Cerium Oxide Nanoparticles Sensitize Pancreatic Cancer to Radiation Therapy through Oxidative Activation of the JNK Apoptotic Pathway
Abstract
1. Introduction
2. Results
2.1. CONPs Do Not Impact RT-Induced DNA Damage in Pancreatic Cancer Cells but Inhibit It in Normal Pancreatic Epithelial Cells
2.2. CONPs Drive RT-Induced JNK Activation in Cancer Cells
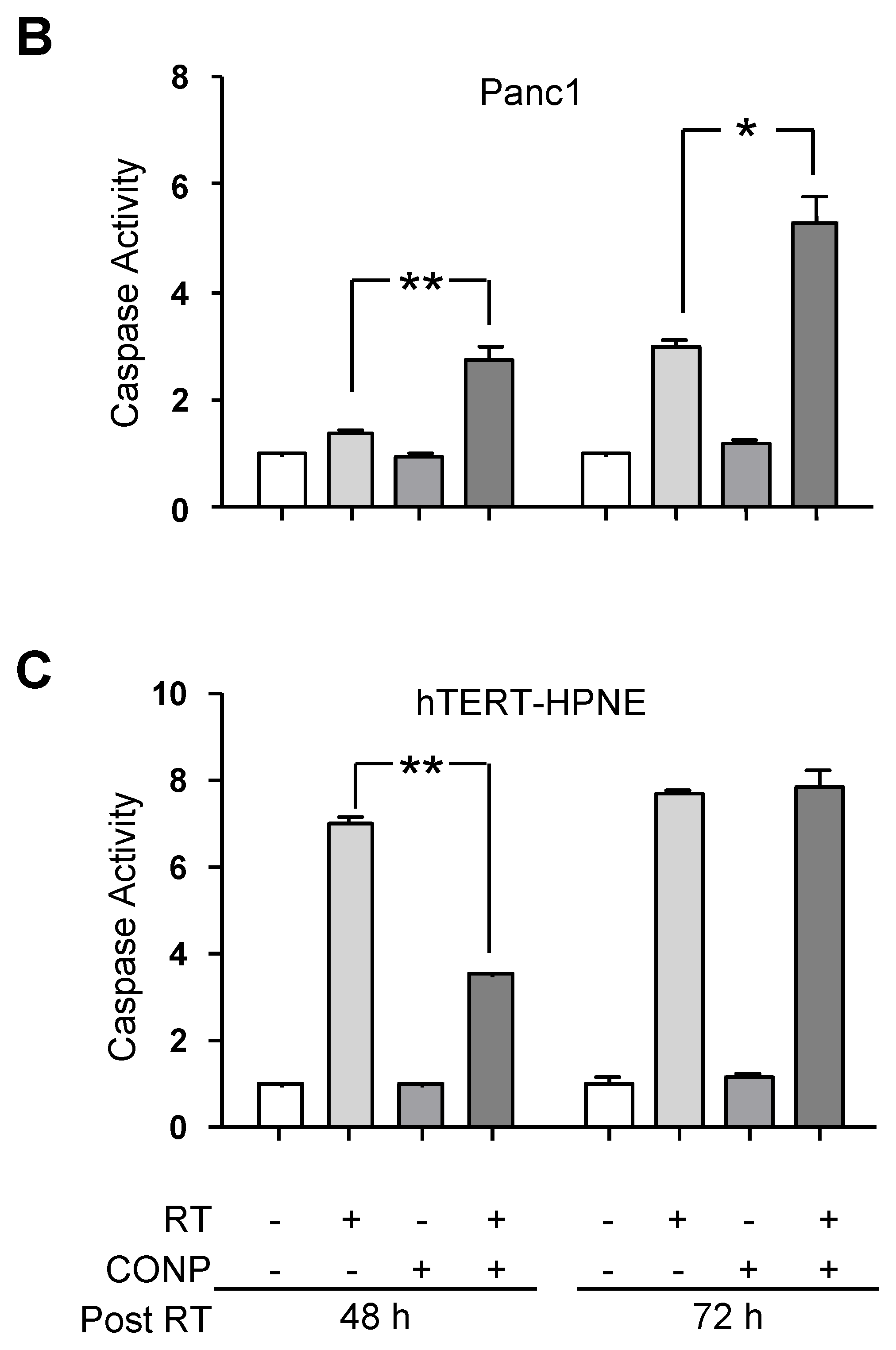
2.3. CONP-Driven JNK Activation Mediates RT-Induced Apoptosis in Pancreatic Cancer Cells
2.4. CONPs Drive RT-Induced ASK1 Activation in Pancreatic Cancer Cells
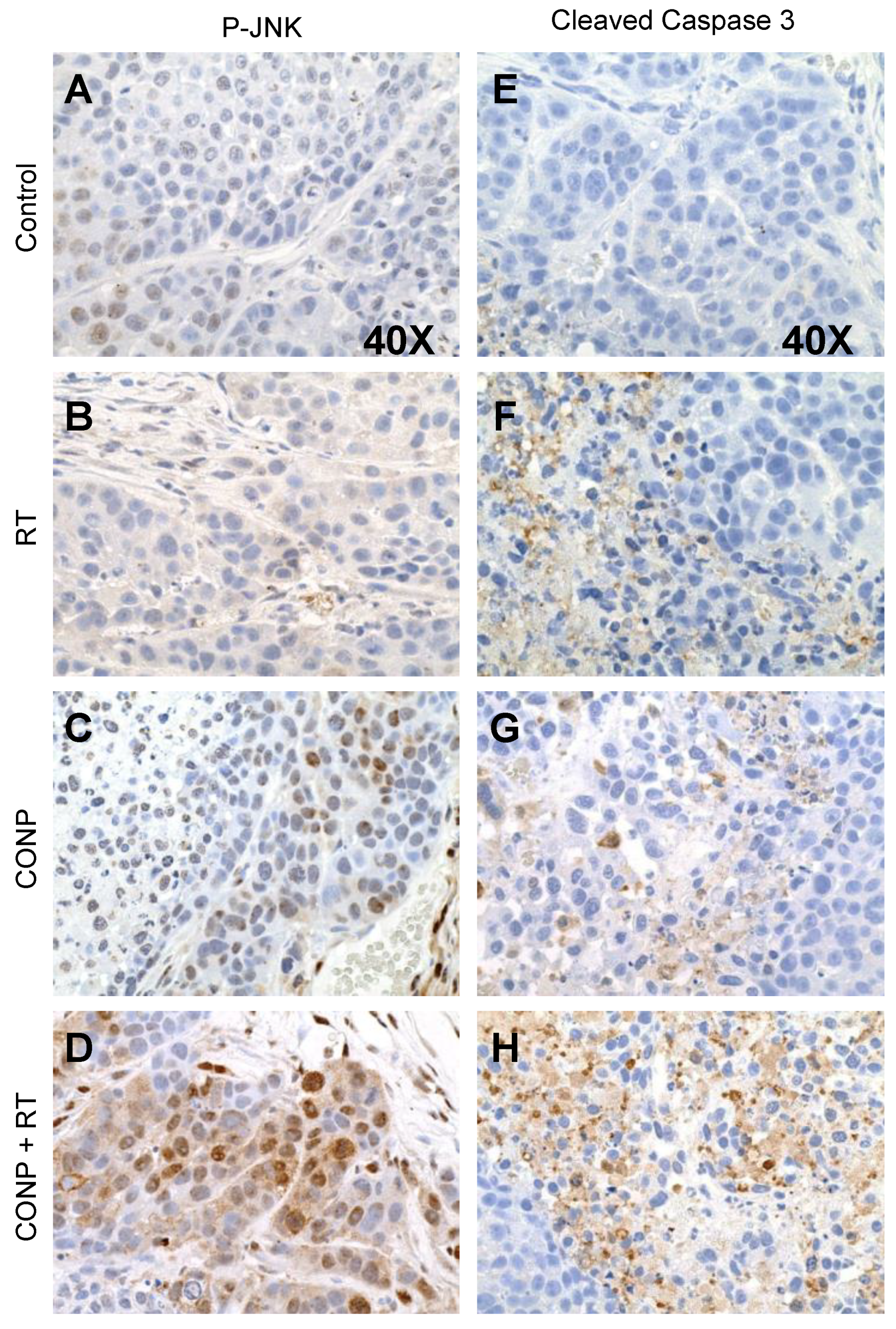
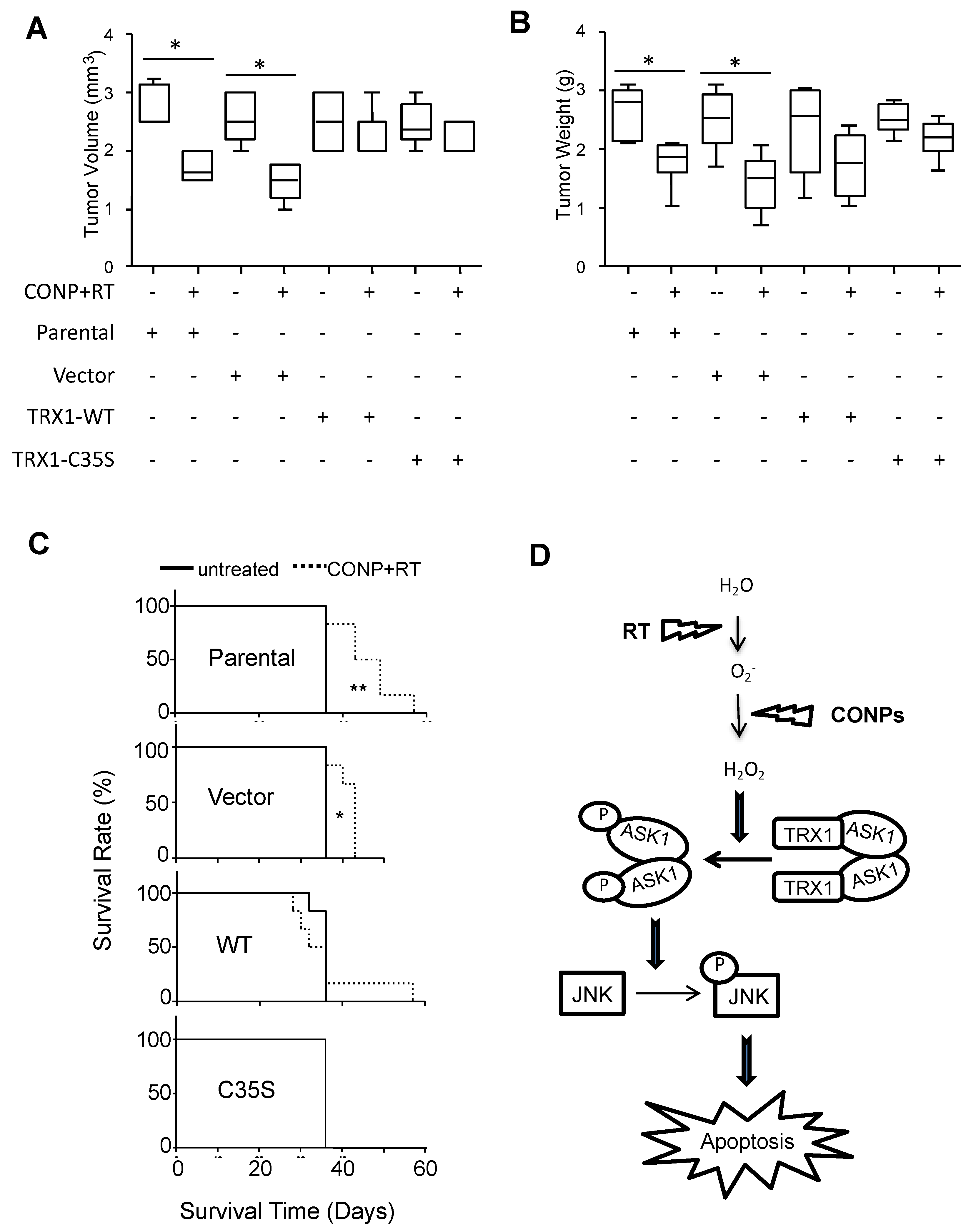
2.5. CONPs Increase JNK Activation and Apoptotic Signaling In Vivo
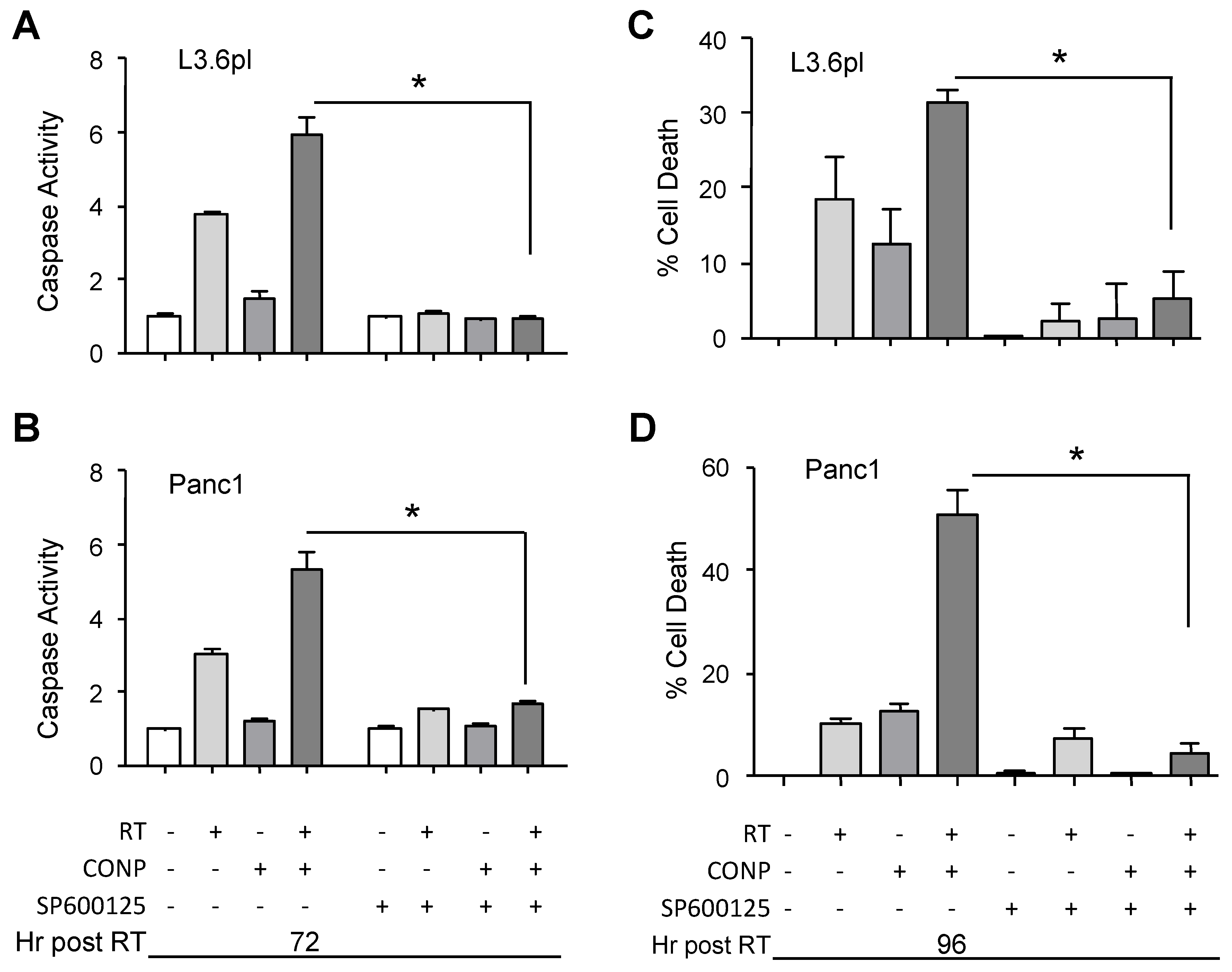
2.6. CONPs Are Unable to Induce Radiosensitization of Pancreatic Cancer Cells in the Absence of JNK Activity
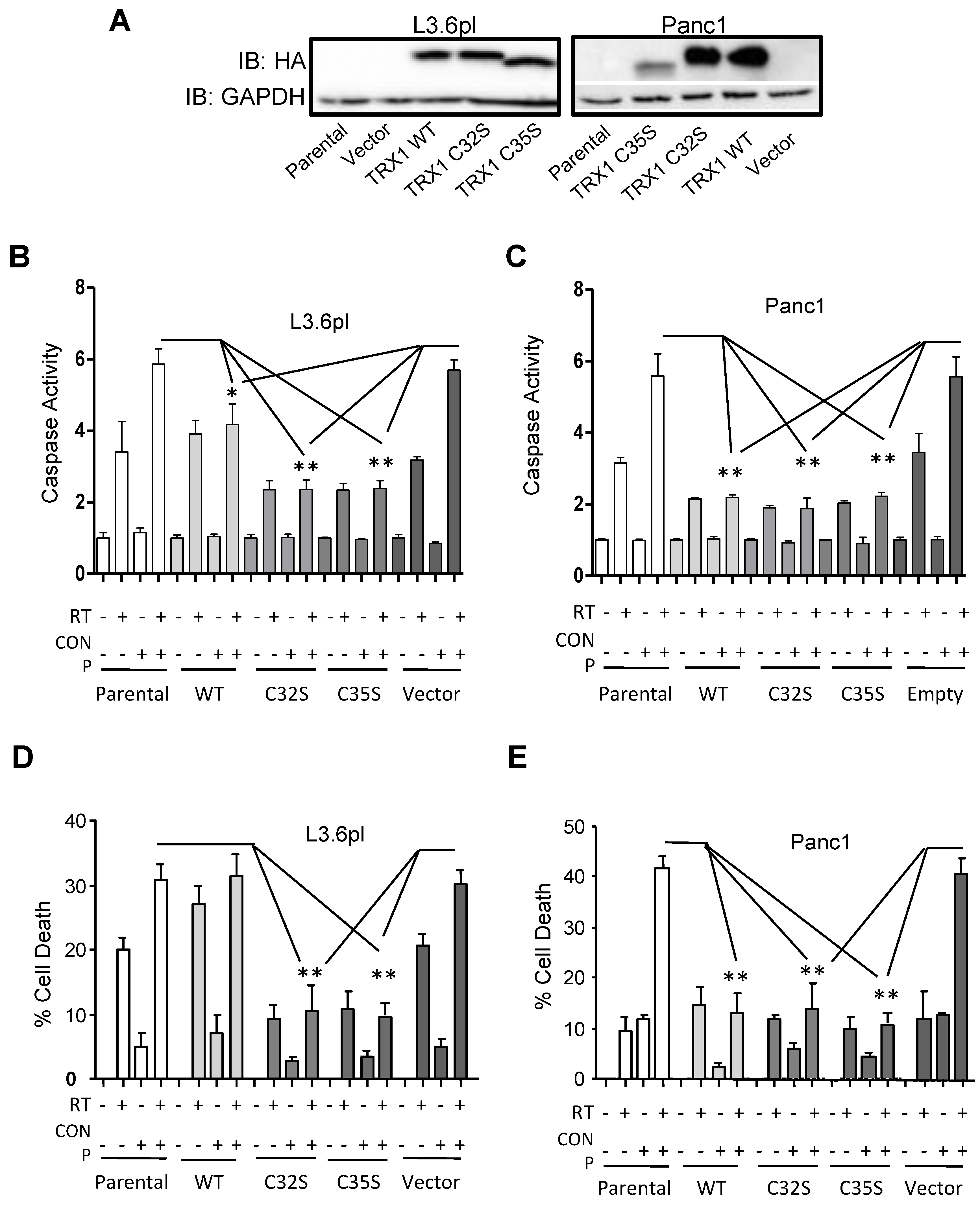
2.7. CONPs Fail to Induced Radiosensitization of Pancreatic Cancer Cells in the Absence of TRX1 Oxidation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Generation of Stable Cell Lines
4.3. Comet Assay
4.4. Phospho-ELISA
4.5. Antibodies, Drugs, and Western Blot
4.6. P-ASK1 Immunofluorescent Staining
4.7. Analysis of Caspase Activation and Cell Viability
4.8. JNK Inhibitor Studies
4.9. Orthotopic Xenograft of Human Pancreatic Cancer Cells in Athymic Mice
4.10. Therapeutic Approach for Human Pancreatic Tumors Bearing Athymic Mice
