Regression Analysis of Protoporphyrin IX Measurements Obtained During Dermatological Photodynamic Therapy
Abstract
1. Introduction
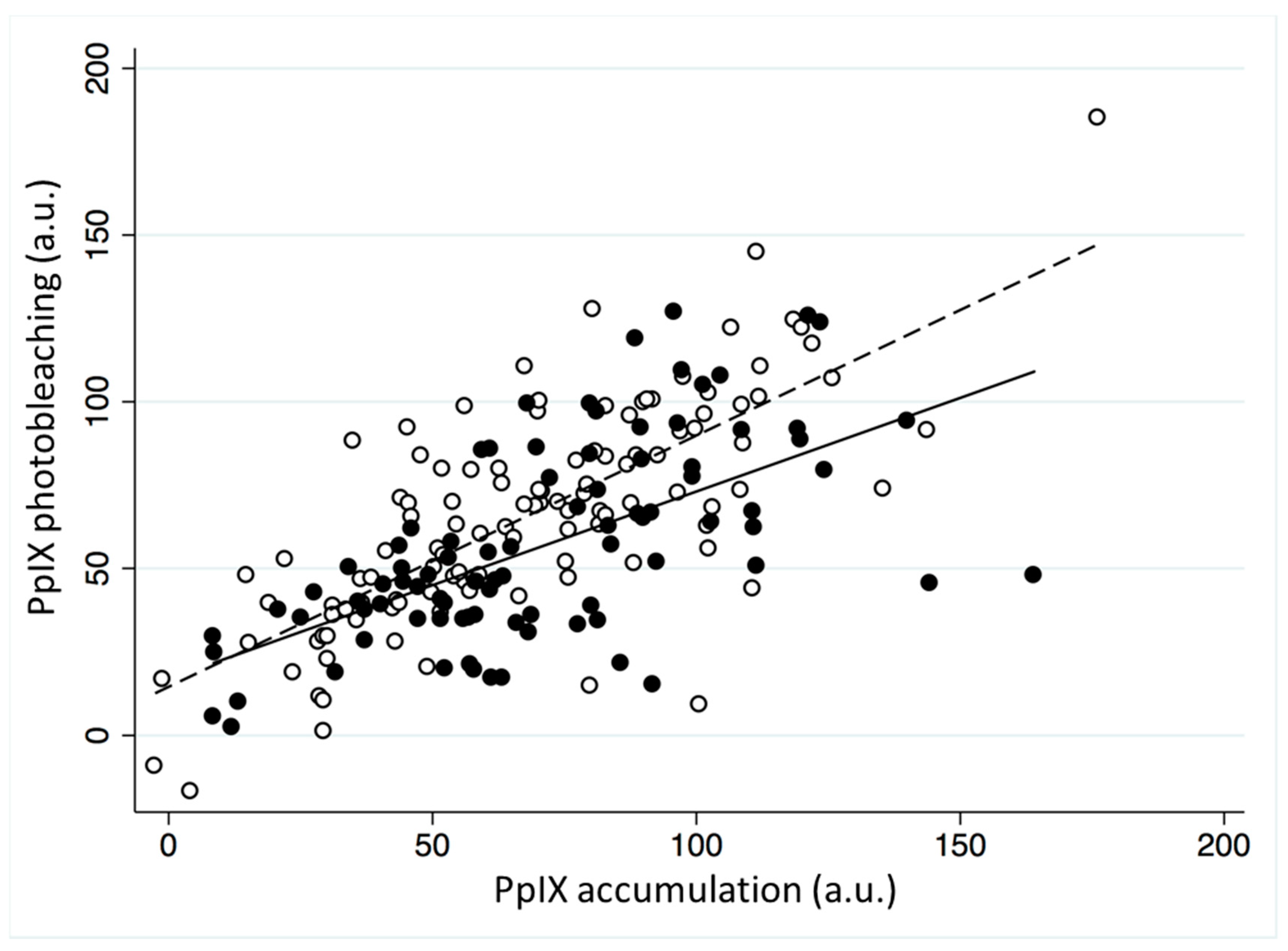
2. Results
2.1. Demographics
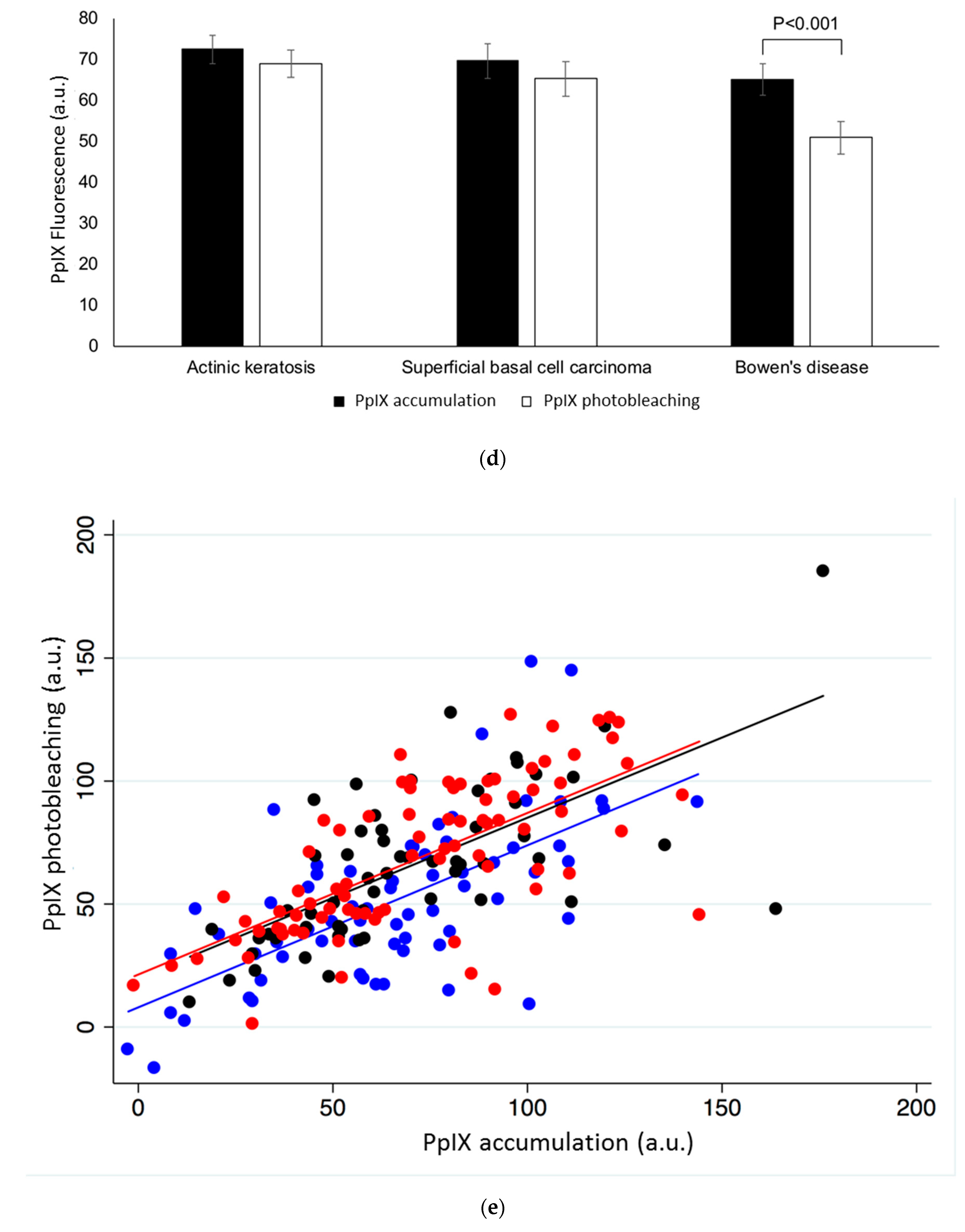
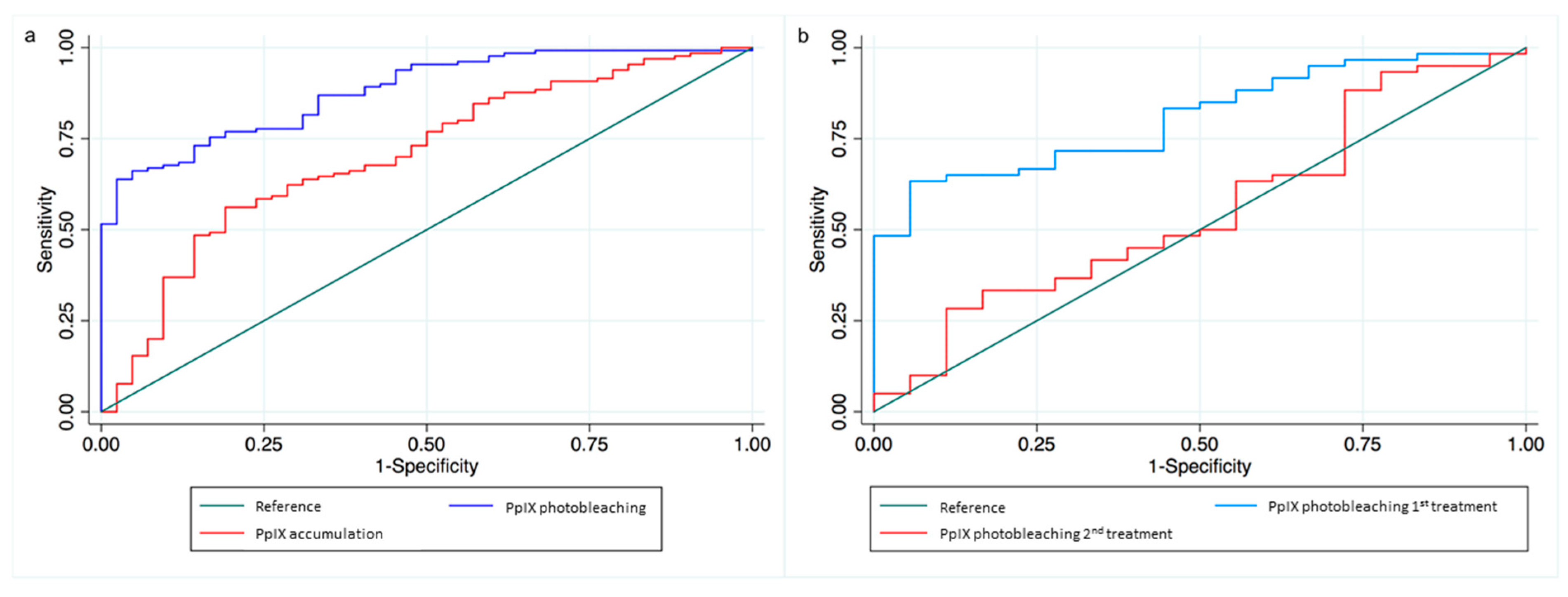
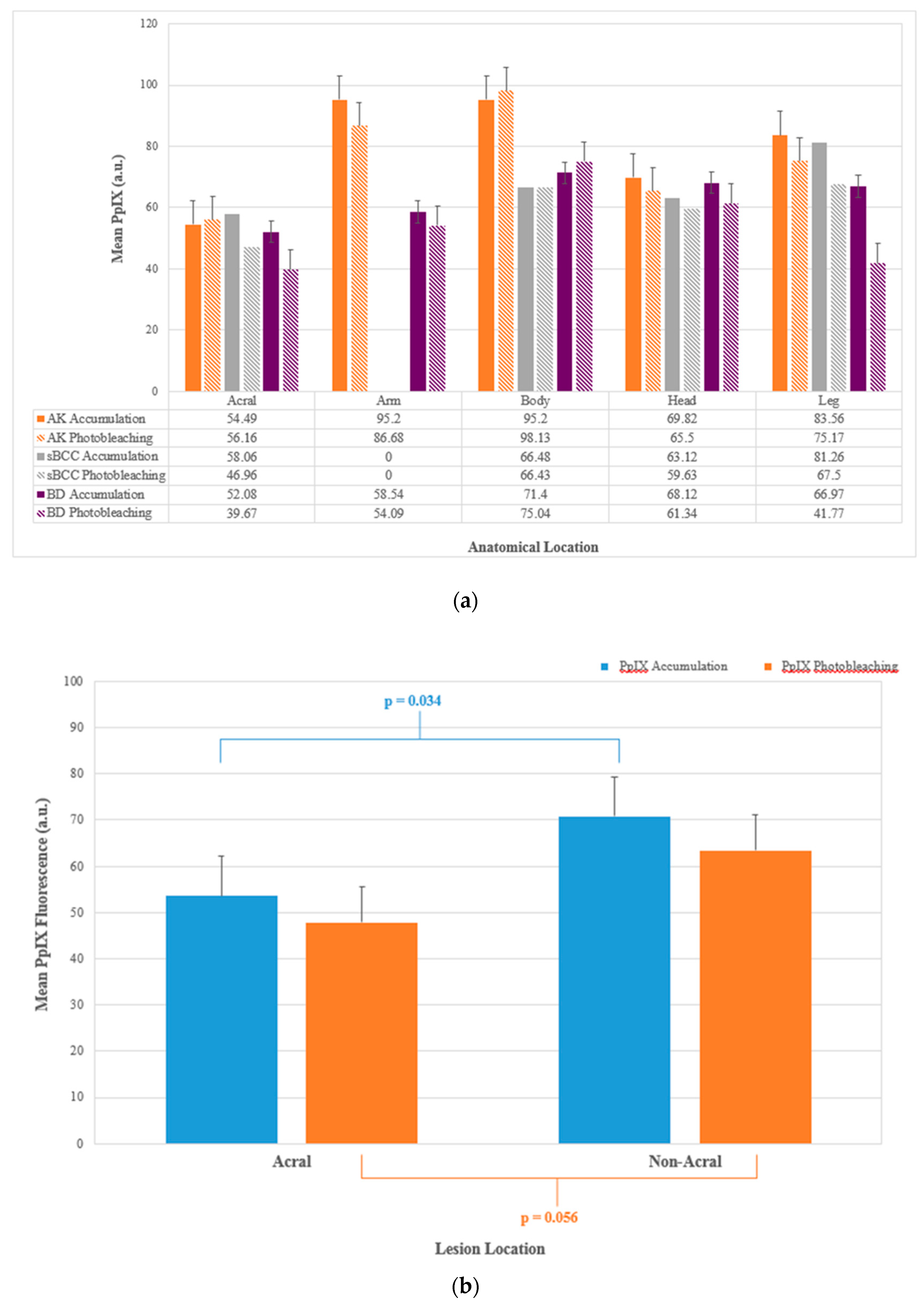
2.2. Different Lesion Types
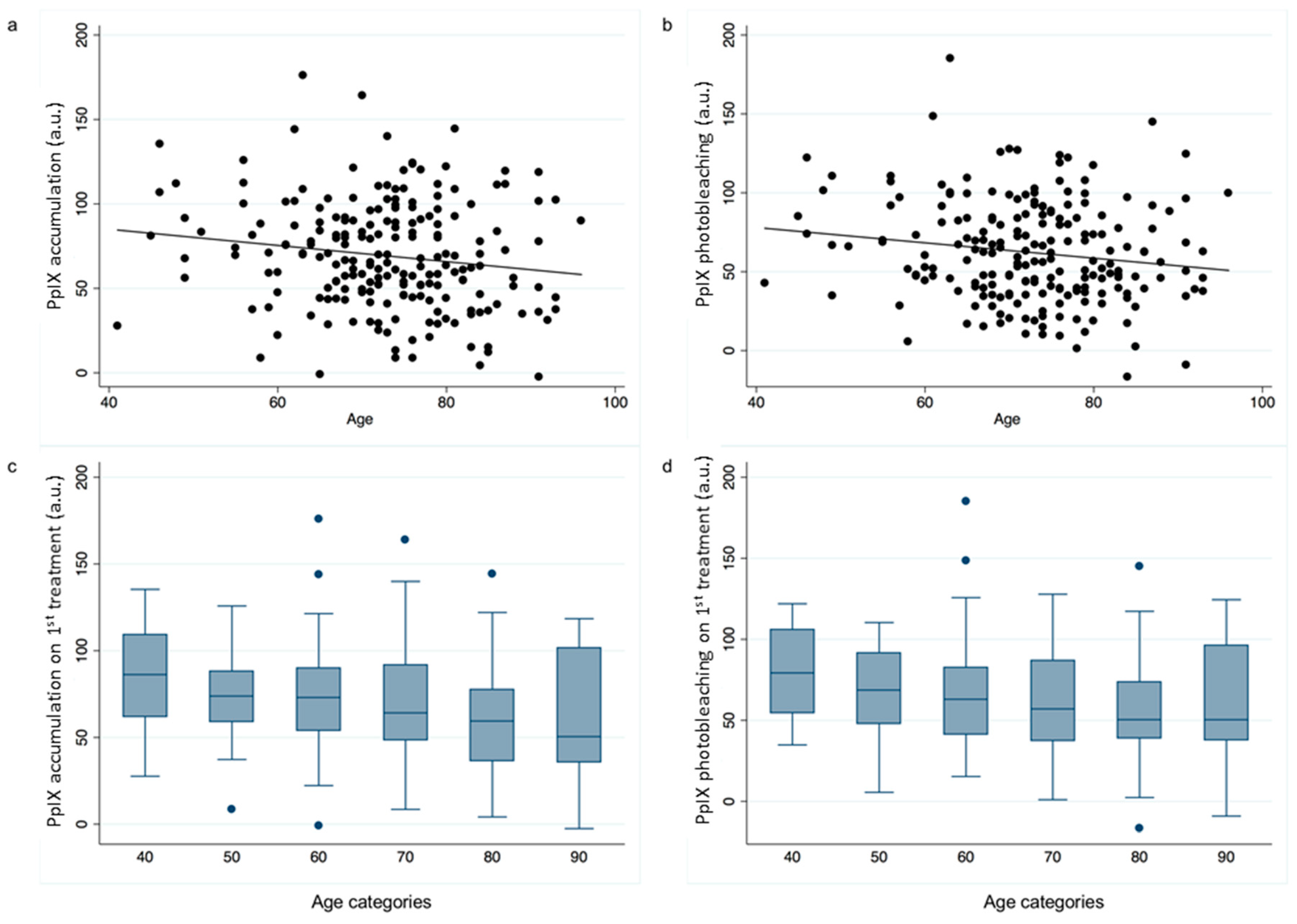
2.3. Age and Gender
2.4. Lesion Location
2.5. ACD Pain Relief
3. Discussion
4. Materials and Methods
4.1. Dermatological MAL-PDT
4.2. Fluorescence Imaging
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taylor, E.L.; Brown, S.B. The advantages of aminolevulinic acid photodynamic therapy in dermatology. J. Dermatol. Treat. 2002, 13, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Steinbauer, J.M.; Schreml, S.; Kohl, E.A.; Karrer, S.; Landthaler, M.; Szeimies, R.M. Photodynamic therapy in dermatology. JDDG J. Dtsch. Dermatol. Ges. 2010, 8, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.; Szeimies, R.; Sidoroff, A.; Braathen, L. European Guidelines for Topical PDT. JEADV 2013, 27, 536–544. [Google Scholar] [PubMed]
- Babilas, P.; Schreml, S.; Landthaler, M.; Szeimies, R.M. Photodynamic therapy in dermatology: State-of-the-art. Photodermatol. Photoimmunol. Photomed. 2010, 26, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Bown, S.G. How mainstream medicine sees photodynamic therapy in the United Kingdom. J. Natl. Compr. Cancer Netw. 2012, 10, S69–S74. [Google Scholar] [CrossRef]
