as a dual mediator by suppressing melanoma cell proliferation and promoting metastasis while highly expressed in malignant melanoma cells [
14]. Furthermore, CD271 was identified as a T cell-suppressive molecule and, therefore, might be interesting for antimelanoma immunotherapy [
15]. However, a study based on xenograft transplantations derived from 7 melanoma patients showed variable expression level of CD271 after propagation of the same tumor into different mice. In addition, no correlation was found between the expression of CD271 and the tumorigenicity of the samples [
16,
17]. Our results show that a higher expression of CD271 is associated with a poor prognosis. The cut-off point for Anti-CD271 was 8 overall IHC score. For patients with an overall IHC score > 8 there is a 12 times greater risk to belong in survival group A and, therefore, to die earlier. The validity of this model is 80%. Because this marker is not commonly accepted, these data have to be taken with caution.
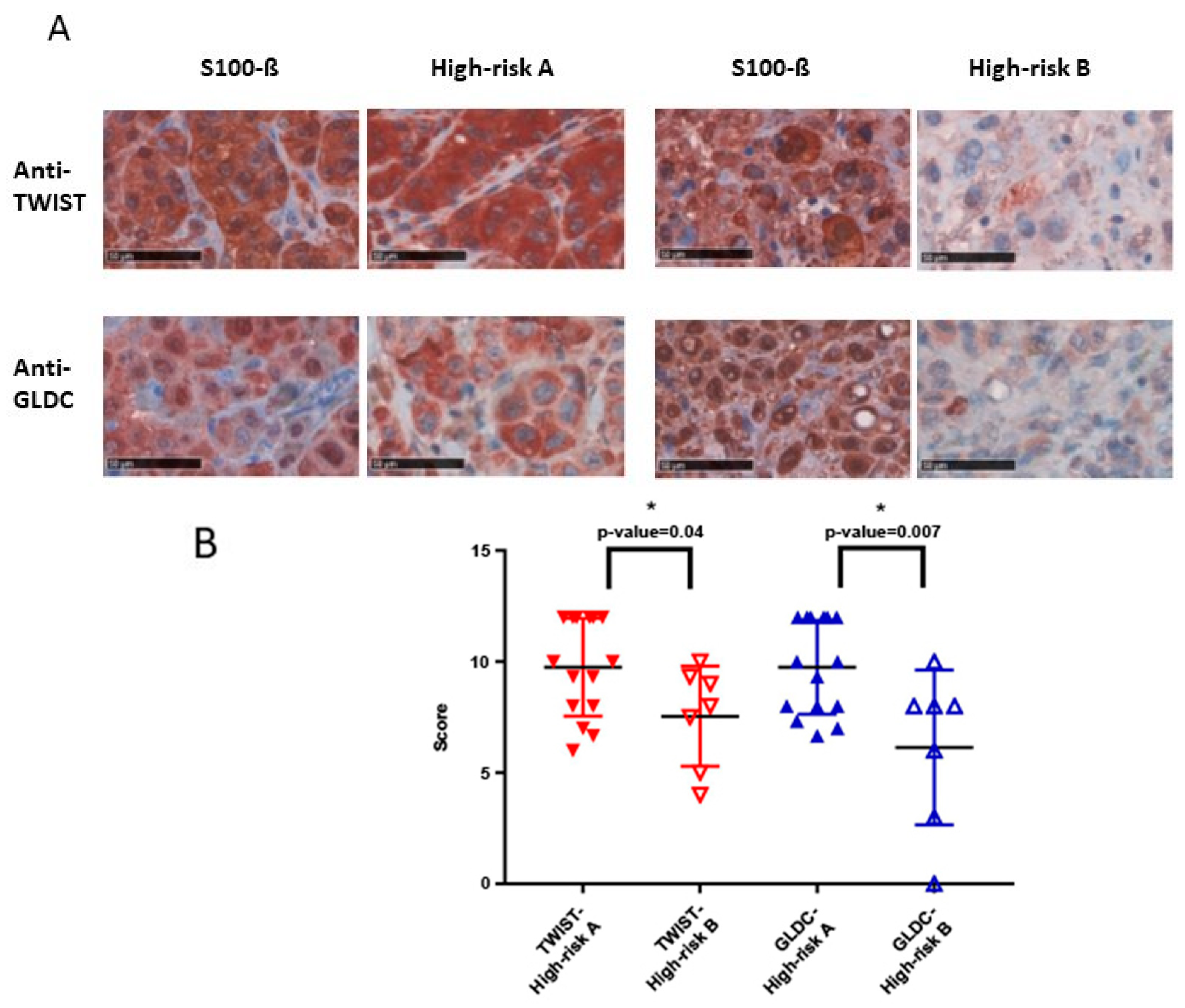
As part of a multienzyme complex, glycine decarboxylase (GLDC) is involved in the biosynthesis of serine. It has been shown that GLDC is a metabolic oncogene and its expression is strongly correlated with rates of cancer proliferation and greater mortality, e.g., in breast cancer [
18,
19]. Our study reveals that a greater expression, which can be seen in a greater overall IHC score, is in line with a poor prognosis. The calculated cut-off point for Anti-GLDC was also 8. Patients with an overall IHC score > 8 are 4.5 times more likely to be part of survival group A. The validity of this model is 73.6%. In addition, our results show that a greater expression of the GLDC protein (IHC score > 9.33) in primary melanoma increases the risk for early metastasis. Patients with an IHC score > 9.33 are 5.7 times more likely to belong to high-risk group A and, therefore, develop a metastasis within a shorter term. The validity of this model is 96%.