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.; DeSantis, C.; Virgo, K.; Stein, K.; Mariotto, A.; Smith, T.; Cooper, D.; Gansler, T.; Lerro, C.; Fedewa, S.; et al. Cancer treatment and survivorship statistics, 2012. CA-Cancer J. Clin. 2012, 62, 220–241. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA-Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xie, K.; Wolff, R.; Abbruzzese, J.L. Pancreatic cancer. Lancet 2004, 363, 1049–1057. [Google Scholar] [CrossRef]
- Eriksson, D.; Stigbrand, T. Radiation-induced cell death mechanisms. Tumour Biol. 2010, 31, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.M. Induction of cell death by radiotherapy. Endocr. Relat. Cancer 1999, 6, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Castedo, M.; Perfettini, J.L.; Roumier, T.; Andreau, K.; Medema, R.; Kroemer, G. Cell death by mitotic catastrophe: A molecular definition. Oncogene 2004, 23, 2825–2837. [Google Scholar] [CrossRef] [PubMed]
- Bold, R.J.; Termuhlen, P.M.; McConkey, D.J. Apoptosis, cancer and cancer therapy. Surg. Oncol. 1997, 6, 133–142. [Google Scholar] [CrossRef]
- Madero-Visbal, R.A.; Alvarado, B.E.; Colon, J.F.; Baker, C.H.; Wason, M.S.; Isley, B.; Seal, S.; Lee, C.M.; Das, S.; Manon, R. Harnessing nanoparticles to improve toxicity after head and neck radiation. Nanomedicine 2012, 8, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Colon, J.; Hsieh, N.; Ferguson, A.; Kupelian, P.; Seal, S.; Jenkins, D.W.; Baker, C.H. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine 2010, 6, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Colon, J.; Herrera, L.; Smith, J.; Patil, S.; Komanski, C.; Kupelian, P.; Seal, S.; Jenkins, D.W.; Baker, C.H. Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomedicine 2009, 5, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Tarnuzzer, R.W.; Colon, J.; Patil, S.; Seal, S. Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett. 2005, 5, 2573–2577. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, A.; Karakoti, A.; Seal, S.; Self, W.T. Unveiling the mechanism of uptake and sub-cellular distribution of cerium oxide nanoparticles. Mol. BioSyst. 2010, 6, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Guo, W.; Han, L.; Chen, E.; Kong, L.; Wang, L.; Ai, W.; Song, N.; Li, H.; Chen, H. Cerium oxide nanoparticles induce cytotoxicity in human hepatoma smmc-7721 cells via oxidative stress and the activation of mapk signaling pathways. Toxicol. In Vitro 2013, 27, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Wason, M.S.; Colon, J.; Das, S.; Seal, S.; Turkson, J.; Zhao, J.; Baker, C.H. Sensitization of pancreatic cancer cells to radiation by cerium oxide nanoparticle-induced ros production. Nanomedicine 2013, 9, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Alili, L.; Sack, M.; Karakoti, A.S.; Teuber, S.; Puschmann, K.; Hirst, S.M.; Reilly, C.M.; Zanger, K.; Stahl, W.; Das, S.; et al. Combined cytotoxic and anti-invasive properties of redox-active nanoparticles in tumor-stroma interactions. Biomaterials 2011, 32, 2918–2929. [Google Scholar] [CrossRef] [PubMed]