- Fayter, D.; Corbett, M.; Heirs, M.; Fox, D.; Eastwood, A. A systematic review of photodynamic therapy in the treatment of pre-cancerous skin conditions, Barrett’s oesphagus and cancers of the biliary tract, brain, head and neck, lung, oesophagus and skin. Health Technol. Assess. 2010, 14, 1–288. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Berg, K.; Moan, J.; Kongshaug, M.; Nesland, J. 5-Aminolevulinic acid-based photodynamic therapy: Principles and experimental research. Photochem. Photobiol. 1997, 65, 235–251. [Google Scholar] [CrossRef]
- Scott, M.A.; Hopper, C.; Sahota, A.; Springett, R.; McIlroy, B.W.; Bown, S.G.; MacRobert, A.J. Fluorescence Photodiagnosis and Photobleaching Studies of Cancerous Lesions using Ratio Imaging and Spectroscopic Techniques. Lasers Med. Sci. 2000, 15, 63–72. [Google Scholar] [CrossRef]
- Wennberg, A.M.; Gudmundson, F.; Stenquist, B.; Ternesten, A.; Molne, L.; Rosen, A.; Larkö, O. In vivo detection of basal cell carcinoma using imaging spectroscopy. Acta Dermato-Venereol. 1999, 79, 54–61. [Google Scholar] [CrossRef]
- Ackermann, G.; Abels, C.; Karrer, S.; Baumler, W.; Landthaler, M.; Szeimies, R.M. Fluorescence-assisted biopsy of basal cell carcinomas. Hautarzt 2000, 51, 920–924. [Google Scholar] [CrossRef]