ERRFI1 is known as a tumor suppressor by directly inhibiting the epidermal-growth-factor-receptor and, therefore, its downstream pathways initializing cell growth [
20,
21]. In addition, ERFFI1 inhibits another receptor family (Erbb), activation of which leads to cell survival, proliferation, migration, and invasion [
22]. It has been shown that ERRFI1 alters negatively invasion and migration processes in malignant melanoma. However, new findings suggest that the role of ERRFI1 depends on the EGFR level. Therefore, the downregulation of ERRFI1 in an EGFR low environment leads to a higher migration rate and promotes cellular growth [
23,
24]. In our work, the EGFR level was not determined, so further investigations are needed to evaluate the validity of ERRFI1 as a prognostic marker in different subtypes of malignant melanoma. The calculated cut-off point for the antibody against ERRFI1 was 9. Patients with an overall IHC score > 9 are 17 times more likely to belong in survival group A. The validity of the model is 92.1%.
MSX1 is a transcription factor, which plays a part in the epithelial-to-mesenchymal transition during the embryonic development of the melanocyte lineage from the neural crest cells [
25]. It could be shown that reactivation of the transcription factor MSX1 in melanoma cells leads to neural crest precursor-like properties, like migration and plasticity [
10]. In our studies, no significant difference could be shown, but patients with a poorer prognosis did show a higher IHC (
p-value = 0.07). A bigger population group would be needed in order to clarify if MSX1 is usable as a significant tool. TWIST, like MSX1, is a transcription factor, which is part of the epithelial-to-mesenchymal transition. It therefore plays an important role in cell movement, invasion, and survival and is often overexpressed in cancer diseases [
26]. It has been shown that TWIST especially promotes metastasis processes and has no influence of primary melanoma growth [
27,
28,
29]. Similar to MSX1, TWIST is involved in metastasis processes. In our study, primary melanomas which are at great risk to metastasis (tumor thickness ≥ 2 mm) with a greater TWIST expression showed earlier metastasis compared to those with lower expression. The calculated cut-off point for TWIST was IHC > 12, the validity for this model is 75.2%. As 12 is the maximal IHC score that can be reached, further investigations are needed to specify the cut-off point as a clinical tool. In addition, there has been a difference for Anti-TWIST between survival group A and survival group B, but without significance. Further investigations are needed in order to display an increasing development of MSX1 and TWIST expression during tumor progression.
Ki67 is not to be found in neural crest cells but is an established proliferation marker, which is expressed in dividing cells [
30]. It is used as a predictive and prognostic marker, e.g., in breast cancer [
7]. As described above, there is a non-significant difference in Ki67 expression between the two groups.
PTPRF encodes the tyrosine-phosphate-receptor-type F, which controls EGFR signaling. PTPRF is overexpressed in primary melanoma and metastasis of malignant melanoma [
31]. The fact that PTPRF is not only overexpressed in metastasis but also in primary melanoma suggests that there is no relevant trend in expression during tumor progression. In our studies, no significant difference in PTPRF expression between the two groups was shown.
TNFRSF21 and TNFRSF12a are members of the tumor necrosis receptor family and contribute to the activation of NF-kappaB, which plays a role in tumorigenesis [
32,
33]. The IHC scores of the two groups for both antibodies suggest a greater expression during tumor progression; therefore, further investigations are needed in order to confirm the validity.
