Abstract
Several tumor entities have been reported to overexpress KCa3.1 potassium channels due to epigenetic, transcriptional, or post-translational modifications. By modulating membrane potential, cell volume, or Ca2+ signaling, KCa3.1 has been proposed to exert pivotal oncogenic functions in tumorigenesis, malignant progression, metastasis, and therapy resistance. Moreover, KCa3.1 is expressed by tumor-promoting stroma cells such as fibroblasts and the tumor vasculature suggesting a role of KCa3.1 in the adaptation of the tumor microenvironment. Combined, this features KCa3.1 as a candidate target for innovative anti-cancer therapy. However, immune cells also express KCa3.1 thereby contributing to T cell activation. Thus, any strategy targeting KCa3.1 in anti-cancer therapy may also modulate anti-tumor immune activity and/or immunosuppression. The present review article highlights the potential of KCa3.1 as an anti-tumor target providing an overview of the current knowledge on its function in tumor pathogenesis with emphasis on vasculo- and angiogenesis as well as anti-cancer immune responses.
1. Introduction
The KCa3.1 channel, also known as SK4 or IK, is activated by a rise of the intracellular Ca2+ concentration [Ca2+]i. Co-assembly of four KCa3.1 pore-forming α subunits is required to form a functional channel. The basis for their Ca2+ sensitivity is conferred by a constitutively bound calmodulin in the C-terminal tail of each α subunit. Through binding to calmodulin, Ca2+ induces a conformational change that permits channel opening. The single channel conductance of the KCa3.1 ranges between 20 to 80 pS, hence intermediary between the single channel conductance of related Ca2+-activated K+ channels with either small (SK1-3 channels with 5–20 pS) or big conductance (100–300 pS) [1,2,3]. KCa3.1 channels conduct K+ across the membrane of excitable and non-excitable cells, but they lack the typical features of a voltage-sensing domain. Additionally, KCa3.1 activity is also regulated by histidine phosphorylation [4,5,6].
Under physiological conditions, KCa3.1 channels are expressed in epithelial, endothelial, and hematopoietic cells [7], whereas their presence in excitable cells such as central neurons and cardiomyocytes has only recently been recognized [8,9,10]. In secretory epithelia such as lung and intestine, KCa3.1 channels contribute to the normal electrochemical gradient for transepithelial secretion of Cl−, Na+ and H2O [11]. KCa3.1 currents were detected in vascular smooth muscle cells derived from murine arteries subjected to the wire injury model of restenosis, but not in those of uninjured control vessels. Importantly, neointima formation was significantly impaired after targeted disruption of KCa3.1 [12]. Basic fibroblast growth factor- (bFGF) and vascular endothelial growth factor (VEGF)-treated human endothelial cells also upregulated KCa3.1 implying a role in the formation of new blood vessels [13]. Finally, a lack of KCa3.1 causes mild hypertension in the dark phase suggesting a significant role in blood pressure control during physical activity [14]. With regard to its role in the immune system, upregulation and activation of KCa3.1 in T cells in response to antigens and mitogens are well established. In this context, KCa3.1 possibly acts upon a nucleoside diphosphate kinase B-mediated histidine phosphorylation to promote T cell activation ultimately resulting in their clonal expansion [15]. A number of additional KCa3.1 functions point to a role in the migration of lipopolysaccharide-(LPS) activated dendritic cells (DCs) [16], proper mast cell activation after IgE binding [17], the pathogenesis of airway inflammation and remodelling in allergic asthma [18], the prevention of hyper-responsiveness to acute stress by modulating the release of corticotropin from the anterior pituitary gland [19], the processing of pain induced by noxious chemical stimuli [20], neuroinflammation in murine stroke models [21], and renal fibroblast proliferation induced by unilateral urethral obstruction in mice [22]. Finally, a number of studies have revealed that KCa3.1 is involved in Ca2+-dependent K+ efflux from erythrocytes, which in combination with Cl− and H2O movement mediates cell shrinkage, a phenomenon referred to as ‘Gardos effect’ [23].
It is becoming increasingly clear that KCa3.1-dependent signaling pathways affect the immune system and mechanisms of cell proliferation and migration; hence, it is not surprising that KCa3.1 plays a role in cancer development and progression. While the details of tumor-related KCa3.1 functions are subject to continued investigation, KCa3.1 channels have emerged as promising targets for immunomodulation in drug-resistant cancers. This review extends previous important works by others [24,25,26] in highlighting the multitude of KCa3.1 physiological functions and their complex role in cancer.
2. Tumor Cell-Specific Functions of KCa3.1
2.1. Molecular Markers and Regulation of KCNN4
Among the known influences that regulate the expression of the KCa3.1-encoding KCNN4 gene are constitutional, epigenetic, and post-transcriptional variations. In this paragraph, we describe some of these effects on KCNN4 expression as they have been reported for a number of different cancer types including breast, lung, endometrial, and pancreatic cancer.
Sequence variations known as single nucleotide polymorphisms (SNP) may impact on gene expression when located in regulatory sites such as non-coding regions. It is therefore of interest that the SNP rs3760982 located at the intergenic region of KCNN4 and LYPD5 (LY6/PLAUR Domain Containing 5, metastasis-associated protein) on chromosome 19q13.31 has been shown to be associated with breast cancer risk [27], a finding that was corroborated in large scale genome wide association studies (GWAS) using data sets of more than 200,000 patients and controls (P = 1.4 × 10−16 [28]). Notably, the association is strongest in patients with tumors expressing estrogen receptors (ER; P = 4 × 10−14) who are predestined to receive anti-hormonal treatment. A number of KCNN4 SNPs reside within the first intron of the gene, some of which may be associated as well with ER-positive breast cancer risk [29], however, whether or not dysregulated KCNN4 expression is the cause of this risk association and which role the genetic control of the KCa3.1 channel plays for breast cancer development is not clear. At the tumor level, the degree of KCNN4 mRNA expression is potentially useful to stratify breast cancer patients into those with shorter and longer survival time. Data from The Cancer Genome Atlas suggests no difference in KCNN4 mRNA expression between normal and breast tumor tissue [30] (Figure 1A), however, higher KCNN4 expression in the tumor tissue might modify patient outcome as indicated by the shorter overall survival in Kaplan–Meier analysis [31] (Figure 1B). In addition, high KCNN4-mRNA levels were also associated with a lower overall survival, shorter progression-free survival, and a high metastatic potential of patients with clear cell renal cell carcinoma (ccRCC) suggesting that KCa3.1 may be of prognostic value in ccRCC [32].
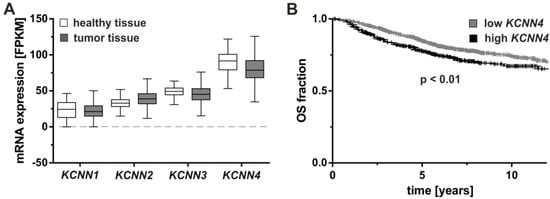
Figure 1.
KCNN4 mRNA expression levels in breast cancer and their association with patient survival. (A) mRNA expression levels of KCNN1-4 coding for SK1-SK3 and KCa3.1 were compared between healthy and breast tumor tissues, measured by RNA sequencing as fragments per kilobase of transcript per million mapped reads (FPKM). Data obtained from The Cancer Genome Atlas [30] revealed no significant difference in a Kruskal–Wallis test with Dunn’s test for multiple comparisons (α = 0.05 for n = 113 healthy and n = 1095 breast tumor tissues). (B) In the Kaplan–Meier plotter [31], significantly prolonged overall survival (OS) was associated with low KCNN4 mRNA levels. Groups were statistically compared by log-rank test (hazard ratio = 1.37 (confidence interval 1.08–1.72) for n = 1030 low and n = 372 high KCNN4-expressing tumors).
Epigenetically, the regulation of gene expression is influenced by chromatin modifications such as acetylation or methylation as well as DNA methylation in the proximity of gene regions. In non-small cell lung cancer, KCNN4 promoter hypomethylation has been observed particularly in advanced-stage tumors. KCNN4 promoter hypomethylation was accompanied by an increase in mRNA expression when compared to normal lung tissue, which was also associated with shorter progression-free and overall survival. Notably, this observation in patients is supported by findings in a model of A549 lung adenocarcinoma cells in which higher KCNN4 mRNA and KCa3.1 protein expression levels, as well as aggressive tumor cell behavior, were observed. Functional tests revealed decreased proliferation and migration upon KCa3.1 inhibition with TRAM-34. Moreover, A549 xenografts in nude mice showed attenuated tumor growth when treated with the KCa3.1 inhibitor senicapoc [33].
The influence of post-transcriptional control via microRNAs (miRNAs) on the expression of KCa3.1 is not well understood. miRNAs are a large family of highly conserved, small non-protein-coding RNA molecules that function as critical regulators of gene expression by triggering either translational repression or degradation of their target mRNAs [34]. Individual miRNAs act either as tumor suppressors by repressing oncogene expression or as oncogenes by repressing tumor suppressor genes. Although KCa3.1 has been observed to be upregulated in pancreatic, breast, and endometrial cancers which affects tumor progression [35,36,37], not much is known about the underlying dysregulation of miRNAs. Yet, in angiosarcoma, miR-497-5p acts in a tumor-suppressive mode as it inhibited cell proliferation and invasion via downregulation of KCa3.1, an observation that highlights both, the regulatory miRNA and the targeted KCa3.1 channel as potential new treatment targets [38]. Similarly, miR-16-5p and miR-375 were identified to have the potential to modulate KCa3.1 expression [39]. MiR-16-5p was among the first downregulated miRNAs identified in chronic lymphocytic leukemia due to frequent deletions [40] and moreover gained wider attention as a regulator of anti-apoptotic BCL2 in prostate and breast cancer [41,42] as well as breast cancer development [43]. Notably, miRNA-16-5p was repressed in MCF-7 breast cancer cells upon 17β-estradiol (E2) treatment, an effect that could be rescued using mimics in order to inhibit E2-induced cell proliferation [44]. The role of KCa3.1 in this process is currently not known. In summary, the available information on KCa3.1 regulation in cancer deserves further attention.
2.2. Tumorigenesis
Cell division is a highly conserved and tightly controlled process that ensures the replication of DNA and its segregation into daughter cells. In tumor cells, control elements of the cell cycle such as growth factors and their receptors or growth-promoting cyclins are often dysregulated due to genetic aberrations. In combination with genetic aberrations affecting tumor suppressor genes, a number of alterations within a single cell may drive uncontrolled proliferation [45,46,47]. Important but often neglected features of the cell cycle are changes in transmembrane ion flux causing fluctuations of the electrical membrane potential or regulatory changes in cell volume. By modulating these processes, KCa3.1 channels may contribute to the abnormal proliferation of tumor cells [47,48,49].
Changes in the cellular resting membrane potential (Vm) are essential for cell cycle progression. When compared to excitable cells, Vm changes in tumor cells occurring during the cell cycle are usually slower and smaller [48,50]. In contrast to proliferating cells, a constant resting Vm is seen for example in striated muscle cells or neurons, which are usually associated with little or no mitotic activity. Activation of K+ channels in the plasma membrane has been shown to cause the more negative membrane potential necessary to initiate this G1/S transition [51]. Hyperpolarization of Vm is also required for G1/S transition, DNA synthesis, and progression through S phase, while Vm depolarization precedes G2/M progression and mitosis entry [47,49]. Accordingly, altered expression and activity of certain K+ channels throughout the different cell cycle phases have been observed [36,48,49,52,53,54]. Studies utilizing a number of K+ channel modulators further emphasize that both temporal and spatial changes in K+ channel activity play a crucial role for the transition from G0 into G1. For example, cell cycle progression was prevented upon blockade of the ATP-sensitive K+ channel or the human Ether-A-Go-Go-Related Protein 1 (KCNH2) in MCF-7 breast tumor cells and in different leukemia cell lines [36,51,52,55,56,57]. With regard to KCa3.1 blockade by the antifungal imidazole, clotrimazole induced MCF-7 cell depolarization and prevented G1/S transition [36]. Furthermore, clotrimazole as well as its more specific analogue TRAM-34 arrested HEC-1-A endometrial cancer cells in G0/G1 phase and suppressed tumor development in nude mice [37]. Moreover, TRAM-34 as well as an RNAi-mediated depletion of KCa3.1 increased the expression of the cyclin-dependent kinase inhibitor p21 important for blockade of G1/S transition and thus suppressed the proliferation of different prostate cancer cell lines. In contrast, the KCa3.1 opener 1-EBIO evoked a clotrimazole-sensitive and concentration-dependent increase in the mitotic cell division [58,59]. In HepG2 hepatocellular carcinoma cells, anti-tumor growth effects of TRAM-34 were linked to a downregulation of ERα and nuclear factor-κB [60,61]. Interestingly, an overexpression of KCa3.1 in the human MDA-MB-231 breast cancer cell line promoted the oncogenic cell growth in an in vivo xenograft model but not in vitro [62]. As the Vm of MDA-MB-231 cells was not altered by KCa3.1 overexpression, pro-tumor functions of this channel in vivo seem to require a crosstalk with microenvironmental factors and/or other signals from non-tumor cells.