- Siewecke, C.; Szeimies, R.M. PDT and fluorescence diagnosis in dermatology. Hospital Pharmacy Europe, 1 May 2004; 49–52. [Google Scholar]
- Bogaards, A.; Sterenborg, H.J.; Trachtenberg, J.; Wilson, B.C.; Lilge, L. In vivo quantification of fluorescent molecular markers in real-time by ratio imaging for diagnostic screening and image-guided surgery. Lasers Surg. Med. 2007, 39, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Smits, T.; Kleinpenning, M.M.; Blokx, W.A.; van de Kerkhof, P.C.; van Erp, P.E.; Gerritsen, M.J. Fluorescence diagnosis in keratinocytic intraepidermal neoplasias. J. Am. Acad. Dermatol. 2007, 57, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Gibson, S.L.; Foster, T.H.; Hilf, R. Effectiveness of delta-aminolevulinic acid-induced protoporphyrin as a photosensitiser for photodynamic therapy in vivo. Cancer Res. 1995, 55, 1723–1731. [Google Scholar] [PubMed]
- Loh, C.S.; Vernon, D.; MacRobert, A.J.; Bedwell, J.; Bown, S.G.; Brown, S.B. Endogenous porphyrin distribution induced by 5-aminolaevulinic acid in the tissue layers of the gastrointestinal tract. J. Photochem. Photobiol. B Biol. 1993, 20, 47–54. [Google Scholar] [CrossRef]
- Tyrrell, J.; Campbell, S.; Curnow, A. Validation of a non-invasive fluorescence imaging system to monitor dermatological PDT. Photodiagn. Photodyn. Ther. 2010, 7, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Jaap de, L.; van der Beek, N.; Neugebauer, W.D.; Bjerring, P.; Neumann, H.A. Fluorescence detection and diagnosis of non-melanoma skin cancer at an early stage. Lasers Surg. Med. 2009, 41, 96–103. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Photodynamic therapy for non-melanoma skin tumours (including premalignant and primary non-metastatic skin lesions). NICE Interv. Proced. Guid. 2006, IPG155, 1–6. [Google Scholar]
- Tyrrell, J.; Campbell, S.; Curnow, A. Monitoring the accumulation and dissipation of the photosensitiser protoporphyrin IX during standard dermatological methyl-aminolevulinate photodynamic therapy utilizing non-invasive fluorescence imaging and quantification. Photodiagn. Photodyn. Ther. 2011, 8, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, J.; Morton, C.; Campbell, S.; Curnow, A. Comparison of PpIX accumulation and destruction during methyl-aminolevulinate photodynamic therapy (MAL-PDT) of skin tumours located at acral and non-acral sites. Br. J. Dermatol. 2011, 164, 1362–1368. [Google Scholar] [CrossRef]
- Tyrrell, J.; Campbell, S.; Curnow, A. The relationship between protoporphyrin IX photobleaching during real-time dermatological methyl-aminolevulinate photodynamic therapy (MAL-PDT) and subsequent clinical outcome. Lasers Surg. Med. 2010, 42, 613–619. [Google Scholar] [CrossRef]
- Nissen, C.V.; Heerfordt, I.M.; Wiegell, S.R.; Mikkelsen, C.S.; Wulf, H.C. Increased protoporphyrin IX accumulation does not improve the effect of photodynamic therapy for actinic keratosis: A randomized controlled trial. Br. J. Dermatol. 2017, 176, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, J.; Campbell, S.; Shore, A.; Curnow, A. The effect of air cooling pain relief on protoporphyrin IX photobleaching during real-time dermatological methyl-aminolevulinate photodynamic therapy. J. Photochem. Photobiol. B Biol. 2011, 103, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Khurana, M.; Moriyama, Y.; Wilson, B. Quantification of in vivo fluorescence decoupled from the effects of tissue optical properties using fiber-optic spectroscopy measurements. J. Biomed. Opt. 2010, 15, 067006. [Google Scholar] [CrossRef]
- Middelburg, T.; Hoy, C.; Neumann, H.; Amelink, A.; Robinson, D. Correction for tissue optical properties enables quantitative skin fluorescence measurements using multi-diameter single fiber reflectance spectroscopy. J. Dermatol. Sci. 2015, 79, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Sunar, U.; Rohrbach, D.J.; Morgan, J.; Zeitouni, N.; Henderson, B.W. Quantification of PpIX concentration in basal cell carcinoma and squamous cell carcinoma models using spatial frequency domain imaging. Biomed. Opt. Express 2013, 4, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Kanick, S.; Davis, S.; Zhao, Y.; Hasan, T.; Maytin, E.; Pogue, B.; Chapman, M. Dual-channel