Finally, IGFBP2 is a tool for proliferation, apoptosis, and migration of cells during embryonic development and tumor cells [
34,
35]. However, Anti-IGFBP2 did not show a significant difference between the two groups investigated in our study.
One limitation of this study is the small-analyzed population size. In order to improve validity of this study, a bigger population group would be needed. Also, gender distribution, especially in the high-risk group, does not fit the normal distribution in Germany [
36]. Here might be a bias as male patients have a shorter overall survival and a greater risk for metastasis compared to women [
37,
38]. In high-risk group A, patients were included which were already metastasized at time of diagnosis. In these cases, it cannot be certainly said how many months have passed between occurrence of tumor and metastasis. However, considering experience with course of disease and tumor parameter thickness, the time in-between is negligible in these cases.
4. Materials and Methods
4.1. Cell Lines
Human melanoma cell lines (C32, HT144, SKmel173, WM266-4, MZ7, and RPMI) were cultured in DMEM (Gibco, Life Technologies, Darmstadt, Germany) with 10% FBS (Biochrom, Berlin, Germany), 0.1 mM β-mercapthoethanol (Gibco, Life Technologies), 1% non-essential amino acids (NEAA) and 1% Penicillin/Streptomycin (Sigma-Aldrich, St. Louis, MO, USA). Normal human melanocytes (NHM) were isolated from donor foreskins according to the ethical regulation (Ethics committee II, University Medical Center Manheim, Mannheim, Germany) and were cultivated in medium 254 (Gibco, Life Technologies) supplemented with 100× human melanocyte growth supplement (HMGS) (Gibco, Life Technologies). Human neural crest cells were derived from hiPSC and cultured in the same medium as for melanoma cell lines.
4.2. RNA Isolation and cDNA Synthesis
Total RNA isolation from all melanoma cell lines (C32, HT144, SKmel173, WM266-4, MZ7, and RPMI), human neural crest cells (NC) and normal human melanocytes (NHM) was done using RNeasy Mini kit (Qiagen, Düsseldorf, Germany) according to the manufacturer’s protocol. The RNA was treated with DNase I on the column. RNA concentration and quality were measured by NanoDrop ND1000 spectrophotometer. cDNA was synthesized using the Revert Aid First Strand cDNA synthesis kit (Thermo scientific, Darmstadt, Germany) according to the manufacturer’s protocol.
4.3. Microarray Gene Expression Profiling
Biotinylated cRNA was hybridized to whole-genome BeadChip Sentrix arrays HumanHT-12 v4 from ILLUMINA (Santa Clara, CA, USA) following the manufacturer’s indications. Microarray scanning was carried out using an iScan array scanner. As test for significance, a Bayes test was used on the bead expression values of the two groups of interest. The average expression value is calculated as mean of the measured expressions of beads together with the standard deviation of the beads. After selecting the genes, which p-values were inferior to 0.05, log2-expression values of the differentially expressed genes were represented.
Gene expression datasets were uploaded on GEO database: GSE123686.
4.4. qPCR
Quantitative real-time PCR (qPCR) was performed using SYBR Green (Applied Biosystems, Life technologies, Darmstadt, Germany) on a 7500 real-time PCR system (Applied Biosystems, Life technologies). In all experiments, rRNA 18s was used as the housekeeping gene and the values were normalized to it. Relative gene expressions were quantified by calculating (∆∆Ct). Primers used are as follow (
Table 5):
4.5. Patient’s Sample
In this study, histopathologic tumor samples (primary melanoma and metastasis) of patients diagnosed with malignant melanoma between 1989 and 2013 were collected and assigned into two groups. All patients did not receive novel immunotherapeutic or targeted therapeutic drugs that have an impact on overall survival. This study was performed in accordance to the ethical vote (2014-835R-MA).
In the first group (survival group A) were included tumor samples of patients who died within 12 months after diagnosis of stage IV melanoma (
n = 12). Only metastases were analyzed. Patients who survived 30 months or longer were included in the second group (
n = 20; survival group B). In survival group A, eight patients were male and four female. The mean age at initial diagnosis in the first group was 52 years. The mean Breslow tumor thickness of primary melanoma in Survival group A was 2.8 mm. In survival group B, the mean age at initial diagnosis was 61 years. Nine patients were male and eleven female. The mean Breslow tumor thickness of primary melanoma in survival group B was 3.3 mm (see also
Table 6). Classifications were made according to system of the American Joint Committee on Cancer (AJCC) of 2009.