The Ca2+ dependence of KCa3.1 activation directly links this channel to an important second messenger and various Ca2+ effector proteins regulating proliferation [63,64]. Ca2+ oscillations occur during G1 phase, G1/S and G2/M transitions as well as between metaphase and anaphase [64,65,66,67]. Cell cycle progression depends on regulated Ca2+ entry. Alterations in [Ca2+]i have, therefore, been associated with abnormal activation of mitogenic pathways in various cancer cell types [65,68]. Through their effect on Vm, K+ channels such as KCa3.1 increase the driving force for Ca2+ influx and thus generate robust [Ca2+]i signals to finally promote tumor cell proliferation [69]. Accordingly, in prostate and pancreatic cancer cells, the Ca2+ influx through transient receptor potential (TRP) vanilloid subfamily member 6 (TRPV6) channels is abolished by blockade of hyperpolarizing KCa3.1 currents. In contrast, escalating extracellular Ca2+ used to artificially increase the driving force for Ca2+ entry stimulated cell proliferation even in the presence of KCa3.1 channel blockers [35,59]. In MCF-7 breast cancer cells, KCa3.1 and Ca2+-permeable canonical TRP subtype 1 (TRPC1) channels accumulate during G1 phase allowing them to interact and regulate basal Ca2+ entry [36,70]. Serum-containing growth factors evoked Ca2+ signals and transition to S phase was suppressed by pharmacological or genetic KCa3.1 blockade in murine breast cancer cells [71]. Recent evidence suggests that ionizing radiation (IR) activates IK channels in glioblastoma cells due to an increase in [Ca2+]i. Accordingly, KCa3.1 inhibition by TRAM-34 suppressed the clonogenic survival of irradiated but not that of unirradiated T98G and U87MG glioblastoma cells. In vivo, co-treatment with TRAM-34 increased the response of an ectopic glioblastoma mouse model to fractionated cancer radiotherapy [72].
Finally, K+ channel pathways may be directly linked to mitogens such as cyclins and immediate early genes. At least in parts, these non-canonical interactions seem to occur independently from K+ permeability changes via yet unknown mechanisms [47,48,49,68,71,73,74,75,76]. In KCa3.1 gene-targeted murine breast cancer cells stimulated with serum, we recently confirmed a significant suppression of c-fos and c-jun mRNA levels. This finding is in accordance with the anti-proliferative and checkpoint functions attributed to KCa3.1 activity [71]. However, K+ permeability and immediate early gene expression have not been assessed directly in KCa3.1-positive versus -negative breast tumor cells and thus the impact of this channel on non-canonical pathways remains largely unclear.
Some studies also challenge the anti-tumorigenic potential of targeting KCa3.1 and its critical role for the proliferation of cancer cells [77,78]. However, it is one serious limitation of the available reports that the applied pharmacological and/or siRNA approaches produce side effects owing to off-target actions. Accordingly, concerns were raised regarding the use of TRAM-34 because this ‘specific’ KCa3.1 blocker was shown to stimulate the proliferation of breast cancer cells via the activation of ERs [79] and, in addition, Agarwal and colleagues suggested that TRAM-34 in low micromolar concentrations may also inhibit multiple cytochrome P450 isoforms [80]. We believe that these discrepancies regarding the proliferative and pharmacological properties of KCa3.1 in cancer cells should be resolved by proof-of-concept studies utilizing, for example, genetically engineered models allowing tumor cell- and tissue-specific knockout or expression of conditionally targeted KCa3.1 alleles in vivo.
2.3. Tumor Cell Apoptosis and Survival
Besides cell cycle progression, the cell cycle control machinery acts to avoid mitotic abnormalities and thereby DNA damage. The latter triggers either its repair or programed cell death ensuring that cells with damaged DNA cannot pass on their genetic information. Tumor cells have developed escape strategies allowing them to avoid cell cycle control and cell death by different mechanisms [81]. In various types of tumor cells, KCa3.1 signaling appears to interfere with apoptotic cell death triggered by transmembrane death receptor and mitochondrial pathways [73,82,83]. Moreover, apoptotic cell death of poorly differentiated triple-negative breast cancer cells was promoted by TRAM-34 [84]. In different melanoma cell lines, TRAM-34 by itself did not elicit apoptosis; however, when applied together with tumor-necrosis-factor-related-apoptosis-inducing-ligand (TRAIL), it stimulated the mitochondrial release of cytochrome c, thereby triggering a cascade of caspase activation. Intriguingly, agonistic TRAIL death receptor expression was found to be upregulated by TRAM-34 suggesting that KCa3.1 plays a key role in sensitizing melanoma cells to TRAIL-induced apoptosis [85]. KCa3.1 channel expression in mitochondria was shown for the HCT116 human colon carcinoma cell line and in HeLa cells where KCa3.1-specific siRNA induced the release of apoptosis-initiating mediators of the intrinsic pathway [78,86]. Apparently, KCa3.1 is also involved in K+ flux across the mitochondrial membrane in tumor cells. As a classical stimulator of the intrinsic apoptotic pathway, staurosporine induced a Ca2+ signal in D54-MG glioma cells that triggered plasma membrane K+ efflux via KCa3.1 resulting in caspase-3 activation and apoptotic volume decrease [83]. In contrast, caspase-3 activity after cisplatin treatment was inhibited by KCa3.1 blockade and amplified under 1-EBIO in epidermoid cancer cells [87]. Kv1.3, a voltage-gated K+ channel that amongst others associates with KCa3.1 for immune activation [88], was already shown to induce mitochondria-dependent apoptosis in lymphocytes [89] but also in cancer cell lines as well as in vivo melanoma and pancreatic cancer models [90,91]. In our previous work, we observed increased histone 2AX (H2AX) phosphorylation on serine 139, an indicator for DNA damage, after irradiating T98G and U87MG glioma cells co-treated with TRAM-34. As KCa3.1 inhibition increased radiosensitivity of glioma cells in vitro and in ectopically growing gliomas in nude mice, we concluded that this channel plays a role in DNA repair processes and thereby in cell survival after radiotherapy [72,92]. In contrast, apoptosis was decreased or even abolished with KCa3.1 inhibition in thymocytes and erythrocytes [93,94]. This discrepancy suggests that KCa3.1 affects the programed cell death either in a cell type-specific manner across cellular differentiation processes even though the anti-apoptotic properties of KCa3.1 seem to dominate.
2.4. Cancer Invasion and Metastasis
One of the biggest challenges in tumor therapy is the local restriction of cancer growth, since approximately 90% of all cancer patients die of secondary tumors [48,81,95]. Migration and infiltration depend on haptotactical and chemotactical signals, Ca2+, cell volume and intracellular signaling cascades modulating cytoskeletal dynamics [63]. K+ channel activity can control any of these processes. As an example, enrichment of a specific splice variant of the big conductance Ca2+- and voltage-activated BK K+ channel has been characterized as crucial factor for the pro-migratory and pro-invasive properties in glioma [96,97,98,99,100]. Likewise, KCa3.1 function has been demonstrated to be required for glioma cell migration and brain infiltration [101,102,103,104,105,106,107]. Beyond, motorizing migration by locally changing the cell volume [108], BK and KCa3.1 K+ channels are part of the Ca2+ signaling complex that programs glioblastoma cell migration [92,98,109,110]. Moreover, BK and KCa3.1 K+ channels are highly expressed in stem-like subpopulations of glioblastoma [111,112,113] where they contribute to the high radioresistance [113] and pronounced migration [111,112] of these cells. Similarly to glioblastoma, K+ channels including KCa3.1 contribute to cell migration and metastasis of extracranial tumors [63,114,115,116].
In particular, KCa3.1 blockade or downregulation in Skov-3 human ovarian cancer cells prevented ATP-induced cell migration possibly due to a loss of interaction between KCa3.1 and the purinergic receptor P2Y2 [117]. Charybdotoxin impaired KCa3.1-mediated locomotion of human A7 and SKMEL28 melanoma cells and it decreased [Ca2+]i and in consequence the polymerization reaction of F-actin [118]. Interfering with KCa3.1 activity by different means resulted in a reduced migration rate of MDA-MB-231 breast cancer cells [84]. However, neither MDA-MB-231 cell division, migration or invasive behaviors were affected by KCa3.1 overexpression or channel activation by 1-EBIO in vitro [62]. Tumor spread in vivo requires a condition of multiple interactions between malignant cells and their environment. Consistent with this understanding, a tumor-promoting microenvironment may amplify the oncogenic properties of the KCa3.1.
3. KCa3.1 in the Tumor Microenvironment
3.1. Tumor Stroma
Cancer-associated fibroblasts (CAF) reportedly communicate with tumor cells and other cells in order to promote tumor growth, angiogenesis, and metastasis [119]. Upregulation of the KCa3.1 by growth factors, especially bFGF and to a minor extent by transforming growth factor-β, was observed in fibroblast-like cell lines, whereas the KCa3.1 status of CAFs is largely unclear so far. In 10T1/2 cells, a murine embryo fibroblast cell line, growth factor-regulated KCa3.1 signaling was linked to the Ras/MEK/ERK pathway and resulted in an accelerated pro-proliferative behavior but diminished myogenic differentiation [120]. In renal fibroblasts, TRAM-34 mitigated the bFGF-induced bromodeoxyuridine (BrdU) incorporation as a marker of cell cycle progression without affecting apoptosis. In a pre-clinical model of renal fibrosis, fibrotic kidneys highly upregulated KCa3.1 transcripts and protein compared to sham-operated kidneys. Furthermore, fibrotic kidneys from KCa3.1 knockout (KO) mice presented with less collagen deposition and fewer α-smooth muscle actin-positive cells as well as a better preservation of functional renal tissue compared to control [22]. In the angiotensin II-stimulated heart, augmented KCa3.1 mRNA and protein levels promoted accumulation of cardiac fibroblasts, an effect which was fully antagonized by TRAM-34 [121,122]. In addition to these growth- and proliferation-stimulating effects, expression and release of pro-inflammatory factors such as interleukin-6 and interleukin-8, monocyte chemotactic protein 1, and matrix metalloproteinase-3 have been linked to KCa3.1 function in synovial fibroblasts that derived from rheumatoid arthritis patients [123]. It needs to be determined how tumor aggressiveness is affected by KCa3.1 function in CAFs. Based on the available studies from other disease models, KCa3.1 in this heterogeneous cell population may have a negative impact on tumor progression and cancer therapy.
3.2. Angiogenesis
In contrast to healthy tissue, growing tumors secure their nutrient and oxygen supply by the induction of angiogenesis, meaning that normally quiescent vessels sprout continuously as part of the so-called angiogenic switch. The developing blood vessels are poorly organized, immature, and not well perfused. Angiogenesis is mostly reached by unbalancing pro-angiogenic and anti-angiogenic factors like VEGF, fibroblast growth factor (FGF) or thrombospondin-1, respectively [81,95,115,124]. The exact mechanisms are poorly understood and possibly depend on the tumor entity, although VEGF and VEGF receptor inhibitors are already applied for the treatment of advanced solid tumors [81,95,125,126].