- Alili, L.; Sack, M.; von Montfort, C.; Giri, S.; Das, S.; Carroll, K.S.; Zanger, K.; Seal, S.; Brenneisen, P. Downregulation of tumor growth and invasion by redox-active nanoparticles. Antioxid. Redox Signal. 2013, 19, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Karakoti, A.; Graham, R.P.; Maguire, J.L.; Reilly, C.M.; Seal, S.; Rattan, R.; Shridhar, V. Nanoceria: A rare-earth nanoparticle as a novel anti-angiogenic therapeutic agent in ovarian cancer. PLoS ONE 2013, 8, e54578. [Google Scholar] [CrossRef] [PubMed]
- Wason, M.S.; Zhao, J. Cerium oxide nanoparticles: Potential applications for cancer and other diseases. Am. J. Transl. Res. 2013, 5, 126–131. [Google Scholar] [PubMed]
- Hayakawa, R.; Hayakawa, T.; Takeda, K.; Ichijo, H. Therapeutic targets in the ask1-dependent stress signaling pathways. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2012, 88, 434–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Min, W. Thioredoxin promotes ask1 ubiquitination and degradation to inhibit ask1-mediated apoptosis in a redox activity-independent manner. Circ. Res. 2002, 90, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, A.; Nishitoh, H.; Tobiume, K.; Takeda, K.; Ichijo, H. Physiological roles of ask1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: Advanced findings from ask1 knockout mice. Antioxid. Redox Signal. 