In addition, tumor samples of patients with tumors ≥ 2 mm showing high risks to metastasize were identified and assigned to two groups. In the first group (high-risk group A), patients were included who developed metastasis within the first 12 month (
n = 16) and the second group contained patients who did not metastasize within 30 months (
n = 7; high-risk group B). In this group, only primary melanomas were analyzed. In high-risk group A, four patients were female and twelve patients male. The mean age in this group at initial diagnosis was 76 years. The mean Breslow tumor thickness of primary melanoma in this group was 5 mm. In high-risk group B, five patients were female and two male. The mean age at initial diagnosis was 68 years and the mean Breslow tumor thickness was 2.5 mm (see also
Table 7).
4.6. Tissue Microarray
Representative tumor areas were detected on HE sections and tissue punch samples (diameter 2 mm) were taken from paraffin-embedded tumor block and displayed on Tissue Microarrays (TMA) according to a previous report [
39]. For one tumor sample up to four punches were taken.
4.7. Immunohistochemistry
An amount of 0.9 µm slices of the in paraffin-embedded tumors on the TMA were stained using standard protocols 11 times with the following antibodies: Anti-GLDC (Atlas Antibodies, Bromma, Sweden, HPA002318), Anti-CD271 (BP Pharmingen, Heidelberg, Germany, 557194), Anti-ERRIF1 (Atlas Antibodies HPA027206), Anti-MSX1 (abcam, Cambridge, UK, ab49153), Anti-TNFRSFR12A (Atlas Antibodies HPA007853), Anti-Ki67 (abcam ab1667), Anti-PTPRF (Atlas Antibodies HPA012710), Anti-TNFRSFR21 (Atlas Antibodies HPA006746), Anti-TWIST (abcam ab50581), Anti-IGFBP2 (Cell signaling, Denver, MA, USA, #3922), and Anti-S100 (Dako, Santa Clara, CA, USA, Z0311). As a negative control, stainings were made according to standard protocol without using primary antibodies. As secondary antibodies, the Dako EnVision™ System-HRP (Dako Kit, Rabbit K4009) was used for Anti-GLDC, Anti-ERRFI1, Anti-MSX1, Anti-TNFRSFR12a, Anti-Ki67, Anti-PTPRF, Anti-TNFRSFR21, Anti-TWIST, Anti-IGFBP2, and Anti-S100. The Dako EnVision™ System-HRP (Dako Kit, Mouse K4005) was used for Anti-CD271. For antibody dilution, Dako Antibody Diluents (Dako S0809) has been used. Antibody dilutions were made according to the manufacturers’ information sheet.
4.8. Analysis of Stained Tumor Samples and Statistics
Stained tumor samples were analyzed and an IHC (immunohistochemistry) score assigned to each sample.
IHC score was defined as the product of the immunopositivity score (0: <10%, 1: 10–25%; 2: 26–50%; 3: 51–75% and 4: >75%) and the immunointensity score (0: negative, 1: mild, 2: moderate, 3: strong). This score was made for each punch (min: 0; max: 12), except for antibody Anti-Ki67. As described above, up to four punches of one tumor sample were taken. Out of IHC scores from each punch, a mean IHC score of each tumor sample was calculated. For an overall IHC score, the mean value form scoring of the two investigators was calculated. For samples stained with Anti-Ki67, the percentage of stained cell nuclei was determined. Two investigators performed scoring independently. One investigation was performed blinded to detect potential bias.
Figure 2a and
Figures S3 and S4 give examples for immunointensity scores for each antibody.
Overall IHC scores were calculated for all samples and data comparison from survival group A and survival group B, also from high-risk group A and high-risk group B were statistically analyzed with two-tailed t-test. As mean and median did not differ much, we could assume data normally distributed. For Anti-Ki67 and Anti-IGFBP in high-risk group A and B, analyses were made with Mann–Whitney U-Test, as in these cases the data was not normally distributed. Equality of variances was tested using f-test. For each significant antibody, a cut-off point was determined by using logistic regression in order to objectify the informative value of the IHC scores. It was shown that patients with an overall IHC above the cut-off point have a greater risk to belong to group A. Therefore, the risk was calculated using odds ratios. In order to validate this prediction, the association of predicted probabilities and observed responses was put in relation.