K+ channels are thought to coordinate angiogenesis by regulation of the Vm and [Ca2+]i as well as by interaction with VEGF or FGF [115]. So far, KCa3.1 activity has not directly been linked to tumor angiogenesis. However, abnormal levels of endothelial cell proliferation are commonly observed in the tumor vasculature [81,95] and bone marrow-derived endothelial progenitor cells expressing KCa3.1 also exhibit a clotrimazole-sensitive K+ current [127]. Furthermore, bFGF and VEGF upregulate KCa3.1 and this was essential for proliferation of HUVEC and HMEC-1 endothelial cells and angiogenesis in vivo. Importantly, bFGF-induced endothelial cell proliferation was sensitive to clotrimazole or TRAM-34, which points to KCa3.1 channels as an important downstream signaling molecule. In an in vivo matrigel plug assay, continuous administration of TRAM-34 for two weeks suppressed angiogenesis in mice [13,128]. KCa3.1 channels were also implicated as important regulators of endothelial cell Vm in human mesenteric endothelium in situ. In the same study, mesenteric arteries from patients with colon cancer showed an increase of endothelial cells expressing KCa3.1 [129]. In response to epidermal growth factor (EGF), both transcriptional and protein levels of KCa3.1 increased in HUVECs, whereas TRAM-34 interfered with the EGF-induced proliferation response, counteracted migration, tube formation, matrix metalloproteinase-2 upregulation and, consequently, it suppressed EGF-mediated angiogenesis in vivo [130]. Upregulation of KCa3.1 promoted platelet-derived growth factor (PDGF)-induced proliferation in vascular smooth muscle cells and, conversely, its modulation by TRAM-34 attenuated the accumulation of cell proliferation markers [131]. Another characteristic of the angiogenic switch refers to bone marrow-derived cells and primarily immune cells that contribute to the building of new vessels via vasculogenesis for example by infiltrating premalignant lesions as well as progressed tumors [81,95]. The potential role(s) of cancer-associated KCa3.1 channels in these cell types will be explained in more detail within the following section. In summary, further evidence is required in order to fully understand how vascular KCa3.1 activity contributes to the blood supply of a tumor in vivo.
3.3. The Immune System
Immune surveillance and tumor-promoting inflammation on the one hand and immune suppression in the tumor microenvironment that may result in tumor immune evasion on the other hand are hallmarks of cancer progression. Inflammation produces factors that stimulate growth and survival, angiogenesis, and epithelial-mesenchymal transition. Additionally, radicals released from immune cells may drive mutagenesis in tumor cells. With regard to immunoediting processes, highly immunogenic tumor cells are detected and eliminated, whereas survival and growth of tumor cell clones that are hardly recognized by the immune system are promoted subsequently. In this context, tumor cells can develop different strategies to avoid immune cell recognition [95]. Both innate and acquired immunity comprise complex mechanisms and a variety of cells that act in concert for rapid and successful defense against foreign and abnormal structures. To this end, immune cells circulate through the body and chemotaxis allows specific immune cell subsets to be recruited to sites of inflammation. Cells of the acquired immune response are primed against an antigen, which is driving their maturation and expansion.
The prominent role of the KCa3.1 channel for proper development and function of the immune system has been recognized by many studies over the last two decades. Together with Kv1.3, KCa3.1 channels are of crucial importance for function of the different T and B cell subsets substantiated by the notion that KCa3.1 channel expression is low in naïve and memory B cells but strongly increases upon their activation [132,133,134]. In primary CD4+ helper T cells expressing tagged KCa3.1, antigen presentation induced recruitment of KCa3.1 to the immunological synapse where it was important for B cell-stimulated [Ca2+]i increase [135]. The effector memory T cell subtype is important for the induction of a rapid secondary immune response. Interestingly, these cells show a low KCa3.1 expression profile in their active state compared to naïve or the central memory T cells [136]. Similar to naïve T cells, regulatory T cells show a rather low KCa3.1 expression [137]. Accordingly, in a murine model of T cell-mediated colitis, the KCa3.1 KO genotype was associated with impaired Ca2+ influx and cytokine production of particular Th0, Th1, and Th2 subsets but had no influence on regulatory T cells or Th17 cells [138].
Moreover, Ca2+ oscillations-mediated TRP melastatin-7 (TRPM7) channel activity has been linked to KCa3.1 function both affecting T cell migration [139]. In addition, KCa3.1 seems to be necessary for the Ca2+-induced apoptotic volume decrease, which is followed by the appearance of phosphatidylserine at the cell surface resulting in T cell depletion [93]. Antigen-dependent differentiation of B cells and germinal center formation require function of the tissue-specific transcriptional coactivator OCA-B and KCNN4 is one of the target genes of OCA-B. Accordingly, OCA-B KO B cells were shown to proliferate less in response to B cell receptor ligation, an effect that involved a strong OCA-B-dependent upregulation of KCNN4 transcription [140]. Additionally, a patient of common variable immunodeficiency carried a KCNN4 gene hypermethylation, whereas its healthy monozygotic twin had no changes regarding KCNN4 methylation status [141]. Another example is provided by a study on chronic lymphocytic leukemia where KCa3.1 mRNA and protein expression were associated with the high proliferation rate of these cells, which could be diminished by TRAM-34 [142].
Regarding antigen-presenting cells, we and others could show that the KCa3.1 contributes to the migration of DCs. Sensitization and stimulation with ovalbumin increased KCa3.1 protein expression in DCs, whereas their chemotaxis in response to the lymphoid chemoattractants CCL19 and CCL21 was abolished with TRAM-34. Accordingly, [Ca2+]i raises were observed upon stimulation of the DCs with either the KCa3.1 activator 1-EBIO, CCL19, or CCL21 [143]. Similarly, KCa3.1 was involved in LPS-derived [Ca2+]i increase, cell swelling and migration of bone marrow-derived DCs in mice [16]. Besides migration, the DC maturation markers CD25 and CD83 were modified by TRAM-34, however, with no impact on the ability of DCs to activate T cells [144]. In addition to antigen presentation, macrophages patrol in the body and degrade foreign structures by phagocytosis. These cells are usually stimulated by factors released by immune cells and they secrete cytokines in order to modulate inflammatory processes. Extracellular ATP, as secreted by many cells during inflammation or infection, was described to provoke KCa3.1-dependent [Ca2+]i oscillations in macrophages. In addition, macrophage stimulation by ATP resulted in transcription of the interleukin-6 (IL-6) gene [145]. Interestingly, LoVo colon cancer cell invasion was stimulated by IL-6 and IL-8, and invasiveness of these cells was enhanced in the presence of tumor-associated macrophages. Most importantly, cancer cell invasion was decreased with depletion of KCa3.1 expression levels in the tumor-associated macrophages [146].
Specialized monocytic cells such as microglia, which are located throughout the brain and spinal cord, show much higher amounts of KCa3.1 mRNA as compared to neurons and astrocytes. LPS stimulation of microglia did not change KCa3.1 expression, but activated their neurotoxic activity, which was sensitive to TRAM-34 [147]. Microglial migration was not promoted by LPS but by IL-4, and this again was blocked by TRAM-34 [148]. In glioma, the grade of malignancy correlates with macrophage and resident microglia infiltration into the tumor and in particular with the presence of M2 macrophages. These cells do not produce pro-inflammatory cytokines, which affects the equilibrium between immune recognition and immune suppression promoting disease progression [149,150]. Along those lines, microglia cultured in glioma-conditioned medium or microglia derived from glioma-bearing mice or human biopsies polarized into an anti-inflammatory and therefore tumor-promoting phenotype. The anti-inflammatory microglia expressed high amounts of KCa3.1 mRNA, and TRAM-34 switched the anti-inflammatory phenotype back to microglia with pro-inflammatory anti-tumor capacity [151].
Natural killer (NK) cells are specialized cytotoxic cells that express both Kv1.3 and KCa3.1 channels with levels independent from their maturation status. Non-adherent NK cells predominantly express Kv1.3, whereas increased KCa3.1 levels are observed in adherent NK cells. TRAM-34 reportedly promoted NK cell proliferation of adherent and non-adherent NK cells and degranulation in adherent NK cells. In vivo, TRAM-34 enhanced the anti-tumor activity of adherent, but not that of non-adherent NK cells. Regarding chemokine receptor expression essential for chemotaxis, a depletion of CX3CR1 was apparent in non-adherent NK cells in the presence of TRAM-34, whereas related receptors such as CCR1, CCR2, CCR5, CXCR3, or CXCR4 as well as cell migration were unaltered in both adherent and non-adherent NK cells [152]. Recently, KCa3.1 mRNA expression was also confirmed in neutrophils, in which the KCa3.1 channel affects cell volume and chemotaxis. KCa3.1 KO mice showed a less effective recruitment of neutrophils to the inflammation site after LPS delivery in the airways. However, TRAM-34 did neither influence Ca2+ entry nor the production of reactive oxygen species in neutrophils [153]. We and others could find evidence for functional KCa3.1 expression in mast cells. Based on β-hexosaminidase as well as histamine release, their degranulation was dependent on KCa3.1 channels. Accordingly, in vivo analysis of KCa3.1 KO mice revealed a lower antigen-provoked decline in body temperature upon IgE challenge as a measure of anaphylactic reaction, when compared to the wildtype mouse [17,154]. Finally, human mast cell migration towards different chemoattractants, but not their proliferation rate, declined with pharmacological KCa3.1 blockade [155].
A comprehensive analysis of peripheral blood revealed no significant difference in blood cell counts between KCa3.1 KO and wildtype mice. Cells tested included erythrocytes as well as total and differential leukocyte count including lymphocytes, eosinophils, neutrophils and monocytes [153]. Importantly, CD19+ B cells, CD4+, and CD8+ T cells as well as CD4+CD25+FOXP3+ cells representing regulatory T cells were not altered by the absence of KCa3.1 [138]. In our recently investigated MMTV-PyMT breast tumor model [71] (Figure 2A), we identified breast tumor-infiltrating leukocytes by using the CD45 pan leukocyte marker. Consistently, a much higher number of CD45+ cells was present in the tumor-surrounding stroma as compared to the tumor itself (Figure 2B). In tumor sections derived from MMTV-PyMT KCa3.1 KO mice, however, CD45+ cells were not detected in the tumor and very rare in the stroma. Together, these data support the notion that inadequate levels of KCa3.1 activity, although not affecting total and differential leukocyte count in vivo, show influence on immune cell maturation and thereby perturb a proper immune cell infiltration of the tumor.
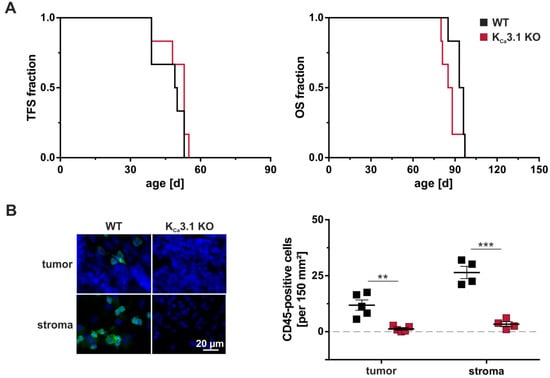
Figure 2.
Tumorigenesis, progression, and CD45 status in MMTV-PyMT WT and KCa3.1 KO mice. Tumor-free survival (TFS) and overall survival (OS) were studied in spontaneous breast cancer-prone MMTV-PyMT wildtype (WT) and KCa3.1 KO mice. At a diameter of 15 mm, tumors were harvested for investigating immune cell infiltration. (A) As previously reported [71], tumorigenesis and tumor progression measured by TFS and OS, respectively, were not dependent on MMTV-PyMT WT or KCa3.1 KO genotypes (n = 6 each). (B) Staining against the CD45 pan leukocyte marker revealed moderate immune cell infiltration in WT tumors (green), which was absent in KCa3.1 KO. Immune cells were generally more abundant in the tumor-surrounding stroma of WT mice, but mostly absent in KCa3.1 KO tumor samples. DAPI labelling was performed to visualize nuclei. Results are presented as means ± SEM for n = 4 stroma sections and n = 5 tumor sections of MMTV-PyMT WT (black squares) or KCa3.1 KO (red squares) genotypes. Unpaired t-tests differentiated between groups with ** p < 0.01 and *** p < 0.001.