2002, 4, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-kinase akt pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Tanno, S.; Tanno, S.; Mitsuuchi, Y.; Altomare, D.A.; Xiao, G.H.; Testa, J.R. Akt activation up-regulates insulin-like growth factor i receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 2001, 61, 589–593. [Google Scholar] [PubMed]
- Boucher, M.J.; Morisset, J.; Vachon, P.H.; Reed, J.C.; Laine, J.; Rivard, N. Mek/erk signaling pathway regulates the expression of bcl-2, bcl-x(l), and mcl-1 and promotes survival of human pancreatic cancer cells. J. Cell. Biochem. 2000, 79, 355–369. [Google Scholar] [CrossRef]
- Garcea, G.; Neal, C.P.; Pattenden, C.J.; Steward, W.P.; Berry, D.P. Molecular prognostic markers in pancreatic cancer: A systematic review. Eur. J. Cancer 2005, 41, 2213–2236. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.S.; West, K.; Streicher, S.; Dennis, P.A. Constitutive and inducible akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol. Cancer Ther. 2002, 1, 707–717. [Google Scholar] [PubMed]
- Wong, C.H.; Iskandar, K.B.; Yadav, S.K.; Hirpara, J.L.; Loh, T.; Pervaiz, S. Simultaneous induction of non-canonical autophagy and apoptosis in cancer cells by ros-dependent erk and jnk activation. PLoS ONE 2010, 5, e9996. [Google Scholar] [CrossRef] [PubMed]