red/blue fluorescence dosimetry with broadband reflectance spectroscopic correction measures protoporphyrin IX production during photodynamic therapy of actinic keratosis. J. Biomed. Opt. 2014, 19, 75002. [Google Scholar] [CrossRef] [PubMed]
- Rollakanti, K.; Kanick, S.; Davis, S.; Pogue, B.; Maytin, E. Techniques for fluorescence detection of protoprophyrin IX in skin cancers associated with photodynamic therapy. Photonics Lasers Med. 2013, 2, 287–303. [Google Scholar] [CrossRef]
- Kulyk, O.; Ibbotson, S.H.; Moseley, H.; Valentine, R.M.; Samuel, I.D. Development of a handheld fluorescence imaging device to investigate the characteristics of protoporphyrin IX fluorescence in healthy and diseased skin. Photodiagn. Photodyn. Ther. 2015, 12, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Juzeniene, A.; Juzenas, P.; Kaalhus, O.; Iani, V.; Moan, J. Temperature effect on accumulation of protoporphyrin IX after topical application of 5-aminolevulinic acid and its methyl ester and hexyl ester derivatives in normal mouse skin. Photochem. Photobiol. 2002, 74, 452–456. [Google Scholar] [CrossRef]
- Cottrell, W.J.; Paquette, A.D.; Keymel, K.R.; Foster, T.H.; Oseroff, A.R. Irradiance-dependent photobleaching and pain in delta-aminolevulinic acid-photodynamic therapy of superficial basal cell carcinomas. Clin. Cancer Res. 2008, 14, 4475–4483. [Google Scholar] [CrossRef] [PubMed]