3.4. Anti-Cancer Therapy with KCa3.1 Modulators
As shown with numerous examples in the previous sections, KCa3.1 inhibition is a powerful approach to interact with malignant cell cycle progression and thus tumor growth, cell migration, and other tumor-promoting features. The pharmacology of the different KCa3.1 inhibitors including the new benzothiazone NS6180 [156] or the most commonly used inhibitors—clotrimazole, TRAM-34, and senicapoc—which are described in more detail in the following sections, is well-known.
Clotrimazole (1-[(2-Chlorphenyl)diphenylmethyl]-1H-imidazol) is a small molecule, which was primarily designed as an anti-mycotic drug. However, further investigations indicated an inhibition of various cytochrome P450 enzymes, in particular CYP3A4 [157], and blockade of the KCa3.1 channel with an IC50 of 70 nM [156,158]. As already mentioned in the previous sections, clotrimazole reduces cell proliferation in a dose-dependent manner in e.g., human melanoma and glioblastoma cell lines [159,160]. Although clotrimazole has inhibitory effects on cancer cells, other inhibitors not interfering with the cytochrome P450 system and more selective for KCa3.1 should be preferred in experimental and pre-/clinical research [59,161].
One of these agents with improved properties is TRAM-34 (1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole) [84], which is a modified triarylmethane pyrazole analogue of the clotrimazole molecule [59,80,134]. TRAM-34 is currently not in clinical use, but indicates a solid KCa3.1 affinity in animals like rats and mice (IC50 20-25 nM) [80,156,162]. Depending on its concentration, TRAM-34 can almost completely inhibit tumor proliferation of pancreatic ductal adenocarcinoma cells and many other tumor cell types as outlined in the previous chapters of this review [35,163,164]. Senicapoc or ICA-17043 (2,2-bis(4-fluorophenyl)-2-phenylacetamide) is similar to TRAM-34 in its chemical structure, but has a higher affinity for KCa3.1 (IC50 11 nM) [165,166]. An advantage is the oral bioavailability of senicapoc, whose half-life of 12.8 days is also much higher than that of TRAM-34 (2 h) [165,167,168]. So far, senicapoc has been mostly investigated as a possible drug in sickle cell anemia treatment, i.e., phase I and II clinical studies on safety and efficacy have been completed [169]. Senicapoc went into randomized phase III clinical trials where it showed beneficial effects by decreasing certain disease markers, e.g., lactate dehydrogenase and bilirubin. Despite these promising effects, the study was terminated ahead of schedule due to a lack of efficacy in the patient cohort [169,170]. Besides, senicapoc has been proven as effective KCa3.1 inhibitor in experimental cancer research. After a six-day therapy in vivo, intrahepatic cholangiocarcinoma tumor volume and weight of the mice were significantly reduced [164]. Together with its safety profile, further research with senicapoc in cancer is indicated.
Besides KCa3.1 inhibition, its activation especially with regard to immune cell functions in general and in the immune cell’s control of malignant diseases also needs further exploration. The classic KCa3.1 channel opener is 1-EBIO (1-ethyl-2-benzimidazolinone) (EC50 30 µM), which was first described in 1996 [171,172]. It shows a proper effect on some Ca2+-activated K+ channels and could for example rescue ionomycin-induced cell death in head and neck squamous cell carcinoma cells shown by Yin et al. [173]. More potent analogues are DC-EBIO (5,6-dichloro-1-ethyl-1,3-dihydro-2H-benzimidazol-2-one) (EC50 1 µM) [174], NS-309 (3-Oxime-6,7-dichloro-1H-indole-2,3-dione) (EC50 20 nM) [172] and SKA-31 (naphtho[1,2-d]thiazol-2-ylamine) (EC50 250 nM) [175]. Except for SKA-31, all these compounds are not completely KCa3.1-selective [176] and need a minimum of Ca2+ to be effective [171,175,177]. Interestingly, clotrimazole and TRAM-34 could abolish 1-EBIO-induced cell proliferation in hepatocellular and in prostate cancer cells [58,178].
4. Conclusions
It has only recently been recognized that KCa3.1 contributes to the malignant cell behaviors seen in cancer. Based on the available data, “oncochannels” such as KCa3.1 as well as dysregulated signaling pathways that depend on these channels may be promising candidates in the therapy of various solid tumors including, among others, glioblastoma, endometrial, prostate, breast, hepatocellular, and cervical carcinoma. Such considerations seem justified, as an effective inhibition of KCa3.1 by genetic and pharmacological means markedly reduces the proliferation of tumor cells and it may also alter the susceptibility of the tumor towards established cancer therapies. In this respect, senicapoc, which proved safe in clinical trials on sickle cell anemia, represents a repurposable candidate drug for future investigations into the anti-tumor action of KCa3.1 inhibition. So far, targeting of KCa3.1 by senicapoc is suggested in combination with existing chemoradiotherapy regimes to tackle the therapy-resistant cancer (stem) cells [102,109,113,179].
Besides adverse effects of KCa3.1 action stemming from the tumor cell itself, KCa3.1 might also be important for the supply of the tumor with pro-tumorigenic factors from cells interacting with the tumor. To adequately and accurately meet the specific challenges of cancer, it will be necessary to better characterize the tumor environment with respect to KCa3.1 channel functions in stromal cell types, tumor microvasculature, and in the immune system. A dysregulation of KCa3.1 in the latter may impair both detection and destruction of aberrant cells, which is in support of the establishment of a tumor in its niche rather than its elimination. Paradoxically, tumor cells that acquire the ability to escape immune recognition further progress by responding to pro-inflammatory factors secreted from invading immune cells. Therefore, it is also tempting to speculate that KCa3.1 inhibition might delay the progression of such immune-evaded tumors (Figure 3).
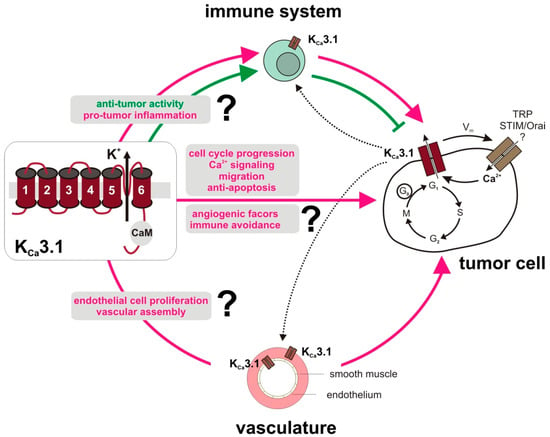
Figure 3.
Role of the KCa3.1 channel in tumor-associated cells. Tumors from different entities and various microenvironmental cell types, i.e., immune cells, vasculature, fibroblasts (not shown) express functional KCa3.1 channels. Physiological roles and tumor behaviors of KCa3.1 are cell type-dependent, but involve proliferation, migration and cancer progression. KCa3.1 channel expression seems to be a crucial determinant of cancer risk and, in established cancers, KCa3.1 upregulation at the end of G1 phase of the cell cycle was seen in various tumor cell types [36]. By its interaction with [Ca2+]i via its constitutively bound calmodulin (CaM), with other ion channels such as TRP or STIM/Orai, with changes in the membrane potential (Vm), and with apoptotic pathways, KCa3.1 may further contribute to aberrant tumor cell signaling. Beyond that, tumor-promoting KCa3.1 activity in stromal cells has been described. Several studies find evidence for KCa3.1 expression in endothelial and in activated smooth muscle cells of the vasculature pointing to its role in tumor angiogenesis and/or vasculogenesis. Moreover, growth factor signaling was linked to KCa3.1 in fibroblasts to promote epithelial-mesenchymal transition in breast cancer (not depicted) [84]. Proper activation and function of various immune cell subsets requires KCa3.1. Therefore, perturbed KCa3.1 signaling may prevent cancer progression and disturb e.g., the immune cell´s pro-angiogenic program, but also its activity to recognize and eliminate tumor cells. Apparently, the impact of a tumor and stromal versus immune cell KCa3.1 inhibition on tumor progression and therapy success and thus also interaction between the different cell types, as indicated by dotted lanes, requires further investigations.
Finally, we should make every effort to intensify the genomic/epigenomic and transcriptomic profiling of putative “oncochannels” within population- and patient-based screenings because this will allow estimations if and how these channels affect cancer development and progression, as well as sensitivity to drug treatment. To this end, such comprehensive data sets should provide important information for the prediction of patient outcome in order to facilitate personalized drug treatments.
Author Contributions
Wrote or contributed to the writing of the manuscript: C.J.M., F.A.S., D.G., and R.L.; contributed to discussion: S.M.H., H.B., W.-Y.L., W.S., and R.H.; edited the manuscript and approved its final version: all authors.
Funding
Work in the authors’ laboratories was funded in parts by the Deutsche Forschungsgemeinschaft with grants to P.R. and R.L., the German Cancer Aid with a grant to P.R. and S.M.H. (70112872, 70113144) and the ICEPHA Graduate Program “Membrane-associated Drug Targets in Personalized Cancer Medicine” (to P.R., H.B., W.S., S.M.H., and R.L.). W.-Y.L. has been a fellow of the FP7 Initial Training Network FightingDrugFailure (GA238132). F.A.S received a scholarship from the Studienstiftung des deutschen Volkes for her PhD studies.
Conflicts of Interest
S.M.H has a research collaboration with Novocure, Haifa, Israel.