- Benhar, M.; Engelberg, D.; Levitzki, A. Ros, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002, 3, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.; Tournier, C. Exploring the function of the jnk (c-jun n-terminal kinase) signalling pathway in physiological and pathological processes to design novel therapeutic strategies. Biochem. Soc. Trans. 2012, 40, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Kargiotis, O.; Geka, A.; Rao, J.S.; Kyritsis, A.P. Effects of irradiation on tumor cell survival, invasion and angiogenesis. J. Neurooncol. 2010, 100, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, Y.W.; Zhou, X.D.; Ma, Y. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int. J. Toxicol. 2006, 25, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bian, Z.C.; Yee, K.; Chen, B.P.; Chien, S.; Guan, J.L. Identification of transcription factor klf8 as a downstream target of focal adhesion kinase in its regulation of cyclin d1 and cell cycle progression. Mol. Cell 2003, 11, 1503–1515. [Google Scholar] [CrossRef]
- Zhao, J.H.; Reiske, H.; Guan, J.L. Regulation of the cell cycle by focal adhesion kinase. J. Cell Biol 1998, 143, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, H.; Urvalek, A.M.; Li, T.; Yu, L.; Lamar, J.; DiPersio, C.M.; Feustel, P.J.; Zhao, J. Klf8 promotes human breast cancer cell invasion and metastasis by transcriptional activation of mmp9. Oncogene 2011, 30, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Hu, L.; Li, T.; Lahiri, S.; Shen, C.; Wason, M.S.; Mukherjee, D.; Xie, H.; Yu, L.; Zhao, J. A novel role of kruppel-like factor 8 in DNA repair in breast cancer cells. J. Biol. Chem. 2012, 287, 43720–43729. [Google Scholar] [CrossRef] [PubMed]