- Boere, I.A.; Robinson, D.J.; de Bruijn, H.S.; van den Boogert, J.; Tilanus, H.W.; Sterenborg, H.J.; de Bruin, R.W.F. Monitoring in situ dosimetry and protoporphyrin IX fluorescence photobleaching in the normal rat esophagus during 5-aminolevulinic acid photodynamic therapy. Photochem. Photobiol. 2003, 78, 271–277. [Google Scholar] [CrossRef]
- Kruijt, B.; de Bruijn, H.S.; van der Ploeg-van den Heuvel, A.; de Bruin, R.W.; Sterenborg, H.J.; Amelink, A.; Robinson, D.J. Monitoring ALA-induced PpIX photodynamic therapy in the rat esophagus using fluorescence and reflectance spectroscopy. Photochem. Photobiol. 2008, 84, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, J.; Thorn, C.; Shore, A.; Campbell, S.; Curnow, A. Oxygen saturation and perfusion changes during dermatological methyl-aminolevulinate photodynamic therapy. Br. J. Dermatol. 2011, 165, 1323–1331. [Google Scholar] [CrossRef] [PubMed]

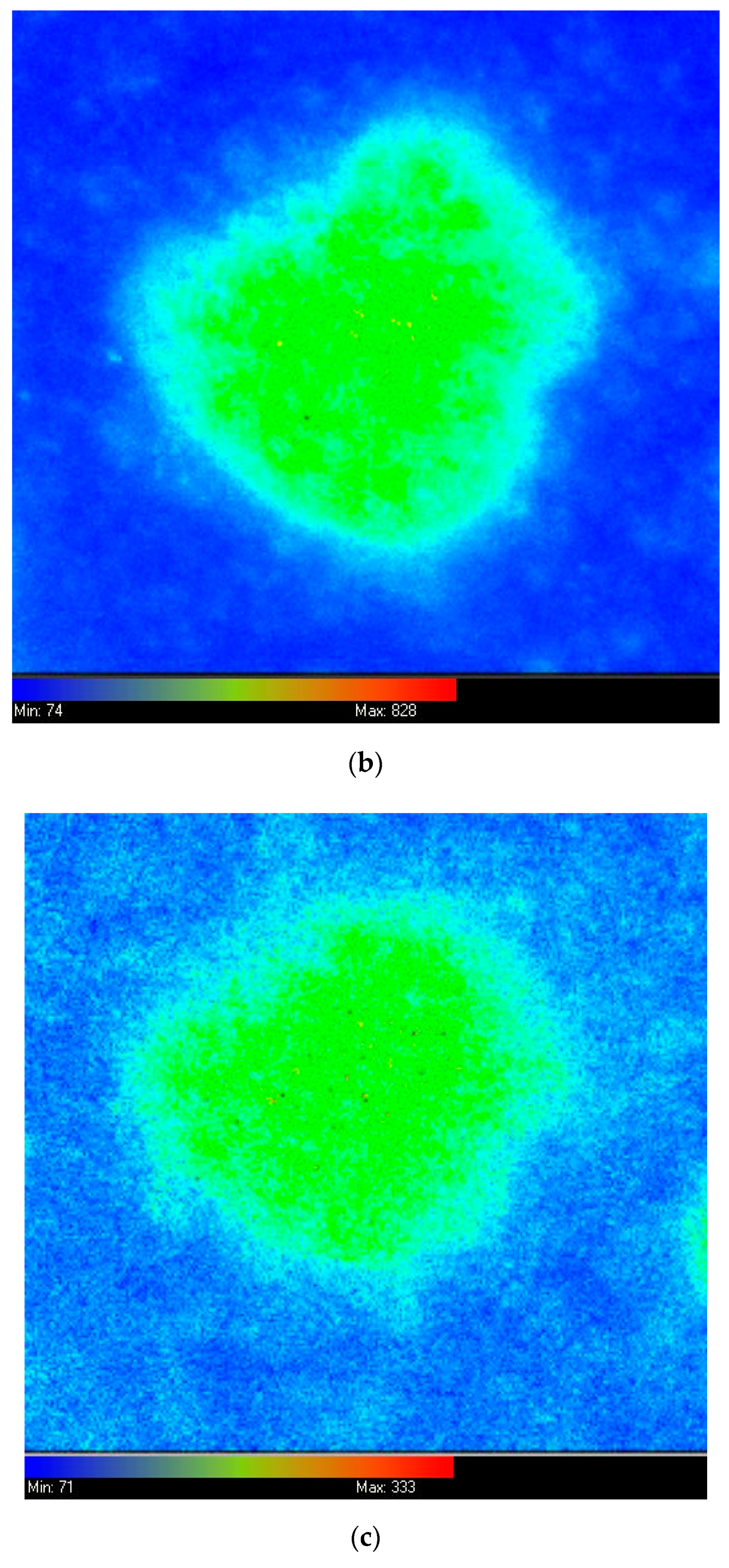
| Demographic | All Patients Included | Patients with Clinical Outcome Data | * P Comparison |
|---|---|---|---|
| N | 207 | 172 | NA |
| Mean age (SD) | 72.7 (10.2) | 73.1 (10.4) | 0.2 |
| Male sex, N (%) | 99 (47.8) | 81 (47.1) | 0.6 |
| Lesion type: | |||
| AK (%) | 82 (39.6) | 64 (37.2) | 0.1 |
| sBCC (%) | 58 (28.0) | 47 (27.3) | |
| BD (%) | 67 (32.4) | 61 (35.5) | |