References
- Vergara, C.; Latorre, R.; Marrion, N.V.; Adelman, J.P. Calcium-activated potassium channels. Curr. Opin. Neurobiol. 1998, 8, 321–329. [Google Scholar] [CrossRef]
- Kohler, M.; Hirschberg, B.; Bond, C.T.; Kinzie, J.M.; Marrion, N.V.; Maylie, J.; Adelman, J.P. Small-conductance, calcium-activated potassium channels from mammalian brain. Science 1996, 273, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Oberhauser, A.; Labarca, P.; Alvarez, O. Varieties of calcium-activated potassium channels. Annu. Rev. Physiol. 1989, 51, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Fanger, C.M.; Ghanshani, S.; Logsdon, N.J.; Rauer, H.; Kalman, K.; Zhou, J.; Beckingham, K.; Chandy, K.G.; Cahalan, M.D.; Aiyar, J. Calmodulin mediates calcium-dependent activation of the intermediate conductance KCa channel, IKCa1. J. Biol. Chem. 1999, 274, 5746–5754. [Google Scholar] [CrossRef] [PubMed]
- Gueguinou, M.; Chantome, A.; Fromont, G.; Bougnoux, P.; Vandier, C.; Potier-Cartereau, M. KCa and Ca(2+) channels: The complex thought. Biochim. Biophys. Acta 2014, 1843, 2322–2333. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Panda, S.; Li, Z.; Fuhs, S.R.; Hunter, T.; Thiele, D.J.; Hubbard, S.R.; Skolnik, E.Y. Histidine phosphorylation relieves copper inhibition in the mammalian potassium channel KCa3.1. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.S.; Strobaek, D.; Olesen, S.P.; Christophersen, P. The Ca2+-activated K+ channel of intermediate conductance: A molecular target for novel treatments? Curr. Drug. Targets 2001, 2, 401–422. [Google Scholar] [CrossRef] [PubMed]
- Engbers, J.D.; Anderson, D.; Asmara, H.; Rehak, R.; Mehaffey, W.H.; Hameed, S.; McKay, B.E.; Kruskic, M.; Zamponi, G.W.; Turner, R.W. Intermediate conductance calcium-activated potassium channels modulate summation of parallel fiber input in cerebellar Purkinje cells. Proc. Natl. Acad. Sci. USA 2012, 109, 2601–2606. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.W.; Kruskic, M.; Teves, M.; Scheidl-Yee, T.; Hameed, S.; Zamponi, G.W. Neuronal expression of the intermediate conductance calcium-activated potassium channel KCa3.1 in the mammalian central nervous system. Pflugers Arch. 2015, 467, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Weisbrod, D.; Peretz, A.; Ziskind, A.; Menaker, N.; Oz, S.; Barad, L.; Eliyahu, S.; Itskovitz-Eldor, J.; Dascal, N.; Khananshvili, D.; et al. SK4 Ca2+ activated K+ channel is a critical player in cardiac pacemaker derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, E1685–E1694. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.S.; Hertz, M.; Christophersen, P.; Madsen, L.S. The Ca2+-activated K+ channel of intermediate conductance: A possible target for immune suppression. Expert Opin. Ther. Targets 2002, 6, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.B.; Feng, Y.X.; Sun, Q.; Lukowski, R.; Qiu, Y.; Spiger, K.; Li, Z.; Ruth, P.; Korth, M.; Skolnik, E.Y.; et al. Nucleoside diphosphate kinase B-activated intermediate conductance potassium channels are critical for neointima formation in mouse carotid arteries. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Grgic, I.; Eichler, I.; Heinau, P.; Si, H.; Brakemeier, S.; Hoyer, J.; Kohler, R. Selective blockade of the intermediate-conductance Ca2+-activated K+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Kohler, R.; Ruth, P. Endothelial dysfunction and blood pressure alterations in K+-channel transgenic mice. Pflugers Arch. 2010, 459, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Panyi, G.; Possani, L.D.; Rodriguez de la Vega, R.C.; Gaspar, R.; Varga, Z. K+ channel blockers: Novel tools to inhibit T cell activation leading to specific immunosuppression. Curr. Pharm. Des. 2006, 12, 2199–2220. [Google Scholar] [CrossRef] [PubMed]
- Grobner, S.; Lukowski, R.; Autenrieth, I.B.; Ruth, P. Lipopolysaccharide induces cell volume increase and migration of dendritic cells. Microbiol. Immunol. 2014, 58, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Shumilina, E.; Lam, R.S.; Wolbing, F.; Matzner, N.; Zemtsova, I.M.; Sobiesiak, M.; Mahmud, H.; Sausbier, U.; Biedermann, T.; Ruth, P.; et al. Blunted IgE-mediated activation of mast cells in mice lacking the Ca2+-activated K.+ channel KCa3.1. J. Immunol. 2008, 180, 8040–8047. [Google Scholar] [CrossRef]
- Yu, Z.H.; Xu, J.R.; Wang, Y.X.; Xu, G.N.; Xu, Z.P.; Yang, K.; Wu, D.Z.; Cui, Y.Y.; Chen, H.Z. Targeted inhibition of KCa3.1 channel attenuates airway inflammation and remodeling in allergic asthma. Am. J. Respir. Cell Mol. Biol. 2013, 48, 685–693. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, L.; McClafferty, H.; Lukowski, R.; MacGregor, D.; King, J.T.; Rizzi, S.; Sausbier, M.; McCobb, D.P.; Knaus, H.G.; et al. Control of hypothalamic-pituitary-adrenal stress axis activity by the intermediate conductance calcium-activated potassium channel, SK4. J. Physiol. 2011, 589, 5965–5986. [Google Scholar] [CrossRef]
- Lu, R.; Flauaus, C.; Kennel, L.; Petersen, J.; Drees, O.; Kallenborn-Gerhardt, W.; Ruth, P.; Lukowski, R.; Schmidtko, A. KCa3.1 channels modulate the processing of noxious chemical stimuli in mice. Neuropharmacology 2017, 125, 386–395. [Google Scholar] [CrossRef]
- Chen, Y.J.; Nguyen, H.M.; Maezawa, I.; Grossinger, E.M.; Garing, A.L.; Kohler, R.; Jin, L.W.; Wulff, H. The potassium channel KCa3.1 constitutes a pharmacological target for neuroinflammation associated with ischemia/reperfusion stroke. J. Cereb. Blood Flow Metab. 2016, 36, 2146–2161. [Google Scholar] [CrossRef]
- Grgic, I.; Kiss, E.; Kaistha, B.P.; Busch, C.; Kloss, M.; Sautter, J.; Muller, A.; Kaistha, A.; Schmidt, C.; Raman, G.; et al. Renal fibrosis is attenuated by targeted disruption of KCa3.1 potassium channels. Proc. Natl. Acad. Sci. USA 2009, 106, 14518–14523. [Google Scholar] [CrossRef] [PubMed]
- Maher, A.D.; Kuchel, P.W. The Gardos channel: A review of the Ca2+-activated K+ channel in human erythrocytes. Int. J. Biochem. Cell Biol. 2003, 35, 1182–1197. [Google Scholar] [CrossRef]
- Gallagher, P.G. Disorders of erythrocyte hydration. Blood 2017, 130, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- Sugunan, S.; Nampoothiri, S.S.; Garg, T.; Krishnamurthy, R.G. Role of KCa3.1 Channels in CNS Diseases: A Concise Review. CNS Neurol. Disord. Drug Targets 2016, 15, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Heyken, W.T.; Wolfle, S.E.; Tysiac, M.; Schubert, R.; Grgic, I.; Vilianovich, L.; Giebing, G.; Maier, T.; Gross, V.; et al. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ. Res. 2006, 99, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, K.; Hall, P.; Gonzalez-Neira, A.; Ghoussaini, M.; Dennis, J.; Milne, R.L.; Schmidt, M.K.; Chang-Claude, J.; Bojesen, S.E.; Bolla, M.K.; et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat.Genet. 2013, 45, 353–361. [Google Scholar] [CrossRef]
- Michailidou, K.; Lindstrom, S.; Dennis, J.; Beesley, J.; Hui, S.; Kar, S.; Lemacon, A.; Soucy, P.; Glubb, D.; Rostamianfar, A.; et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017, 551, 92–94. [Google Scholar] [CrossRef]
- Lo, W.Y.; Mohr, C.; Steudel, F.; Schmidt, M.; Easton, D.; Hoppe, R.; Schroth, W.; Ruth, P.; Lukowski, R.; Brauch, H.; et al. The role of genetic variation in calcium-activated potassium channels in breast cancer patients treated with tamoxifen. Cancer Res. 2016, 76, 2030. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Nextwork; Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Gyorffy, B.; Lanczky, A.; Eklund, A.C.; Denkert, C.; Budczies, J.; Li, Q.; Szallasi, Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res. Treat. 2010, 123, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Rabjerg, M.; Olivan-Viguera, A.; Hansen, L.K.; Jensen, L.; Sevelsted-Moller, L.; Walter, S.; Jensen, B.L.; Marcussen, N.; Kohler, R. High expression of KCa3.1 in patients with clear cell renal carcinoma predicts high metastatic risk and poor survival. PLoS ONE 2015, 10, e0122992. [Google Scholar] [CrossRef] [PubMed]
- Bulk, E.; Ay, A.S.; Hammadi, M.; Ouadid-Ahidouch, H.; Schelhaas, S.; Hascher, A.; Rohde, C.; Thoennissen, N.H.; Wiewrodt, R.; Schmidt, E.; et al. Epigenetic dysregulation of KCa 3.1 channels induces poor prognosis in lung cancer. Int. J. Cancer 2015, 137, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Jager, H.; Dreker, T.; Buck, A.; Giehl, K.; Gress, T.; Grissmer, S. Blockage of intermediate-conductance Ca2+-activated K+ channels inhibit human pancreatic cancer cell growth in vitro. Mol. Pharmacol. 2004, 65, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Ouadid-Ahidouch, H.; Roudbaraki, M.; Delcourt, P.; Ahidouch, A.; Joury, N.; Prevarskaya, N. Functional and molecular identification of intermediate-conductance Ca(2+)-activated K(+) channels in breast cancer cells: Association with cell cycle progression. Am. J. Physiol. Cell Physiol. 2004, 287, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Shen, B.; Yao, H.L.; Jia, Y.C.; Ren, J.; Feng, Y.J.; Wang, Y.Z. Blockage of intermediate-conductance-Ca(2+)-activated K(+) channels inhibits progression of human endometrial cancer. Oncogene 2007, 26, 5107–5114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kuang, D.; Zhao, X.; Chen, D.; Wang, X.; Yang, Q.; Wan, J.; Zhu, Y.; Wang, Y.; Zhang, S.; et al. miR-497-5p inhibits cell proliferation and invasion by targeting KCa3.1 in angiosarcoma. Oncotarget 2016, 7, 58148–58161. [Google Scholar] [CrossRef] [PubMed]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent deletions and downregulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef]
- Bonci, D.; Coppola, V.; Musumeci, M.; Addario, A.; Giuffrida, R.; Memeo, L.; D’Urso, L.; Pagliuca, A.; Biffoni, M.; Labbaye, C.; et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat. Med. 2008, 14, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Cittelly, D.M.; Das, P.M.; Salvo, V.A.; Fonseca, J.P.; Burow, M.E.; Jones, F.E. Oncogenic HER2{Delta}16 suppresses miR-15a/16 and deregulates BCL-2 to promote endocrine resistance of breast tumors. Carcinogenesis 2010, 31, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.A.; Venturutti, L.; Huang, Y.W.; Schillaci, R.; Huang, T.H.; Elizalde, P.V. Downregulation of the tumor-suppressor miR-16 via progestin-mediated oncogenic signaling contributes to breast cancer development. Breast Cancer Res. 2012, 14, R77. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, X.; Dhakal, I.B.; Beggs, M.; Kadlubar, S.; Luo, D. Induction of cell proliferation and survival genes by estradiol-repressed microRNAs in breast cancer cells. BMC Cancer 2012, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Pines, J. Cell proliferation and control. Curr. Opin. Cell Biol. 1992, 4, 144–148. [Google Scholar] [CrossRef]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef]