- Karakoti, A.S.; Kuchibhatla, S.V.N.T.; Suresh Babu, K.; Seal, S. Direct synthesis of nanoceria in aqueous polyhydroxyl solutions. J. Phys. Chem. C 2007, 111, 17232–17240. [Google Scholar] [CrossRef]
- Li, T.; Lu, H.; Mukherjee, D.; Lahiri, S.K.; Shen, C.; Yu, L.; Zhao, J. Identification of epidermal growth factor receptor and its inhibitory microrna141 as novel targets of kruppel-like factor 8 in breast cancer. Oncotarget 2015, 6, 21428–21442. [Google Scholar] [PubMed]
- Bennett, B.L.; Sasaki, D.T.; Murray, B.W.; O’Leary, E.C.; Sakata, S.T.; Xu, W.; Leisten, J.C.; Motiwala, A.; Pierce, S.; Satoh, Y.; et al. Sp600125, an anthrapyrazolone inhibitor of jun n-terminal kinase. Proc. Natl. Acad. Sci. USA 2001, 98, 13681–13686. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.H.; Kedar, D.; McCarty, M.F.; Tsan, R.; Weber, K.L.; Bucana, C.D.; Fidler, I.J. Blockade of epidermal growth factor receptor signaling on tumor cells and tumor-associated endothelial cells for therapy of human carcinomas. Am. J. Pathol. 2002, 161, 929–938. [Google Scholar] [CrossRef]
- Baker, C.H.; Solorzano, C.C.; Fidler, I.J. Blockade of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling for therapy of metastatic human pancreatic cancer. Cancer Res. 2002, 62, 1996–2003. [Google Scholar] [PubMed]