| Lesion location: | 0.9 | ||
| Acral (%) | 17 (8.2) | 15 (8.7) | |
| Arms (%) | 11 (5.3) | 8 (4.7) | |
| Body (%) | 47 (22.7) | 39 (22.7) | |
| Head (%) | 81 (39.1) | 68 (39.5) | |
| Legs (%) | 51 (24.6) | 42 (24.4) | |
| Mean PpIX accumulation in arbitrary units (SD) | 69.3 (32.1) | 69.9 (31.7) | 0.6 |
| Mean PpIX photobleaching in arbitrary units (SD) | 62.1 (32.2) | 61.5 (30.2) | 0.6 |
| Pain relief used, N (%) | 90 (43.5) | 77 (44.8) | 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyrrell, J.; Paterson, C.; Curnow, A. Regression Analysis of Protoporphyrin IX Measurements Obtained During Dermatological Photodynamic Therapy. Cancers 2019, 11, 72. https://doi.org/10.3390/cancers11010072
Tyrrell J, Paterson C, Curnow A. Regression Analysis of Protoporphyrin IX Measurements Obtained During Dermatological Photodynamic Therapy. Cancers. 2019; 11(1):72. https://doi.org/10.3390/cancers11010072
Chicago/Turabian StyleTyrrell, Jessica, Cheryl Paterson, and Alison Curnow. 2019. "Regression Analysis of Protoporphyrin IX Measurements Obtained During Dermatological Photodynamic Therapy" Cancers 11, no. 1: 72. https://doi.org/10.3390/cancers11010072
APA StyleTyrrell, J., Paterson, C., & Curnow, A. (2019). Regression Analysis of Protoporphyrin IX Measurements Obtained During Dermatological Photodynamic Therapy. Cancers, 11(1), 72. https://doi.org/10.3390/cancers11010072