- Urrego, D.; Tomczak, A.P.; Zahed, F.; Stuhmer, W.; Pardo, L.A. Potassium channels in cell cycle and cell proliferation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130094. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jan, L.Y. Targeting potassium channels in cancer. J. Cell Biol. 2014, 206, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Blackiston, D.J.; McLaughlin, K.A.; Levin, M. Bioelectric controls of cell proliferation: Ion channels, membrane voltage and the cell cycle. Cell Cycle 2009, 8, 3527–3536. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.A.; Iliev, I.G.; Schwalke, M.A.; Gonzalez, E.; Marler, K.C.; Flanagan, C.A. Association between cell membrane potential and breast cancer. Tumor Biol. 1994, 15, 82–89. [Google Scholar] [CrossRef]
- Woodfork, K.A.; Wonderlin, W.F.; Peterson, V.A.; Strobl, J.S. Inhibition of ATP-sensitive potassium channels causes reversible cell-cycle arrest of human breast cancer cells in tissue culture. J. Cell. Physiol. 1995, 162, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Jehle, J.; Schweizer, P.A.; Katus, H.A.; Thomas, D. Novel roles for hERG K(+) channels in cell proliferation and apoptosis. Cell Death Dis. 2011, 2, e193. [Google Scholar] [CrossRef] [PubMed]
- Crociani, O.; Guasti, L.; Balzi, M.; Becchetti, A.; Wanke, E.; Olivotto, M.; Wymore, R.S.; Arcangeli, A. Cell cycle-dependent expression of HERG1 and HERG1B isoforms in tumor cells. J. Biol. Chem. 2003, 278, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Ouadid-Ahidouch, H.; Roudbaraki, M.; Ahidouch, A.; Delcourt, P.; Prevarskaya, N. Cell-cycle-dependent expression of the large Ca2+-activated K+ channels in breast cancer cells. Biochem. Biophys. Res. Commun. 2004, 316, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Wonderlin, W.F.; Strobl, J.S. Potassium channels, proliferation and G1 progression. J. Membr. Biol. 1996, 154, 91–107. [Google Scholar] [CrossRef]
- Wonderlin, W.F.; Woodfork, K.A.; Strobl, J.S. Changes in membrane potential during the progression of MCF-7 human mammary tumor cells through the cell cycle. J. Cell. Physiol. 1995, 165, 177–185. [Google Scholar] [CrossRef]
- Pillozzi, S.; Brizzi, M.F.; Balzi, M.; Crociani, O.; Cherubini, A.; Guasti, L.; Bartolozzi, B.; Becchetti, A.; Wanke, E.; Bernabei, P.A.; et al. HERG potassium channels are constitutively expressed in primary human acute myeloid leukemias and regulate cell proliferation of normal and leukemic hemopoietic progenitors. Leukemia 2002, 16, 1791–1798. [Google Scholar] [CrossRef]
- Parihar, A.S.; Coghlan, M.J.; Gopalakrishnan, M.; Shieh, C.C. Effects of intermediate-conductance Ca2+-activated K+ channel modulators on human prostate cancer cell proliferation. Eur. J. Pharmacol. 2003, 471, 157–164. [Google Scholar] [CrossRef]
- Lallet-Daher, H.; Roudbaraki, M.; Bavencoffe, A.; Mariot, P.; Gackiere, F.; Bidaux, G.; Urbain, R.; Gosset, P.; Delcourt, P.; Fleurisse, L.; et al. Intermediate-conductance Ca2+-activated K+ channels (IKCa1) regulate human prostate cancer cell proliferation through a close control of calcium entry. Oncogene 2009, 28, 1792–1806. [Google Scholar] [CrossRef]
- Freise, C.; Ruehl, M.; Seehofer, D.; Hoyer, J.; Somasundaram, R. The inhibitor of Ca(2+)-dependent K+ channels TRAM-34 blocks growth of hepatocellular carcinoma cells via downregulation of estrogen receptor alpha mRNA and nuclear factor-kappaB. Investig. New Drugs 2013, 31, 452–457. [Google Scholar] [CrossRef]
- Yang, X.W.; Liu, J.W.; Zhang, R.C.; Yin, Q.; Shen, W.Z.; Yi, J.L. Inhibitory effects of blockage of intermediate conductance Ca(2+)-activated K (+) channels on proliferation of hepatocellular carcinoma cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Thurber, A.E.; Nelson, M.; Frost, C.L.; Levin, M.; Brackenbury, W.J.; Kaplan, D.L. IK channel activation increases tumor growth and induces differential behavioral responses in two breast epithelial cell lines. Oncotarget 2017, 8, 42382–42397. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.M. Oncochannels. Cell Calcium 2013, 53, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Kahl, C.R.; Means, A.R. Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr. Rev. 2003, 24, 719–736. [Google Scholar] [CrossRef] [PubMed]
- Roderick, H.L.; Cook, S.J. Ca2+ signaling checkpoints in cancer: Remodelling Ca2+ for cancer cell proliferation and survival. Nat. Rev. Cancer 2008, 8, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Santella, L. The role of calcium in the cell cycle: Facts and hypotheses. Biochem. Biophys. Res.Commun. 1998, 244, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Calcium signaling and cell proliferation. Bioessays 1995, 17, 491–500. [Google Scholar] [CrossRef]
- Cook, S.J.; Lockyer, P.J. Recent advances in Ca(2+)-dependent Ras regulation and cell proliferation. Cell Calcium 2006, 39, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Ouadid-Ahidouch, H.; Ahidouch, A. K(+) channels and cell cycle progression in tumor cells. Front. Physiol. 2013, 4, 220. [Google Scholar] [CrossRef]
- Faouzi, M.; Hague, F.; Geerts, D.; Ay, A.S.; Potier-Cartereau, M.; Ahidouch, A.; Ouadid-Ahidouch, H. Functional cooperation between KCa3.1 and TRPC1 channels in human breast cancer: Role in cell proliferation and patient prognosis. Oncotarget 2016, 7, 36419–36435. [Google Scholar] [CrossRef]
- Steudel, F.A.; Mohr, C.J.; Stegen, B.; Nguyen, H.Y.; Barnert, A.; Steinle, M.; Beer-Hammer, S.; Koch, P.; Lo, W.Y.; Schroth, W.; et al. SK4 channels modulate Ca2+ signaling and cell cycle progression in murine breast cancer. Mol. Oncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Stegen, B.; Butz, L.; Klumpp, L.; Zips, D.; Dittmann, K.; Ruth, P.; Huber, S.M. Ca2+-Activated IK K+ Channel Blockade Radiosensitizes Glioblastoma Cells. Mol. Cancer Res. 2015, 13, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Roles of K+ channels in regulating tumor cell proliferation and apoptosis. Pflugers Arch. 2004, 448, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Strobl, J.S.; Wonderlin, W.F.; Flynn, D.C. Mitogenic signal transduction in human breast cancer cells. Gen. Pharmacol. 1995, 26, 1643–1649. [Google Scholar] [CrossRef]
- Sheng, M.; Greenberg, M.E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 1990, 4, 477–485. [Google Scholar] [CrossRef]
- Millership, J.E.; Devor, D.C.; Hamilton, K.L.; Balut, C.M.; Bruce, J.I.; Fearon, I.M. Calcium-activated K+ channels increase cell proliferation independent of K+ conductance. Am. J. Physiol. Cell Physiol. 2011, 300, C792–C802. [Google Scholar] [CrossRef] [PubMed]
- Abdullaev, I.F.; Rudkouskaya, A.; Mongin, A.A.; Kuo, Y.H. Calcium-activated potassium channels BK and IK1 are functionally expressed in human gliomas but do not regulate cell proliferation. PLoS ONE 2010, 5, e12304. [Google Scholar] [CrossRef]
- Sassi, N.; De Marchi, U.; Fioretti, B.; Biasutto, L.; Gulbins, E.; Franciolini, F.; Szabo, I.; Zoratti, M. An investigation of the occurrence and properties of the mitochondrial intermediate-conductance Ca2+-activated K+ channel mtKCa3.1. Biochim. Biophys. Acta 2010, 1797, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.W.; Cowley, E.A.; Blay, J.; Linsdell, P. The intermediate conductance Ca2+-activated K+ channel inhibitor TRAM-34 stimulates proliferation of breast cancer cells via activation of estrogen receptors. Br. J. Pharmacol. 2010, 159, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, J.J.; Zhu, Y.; Zhang, Q.Y.; Mongin, A.A.; Hough, L.B. TRAM-34, a putatively selective blocker of intermediate-conductance, calcium-activated potassium channels, inhibits cytochrome P450 activity. PLoS ONE 2013, 8, e63028. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Burg, E.D.; Remillard, C.V.; Yuan, J.X. K+ channels in apoptosis. J. Membr. Biol. 2006, 209, 3–20. [Google Scholar] [CrossRef] [PubMed]
- McFerrin, M.B.; Turner, K.L.; Cuddapah, V.A.; Sontheimer, H. Differential role of IK and BK potassium channels as mediators of intrinsic and extrinsic apoptotic cell death. Am. J. Physiol. Cell Physiol. 2012, 303, C1070–C1078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, X.; Yin, Q.; Yi, J.; Shen, W.; Zhao, L.; Zhu, Z.; Liu, J. Inhibition of SK4 Potassium Channels Suppresses Cell Proliferation, Migration and the Epithelial-Mesenchymal Transition in Triple-Negative Breast Cancer Cells. PLoS ONE 2016, 11, e0154471. [Google Scholar] [CrossRef] [PubMed]
- Quast, S.A.; Berger, A.; Buttstadt, N.; Friebel, K.; Schonherr, R.; Eberle, J. General Sensitization of melanoma cells for TRAIL-induced apoptosis by the potassium channel inhibitor TRAM-34 depends on release of SMAC. PLoS ONE 2012, 7, e39290. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, U.; Sassi, N.; Fioretti, B.; Catacuzzeno, L.; Cereghetti, G.M.; Szabo, I.; Zoratti, M. Intermediate conductance Ca2+-activated potassium channel (KCa3.1) in the inner mitochondrial membrane of human colon cancer cells. Cell Calcium 2009, 45, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.L.; Hasegawa, Y.; Shimizu, T.; Okada, Y. IK1 channel activity contributes to cisplatin sensitivity of human epidermoid cancer cells. Am. J. Physiol. Cell Physiol. 2008, 294, C1398–C1406. [Google Scholar] [CrossRef]
- Lam, J.; Wulff, H. The Lymphocyte Potassium Channels Kv1.3 and KCa3.1 as Targets for Immunosuppression. Drug. Dev. Res. 2011, 72, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Szabo, I.; Bock, J.; Grassme, H.; Soddemann, M.; Wilker, B.; Lang, F.; Zoratti, M.; Gulbins, E. Mitochondrial potassium channel Kv1.3 mediates Bax-induced apoptosis in lymphocytes. Proc. Natl. Acad. Sci. USA 2008, 105, 14861–14866. [Google Scholar] [CrossRef]
- Leanza, L.; Henry, B.; Sassi, N.; Zoratti, M.; Chandy, K.G.; Gulbins, E.; Szabo, I. Inhibitors of mitochondrial Kv1.3 channels induce Bax/Bak-independent death of cancer cells. EMBO Mol. Med. 2012, 4, 577–593. [Google Scholar] [CrossRef]
- Leanza, L.; Romio, M.; Becker, K.A.; Azzolini, M.; Trentin, L.; Manago, A.; Venturini, E.; Zaccagnino, A.; Mattarei, A.; Carraretto, L.; et al. Direct Pharmacological Targeting of a Mitochondrial Ion Channel Selectively Kills Tumor Cells In Vivo. Cancer Cell 2017, 31, 516–531.e10. [Google Scholar] [CrossRef] [PubMed]
- Stegen, B.; Klumpp, L.; Misovic, M.; Edalat, L.; Eckert, M.; Klumpp, D.; Ruth, P.; Huber, S.M. K(+) channel signaling in irradiated tumor cells. Eur. Biophys. J. 2016, 45, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.I.; Higgins, C.F. IKCa1 activity is required for cell shrinkage, phosphatidylserine translocation and death in T lymphocyte apoptosis. EMBO Rep. 2003, 4, 189–194. [Google Scholar] [CrossRef]