- Konduri, S.; Colon, J.; Baker, C.H.; Safe, S.; Abbruzzese, J.L.; Abudayyeh, A.; Basha, M.R.; Abdelrahim, M. Tolfenamic acid enhances pancreatic cancer cell and tumor response to radiation therapy by inhibiting survivin protein expression. Mol. Cancer Ther. 2009, 8, 533–542. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wason, M.S.; Lu, H.; Yu, L.; Lahiri, S.K.; Mukherjee, D.; Shen, C.; Das, S.; Seal, S.; Zhao, J. Cerium Oxide Nanoparticles Sensitize Pancreatic Cancer to Radiation Therapy through Oxidative Activation of the JNK Apoptotic Pathway. Cancers 2018, 10, 303. https://doi.org/10.3390/cancers10090303
Wason MS, Lu H, Yu L, Lahiri SK, Mukherjee D, Shen C, Das S, Seal S, Zhao J. Cerium Oxide Nanoparticles Sensitize Pancreatic Cancer to Radiation Therapy through Oxidative Activation of the JNK Apoptotic Pathway. Cancers. 2018; 10(9):303. https://doi.org/10.3390/cancers10090303
Chicago/Turabian StyleWason, Melissa S., Heng Lu, Lin Yu, Satadru K. Lahiri, Debarati Mukherjee, Chao Shen, Soumen Das, Sudipta Seal, and Jihe Zhao. 2018. "Cerium Oxide Nanoparticles Sensitize Pancreatic Cancer to Radiation Therapy through Oxidative Activation of the JNK Apoptotic Pathway" Cancers 10, no. 9: 303. https://doi.org/10.3390/cancers10090303
APA StyleWason, M. S., Lu, H., Yu, L., Lahiri, S. K., Mukherjee, D., Shen, C., Das, S., Seal, S., & Zhao, J. (2018). Cerium Oxide Nanoparticles Sensitize Pancreatic Cancer to Radiation Therapy through Oxidative Activation of the JNK Apoptotic Pathway. Cancers, 10(9), 303. https://doi.org/10.3390/cancers10090303