- Lang, P.A.; Kaiser, S.; Myssina, S.; Wieder, T.; Lang, F.; Huber, S.M. Role of Ca2+-activated K+ channels in human erythrocyte apoptosis. Am. J. Physiol. Cell Physiol. 2003, 285, C1553–C1560. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Ge, L.; Hoa, N.T.; Wilson, Z.; Arismendi-Morillo, G.; Kong, X.T.; Tajhya, R.B.; Beeton, C.; Jadus, M.R. Big Potassium (BK) ion channels in biology, disease and possible targets for cancer immunotherapy. Int. Immunopharmacol. 2014, 22, 427–443. [Google Scholar] [CrossRef]
- Wondergem, R.; Bartley, J.W. Menthol increases human glioblastoma intracellular Ca2+, BK channel activity and cell migration. J. Biomed. Sci. 2009, 16, 90. [Google Scholar] [CrossRef]
- Steinle, M.; Palme, D.; Misovic, M.; Rudner, J.; Dittmann, K.; Lukowski, R.; Ruth, P.; Huber, S.M. Ionizing radiation induces migration of glioblastoma cells by activating BK K(+) channels. Radiother. Oncol. 2011, 101, 122–126. [Google Scholar] [CrossRef]
- Edalat, L.; Stegen, B.; Klumpp, L.; Haehl, E.; Schilbach, K.; Lukowski, R.; Kuhnle, M.; Bernhardt, G.; Buschauer, A.; Zips, D.; et al. BK K+ channel blockade inhibits radiation-induced migration/brain infiltration of glioblastoma cells. Oncotarget 2016, 7, 14259–14278. [Google Scholar] [CrossRef]
- Rosa, P.; Catacuzzeno, L.; Sforna, L.; Mangino, G.; Carlomagno, S.; Mincione, G.; Petrozza, V.; Ragona, G.; Franciolini, F.; Calogero, A. BK channels blockage inhibits hypoxia-induced migration and chemoresistance to cisplatin in human glioblastoma cells. J. Cell Physiol. 2018, 233, 6866–6877. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Catalano, M.; Sciaccaluga, M.; Chece, G.; Cipriani, R.; Rosito, M.; Grimaldi, A.; Lauro, C.; Cantore, G.; Santoro, A.; et al. KCa3.1 channels are involved in the infiltrative behavior of glioblastoma in vivo. Cell Death Dis. 2013, 4, e773. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, G.; Limatola, C.; Catalano, M. Functional Roles of the Ca2+-activated K+ Channel, KCa3.1, in Brain Tumors. Curr. Neuropharmacol. 2018, 16, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Catacuzzeno, L.; Fioretti, B.; Franciolini, F. Expression and Role of the Intermediate-Conductance Calcium-Activated Potassium Channel KCa3.1 in Glioblastoma. J. Signal Transduct. 2012, 2012, 421564. [Google Scholar] [CrossRef] [PubMed]
- Catacuzzeno, L.; Aiello, F.; Fioretti, B.; Sforna, L.; Castigli, E.; Ruggieri, P.; Tata, A.M.; Calogero, A.; Franciolini, F. Serum-activated K and Cl currents underlay U87-MG glioblastoma cell migration. J. Cell Physiol. 2011, 226, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Sciaccaluga, M.; Fioretti, B.; Catacuzzeno, L.; Pagani, F.; Bertollini, C.; Rosito, M.; Catalano, M.; D’Alessandro, G.; Santoro, A.; Cantore, G.; et al. CXCL12-induced glioblastoma cell migration requires intermediate conductance Ca2+-activated K+ channel activity. Am. J. Physiol. Cell Physiol. 2010, 299, C175–C184. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.L.; Honasoge, A.; Robert, S.M.; McFerrin, M.M.; Sontheimer, H. A proinvasive role for the Ca(2+)-activated K(+) channel KCa3.1 in malignant glioma. Glia 2014, 62, 971–981. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Turner, K.L.; Seifert, S.; Sontheimer, H. Bradykinin-induced chemotaxis of human gliomas requires the activation of KCa3.1 and ClC-3. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 1427–1440. [Google Scholar] [CrossRef]
- Watkins, S.; Sontheimer, H. Hydrodynamic cellular volume changes enable glioma cell invasion. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 17250–17259. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Franciolini, F. Role of KCa3.1 Channels in Modulating Ca(2+) Oscillations during Glioblastoma Cell Migration and Invasion. Int. J. Mol. Sci. 2018, 19, 2970. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Caramia, M.; Sforna, L.; Belia, S.; Guglielmi, L.; D’Adamo, M.C.; Pessia, M.; Franciolini, F. Reconciling the discrepancies on the involvement of large-conductance Ca(2+)-activated K channels in glioblastoma cell migration. Front. Cell Neurosci. 2015, 9, 152. [Google Scholar] [CrossRef]
- Rosa, P.; Sforna, L.; Carlomagno, S.; Mangino, G.; Miscusi, M.; Pessia, M.; Franciolini, F.; Calogero, A.; Catacuzzeno, L. Overexpression of Large-Conductance Calcium-Activated Potassium Channels in Human Glioblastoma Stem-Like Cells and Their Role in Cell Migration. J. Cell Physiol. 2017, 232, 2478–2488. [Google Scholar] [CrossRef]
- Ruggieri, P.; Mangino, G.; Fioretti, B.; Catacuzzeno, L.; Puca, R.; Ponti, D.; Miscusi, M.; Franciolini, F.; Ragona, G.; Calogero, A. The inhibition of KCa3.1 channels activity reduces cell motility in glioblastoma derived cancer stem cells. PloS ONE 2012, 7, e47825. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, L.; Sezgin, E.C.; Skardelly, M.; Eckert, F.; Huber, S.M. KCa3.1 Channels and Glioblastoma: In Vitro Studies. Curr. Neuropharmacol. 2018, 16, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Nechyporuk-Zloy, V.; Fabian, A.; Stock, C. Cells move when ions and water flow. Pflugers Arch. 2007, 453, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels and the hallmarks of cancer. Trends Mol. Med. 2010, 16, 107–121. [Google Scholar] [CrossRef]
- Schwab, A.; Fabian, A.; Hanley, P.J.; Stock, C. Role of ion channels and transporters in cell migration. Physiol. Rev. 2012, 92, 1865–1913. [Google Scholar] [CrossRef]
- Robles-Martinez, L.; Garay, E.; Martel-Gallegos, M.G.; Cisneros-Mejorado, A.; Perez-Montiel, D.; Lara, A.; Arellano, R.O. Kca3.1 Activation Via P2y2 Purinergic Receptors Promotes Human Ovarian Cancer Cell (Skov-3) Migration. Sci. Rep. 2017, 7, 4340. [Google Scholar] [CrossRef]
- Schwab, A.; Reinhardt, J.; Schneider, S.W.; Gassner, B.; Schuricht, B. K(+) channel-dependent migration of fibroblasts and human melanoma cells. Cell. Physiol. Biochem. 1999, 9, 126–132. [Google Scholar] [CrossRef]
- Qiao, A.; Gu, F.; Guo, X.; Zhang, X.; Fu, L. Breast cancer-associated fibroblasts: Their roles in tumor initiation, progression and clinical applications. Front. Med. 2016, 10, 33–40. [Google Scholar] [CrossRef]
- Pena, T.L.; Chen, S.H.; Konieczny, S.F.; Rane, S.G. Ras/MEK/ERK Upregulation of the fibroblast KCa channel FIK is a common mechanism for basic fibroblast growth factor and transforming growth factor-beta suppression of myogenesis. J. Biol. Chem. 2000, 275, 13677–13682. [Google Scholar] [CrossRef]
- Wang, L.P.; Wang, Y.; Zhao, L.M.; Li, G.R.; Deng, X.L. Angiotensin II upregulates K(Ca)3.1 channels and stimulates cell proliferation in rat cardiac fibroblasts. Biochem. Pharmacol. 2013, 85, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.M.; Zhang, W.; Wang, L.P.; Li, G.R.; Deng, X.L. Advanced glycation end products promote proliferation of cardiac fibroblasts by upregulation of KCa3.1 channels. Pflugers Arch. 2012, 464, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Friebel, K.; Schonherr, R.; Kinne, R.W.; Kunisch, E. Functional role of the KCa3.1 potassium channel in synovial fibroblasts from rheumatoid arthritis patients. J. Cell. Physiol. 2015, 230, 1677–1688. [Google Scholar] [CrossRef] [PubMed]
- Viallard, C.; Larrivee, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Bachelot, T.; Hudis, C.A.; Curigliano, G.; Reynolds, A.R.; Petrioli, R.; Generali, D. The role of bevacizumab in solid tumors: A literature based meta-analysis of randomised trials. Eur. J. Cancer 2017, 75, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xia, J.; Yang, X.; Huang, X.; Gao, D.; Zhou, J.; Lian, J.; Zhou, J. Intermediate-conductance Ca(2+)-activated potassium and volume-sensitive chloride channels in endothelial progenitor cells from rat bone marrow mononuclear cells. Acta Physiol. 2012, 205, 302–313. [Google Scholar] [CrossRef]
- Cheong, A.; Bingham, A.J.; Li, J.; Kumar, B.; Sukumar, P.; Munsch, C.; Buckley, N.J.; Neylon, C.B.; Porter, K.E.; Beech, D.J.; et al. Downregulated REST transcription factor is a switch enabling critical potassium channel expression and cell proliferation. Mol. Cell 2005, 20, 45–52. [Google Scholar] [CrossRef]
- Kohler, R.; Degenhardt, C.; Kuhn, M.; Runkel, N.; Paul, M.; Hoyer, J. Expression and function of endothelial Ca(2+)-activated K(+) channels in human mesenteric artery: A single-cell reverse transcriptase-polymerase chain reaction and electrophysiological study in situ. Circ. Res. 2000, 87, 496–503. [Google Scholar] [CrossRef]
- Yang, H.; Li, X.; Ma, J.; Lv, X.; Zhao, S.; Lang, W.; Zhang, Y. Blockade of the intermediate-conductance Ca(2+)-activated K+ channel inhibits the angiogenesis induced by epidermal growth factor in the treatment of corneal alkali burn. Exp. Eye Res. 2013, 110, 76–87. [Google Scholar] [CrossRef]
- Bi, D.; Toyama, K.; Lemaitre, V.; Takai, J.; Fan, F.; Jenkins, D.P.; Wulff, H.; Gutterman, D.D.; Park, F.; Miura, H. The intermediate conductance calcium-activated potassium channel KCa3.1 regulates vascular smooth muscle cell proliferation via controlling calcium-dependent signaling. J. Biol. Chem. 2013, 288, 15843–15853. [Google Scholar] [CrossRef] [PubMed]
- Ghanshani, S.; Wulff, H.; Miller, M.J.; Rohm, H.; Neben, A.; Gutman, G.A.; Cahalan, M.D.; Chandy, K.G. Upregulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J. Biol. Chem. 2000, 275, 37137–37149. [Google Scholar] [CrossRef]
- Khanna, R.; Chang, M.C.; Joiner, W.J.; Kaczmarek, L.K.; Schlichter, L.C. hSK4/hIK1, a calmodulin-binding KCa channel in human T lymphocytes. Roles in proliferation and volume regulation. J. Biol. Chem. 1999, 274, 14838–14849. [Google Scholar] [CrossRef] [PubMed]
- Wulff, H.; Knaus, H.G.; Pennington, M.; Chandy, K.G. K+ channel expression during B cell differentiation: Implications for immunomodulation and autoimmunity. J Immunol 2004, 173, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, S.A.; Neumeier, L.; Peng, Y.; Devor, D.C.; Conforti, L. The Ca(2+)-activated K(+) channel KCa3.1 compartmentalizes in the immunological synapse of human T lymphocytes. Am. J. Physiol. Cell Physiol. 2007, 292, C1431–C1439. [Google Scholar] [CrossRef] [PubMed]
- Wulff, H.; Calabresi, P.A.; Allie, R.; Yun, S.; Pennington, M.; Beeton, C.; Chandy, K.G. The voltage-gated Kv1.3 K(+) channel in effector memory T cells as new target for MS. J. Clin. Investig. 2003, 111, 1703–1713. [Google Scholar] [CrossRef]
- Varga, Z.; Csepany, T.; Papp, F.; Fabian, A.; Gogolak, P.; Toth, A.; Panyi, G. Potassium channel expression in human CD4+ regulatory and naive T cells from healthy subjects and multiple sclerosis patients. Immunol. Lett. 2009, 124, 95–101. [Google Scholar] [CrossRef]
- Di, L.; Srivastava, S.; Zhdanova, O.; Ding, Y.; Li, Z.; Wulff, H.; Lafaille, M.; Skolnik, E.Y. Inhibition of the K+ channel KCa3.1 ameliorates T cell-mediated colitis. Proc. Natl. Acad. Sci. USA 2010, 107, 1541–1546. [Google Scholar] [CrossRef]
- Kuras, Z.; Yun, Y.H.; Chimote, A.A.; Neumeier, L.; Conforti, L. KCa3.1 and TRPM7 channels at the uropod regulate migration of activated human T cells. PLoS ONE 2012, 7, e43859. [Google Scholar] [CrossRef]
- Kim, U.; Siegel, R.; Ren, X.; Gunther, C.S.; Gaasterland, T.; Roeder, R.G. Identification of transcription coactivator OCA-B-dependent genes involved in antigen-dependent B cell differentiation by cDNA array analyses. Proc. Natl. Acad. Sci. USA 2003, 100, 8868–8873. [Google Scholar] [CrossRef]
- Rodriguez-Cortez, V.C.; Del Pino-Molina, L.; Rodriguez-Ubreva, J.; Ciudad, L.; Gomez-Cabrero, D.; Company, C.; Urquiza, J.M.; Tegner, J.; Rodriguez-Gallego, C.; Lopez-Granados, E.; et al. Monozygotic twins discordant for common variable immunodeficiency reveal impaired DNA demethylation during naive-to-memory B-cell transition. Nat. Commun. 2015, 6, 7335. [Google Scholar] [CrossRef] [PubMed]
- Grossinger, E.M.; Weiss, L.; Zierler, S.; Rebhandl, S.; Krenn, P.W.; Hinterseer, E.; Schmolzer, J.; Asslaber, D.; Hainzl, S.; Neureiter, D.; et al. Targeting proliferation of chronic lymphocytic leukemia (CLL) cells through KCa3.1 blockade. Leukemia 2014, 28, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Makinde, T.O.; Agrawal, D.K. Calcium-activated potassium channel KCa3.1 in lung dendritic cell migration. Am. J. Respir. Cell Mol. Biol. 2011, 45, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Crottes, D.; Felix, R.; Meley, D.; Chadet, S.; Herr, F.; Audiger, C.; Soriani, O.; Vandier, C.; Roger, S.; Angoulvant, D.; et al. Immature human dendritic cells enhance their migration through KCa3.1 channel activation. Cell Calcium 2016, 59, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Hanley, P.J.; Musset, B.; Renigunta, V.; Limberg, S.H.; Dalpke, A.H.; Sus, R.; Heeg, K.M.; Preisig-Muller, R.; Daut, J. Extracellular ATP induces oscillations of intracellular Ca2+ and membrane potential and promotes transcription of IL-6 in macrophages. Proc. Natl. Acad. Sci. USA 2004, 101, 9479–9484. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lai, W.; Zhang, Y.; Liu, L.; Luo, X.; Zeng, Y.; Wu, H.; Lan, Q.; Chu, Z. Tumor-associated macrophage-derived IL-6 and IL-8 enhance invasive activity of LoVo cells induced by PRL-3 in a KCNN4 channel-dependent manner. BMC Cancer 2014, 14, 330. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, V.; Koeberle, P.D.; Wang, Y.; Schlichter, L.C. The Ca2+-activated K+ channel KCNN4/KCa3.1 contributes to microglia activation and nitric oxide-dependent neurodegeneration. J. Neurosci. 2007, 27, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Lively, S.; Schlichter, L.C. IL-4 type 1 receptor signaling upregulates KCNN4 expression, and increases the KCa3.1 current and its contribution to migration of alternative-activated microglia. Front. Cell. Neurosci. 2014, 8, 183. [Google Scholar] [CrossRef]
- Komohara, Y.; Ohnishi, K.; Kuratsu, J.; Takeya, M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J. Pathol. 2008, 216, 15–24. [Google Scholar] [CrossRef]
- Gabrusiewicz, K.; Ellert-Miklaszewska, A.; Lipko, M.; Sielska, M.; Frankowska, M.; Kaminska, B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS ONE 2011, 6, e23902. [Google Scholar] [CrossRef]
- Grimaldi, A.; D’Alessandro, G.; Golia, M.T.; Grossinger, E.M.; Di Angelantonio, S.; Ragozzino, D.; Santoro, A.; Esposito, V.; Wulff, H.; Catalano, M.; et al. KCa3.1 inhibition switches the phenotype of glioma-infiltrating microglia/macrophages. Cell Death Dis. 2016, 7, e2174. [Google Scholar] [CrossRef] [PubMed]
- Koshy, S.; Wu, D.; Hu, X.; Tajhya, R.B.; Huq, R.; Khan, F.S.; Pennington, M.W.; Wulff, H.; Yotnda, P.; Beeton, C. Blocking KCa3.1 channels increases tumor cell killing by a subpopulation of human natural killer lymphocytes. PLoS ONE 2013, 8, e76740. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, C.; Riquelme, T.T.; Vera, D.; Julio-Kalajzic, F.; Ehrenfeld, P.; Melvin, J.E.; Figueroa, C.D.; Sarmiento, J.; Flores, C.A. The calcium-activated potassium channel KCa3.1 plays a central role in the chemotactic response of mammalian neutrophils. Acta Physiol. 2016, 216, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Mark Duffy, S.; Berger, P.; Cruse, G.; Yang, W.; Bolton, S.J.; Bradding, P. The K+ channel iKCA1 potentiates Ca2+ influx and degranulation in human lung mast cells. J. Allergy Clin. Immunol. 2004, 114, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Cruse, G.; Duffy, S.M.; Brightling, C.E.; Bradding, P. Functional KCa3.1 K+ channels are required for human lung mast cell migration. Thorax 2006, 61, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Wulff, H.; Miller, M.J.; Hansel, W.; Grissmer, S.; Cahalan, M.D.; Chandy, K.G. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: A potential immunosuppressant. Proc. Natl. Acad. Sci. USA 2000, 97, 8151–8156. [Google Scholar] [CrossRef]
- McNaughton-Smith, G.A.; Burns, J.F.; Stocker, J.W.; Rigdon, G.C.; Creech, C.; Arrington, S.; Shelton, T.; de Franceschi, L. Novel inhibitors of the Gardos channel for the treatment of sickle cell disease. J. Med. Chem. 2008, 51, 976–982. [Google Scholar] [CrossRef]
- Ishii, T.M.; Silvia, C.; Hirschberg, B.; Bond, C.T.; Adelman, J.P.; Maylie, J. A human intermediate conductance calcium-activated potassium channel. Proc. Natl. Acad. Sci. USA 1997, 94, 11651–11656. [Google Scholar] [CrossRef]
- Benzaquen, L.R.; Brugnara, C.; Byers, H.R.; Gatton-Celli, S.; Halperin, J.A. Clotrimazole inhibits cell proliferation in vitro and in vivo. Nat. Med. 1995, 1, 534–540. [Google Scholar] [CrossRef]
- Khalid, M.H.; Shibata, S.; Hiura, T. Effects of clotrimazole on the growth, morphological characteristics, and cisplatin sensitivity of human glioblastoma cells in vitro. J. Neurosurg. 1999, 90, 918–927. [Google Scholar] [CrossRef]
- Jensen, B.S.; Odum, N.; Jorgensen, N.K.; Christophersen, P.; Olesen, S.P. Inhibition of T cell proliferation by selective block of Ca(2+)-activated K(+) channels. Proc. Natl. Acad. Sci. USA 1999, 96, 10917–10921. [Google Scholar] [CrossRef] [PubMed]
- Toyama, K.; Wulff, H.; Chandy, K.G.; Azam, P.; Raman, G.; Saito, T.; Fujiwara, Y.; Mattson, D.L.; Das, S.; Melvin, J.E.; et al. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J. Clin. Investig. 2008, 118, 3025–3037. [Google Scholar] [CrossRef] [PubMed]
- Bonito, B.; Sauter, D.R.; Schwab, A.; Djamgoz, M.B.; Novak, I. KCa3.1 (IK) modulates pancreatic cancer cell migration, invasion and proliferation: Anomalous effects on TRAM-34. Pflugers Arch. 2016, 468, 1865–1875. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Du, Y.; Song, W.; Chen, H.; Xuan, Z.; Zhao, L.; Chen, J.; Chen, J.; Guo, D.; Jin, C.; et al. KCa3.1 as an Effective Target for Inhibition of Growth and Progression of Intrahepatic Cholangiocarcinoma. J. Cancer 2017, 8, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Ataga, K.I.; Orringer, E.P.; Styles, L.; Vichinsky, E.P.; Swerdlow, P.; Davis, G.A.; Desimone, P.A.; Stocker, J.W. Dose-escalation study of ICA-17043 in patients with sickle cell disease. Pharmacotherapy 2006, 26, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Stocker, J.W.; De Franceschi, L.; McNaughton-Smith, G.A.; Corrocher, R.; Beuzard, Y.; Brugnara, C. ICA-17043, a novel Gardos channel blocker, prevents sickled red blood cell dehydration in vitro and in vivo in SAD mice. Blood 2003, 101, 2412–2418. [Google Scholar] [CrossRef]
- Chen, Y.J.; Raman, G.; Bodendiek, S.; O’Donnell, M.E.; Wulff, H. The KCa3.1 blocker TRAM-34 reduces infarction and neurological deficit in a rat model of ischemia/reperfusion stroke. J. Cereb. Blood Flow Metab. 2011, 31, 2363–2374. [Google Scholar] [CrossRef]
- Ataga, K.I.; Smith, W.R.; De Castro, L.M.; Swerdlow, P.; Saunthararajah, Y.; Castro, O.; Vichinsky, E.; Kutlar, A.; Orringer, E.P.; Rigdon, G.C.; et al. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood 2008, 111, 3991–3997. [Google Scholar] [CrossRef]
- Nagalla, S.; Ballas, S.K. Drugs for preventing red blood cell dehydration in people with sickle cell disease. Cochrane Database Syst. Rev. 2018, 10, CD003426. [Google Scholar] [CrossRef]
- Ataga, K.I.; Reid, M.; Ballas, S.K.; Yasin, Z.; Bigelow, C.; James, L.S.; Smith, W.R.; Galacteros, F.; Kutlar, A.; Hull, J.H.; et al. Improvements in hemolysis and indicators of erythrocyte survival do not correlate with acute vaso-occlusive crises in patients with sickle cell disease: A phase III randomized, placebo-controlled, double-blind study of the Gardos channel blocker senicapoc (ICA-17043). Br. J. Hematol. 2011, 153, 92–104. [Google Scholar] [CrossRef]
- Devor, D.C.; Singh, A.K.; Frizzell, R.A.; Bridges, R.J. Modulation of Cl- secretion by benzimidazolones. I. Direct activation of a Ca(2+)-dependent K+ channel. Am. J. Physiol. 1996, 271, L775–L784. [Google Scholar] [CrossRef] [PubMed]
- Coleman, N.; Brown, B.M.; Olivan-Viguera, A.; Singh, V.; Olmstead, M.M.; Valero, M.S.; Kohler, R.; Wulff, H. New positive Ca2+-activated K+ channel gating modulators with selectivity for KCa3.1. Mol. Pharmacol. 2014, 86, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.Z.; Park, S.W.; Kang, T.W.; Kim, K.S.; Yoo, H.Y.; Lee, J.; Hah, J.H.; Sung, M.H.; Kim, S.J. Activation of K(+) channel by 1-EBIO rescues the head and neck squamous cell carcinoma cells from Ca(2+) ionophore-induced cell death. Korean J. Physiol. Pharmacol. 2016, 20, 25–33. [Google Scholar] [CrossRef]
- King, B.; Rizwan, A.P.; Asmara, H.; Heath, N.C.; Engbers, J.D.; Dykstra, S.; Bartoletti, T.M.; Hameed, S.; Zamponi, G.W.; Turner, R.W. IKCa channels are a critical determinant of the slow AHP in CA1 pyramidal neurons. Cell Rep. 2015, 11, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, A.; Raman, G.; Busch, C.; Schultz, T.; Zimin, P.I.; Hoyer, J.; Kohler, R.; Wulff, H. Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol. Pharmacol. 2009, 75, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Morimura, K.; Yamamura, H.; Ohya, S.; Imaizumi, Y. Voltage-dependent Ca2+-channel block by openers of intermediate and small conductance Ca2+-activated K+ channels in urinary bladder smooth muscle cells. J. Pharmacol. Sci. 2006, 100, 237–241. [Google Scholar] [CrossRef]
- Schroder, R.L.; Jensen, B.S.; Strobaek, D.; Olesen, S.P.; Christophersen, P. Activation of the human, intermediate-conductance, Ca2+-activated K+ channel by methylxanthines. Pflugers Arch. 2000, 440, 809–818. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, L.; Ma, W.; Cao, X.; Chen, H.; Feng, D.; Liang, J.; Yin, K.; Jiang, X. The Blockage of KCa3.1 Channel Inhibited Proliferation, Migration and Promoted Apoptosis of Human Hepatocellular Carcinoma Cells. J. Cancer 2015, 6, 643–651. [Google Scholar] [CrossRef]
- Brown, B.M.; Pressley, B.; Wulff, H. KCa3.1 Channel Modulators as Potential Therapeutic Compounds for Glioblastoma. Curr. Neuropharmacol. 2018, 16, 618–626. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).